Abstract
Surface modification of nucleic acid-based electrochemical biosensors has been at the forefront of research since their inception. Effective modification ensures the optimization of the sensitivity, specificity, and stability of modern biosensors. Recent advances in DNA nanotechnology have enabled the development of novel electrochemical biosensor interfaces with precise assembly and high biocompatibility. In this review, we explore three strategies for enhancing biosensor performance: the integration of tetrahedral DNA nanostructures (TDNs), self-assembled monolayers (SAMs), and DNA-based hydrogels. TDNs offer well-defined geometry and controlled spatial presentation of capture probes, significantly reducing background noise and improving target accessibility. SAMs provide a robust and tunable platform for anchoring these nanostructures, enabling reproducible and chemically stable interfaces. DNA hydrogels serve as a responsive and flexible scaffold capable of signal amplification and analyte retention. These surface architectures enhance sensitivity and minimize non-specific adsorption (NSA). We discuss recent applications and experimental outcomes, highlighting how each component is driving the next generation of nucleic acid-based biosensors.
1. Introduction
Early detection of complex diseases remains a cornerstone of modern healthcare, with the potential to significantly improve patient outcomes and reduce global mortality. Among emerging technologies, DNA nanostructure-based biosensors stand out for their ability to detect a wide range of molecular targets, including DNA, RNA, miRNA, proteins, and other small molecules [1], making them especially valuable for diagnosing cancer, cardiovascular diseases, and infectious diseases [2]. Foundational literature has extensively explored the principles and applications of DNA nanostructures and electrochemical DNA biosensors, providing a basis for understanding their design and functionality [3]. It is important to note that nucleic acids are highly heterogeneous analytes, ranging from short oligonucleotides (<100 bases) to long DNA and RNA with several kilobases to megabases in length, and the applicability of each detection approach often depends on the length and structure of the target sequence.
Electrochemical nucleic-acid-based biosensors work by immobilizing a single-stranded DNA (ssDNA) probe onto an electrode surface to recognize and hybridize with its complementary target sequence [4]. This hybridization event is converted into an electrical signal through oxidation of DNA bases, redox reactions of electroactive markers, or charge transport across π-stacked base pairs. Typical electrode materials include gold, carbon nanotubes (CNTs), graphene, and conductive polymers, which provide large surface areas and high electron-transfer efficiency. The general architecture of these biosensors consists of an electrode substrate, a DNA probe serving as the biorecognition element, and an electrochemical transducer that translates the hybridization process into measurable current or potential changes. Detection can be achieved through direct (label-free) methods using the intrinsic electroactivity of guanine or adenine, or through indirect (label-based) methods employing electroactive compounds such as dyes, metal complexes, or enzymes. These platforms have been widely applied to detect genetic mutations, pathogenic DNA, DNA damage, small biomolecules, and ions [5].
Traditional nucleic acid-based biosensor platforms often suffer from critical limitations, including inadequate control over probe orientation and density, as well as high levels of non-specific adsorption (NSA), which leads to inherently low sensitivity and specificity [1]. These challenges are particularly significant given target nucleic acids frequently exist at exceedingly low concentrations, making robust detection even more challenging. Traditional biosensor platforms often suffer from critical limitations related to the immobilization of biorecognition elements. Common strategies for developing biorecognition surfaces include physical adsorption, biotin–avidin affinity interactions, and covalent bonding [6,7,8]. Physical adsorption is a simple method but generally considered the least effective due to weak interactions and poor stability of the immobilized biomolecules [8]. In contrast, the biotin–avidin system offers strong and specific binding, enabling oriented immobilization of biorecognition elements, which enhances biosensor performance [7]. This approach also reduces non-specific adsorption (NSA), a crucial factor when working with complex biological samples such as serum. Covalent bonding strategies involve immobilizing biorecognition elements via chemical linkers, which can result in random orientation [8]. Although various linkers with antifouling properties have been reported, they may still suffer from NSA. Table 1 provides a few examples of these strategies and their limitation of detection (LOD).

Table 1.
Examples of immobilization techniques for developing electrochemical DNA-based biosensors.
This review highlights three advanced surface modification approaches for nucleic acid detection: tetrahedral DNA nanostructures (TDNs), self-assembled monolayers (SAMs), and DNA hydrogels (Table 1). Each offers a unique mechanism for probe presentation and signal amplification, with varying levels of programmability, biocompatibility, and integration into diagnostic systems. TDNs function as rigid scaffolds that promote upright probe orientation and minimize nonspecific adsorption. SAMs offer chemically tunable monolayers that enable dense and selective DNA immobilization. DNA hydrogels, on the other hand, serve as dynamic 3D-networks that can both recognize targets and transduce signals through structural transformation. Together, these surface engineering strategies represent the evolving frontier of nucleic acid biosensing technology.
