Catalase Like-Activity of Metal NPs–Enzyme Biohybrids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Techniques Used
2.3. Synthesis of CuNPs@CALB Bionanohybrids
2.4. Synthesis of MnNPs@CALB Bionanohybrids
2.4.1. Method 1
2.4.2. Method 2
2.5. Synthesis of MeNPs@CATb Bionanohybrids
2.6. Catalase-like Activity of Metal Bionanohybrids
3. Results
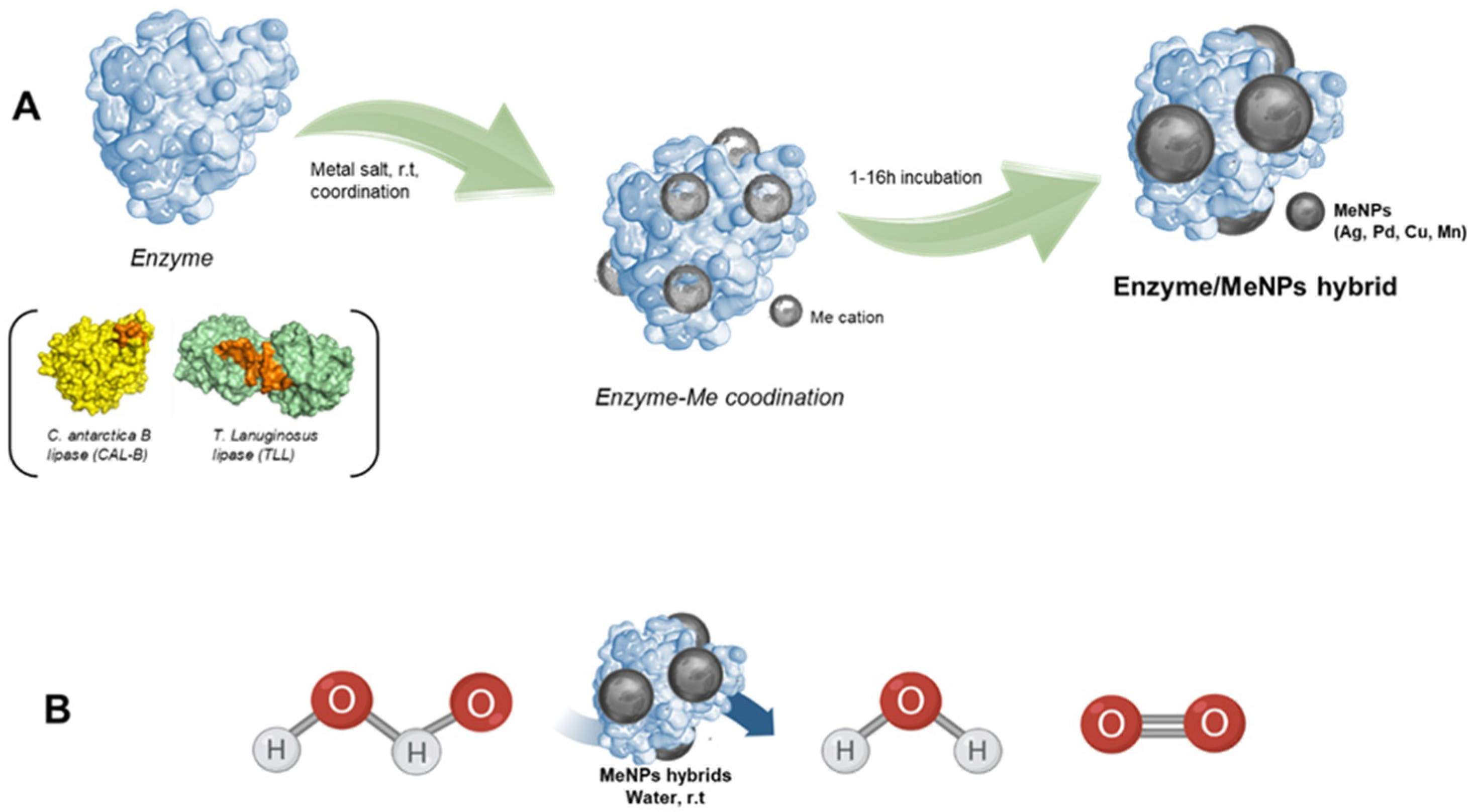
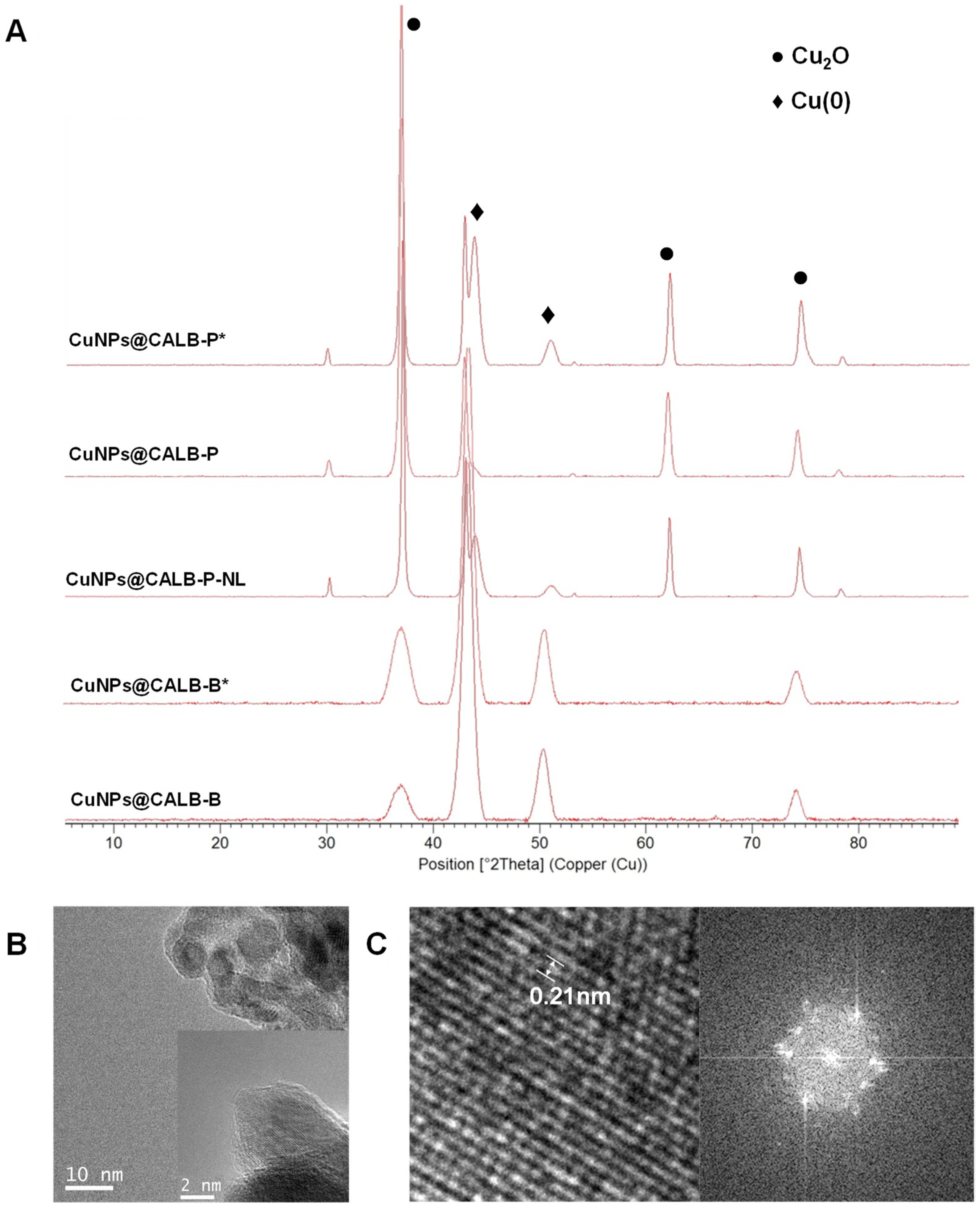
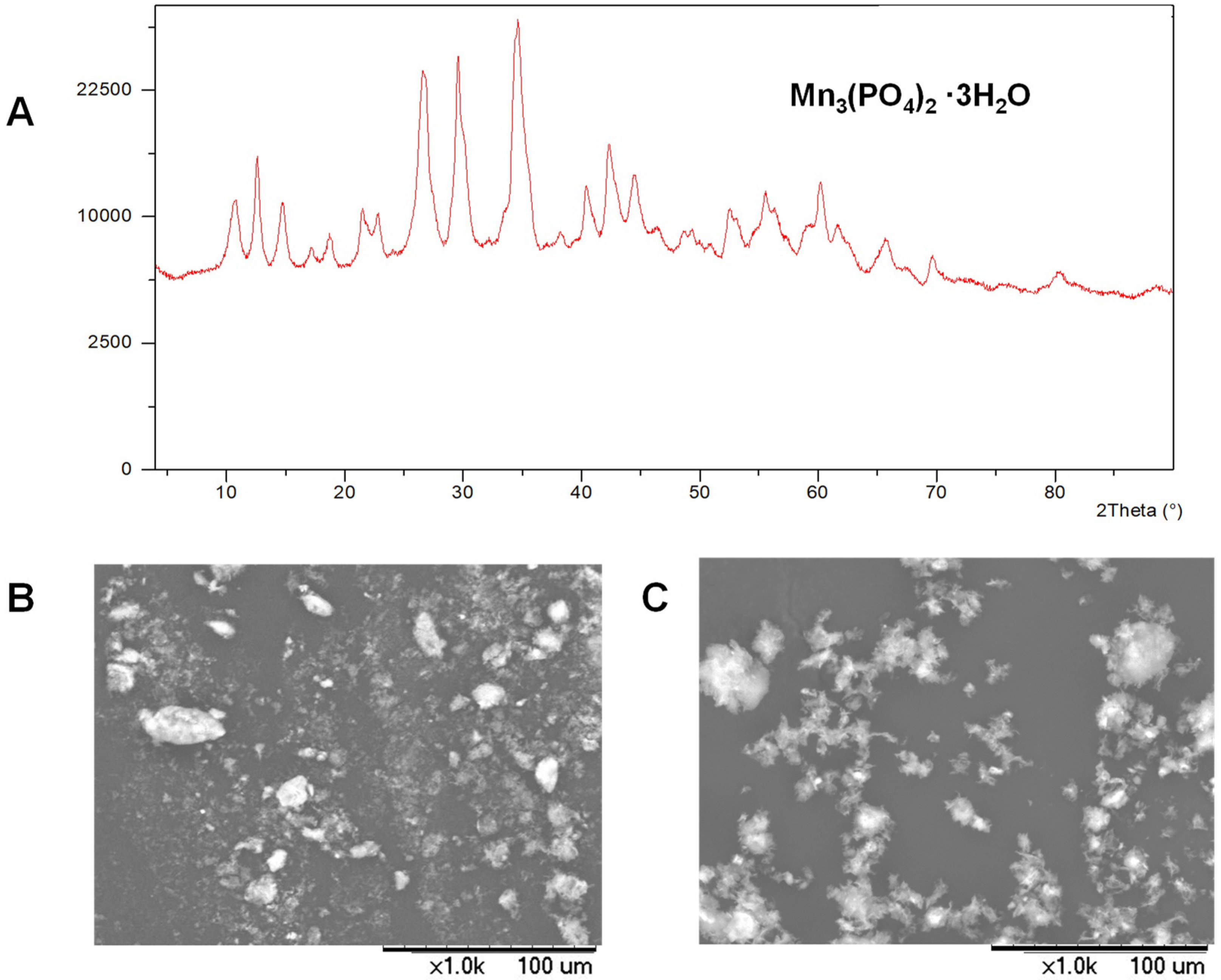
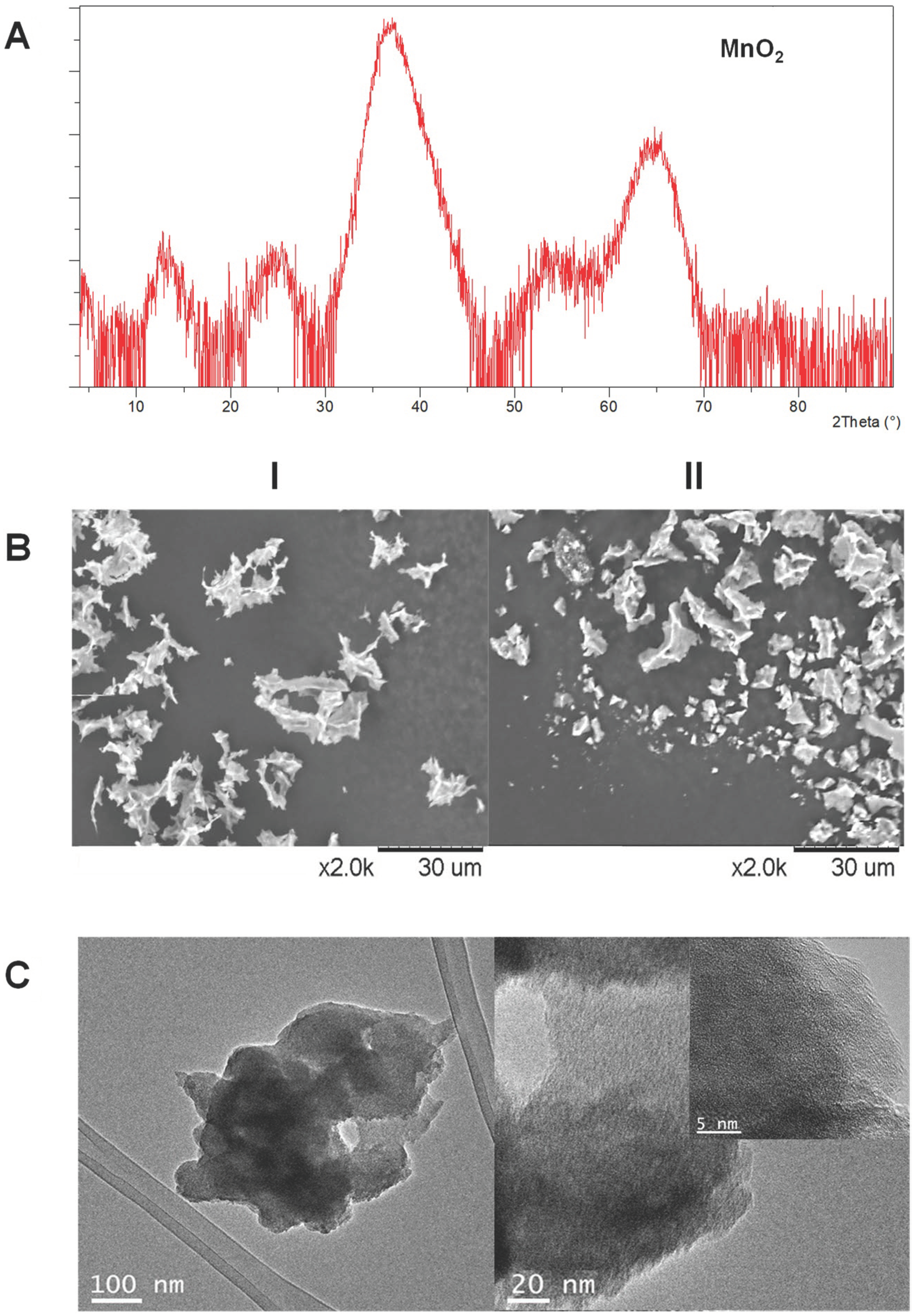
3.1. Synthesis and Characterization of MeNPs@Enzyme Biohybrids
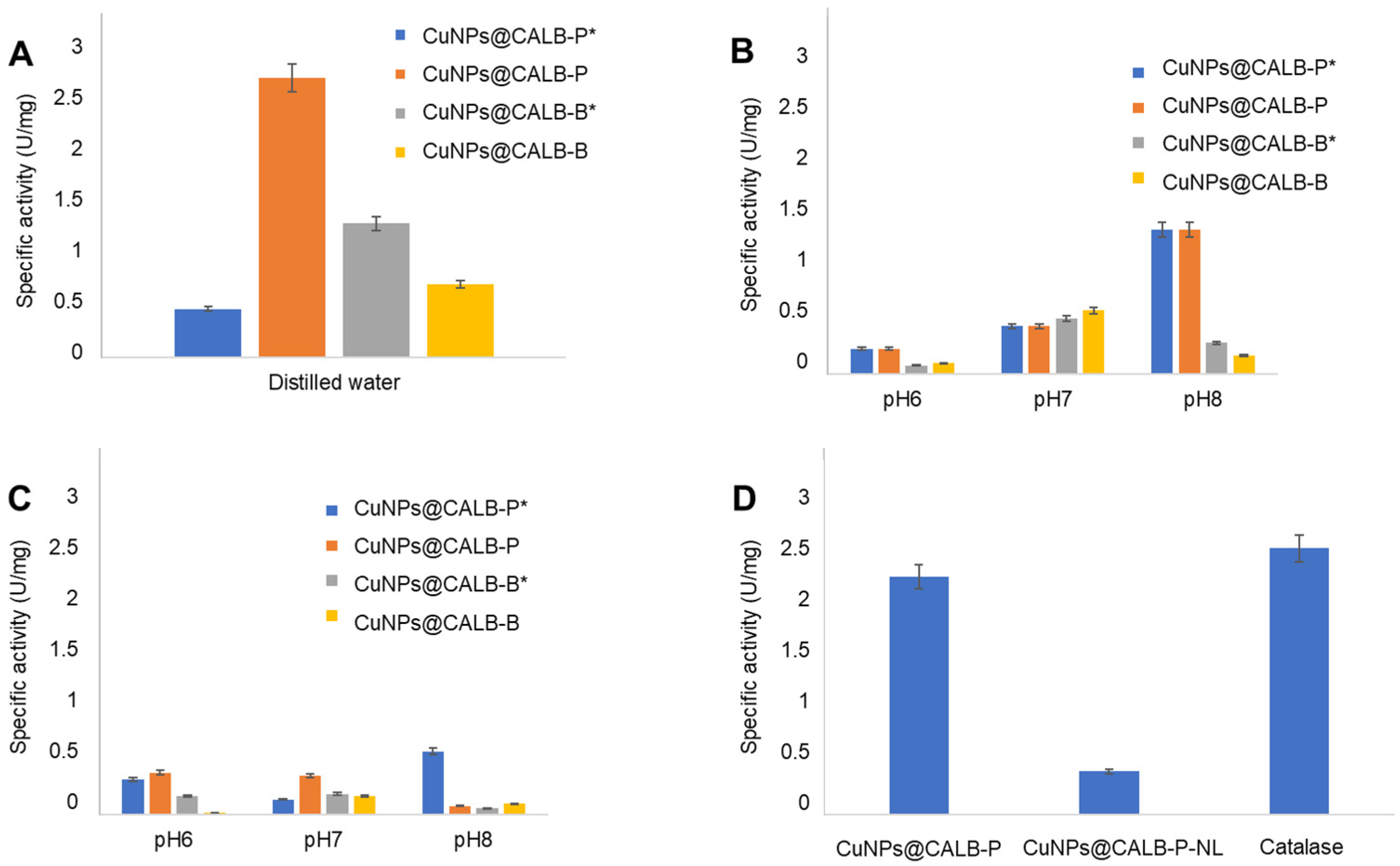
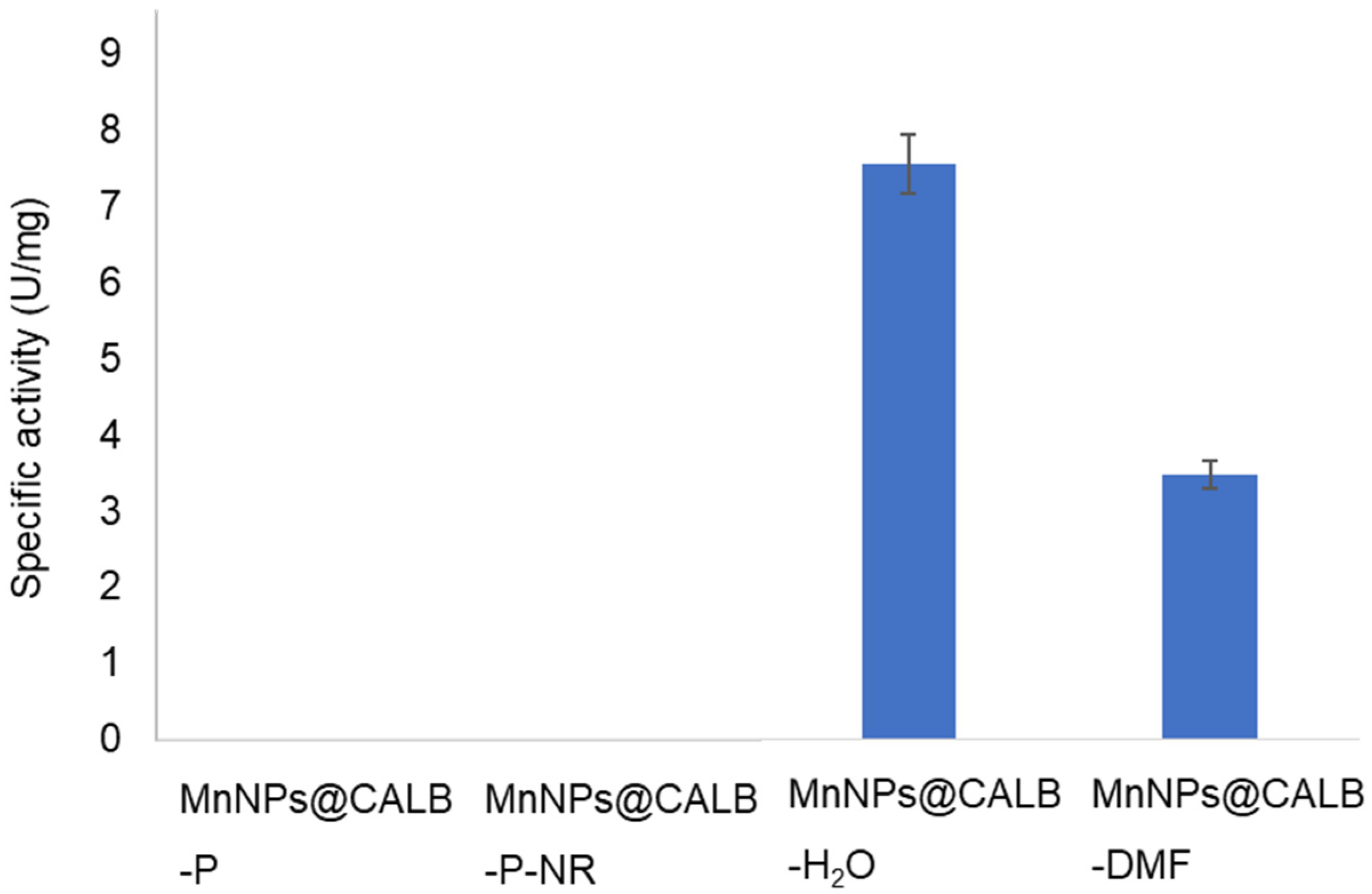
3.2. Catalase-like Activity of Different MeNPs Biohybrids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Z.; Liu, M.; Liu, Z.; Tian, Y. Real-time imaging and simultaneous quantification of mitochondrial H2O2 and ATP in neurons with a single two-photon fluorescence-lifetime-based probe. J. Am. Chem. Soc. 2020, 142, 7532–7541. