A New Hope for All-Diamond Electrodes? The Interdigitated Double Diamond Electrode
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Ceramic-Based Planar Diamond Electrodes
2.2. Characterization of the BDD Electrode
2.3. Electrochemical Testing Setup
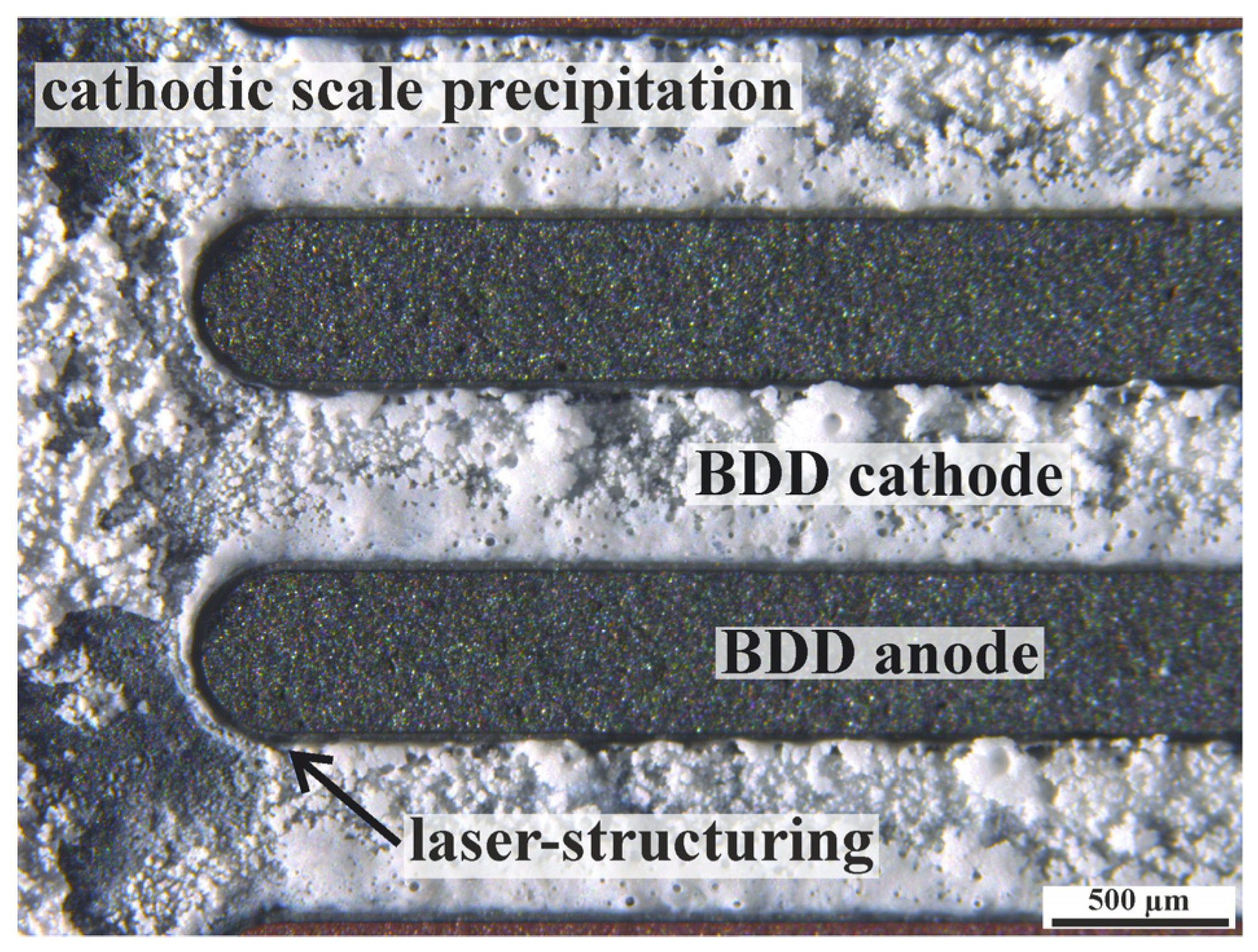
3. Results and Discussion
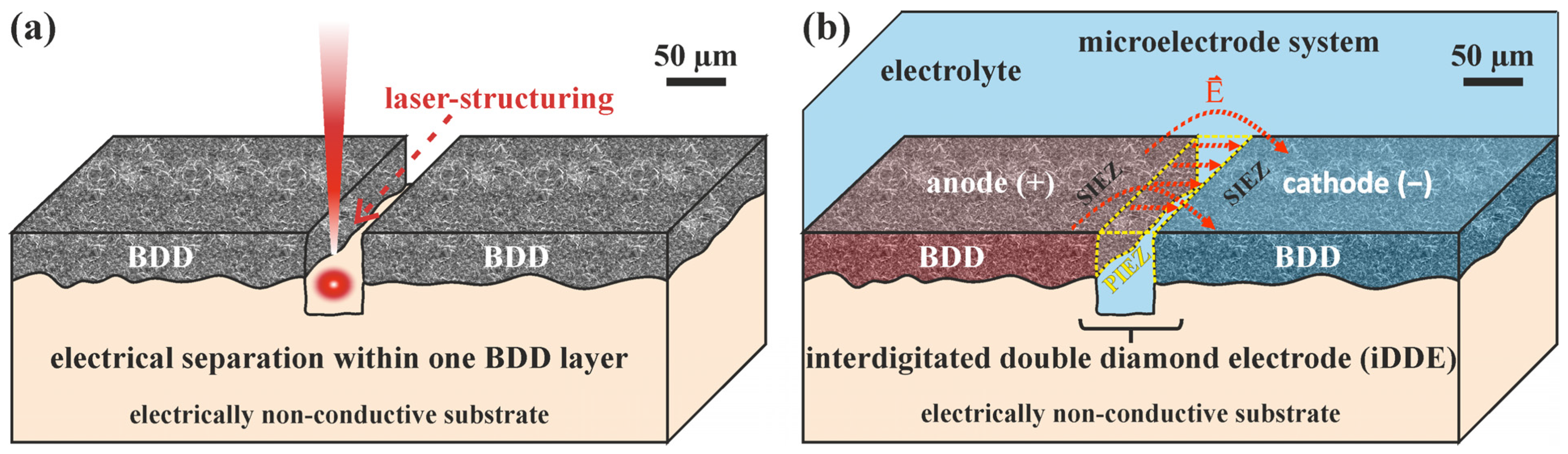
3.1. The Concept of an Interdigitated Double Diamond Electrode (iDDE)
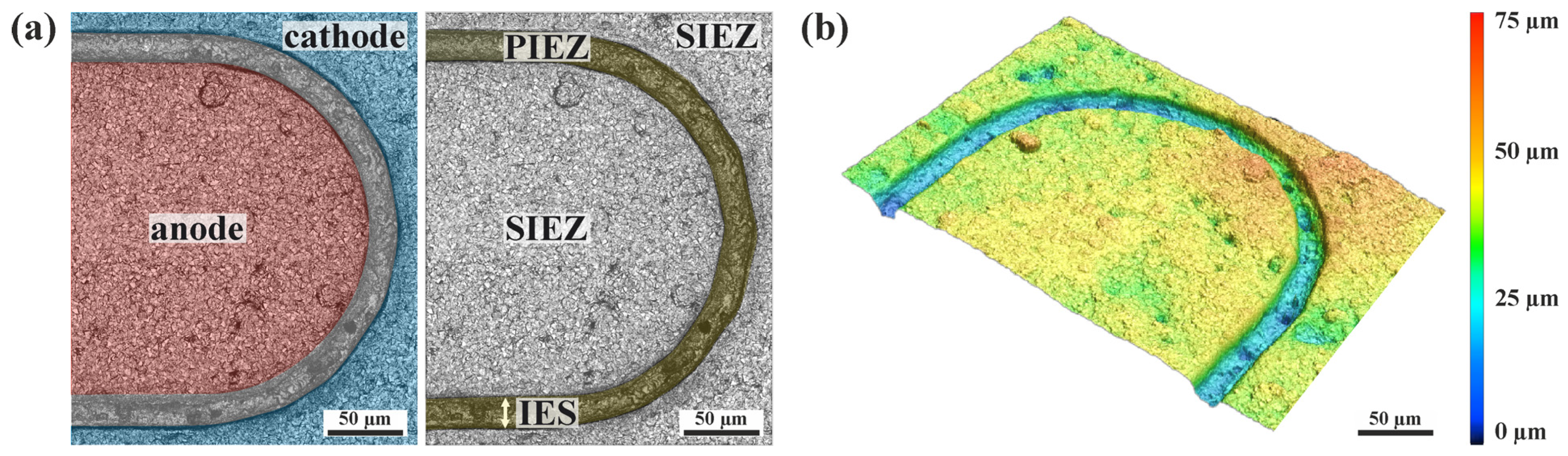
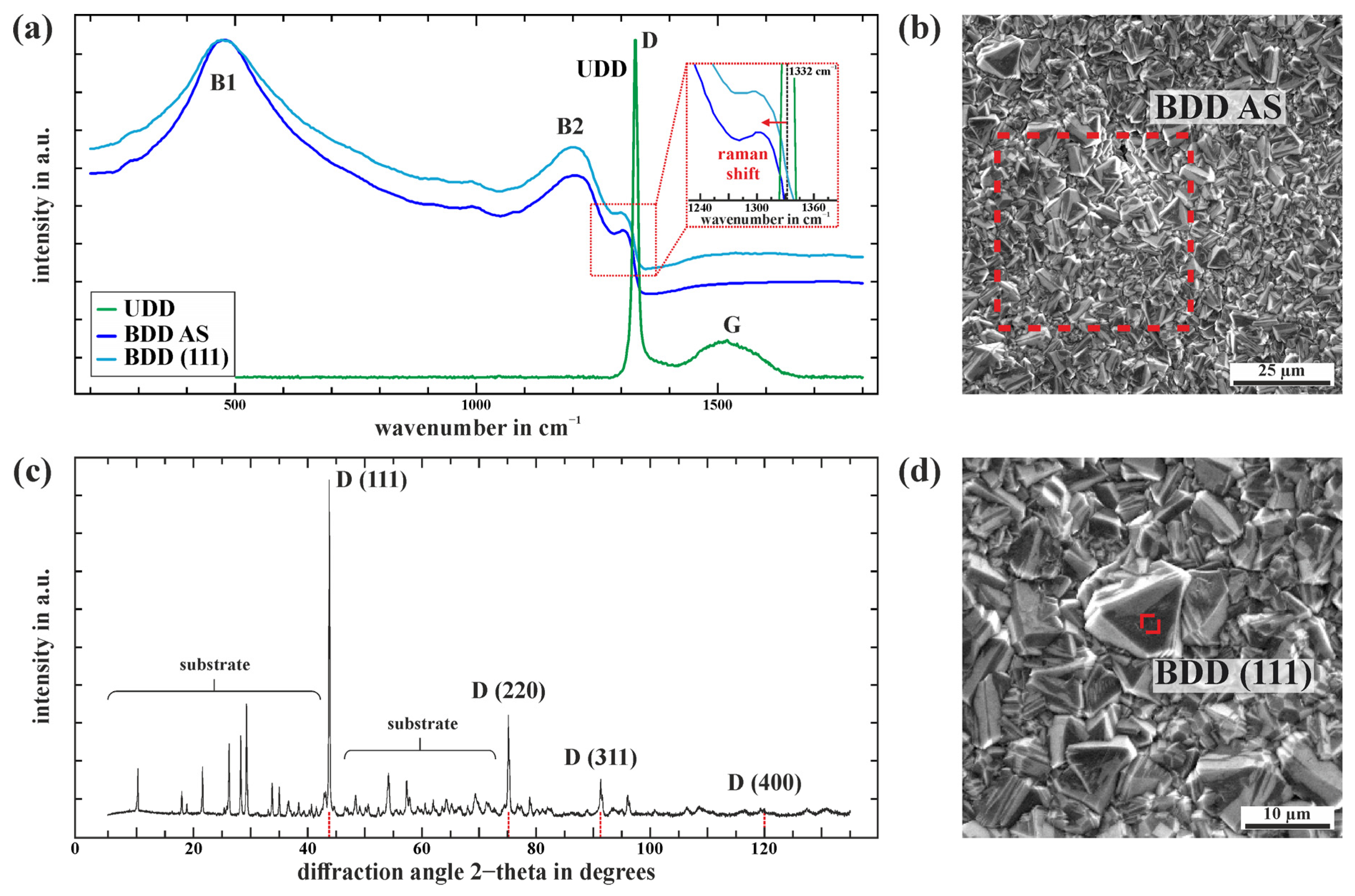
3.2. Characterization of the iDDE
3.3. Local Current Density Distribution at the iDDE
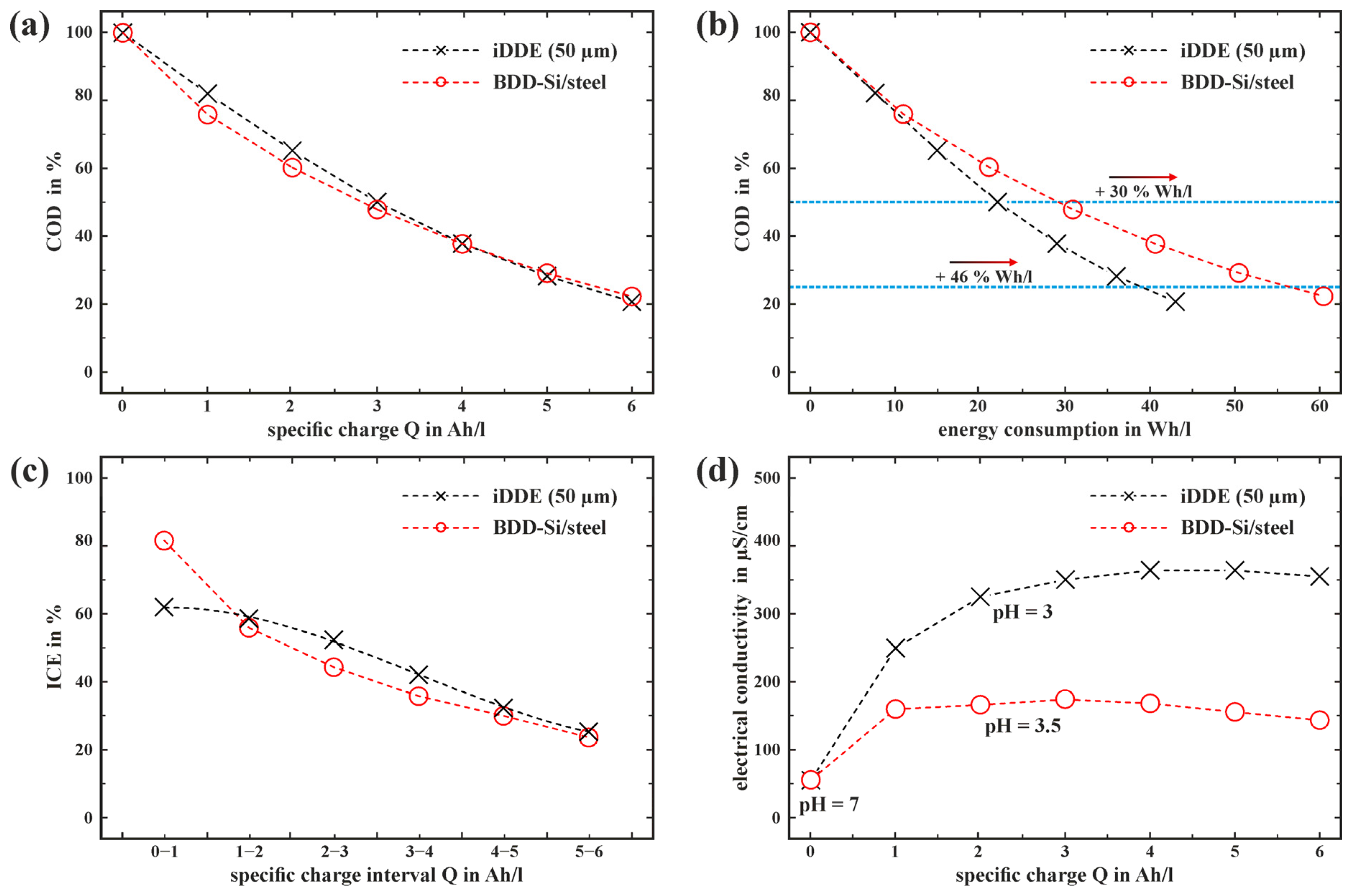
3.4. Capability of Glucose Oxidation with an iDDE
3.5. Feasability and Applicability
3.6. Challenges and Outlook
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AOP | Advanced oxidation process |
| BDD | Boron-doped diamond |
| COD | Chemical oxygen demand |
| CVD | Chemical vapor deposition |
| EAOPs | Electrochemical advanced oxidation processes |
| HFCVD | Hot-filament chemical vapor deposition |
| iDDE | Interdigitated double diamond electrode |
| IES | Interelectrode spacing |
| LSM | Laser scanning microscope |
| PIEZ | Primary interelectrode zone |
| SEM | Scanning electron microscope |
| SIEZ | Secondary interelectrode zone |
| UDD | Undoped diamond |
| XRD | X-ray diffraction |
References
- Styszko, K.; Proctor, K.; Castrignanò, E.; Kasprzyk-Hordern, B. Occurrence of pharmaceutical residues, personal care products, lifestyle chemicals, illicit drugs and metabolites in wastewater and receiving surface waters of Krakow agglomeration in South Poland. Sci. Total Environ. 2021, 768, 144360. [Google Scholar] [CrossRef]
- Falgenhauer, L.; Schwengers, O.; Schmiedel, J.; Baars, C.; Lambrecht, O.; Heß, S.; Berendonk, T.U.; Falgenhauer, J.; Chakraborty, T.; Imirzalioglu, C. Multidrug-Resistant and Clinically Relevant Gram-Negative Bacteria Are Present in German Surface Waters. Front. Microbiol. 2019, 10, 2779. [Google Scholar] [CrossRef]
- Ahrens, L.; Felizeter, S.; Sturm, R.; Xie, Z.; Ebinghaus, R. Polyfluorinated compounds in waste water treatment plant effluents and surface waters along the River Elbe, Germany. Mar. Pollut. Bull. 2009, 58, 1326–1333. [Google Scholar] [CrossRef]