In recent years, research has focused on engineering functional interfaces that improve analyte recognition while enhancing the overall biosensor response. For instance, the TDN has been used in the fabrication of a biosensor for hepatocellular carcinoma diagnosis, the most common form of liver cancer, through alpha-fetoprotein and miRNA-122 (22 nucleotides) detection [3]. A biosensor using a SAM structure has been reported for early diagnosis of head and neck cancer by detecting MGMT gene methylation [12]. DNA hydrogels have also demonstrated effectiveness in detecting creatine kinase isoenzyme (CK-MB, 41–43 kDa) levels, a cardiac biomarker used before the detection of cardiac troponin [13,14].
These next-generation devices are paving the way for early detection and personalized medicine on a global scale [1]. The success of these biosensors depends heavily on their surface modification and architecture. Surface modifications are the building blocks of any biosensor, which are responsible for the specificity, sensitivity, and reliability of biosensors, especially those designed to detect nucleic acids, such as microRNAs, viral RNA, and circulating tumor DNA. The ability to immobilize probes in a controlled and reproducible manner directly impacts hybridization efficiency and signal output.
Table 1 shows examples of different surface engineering, including DNA nanostructure strategies for developing electrochemical biosensors for detecting DNA and RNA. The sensitivity of the sensor depends on the target analyte, sample, and the surface modification strategy. This article compares these emerging strategies, outlines their construction and mechanism, evaluates their benefits and limitations, and discusses how they are shaping the next generation of biosensors. By comparing these emerging strategies, we aim to provide a comprehensive overview of current innovations in surface modification for nucleic acid-based electrochemical biosensors.
2. Tetrahedral DNA Nanostructures
2.1. Overview
A key element of biosensor performance is the consistent and uniform attachment of biorecognition elements onto the sensing surface to provide repeatable and reproducible results [1]. Traditional nucleic acid biosensors that anchor single-stranded DNA (ssDNA) to the surface may lack such a system due to random immobilization [2,15]. However, three-dimensional DNA-based nanostructures like Tetrahedral DNA Nanostructures (TDNs) provide a compelling alternative to combat this problem. Inserted between the recognition elements and the sensing interface, TDNs provide systematic organization across the surface by acting as a scaffold for recognition elements [1,2,15]. They are particularly effective in this task, as their rigid three-dimensional structure ensures adequate spacing and stabilizes the probes for consistent binding to target nucleic acids.
TDNs are composed of four individual oligonucleotides arranged in a pyramidal configuration, which is responsible for their stable structure [16]. Beyond this physical strength, they are extremely versatile, offering programmability, biocompatibility, and predictable spatial arrangement across the surface of a sensor, all while providing an extremely functionalize interface [17,18]. TDNs may be used in combination with a variety of different capture technologies to offer customized surface modification. From simple systems using only a short nucleic acid strand, to more complex arrangements involving enzymes and multiple oligonucleotides, the incorporation of a TDN scaffold stabilizes detection elements for optimized sensitivity [15,18,19]. Their ubiquity has led them to be used in a host of nucleic acid-based sensors with great success, including in the detection of disease-related cell-free DNA (cfDNA) [19], circulating tumor DNA (ctDNA) [20], micro RNAs (miRNAs) [21], viral DNA [22], and circular RNAs (circRNAs) [23]. Their high compatibility with various surface materials further expands their range of applications. While gold is commonly used, graphene and titanium have also been demonstrated to be effective choices [18,19,21,24].
2.2. Structure and Assembly
The first step in the synthesis is the design of the four precursor molecules used in the assembly of the tetrahedron. One key consideration is the length of the oligonucleotides, which is typically between 40 and 60 bases. This serves as the optimal range for balancing structural integrity and functional performance. Strands that are too long and bulky can introduce steric hindrance, reduce probe accessibility, and thereby decrease sensitivity. Conversely, strands that are too short may not provide sufficient spacing between probes, resulting in random probe distribution and increased nonspecific adsorption. This leads to reduced reliability and a shorter operational lifespan of the sensor [25]. The length of oligonucleotides also affects the stability of the TDN itself. Longer strands are more vulnerable to nuclease degradation in biological samples, with reports of up to a fourfold increase in enzymatic breakdown when the strand length increases from about 40 to 60 nucleotides [26].
These structural concerns are addressed at the design stage. Keeping in mind the size restrictions, each of the four oligonucleotide strands is typically divided into three domains of 10 and 20 bases each [16]. While all three are often equivalent in length to ensure uniformity of the tetrahedral nanostructure, they can be customized such that the individual domain lengths can be altered to make the TDN wider or taller [16,27]. This allows for flexibility in spacing configurations to accommodate biorecognition elements without compromising the overall length of each strand.
In traditional four-strand construction, the tetrahedral structure forms through the hybridization of complementary domains on the four separate oligonucleotides. It is more common for two of these domains to exist as unbroken segments while the third domain is split between the two ends of the strand, creating overhangs complementary to an unbroken domain of a different strand (Figure 1). These overhangs facilitate rapid and efficient self-assembly while minimizing unwanted secondary structures [16,27]. Modern synthesis is often aided by computational software that provides a map of base-pairing patterns, ensuring a consistent GC content across all sides, and screening for undesired features such as hairpins, loops, or other secondary structures [28]. Such optimization improves the structural stability of the TDN by maintaining uniform melting behavior and reducing susceptibility to degradation.