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Bai, S.; He, N.; Wang, R.; Xing, Y.; Lv, C.; Yu, F. Real-time evaluation of hydrogen peroxide injuries in pulmonary fibrosis mice models with a mitochondria-targeted near-infrared fluorescent probe. ACS Sen. 2021, 6, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Filip, C.; Albu, E. (Eds.) Reactive Oxygen Species (ROS) in Living Cells; IntechOpen: London, UK, 2018. [Google Scholar]
- Bai, Z.; Li, G.; Liang, J.; Su, J.; Zhang, Y.; Chen, H.; Huang, Y.; Sui, W.; Zhao, Y. Non-enzymatic electrochemical biosensor based on PtNPs/RGO-CS-Fc nano-hybrids for the detection of hydrogen peroxide in living cells. Biosens. Bioelectron. 2016, 82, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, R.T.; Mansoor, H.S.; Abdullah, T.A.; Juzsakova, T.; Al-Jammal, N.; Salman, A.D.; Al-Shaikhly, R.R.; Le, P.C.; Domokos, E.; Abdulla, T.A. Synthesis, characterization of V2O5 nanoparticles and determination of catalase mimetic activity by new colorimetric method. J. Therm. Anal. Calorim. 2021, 145, 297–307. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxid. Med. Cell. Longev. 