- Giwa, A.; Yusuf, A.; Balogun, H.A.; Sambudi, N.S.; Bilad, M.R.; Adeyemi, I.; Chakraborty, S.; Curcio, S. Recent advances in advanced oxidation processes for removal of contaminants from water: A comprehensive review. Process Saf. Environ. Prot. 2021, 146, 220–256. [Google Scholar] [CrossRef]
- Knozowski, D.; Gmurek, M. Non-active anodes based on boron-doped diamond, PbO2 and SnO2-Sb for anodic oxidation of water contaminants: Synthesis, properties, and recent advances. Desalination Water Treat. 2024, 320, 100655. [Google Scholar] [CrossRef]
- Comninellis, C.; Pulgarin, C. Electrochemical oxidation of phenol for wastewater treatment using SnO2, anodes. J. Appl. Electrochem. 1993, 23, 108–112. [Google Scholar] [CrossRef]
- Comninellis, C.; de Battisti, A. Electrocatalysis in anodic oxidation of organics with simultaneous oxygen evolution. J. Chim. Phys. 1996, 93, 673–679. [Google Scholar] [CrossRef]
- Fóti, G.; Gandini, D.; Comninellis, C.; Perret, A.; Haenni, W. Oxidation of Organics by Intermediates of Water Discharge on IrO2 and Synthetic Diamond Anodes. Electrochem. Solid-State Lett. 1999, 2, 228–230. [Google Scholar] [CrossRef]
- Oliveira, K.S.; dos Santos, E.V.; Loor-Urgilés, L.D.; Shabanloo, A.; Martínez-Huitle, C.A. The world impact of boron doped diamond electrodes and low-cost strategies for novel production systems for sustainable wastewater treatment. Curr. Opin. Electrochem. 2025, 50, 101648. [Google Scholar] [CrossRef]
- Nzeh, N.S.; Popoola, A.; Adeleke, A.A.; Adeosun, S. Factors and challenges in the recovery of niobium and tantalum from mineral deposits, recommendations for future development—A review. Mater. Today Proc. 2022, 65, 2184–2191. [Google Scholar] [CrossRef]
- Isidro, J.; Llanos, J.; Sáez, C.; Brackemeyer, D.; Cañizares, P.; Matthee, T.; Rodrigo, M.A. Can CabECO® technology be used for the disinfection of highly faecal-polluted surface water? Chemosphere 2018, 209, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Isidro, J.; Brackemeyer, D.; Sáez, C.; Llanos, J.; Lobato, J.; Cañizares, P.; Matthée, T.; Rodrigo, M. Electro-disinfection with BDD-electrodes featuring PEM technology. Sep. Purif. Technol. 2020, 248, 117081. [Google Scholar] [CrossRef]