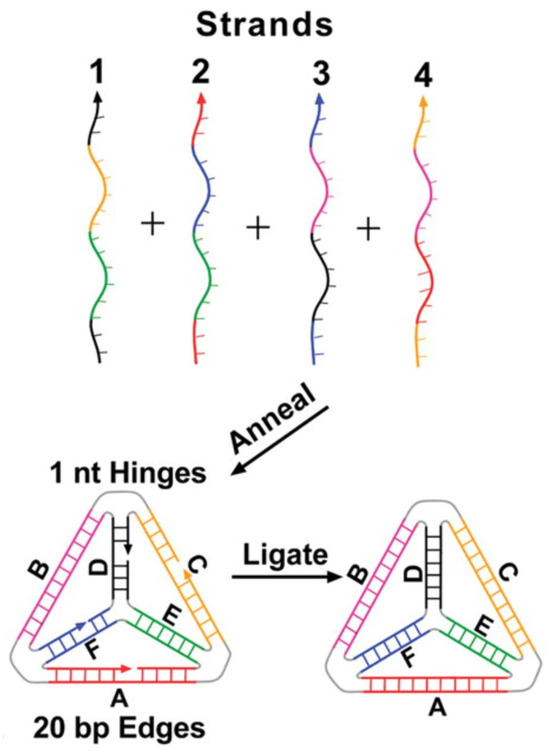
Figure 1.
Schematic diagram of TDN assembly from four oligonucleotides. Sides B and E are unnicked, as they are made of a single unbroken strand, while A, C, D, and F are linked as a result of the complementary base-pairing. Corresponding colors on different strands hybridize with each other in the annealing stage. Image was reprinted with permission from [16] under Copyright © 2005, The American Association for the Advancement of Science.
Although TDNs were first reported nearly twenty years ago, the synthetic procedure for their construction has remained largely unchanged [16,18]. This is typically achieved by mixing the oligonucleotides in equimolar ratios in a hybridization buffer that mimics physiological ionic strength and pH. The mixture is heated to 95 °C to fully denature any mismatched base-pairing, followed by gradual cooling to 4 °C, which promotes accurate hybridization between the complementary strands [16]. The rate-determining step is the annealing of the unnicked sides because they are the largest continuous stretches of DNA. They hybridize more efficiently, allowing the other strands of ssDNA into position through spontaneous self-assembly. The nicks produced by the joining of the broken-end domain may then be repaired by a DNA Ligase to reduce nuclease-mediated degradation (Figure 1) [16]. The self-assembling nature of the procedure makes it incredibly efficient, both in terms of time and yield, as the entire process is over 95% efficient [16].
The second key step in TDN synthesis is functionalization. While modification can occur either at an edge or a vertex of a tetrahedron, applications in nucleic acid biosensing typically restrict functionalization to a vertex [18,24,28]. The preference arises from steric effects and the role of the TDN in biosensor development; attaching probes along an edge can create interference with neighboring units, obstructing analyte binding and disrupting probe orientation on the sensor surface. To ensure stability in its three-dimensional structure, ssDNA is often attached at a vertex via an overhang from one of the four oligonucleotides. This attachment is typically limited to the upward-facing vertex to prevent steric clashes between probes. The attachment of biorecognition elements onto the vertex does not occur until after the assembly of TDNs, as interference created by their presence would hinder the binding of the complementary bases and impede the formation of the structure [29]. The other three vertices of the TDN are generally functionalized with anchoring groups instead of probes (Figure 2). This anchoring group is commonly a thiol, specifically in the case of a gold surface [28,30]. However, other surfaces may require different chemistries, for example, pyrene butanoic acid succinimidyl ester (PBASE) for carbon nanotube surfaces [29]. These anchoring groups are incorporated before assembly so that, upon completion, the three predetermined bottom vertices can covalently bond to the sensor surface, positioning the probe correctly for detection [31].
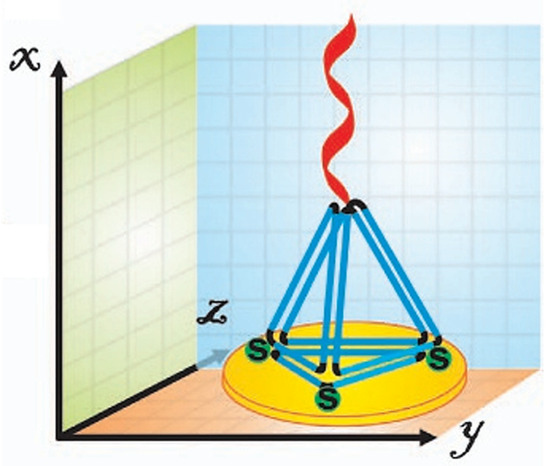
Figure 2.
Schematic illustration of tetrahedral DNA nanostructure (TDN) functionalization in a biosensor. Three vertices are modified with thiol groups (green) for surface anchoring, while the upward-facing vertex carries capture DNA (red) for target recognition. Reproduced from [28] under the terms of the Creative Commons Attribution-Non-Commercial License (CC BY-NC).