2019, 13, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Ali, F.; Manzoor, U.; Khan, F.I.; Lai, D.; Khan, M.K.A.; Chandrashekharaiah, K.S.; Singh, L.R.; Dar, T.A. Effect of polyol osmolytes on the structure-function integrity and aggregation propensity of catalase: A comprehensive study based on spectroscopic and molecular dynamic simulation measurements. Int. J. Biol. Macromol. 2022, 209, 198–210. [Google Scholar] [CrossRef]
- Nantapong, N.; Murata, R.; Trakulnaleamsai, S.; Kataoka, N.; Yakushi, T.; Matsushita, K. The effect of reactive oxygen species (ROS) and ROS-scavenging enzymes, superoxide dismutase and catalase, on the thermotolerant ability of Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2019, 103, 5355–5366. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Stabilization of multimeric enzymes: Strategies to prevent subunit dissociation. Enzyme Microb. Technol. 2009, 45, 405–418. [Google Scholar] [CrossRef]
- Hromic-Jahjefendic, A. Testing temperature and pH stability of the catalase enzyme in the presence of inhibitors. Period. Eng. Nat. Sci. 2022, 10, 18–29. [Google Scholar] [CrossRef]
- Cormode, D.P.; Gao, L.; Koo, H. Emerging biomedical applications of enzyme-like catalytic nanomaterials. Trends Biotechnol. 2018, 36, 15–29. [Google Scholar] [CrossRef]
- Jiao, M.; Li, Z.; Li, X.; Zhang, Z.; Yuan, Q.; Vriesekoop, F.; Liang, H.; Liu, J. Solving the H2O2 by-product problem using a catalase-mimicking nanozyme cascade to enhance glycolic acid oxidase. Chem. Eng. J. 2020, 388, 124249. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, K.L. In situ formation and immobilization of gold nanoparticles on polydimethylsiloxane (PDMS) exhibiting catalase-mimetic activity. Chem. Commun. 2020, 56, 6416–6419. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, X.; Tang, D.; Wu, S.; Hou, X.; Liu, J.; Wu, P. Phosphorescent carbon dots for highly efficient oxygen photosensitization and as photo-oxidative nanozymes. ACS Appl. Mater. Interfaces 2018, 10, 40808–40814. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Seiber, J.N.; Hotze, M. ACS select on nanotechnology in food and agriculture: A perspective on implications and applications. J. Agric. Food Chem. 2014, 62, 1209–1212. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, J.; Feng, Y.B.; Hong, L.; Chen, Q.Y.; Wu, L.F.; Lin, X.