- Mordačíková, E.; Vojs, M.; Grabicová, K.; Marton, M.; Michniak, P.; Řeháček, V.; Bořík, A.; Grabic, R.; Bruncko, J.; Mackuľak, T.; et al. Influence of boron doped diamond electrodes properties on the elimination of selected pharmaceuticals from wastewater. J. Electroanal. Chem. 2020, 862, 114007. [Google Scholar] [CrossRef]
- Kuchtová, G.; Herink, P.; Herink, T.; Chýlková, J.; Mikulášek, P.; Dušek, L. From lab-scale to pilot-scale treatment of real wastewater from the production of rayon fiber. Process Saf. Environ. Prot. 2023, 171, 834–846. [Google Scholar] [CrossRef]
- Pagels, M.; Hall, C.E.; Lawrence, N.S.; Meredith, A.; Jones, T.G.J.; Godfried, H.P.; Pickles, C.S.J.; Wilman, J.; Banks, C.E.; Compton, R.G.; et al. All-diamond microelectrode array device. Anal. Chem. 2005, 77, 3705–3708. [Google Scholar] [CrossRef]
- Smirnov, W.; Yang, N.; Hoffmann, R.; Hees, J.; Obloh, H.; Müller-Sebert, W.; Nebel, C.E. Integrated all-diamond ultramicroelectrode arrays: Optimization of faradaic and capacitive currents. Anal. Chem. 2011, 83, 7438–7443. [Google Scholar] [CrossRef]
- Vahidpour, F.; Curley, L.; Biró, I.; McDonald, M.; Croux, D.; Pobedinskas, P.; Haenen, K.; Giugliano, M.; Živcová, Z.V.; Kavan, L.; et al. All-diamond functional surface micro-electrode arrays for brain-slice neural analysis. Phys. Status Solidi A 2017, 214, 1532347. [Google Scholar] [CrossRef]
- Sbartai, A.; Namour, P.; Errachid, A.; Krejči, J.; Šejnohová, R.; Renaud, L.; Hamlaoui, M.L.; Loir, A.-S.; Garrelie, F.; Donnet, C.; et al. Electrochemical boron-doped diamond film microcells micromachined with femtosecond laser: Application to the determination of water framework directive metals. Anal. Chem. 2012, 84, 4805–4811. [Google Scholar] [CrossRef]
- Asai, K.; Einaga, Y. Fabrication of an all-diamond microelectrode using a chromium mask. Chem. Commun. 2019, 55, 897–900. [Google Scholar] [CrossRef]