Recent advances have identified numerous novel nucleic acid biomarkers, leading to the development of biosensors capable of detecting multiple analytes simultaneously [21]. In these systems, each TDN still carries only a single probe attached to the upward-facing vertex, while the remaining three vertices are functionalized to anchor the structure to the sensor surface. Multiplexing is achieved not by loading multiple probes onto one nanostructure, but by using different populations of TDNs, each functionalized with a distinct biorecognition element and operating independently [21]. Although it is theoretically possible to functionalize more than one vertex with probes, this approach reduces immobilization efficiency. The three anchoring points of a TDN form a plane parallel to the sensor surface, which enhances structural stability and improves signal strength [29].
2.3. Signal Amplification Methods
A major barrier to the widespread application of nucleic acid biosensors as rapid diagnostic tools is the typically low concentration of analytes in a biological system. While genomic DNA is abundant, specific markers like circular DNA (circDNA) and characteristic microRNAs (miRNAs) are present at much lower levels [23,32,33]. Although detecting systems could be designed to detect these biomolecules, they often fail to generate signals large enough for reliable quantification. This has left known biomarkers detectable but without a reliable pathway for clinical quantification.
Structural optimizations play a critical role in enhancing signal output. By promoting uniform and complete surface filling, these approaches directly contribute to signal amplification through improved hybridization efficiency and reduced background noise, which in turn amplifies the electrochemical signal. The rigid, three-dimensional structure of the TDN creates a surface that can anchor probes, precisely controlling their spatial arrangement. This improves the efficiency of reactions and signal amplification.
Polymerase chain reaction (PCR) has commonly been used to detect the target DNA by amplifying a segment of DNA; however, it is a time and resource-intensive method, making it unsuitable for many diagnostic applications. Moreover, it tends to favor nucleic acids present at higher concentrations, introducing bias and reducing reliability compared to direct measurement [34]. Unlike PCR, which amplifies the target nucleic acid itself, TDNs enhance biosensor performance by improving probe stability, reducing background noise, and facilitating efficient target capture. This signal optimization capability makes TDNs increasingly valuable in biosensing applications, particularly where direct detection methods are preferred.
The rigid molecular architecture of TDNs, combined with their three thiol linkages to the sensor surface, promotes a planar orientation that is highly resistant to structural rearrangement and probe displacement during hybridization or washing steps. This arrangement ensures uniform immobilization, prevents surface defects, and enhances sensor accuracy (Figure 2) [28]. Because probes are attached via overhangs, the structural stability of the TDN is transferred to the biorecognition elements, which are held in a secure and fixed position [35]. As a result, the likelihood of false-positive signals arising from nonspecific interactions is reduced. Moreover, background noise is minimized, since conformational changes of the probe are restricted and occur only during hybridization with the target nucleic acid [36]. This improved understanding of the mechanism of signal production has driven the widespread incorporation of TDNs into nucleic acid biosensors. Compared to traditional ssDNA probes or DNA-sandwich configurations, TDN-based sensors consistently demonstrate drastic improvements in sensitivity, lowering the limit of detection by three to four orders of magnitude [12,31]. Attempts to detect miRNA 196a, a known marker of pancreatic carcinoma [21], had previously been constrained to a limit of detection of 10 pM when utilizing ssDNA to capture the analyte [37]. The incorporation of a TDN enabled a dramatic improvement, reducing the LOD to 3.1 aM, over six orders of magnitude lower, due to enhanced stability and spatial orientation of the biorecognition elements [36]. Similarly, in electrochemical biosensing of miRNA in serum, a DNA-super-sandwich strategy achieved an LOD of 0.6 pM, whereas a TDN-based sensor reached an LOD of 0.04 fM, representing a ~15,000-fold improvement [38,39].
This enhancement is largely attributed to their well-defined three-dimensional architecture, which promotes uniform probe orientation and spacing, thereby reducing steric hindrance and minimizing surface defects. In addition, the rigid tetrahedral framework stabilizes the attached probes, preventing unwanted conformational changes and reducing background noise. Together, these features extend the dynamic range of detection, enabling more accurate quantification across a broader range of analyte concentrations. As a result, sensors that were previously restricted to the micromolar or nanomolar range can now reliably detect targets at picomolar or even femtomolar levels [12,31,38,40]. Unlike PCR-based strategies, which rely on amplification of the analyte itself and often introduce bias toward more abundant nucleic acids, TDN-based systems amplify the signal generated upon target binding. This distinction allows TDN-inclusive biosensors to achieve highly sensitive and quantitative detection without requiring additional enzymatic steps, positioning them as strong candidates for rapid and resource-efficient diagnostics.