-H.; Xia, X.-H. Peroxidase-like activity of water-soluble cupric oxide nanoparticles and its analytical application for detection of hydrogen peroxide and glucose. Analyst 2012, 137, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wang, X.; Xiong, C.; Yao, L. Recent advances in biological detection with magnetic nanoparticles as a useful tool. Sci. China Chem. 2015, 58, 793–809. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, H.; Zhang, Z.; Wang, E.; Dong, S. Nanozyme: An emerging alternative to natural enzyme for biosensing and immunoassay. Trends Analyt. Chem. 2018, 105, 218–224. [Google Scholar]
- Wu, J.J.; Wang, X.Y.; Wang, Q.; Lou, Z.P.; Li, S.R.; Zhu, Y.Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Losada-Garcia, N.; Jimenez-Alesanco, A.; Velazquez-Campoy, A.; Abian, O.; Palomo, J.M. Enzyme/nanocopper hybrid nanozymes: Modulating enzyme-like activity by the protein structure for biosensing and tumor catalytic therapy. ACS Appl. Mater. Interfaces 2021, 13, 5111–5124. [Google Scholar] [CrossRef]
- Palomo, J.M. Nanobiohybrids: A new concept for metal nanoparticles synthesis. Chem. Commun. 2019, 55, 9583–9589. [Google Scholar] [CrossRef] [Green Version]
- Filice, M.; Losada-Garcia, N.; Perez-Rizquez, C.; Marciello, M.; Morales, M.D.P.; Palomo, J.M. Palladium-nanoparticles BioHybrids in applied chemistry. Appl. Nano 2020, 2, 1–13. [Google Scholar] [CrossRef]
- Losada-Garcia, N.; Rodriguez-Otero, A.; Palomo, J.M. Tailorable synthesis of heterogeneous enzyme–copper nanobiohybrids and their application in the selective oxidation of benzene to phenol. Catal. Sci. Technol. 2020, 10, 196–206. [Google Scholar] [CrossRef] [Green Version]
- Munyemana, J.C.; He, H.; Ding, S.; Yin, J.; Xi, P.; Xiao, J. Synthesis of manganese phosphate hybrid nanoflowers by collagen-templated biomineralization. RSC Adv. 2018, 8, 2708–2713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Xiao, L.; Liu, F.; Dou, Y.; Liu, S.; Fan, Y.; Cheng, G.; Song, W.; Zhou, J. Core-shell structure Ag@Pd nanoparticles supported on layered MnO2 substrate as toluene oxidation catalyst. J. Nanopart. Res. 2019, 21, 28. [Google Scholar] [CrossRef]