- Silva, E.L.; Gouvêa, C.P.; Quevedo, M.C.; Neto, M.A.; Archanjo, B.S.; Fernandes, A.J.S.; Achete, C.A.; Silva, R.F.; Zheludkevich, M.L.; Oliveira, F.J. All-Diamond Microelectrodes as Solid State Probes for Localized Electrochemical Sensing. Anal. Chem. 2015, 87, 6487–6492. [Google Scholar] [CrossRef]
- Henquín, E.R.; Colli, A.N.; Bergmann, M.; Bisang, J. Characterization of a bipolar parallel-plate electrochemical reactor for water disinfection using low conductivity drinking water. Chem. Eng. Process. Process Intensif. 2013, 65, 45–52. [Google Scholar] [CrossRef]
- Xu, L.; Hu, C.; Huang, Q.; Jin, K.; Zhao, P.; Wang, D.; Hou, W.; Dong, L.; Hu, S.; Ma, H. Trends and recent development of the microelectrode arrays (MEAs). Biosens. Bioelectron. 2021, 175, 112854. [Google Scholar] [CrossRef] [PubMed]
- Zulla, M.; Vierheilig, V.; Koch, M.; Burkovski, A.; Karl, M.; Rosiwal, S. Diamond as Insulation for Conductive Diamond—A Spotted Pattern Design for Miniaturized Disinfection Devices. C 2023, 9, 78. [Google Scholar] [CrossRef]
- Ager, J.W.; Walukiewicz, W.; McCluskey, M.; Plano, M.A.; Landstrass, M.I. Fano interference of the Raman phonon in heavily boron-doped diamond films grown by chemical vapor deposition. Appl. Phys. Lett. 1995, 66, 616–618. [Google Scholar] [CrossRef]
- Anastassakis, E. Strain characterization of polycrystalline diamond silicon systems. J. Appl. Phys. 1999, 86, 249–258. [Google Scholar] [CrossRef]
- Göltz, M.; Helmreich, T.; Börner, R.; Kupfer, T.; Schubert, A.; Rosiwal, S. Spatial distribution of thermally induced residual stresses in HF-CVD diamond coatings on microstructured steel surfaces. Diam. Relat. Mater. 2023, 136, 109931. [Google Scholar] [CrossRef]
- Ferreira, N.G.; Abramof, E.; Leite, N.F.; Corat, E.J.; Trava-Airoldi, V.J. Analysis of residual stress in diamond films by x-ray diffraction and micro-Raman spectroscopy. J. Appl. Phys. 2002, 91, 2466–2472. [Google Scholar] [CrossRef]
- Guo, L.; Chen, G. Long-Term Stable Ti/BDD Electrode Fabricated with HFCVD Method Using Two-Stage Substrate Temperature. J. Electrochem. Soc. 2007, 154, D657–D661. [Google Scholar] [CrossRef]
- Wurzinger, P.; Pongratz, P.; Hartmann, P.; Haubner, R.; Lux, B. Investigation of the boron incorporation in polycrystalline CVD diamond films by TEM, EELS and Raman spectroscopy. Diam. Relat. Mater. 1997, 6, 763–768. [Google Scholar] [CrossRef]