2.4. Biocompatibility
TDNs display high biocompatibility, making them a promising candidate for in vivo sensing applications. Unlike many other DNA-based sensing platforms that struggle with stability and limited lifetimes under biological conditions, TDNs demonstrate enhanced durability and functional integrity in complex biological environments. TDNs exhibit strong resistance to nucleases, which typically degrade other structural forms of DNA. In human serum, TDNs were found to have a half-life of over ten hours, which is approximately ten times longer than that of conventional cell-free DNA [41,42]. This is largely due to the compact nature of TDNs, as opposed to the looser and linear structure of most cell-free DNAs, which makes them more susceptible to nuclease-induced degradation. Beyond their stability, TDNs are also extremely safe. They show no observable cytotoxicity and do not trigger an immune response, unlike other molecules such as proteins that may provoke such reactions [43]. The combination of extended lifespan and excellent biocompatibility indicates that TDNs have immense potential for integration into wearable biosensors designed to detect various nucleic acids.
2.5. Future Research and Limitations
While TDNs offer significant advantages over traditional surface modification strategies, several challenges must be addressed before their broader application in biosensing and diagnostics. The most prominent limitation is the cost of synthesis. Because the self-assembly process requires highly pure oligonucleotides, production remains expensive. Current estimates place the cost of analogous DNA nanostructure synthesis at approximately €125 per milligram, an impractical expense for large-scale or disposable clinical devices, particularly in wearable sensors that require frequent replacement due to TDN degradation [42]. There has been recent development in the synthesis of these oligonucleotides, bringing the cost down by nearly three orders of magnitude, but future research must be conducted to determine the viability of using this method for TDN synthesis [44]. Future research could also decrease the cost by further reducing the rate of nuclease degradation. This would increase their lifespan in a biological environment and make replacements less frequent. While the ten-hour lifespan is significantly higher than other DNA-based structures, it is still a concern for wearable devices [41,42]. Constantly replacing components of the sensor would both inflate the price and reduce the convenience, as backup units must always be on hand. One potential avenue for future research is the viability of a synthetic coating to protect the DNA. Though silicon-based coatings are an effective method of protecting other DNA nanostructures, it has not yet been explored for use in shielding TDNs [45]. Investigating such protective approaches, alongside innovations in low-cost synthesis and improved nuclease resistance, will be crucial to unlocking the full potential of TDNs for practical, real-world biosensing applications.
3. Self-Assembled Monolayers
3.1. Overview
Self-Assembled Monolayers (SAMs) have long served as a foundational strategy in biosensor surface engineering, offering precise control over molecular orientation and density. While traditional SAMs rely on small molecules like thiols, recent advances have explored the use of DNA strands, particularly thiolated DNA, as a means of forming DNA-based SAMs, bridging the gap between conventional surface chemistry and DNA nanostructure integration. SAMs are defined as single layers of organized molecules that form on a surface through chemical bonding (Figure 3). In broad terms, these molecules align into a densely packed, ordered layer due to surface interactions and intermolecular forces. SAMs have become a widely used method for surface modification, as they can be easily formed by attaching specific molecules to a variety of surfaces such as gold, silver, platinum, and palladium [46]. Gold in particular is commonly used in biological settings due to its chemical stability and biocompatibility. The surface properties of SAMs can be precisely adjusted, enabling tailored interfaces for various sensing and biomedical applications. The structure of a SAM usually consists of three components: the head group, the spacer, and the terminal functional group (Figure 3). The headgroup is chosen based on its ability to bind to a specific substrate of the sensing surface. Common head groups include thiols, silanes, imidazoles, or phosphonates. Thiol headgroups are mostly preferred for gold and other noble metal surfaces due to their strong and stable attachment [47].
The middle component, spacer, is typically composed of an alkyl chain that influences the thickness of the monolayer and packing density through van der Waals interactions [48]. The final component is the terminal functional group, which can be chemically modified to enable interactions with recognition elements or biological molecules. In biological applications, common terminal groups include amino groups (or their protonated forms), carboxylic acids, Hydroxyl and Polyethylene Glycol, and thiols [49,50,51]. These groups facilitate covalent or electrostatic interactions with biomolecules such as proteins and nucleic acids. Thiol groups play a dual role in SAM design: while they are commonly used as head groups for anchoring molecules to gold surfaces due to their strong and stable Au–S bond, they can also serve as terminal groups in more complex SAM architectures. This versatility makes thiols particularly valuable in biosensor surface engineering, especially when integrating DNA nanostructures that require robust and reliable attachment to metal substrates.
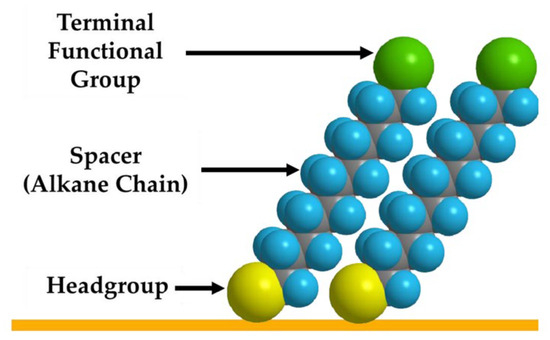
Figure 3.
Schematic representation of the three essential parts of a Self-Assembled Monolayer (SAM). Image was reprinted with permission from [51] under the terms of the Creative Commons Attribution License (CC BY 4.0).