- Manepalli, R.K.N.R.; Madhav, B.T.P.; Giridhar, G.; Srinivasulu, M.; Tejaswi, M.; Sivaram, K.; Jayaprada, P.; Pisipati, V.G.K.M. Characterisation and mesomorphic behaviour of liquid crystals with dispersed PdCl2 nanoparticles. Liq. Cryst. Today 2017, 26, 32–38. [Google Scholar] [CrossRef]
- Chaibakhsh, N.; Moradi-Shoeili, Z. Enzyme mimetic activities of spinel substituted nanoferrites (MFe2O4): A review of synthesis, mechanism and potential applications. Mater. Sci. Eng. C 2019, 99, 1424–1447. [Google Scholar] [CrossRef]
- Marr, K.M.; Chen, B.; Mootz, E.J.; Geder, J.; Pruessner, M.; Melde, B.J.; Vanfleet, R.R.; Mendintz, I.L.; Iverson, B.D.; Claussen, J.C. High aspect ratio carbon nanotube membranes decorated with Pt nanoparticle urchins for micro underwater vehicle propulsion via H2O2 decomposition. ACS Nano 2015, 9, 7791–7803. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Deng, X.; Jiao, C.; Lu, L.; Zhang, S. Preparation and catalytic activities for H2O2 decomposition of Rh/Au bimetallic nanoparticles. Mater. Res. Bull. 2016, 79, 29–35. [Google Scholar] [CrossRef]