- Ushizawa, K.; Watanabe, K.; Ando, T.; Sakaguchi, I.; Nishitani-Gamo, M.; Sato, Y.; Kanda, H. Boron concentration dependence of Raman spectra on {100} and {111} facets of B-doped CVD diamond. Diam. Relat. Mater. 1998, 7, 1719–1722. [Google Scholar] [CrossRef]
- Pleskov, Y.; Evstefeeva, Y.; Krotova, M.D.; Varnin, V.; Teremetskaya, I. Synthetic semiconductor diamond electrodes: Electrochemical behaviour of homoepitaxial boron-doped films orientated as (111), (110), and (100) faces. J. Electroanal. Chem. 2006, 595, 168–174. [Google Scholar] [CrossRef]
- Ivandini, T.A.; Watanabe, T.; Matsui, T.; Ootani, Y.; Iizuka, S.; Toyoshima, R.; Kodama, H.; Kondoh, H.; Tateyama, Y.; Einaga, Y. Influence of Surface Orientation on Electrochemical Properties of Boron-Doped Diamond. J. Phys. Chem. C 2019, 123, 5336–5344. [Google Scholar] [CrossRef]
- Tichter, T.; Marshall, A.T. Electrochemical characterisation of macroporous electrodes: Recent advances and hidden pitfalls. Curr. Opin. Electrochem. 2022, 34, 101027. [Google Scholar] [CrossRef]
- Kodým, R.; Bergmann, M.H.; Bouzek, K. First results of modelling geometry factors in electrolysis cells for direct drinking water disinfection. In Proceedings of the 56th Annual Meeting of the International Society of Electrochemistry, Busan, Republic of Korea, 26–30 September 2005; p. 896. [Google Scholar]
- Foller, P.C.; Tobias, C.W. The Anodic Evolution of Ozone. J. Electrochem. Soc. 1982, 129, 506–515. [Google Scholar] [CrossRef]
- Grimmig, R.; Gillemot, P.; Stucki, S.; Günther, K.; Baltruschat, H.; Witzleben, S. Operating an ozone-evolving PEM electrolyser in tap water: A case study of water and ion transport. Sep. Purif. Technol. 2022, 292, 121063. [Google Scholar] [CrossRef]
- Watanabe, T.; Honda, Y.; Kanda, K.; Einaga, Y. Tailored design of boron-doped diamond electrodes for various electrochemical applications with boron-doping level and sp 2 -bonded carbon impurities. Phys. Status Solidi A 2014, 211, 2709–2717. [Google Scholar] [CrossRef]