SAMs offer a customizable platform for incorporating functional compounds into diagnostic devices and have become essential for achieving reliable, high-performance biosensing. Through advancing research, SAMs have become a foundational tool in the development of sensitive and label-free detection technologies. In nucleic acid-based biosensors, SAMs are commonly used to immobilize single-stranded DNA or RNA probes that can recognize and bind to complementary sequences in a given sample. The resulting hybridization event can then be detected using methods, including electrochemical, optical, or acoustic wave biosensors. When properly assembled, SAMs ensure high probe coverage and minimize NSA, which are critical for improving the accuracy and sensitivity of the sensor [51].
3.2. Signal Amplification Methods
SAMs can be chemically or physically engineered to optimize their interaction with biorecognition elements, thereby enhancing the effectiveness in nucleic acid detection. These modifications aim to improve signal strength, increase hybridization efficiency, stabilize probe attachment, and minimize NSA. A common approach involves immobilizing DNA via thiol-gold linkages and using small thiol molecules such as mercaptohexanol to “backfill” the unreacted or unoccupied regions of the sensing surface. This strategy helps space out the DNA probes and promotes an upright orientation, which improves accessibility for target binding and reduces background interference.
A new approach was introduced for forming stable, multifunctional monolayers on carbon surfaces using protected aryldiazonium ions [52]. As shown in Figure 4, this approach involves a three-step modification process to facilitate the covalent attachment of ethynyl-, amino-, and carboxy-terminated tethers in monolayers, which were then back-filled with complementary amine derivatives to increase the functional group density. Electrochemical analysis revealed that the backfilled monolayers achieved a fractional surface coverage of approximately 0.4, compared to 0.2 for layers without backfilling. The doubled surface coverage monolayers demonstrated strong potential for further surface modification, with promising applications in biosensing, molecular electronics, and catalysis.
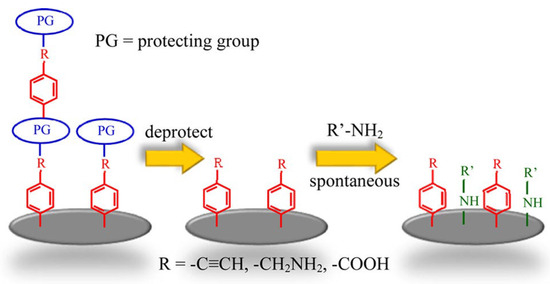
Figure 4.
The figure shows a three-step process for forming multifunctional monolayers on carbon surfaces. First, protected aryldiazonium salts are used to attach functional groups (R) to the surface. The protecting groups (PG) are removed, exposing reactive sites. Finally, these sites react with amine derivatives (R’-NH2) through nucleophilic addition, resulting in a backfilled monolayer with increased surface coverage. Image was reprinted with permission from [52] using Copyright © 2016, American Chemical Society.
Spacers like polyethylene glycol (PEG) chains or alkyl groups are often introduced between the thiol anchor and the functional group to enhance the performance of biosensors. These spacers increase molecular flexibility, reduce steric hindrance, reduce the NSA, and improve the kinetics of hybridization. A study investigated how different spacers affect the hybridization efficiency of self-assembled mixed DNA films used in biosensors. [53]. In this study, various mercaptohexyl (C6) and mercaptoundecyl (C11) spacer groups were used to attach a thiol at the 5′ end of a certain DNA sequence (Figure 5) derived from the stx2 gene of E. coli. [53].
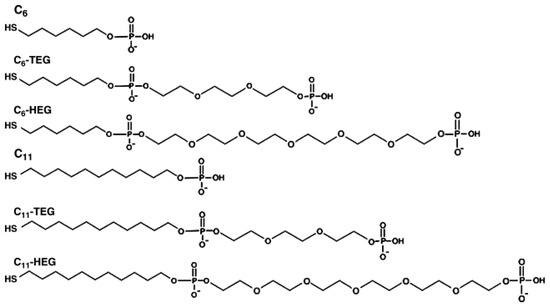
Figure 5.
Chemical structure of the different spacers coupled to the 5′ end of DNA capture probes. Image was reprinted with permission from [53] under Copyright © 2008 Elsevier B.V.
Using surface plasmon resonance (SPR) analysis, the researchers found that the longer C11 spacer exhibited a sensitivity signal of 99.2 RU/nM while the shorter C6 presented a signal of 3.6 RU/nM. These results indicated that the sensitivity improved by more than 25 times when the longer spacer was used. The C6 spacer exhibited a higher detection limit of 21.6 nM, compared to the 0.3 nM result from the C11 spacer, which also displayed better reproducibility. These findings underscore the critical role of spacer length in optimizing biosensor performance.