- Yesmurzayeva, N.N.; Nurakhmetova, Z.A.; Tatykhanova, G.S.; Selenova, B.S.; Kudaibergenov, S.E. Catalytic activity of gold and silver nanoparticles supported on zinc oxide. Supramol. Catal. 2015, 2, 1–8. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Losada-Garcia, N.; Rodriguez-Otero, A.; Ortega-Nieto, C.; Azarmi, A.; Palomo, J.M. Catalase Like-Activity of Metal NPs–Enzyme Biohybrids. Appl. Nano 2022, 3, 149-159. https://doi.org/10.3390/applnano3030011
Losada-Garcia N, Rodriguez-Otero A, Ortega-Nieto C, Azarmi A, Palomo JM. Catalase Like-Activity of Metal NPs–Enzyme Biohybrids. Applied Nano. 2022; 3(3):149-159. https://doi.org/10.3390/applnano3030011
Chicago/Turabian StyleLosada-Garcia, Noelia, Alba Rodriguez-Otero, Clara Ortega-Nieto, Ariane Azarmi, and Jose M. Palomo. 2022. "Catalase Like-Activity of Metal NPs–Enzyme Biohybrids" Applied Nano 3, no. 3: 149-159. https://doi.org/10.3390/applnano3030011
APA StyleLosada-Garcia, N., Rodriguez-Otero, A., Ortega-Nieto, C., Azarmi, A., & Palomo, J. M. (2022). Catalase Like-Activity of Metal NPs–Enzyme Biohybrids. Applied Nano, 3(3), 149-159. https://doi.org/10.3390/applnano3030011