- Kodým, R.; Bergmann, M.H.; Bouzek, K. Results of modelling electrodes and reactors for the direct electrochemical drinking water electrolysis. In Proceedings of the 57th Annual Meeting of the International Society of Electrochemistry, Edinburgh, UK, 27 August–1 September 2006; pp. S5–P16. [Google Scholar]
- Popov, K.I.; Djokić, S.S.; Grgur, B.N. Optimum Conditions for Electroplating. In Fundamental Aspects of Electrometallurgy; Kluwer Academic Publishers: Boston, MA, USA, 2002; pp. 191–196. [Google Scholar]
- Bergmann, H. Electrochemical disinfection—State of the art and tendencies. Curr. Opin. Electrochem. 2021, 28, 100694. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Direct and mediated anodic oxidation of organic pollutants. Chem. Rev. 2009, 109, 6541–6569. [Google Scholar] [CrossRef]
- Medeiros de Araújo, D.; Cañizares, P.; Martínez-Huitle, C.A.; Rodrigo, M.A. Electrochemical conversion/combustion of a model organic pollutant on BDD anode: Role of sp 3/sp 2 ratio. Electrochem. Commun. 2014, 47, 37–40. [Google Scholar] [CrossRef]
- Santos, G.O.; Eguiluz, K.I.; Salazar-Banda, G.R.; Sáez, C.; Rodrigo, M.A. Understanding the electrolytic generation of sulfate and chlorine oxidative species with different boron-doped diamond anodes. J. Electroanal. Chem. 2020, 857, 113756. [Google Scholar] [CrossRef]
- Brito, C.d.N.; de Araújo, D.M.; Martínez-Huitle, C.A.; Rodrigo, M.A. Understanding active chlorine species production using boron doped diamond films with lower and higher sp3/sp2 ratio. Electrochem. Commun. 2015, 55, 34–38. [Google Scholar] [CrossRef]
- Bogdanowicz, R.; Ryl, J. Structural and electrochemical heterogeneities of boron-doped diamond surfaces. Curr. Opin. Electrochem. 2022, 31, 100876. [Google Scholar] [CrossRef]
- Ryl, J.; Burczyk, L.; Bogdanowicz, R.; Sobaszek, M.; Darowicki, K. Study on surface termination of boron-doped diamond electrodes under anodic polarization in H2SO4 by means of dynamic impedance technique. Carbon 2016, 96, 1093–1105. [Google Scholar] [CrossRef]
- Souza, F.L.; Saéz, C.; Lanza, M.; Sobaszek, M.; Darowicki, K. The effect of the sp3/sp2 carbon ratio on the electrochemical oxidation of 2,4-D with p-Si BDD anodes. Electrochim. Acta 2016, 187, 119–124. [Google Scholar] [CrossRef]