Szymczyk-Drozd et al. reported on a comprehensive study of the effect of SAM formation on the performance of their developed DNA-based electrochemical biosensor. The methylene blue-labeled SAM-based sensors fabricated on planar gold transducers achieved reliable SARS-CoV-2 detection in untreated post-PCR samples at concentrations below 10 nM, with stable signal responses and clear discrimination between positive and negative samples [54]. A DNA origami nanostructure using mixed pDNA/MCP (3-Mercapto-1-propanol) SAM was used to enhance the electrochemical signal of the developed label-free electrochemical biosensor. This design improved the limit of detection by two orders of magnitude compared to a conventional label-free e-DNA biosensor while achieving a high degree of strand selectivity in a challenging DNA-rich environment [55]. These studies highlight the critical role of SAM organization in achieving low detection limits and reproducible biosensor performance.
Redox-active molecules such as ferrocene and methylene blue are often incorporated into DNA probes to enhance signal output. These labeling molecules undergo redox reactions when hybridization occurs, amplifying the biosensor’s response and improving sensitivity. A study explored a redox-tagged peptide designed for capacitive biosensing to evaluate the effects on improving detection [13]. The peptide, tagged with ferrocene, self-assembled onto gold electrodes and enabled highly sensitive detection of C-reactive protein (CRP), a key inflammation biomarker. The electroactive labeled peptide SAM achieved a lower detection limit of 0.80 nM compared to the 0.24 nM result from the non-labeled mixed thiolated SAM [13].
Creating nanostructured surfaces, such as nanopillars or nanogaps, further enhances biosensors’ function by increasing the effective surface area. This allows for the immobilization of a greater number of probes and amplifies the sensor’s signal response. Additionally, incorporating nanoparticles or textured surfaces can enhance local electric fields, particularly in electrochemical and plasmonic biosensors, thereby improving the sensitivity of biosensors. A study by Cortés et al. demonstrated that nanostructured gold substrates provide significant advantages for the stability and performance of alkanethiolate SAMs compared to conventional flat gold surfaces [56]. The nanostructured gold, composed of nanocolumns with diameters ranging from 10 to 20 nanometers, showed no significant degradation of thiolate coverage after 45 days of immersion in ethanol under dark conditions. In contrast, the polycrystalline oriented gold surface lost 40% of thiolate species within 2 weeks under the same conditions. This enhanced stability is linked to the stronger binding affinity between thiol groups and the low-coordinated gold atoms present in surface defects of the nanostructured material. These findings highlight the potential of nanostructured gold as a robust and sensitive platform for SAM-based biosensors and other biosensors.
SAMs can also serve as effective platforms for enzyme-free signal amplification strategies. These approaches rely on the formation of long DNA structures that hybridize to immobilized probes on the surface, resulting in a measurable increase in mass or charge and thereby strengthening the signal. A recent review article highlights the effectiveness of DNA hybridized chain reaction (HCR) and DNA supersandwich self-assembly (SSA) as two enzyme-free amplification strategies for the ultrasensitive detection of various biomarkers, including nucleic acids, proteins, and small molecules [57]. These amplification strategies offer several advantages, including high specificity, operational simplicity, and compatibility with various detection formats. The authors suggested that combining DNA HCR and SSA with other amplification strategies could further advance the performance of biosensors, particularly those based on SAM-functionalized surfaces.
3.3. Applications of SAMs
SAM strategies are used across a wide range of applications, including biomedical diagnostics, environmental monitoring, and food safety. One of the most prominent applications is in medical diagnostics, where SAM-based biosensors play a key role in detecting viral RNA and disease-associated genetic mutations. In a study, Orozco et al. reported on the development of an electrochemical genosensor for SARS-CoV-2 that used thiolated DNA probes immobilized via SAM chemistry on maleimide-coated particles [58]. The device employed a sandwich hybridization format and achieved highly sensitive detection of viral RNA in clinical samples, highlighting the effectiveness of SAMs in real-world diagnostic applications. SAM strategies are also applied in microarrays and lab-on-a-chip technologies, enabling high-throughput genetic analysis and multiplexed biosensing.
In DNA microarrays, thiol-gold SAMs are used to immobilize different probes in discrete spots on a chip. Lee et al. demonstrated that using mixed DNA/alkanethiol SAMs with controlled probe spacing improves hybridization efficiency by reducing steric hindrance, leading to more reliable detection of single-nucleotide variants [59]. Similarly, SAMs are integral to lab-on-a-chip devices, where they support miniaturized, automated biosensing. A microfluidic chip was developed using a nanoporous gold electrode array, each functionalized with a unique thiolated DNA probe via SAM electro-grafting [60]. This setup allowed simultaneous detection of three cancer biomarkers (BRCA1, BRCA2, and p53) from a single sample, while reducing reagent consumption and speeding up analysis. These studies highlight how SAMs enhance the precision, efficiency, and scalability of genomic screening tools.
SAM-based biosensors are also widely used in environmental and food monitoring due to their high sensitivity and specificity. They are particularly effective for detecting microbial contamination and Genetically Modified Organism (GMO)-related genes in complex samples. In food safety, SAM-modified electrodes have been employed to identify pathogens such as Salmonella and E. coli. An electrochemical DNA sensor for detecting Salmonella in milk was reported using a thiolated probe immobilized on a gold nanoparticle-modified surface [61]. The biosensor achieved an ultralow detection limit of 10−18 M for detecting Salmonella DNA in spiked milk samples [61].