- Gałaś, A.; Krzak, M.; Szlugaj, J. Niobium—A critical and conflict raw material of great economic significance—The state of the art. Gospod. Surowcami Miner.-Miner. Resour. Manag. 2024, 40, 47–67. [Google Scholar] [CrossRef]
- Fu, Y.; Yan, B.; Loh, N.L.; Sun, C.Q.; Hing, P. Hydrogen embrittlement of titanium during microwave plasma assisted CVD diamond deposition. Surf. Eng. 2000, 16, 355–360. [Google Scholar] [CrossRef]
- Damm, D.; Contin, A.; Barbieri, F.; Trava-Airoldi, V.J.; Barquete, D.M.; Corat, E.J. Interlayers Applied to CVD Diamond Deposition on Steel Substrate: A Review. Coatings 2017, 7, 141. [Google Scholar] [CrossRef]
- Sirés, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ. Sci. Pollut. Res. Int. 2014, 21, 8336–8367. [Google Scholar] [CrossRef]
- Koch, M.; Burkovski, A.; Zulla, M.; Rosiwal, S.; Geißdörfer, W.; Dittmar, R.; Grobecker-Karl, T. Pilot Study on the Use of a Laser-Structured Double Diamond Electrode (DDE) for Biofilm Removal from Dental Implant Surfaces. J. Clin. Med. 2020, 9, 3036. [Google Scholar] [CrossRef]
- Arenas, L.F.; Ponce de León, C.; Walsh, F.C. Critical Review—The Versatile Plane Parallel Electrode Geometry: An Illustrated Review. J. Electrochem. Soc. 2020, 167, 23504. [Google Scholar] [CrossRef]
- Bergmann, M.H.; Rollin, J. Product and by-product formation in laboratory studies on disinfection electrolysis of water using boron-doped diamond anodes. Catal. Today 2007, 124, 198–203. [Google Scholar] [CrossRef]
- Bergmann, M.H.; Rollin, J.; Iourtchouk, T. The occurrence of perchlorate during drinking water electrolysis using BDD anodes. Electrochim. Acta 2009, 54, 2102–2107. [Google Scholar] [CrossRef]
- Azizi, O.; Hubler, D.; Schrader, G.; Farrell, J.; Chaplin, B.P. Mechanism of perchlorate formation on boron-doped diamond film anodes. Environ. Sci. Technol. 2011, 45, 10582–10590. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Interim Drinking Water Health Advisory for Perchlorate; USEPA: Washington, DC, USA, 2005.
- Wilbur, S.B.; Diamond, G.L.; Fernando, L.; Odin, M.; Citra, M.; Plewak, D.; Tunkel, J. Toxicological profile for perchlorates. Agency for Toxic Substances and Disease Registry. 2008. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp162.pdf (accessed on 31 October 2025).
- Leung, A.M.; Pearce, E.N.; Braverman, L.E. Perchlorate, iodine and the thyroid. Best Pr. Res. Clin. Endocrinol. Metab. 2010, 24, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Alfredo, K.; Stanford, B.; Roberson, J.A.; Eaton, A. Chlorate Challenges for Water Systems. J. AWWA 2015, 107, E187–E196. [Google Scholar] [CrossRef]
- Llorente-Esteban, A.; Manville, R.W.; Reyna-Neyra, A.; Abbott, G.W.; Amzel, L.M.; Carrasco, N. Allosteric regulation of mammalian Na+/I- symporter activity by perchlorate. Nat. Struct. Mol. Biol. 2020, 27, 533–539. [Google Scholar] [CrossRef]
- Bergmann, M.; Rollin, J.; Koparal, A.S. Chlorate and perchlorate—New criterions for environmentally-friendly processes in Advanced Oxidation. Water Pract. Technol. 2010, 5, 2101–2107. [Google Scholar] [CrossRef]


| Global | PIEZ | SIEZ | |
| Aanode (+) in cm2 | 49.00 | 0.33 ± 0.02 | 47.83 |
| I in A | 0.50 | 0.40 | 0.10 |
| j in A/cm2 | 0.01 | 1.22 ± 0.08 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulla, M.; Messerschmidt, C.; Ghanem, H.; Bähr, J.; Hegemann, L.; Rosiwal, S. A New Hope for All-Diamond Electrodes? The Interdigitated Double Diamond Electrode. Electrochem 2025, 6, 41. https://doi.org/10.3390/electrochem6040041
Zulla M, Messerschmidt C, Ghanem H, Bähr J, Hegemann L, Rosiwal S. A New Hope for All-Diamond Electrodes? The Interdigitated Double Diamond Electrode. Electrochem. 2025; 6(4):41. https://doi.org/10.3390/electrochem6040041
Chicago/Turabian StyleZulla, Manuel, Carolin Messerschmidt, Hanadi Ghanem, Johannes Bähr, Lukas Hegemann, and Stefan Rosiwal. 2025. "A New Hope for All-Diamond Electrodes? The Interdigitated Double Diamond Electrode" Electrochem 6, no. 4: 41. https://doi.org/10.3390/electrochem6040041
APA StyleZulla, M., Messerschmidt, C., Ghanem, H., Bähr, J., Hegemann, L., & Rosiwal, S. (2025). A New Hope for All-Diamond Electrodes? The Interdigitated Double Diamond Electrode. Electrochem, 6(4), 41. https://doi.org/10.3390/electrochem6040041