In forensic science, SAM-based biosensors are increasingly explored for detecting trace DNA at crime scenes. These biosensors offer high sensitivity, making them ideal for minimal or degraded samples. One study used Diamond™ Nucleic Acid Dye on SAM-coated surfaces to visualize both fingerprint patterns and the DNA present [62]. This dual-purpose approach highlights how SAMs can support rapid, on-site DNA detection. Future kits may use similar SAM-coated slides for real-time forensic analysis without requiring full lab processing.
In drug discovery, SAM-based biosensors are used to evaluate how small molecules interact with nucleic acids. Ozkan et al. developed an electrochemical sensor with thiol-linked double-stranded DNA immobilized on a nanoparticle-modified electrode [63]. They tested anthracycline drugs like doxorubicin and observed changes in guanine and adenine oxidation signals, indicating intercalation into the DNA helix. This approach offers a simplified, sensitive method for analyzing drug–DNA interactions.
3.4. Limitations
Despite their wide applicability, SAM-based systems face certain limitations. Stability is a key concern, particularly for SAMs formed via thiol-gold chemistry, as highlighted by Srisombat et al. [64]. While SAMs are widely used in biosensors due to their ease of fabrication and chemical versatility, their long-term performance is hindered by several factors. Environmental conditions such as oxidation, UV exposure, heat, and chemical stress can affect the gold–thiol bond, leading to degradation or molecular exchange. An example of the extent of degradation was explored by Yang et al. [65]. In this research, C18 thiol SAMs were heated at 80 °C, showing minimal changes after one hour. Comparatively, the same compound demonstrated substantial degradation after 16 h of heating, as evidenced by water-contact-angle and XPS analysis, with an increase in oxygenated species. Similarly, Willey et al. proposed that alkanethiol SAMs stored in ambient laboratory air were found to undergo rapid oxidation [66]. This was discovered when a freshly prepared SAM with only bound thiolates (~0% oxidized S) was compared to an identically prepared SAM that had been exposed to air and light. The exposed SAM had ~97% of its sulfur in an oxidized state, suggesting that the gold–thiol bond is susceptible to oxidation by oxygen and other oxidants. Besides heat and oxidation, structural features such as the length of the alkyl chain and the polarity of the terminal groups reduce molecular packing density, making the monolayer more vulnerable to environmental degradation. To overcome these issues, researchers have proposed strategies such as using longer alkyl chains, hydrophobic terminal groups, multidentate headgroups, and crosslinking techniques. These strategies are essential for improving SAM durability and ensuring their effectiveness in biosensing applications. Surface defects are another key challenge, particularly in applications requiring high resolution and reproducibility. Gannon, G. et al. used molecular dynamics simulations to prove that domain boundaries and surface irregularities disrupt the uniform packing of SAMs [67]. This study addresses a key challenge in nanofabrication, controlling molecular diffusion during microcontact printing to prevent smudging in nanopatterns. Using large-scale atomistic simulations, researchers found that domain boundaries in SAMs can trap excess ink molecules and act as diffusion barriers, helping limit unwanted spreading. These findings offer insights into improving pattern resolution and suggest mechanisms for SAM self-repair at the atomic level. The study underscores the importance of controlling surface defects to ensure the reliability and consistency of SAM-based sensors [67].
Limited multiplexing remains another challenge in SAM-based biosensors, as immobilizing multiple DNA probes on a single surface can lead to cross-interference and reduced probe activity. While traditional microarrays address this by placing each probe in distinct spots, attempts to mix multiple probes within a single monolayer on the sensing surface require precise control of probe spacing. Without sufficient lateral separation, probes can interfere with each other’s binding efficiency. As a result, most reliable multiplexing strategies use compartmentalized formats, such as separate electrodes or printed spots, to maintain the functionality and specificity of each probe, ultimately avoiding cross-reactivity [68].
Another limitation in SAM-based biosensors is the sensitivity plateau, where signal output stops increasing despite higher analyte concentrations. Although sensitivity plateaus are commonly seen in most biosensors when analyte binding sites become saturated, SAM-based biosensors can experience this effect even earlier due to additional interfacial factors such as probe crowding, steric hindrance, and uneven probe orientation, which limit target accessibility and signal gain. In studies using electrochemical DNA biosensors, researchers observed that even when all accessible probes on the sensor surface had hybridized, a residual signal remained. This suggests that a portion of probes may be inaccessible due to crowding or poor orientation, preventing full target binding. As a result, the signal levels off, indicating that the sensor has reached its maximum response. This plateau effect highlights how surface saturation and steric hindrance can limit dynamic range, even when amplification strategies are employed [69].
4. DNA-Based Hydrogels
4.1. Overview
Hydrogels are yet another example of intriguing nanostructures with the capacity to be utilized in the surface engineering of biosensors. Although DNA hydrogels can exhibit micrometer-scale thickness, their architecture in biosensor applications is composed of nanometer-scale features, which is why they are commonly classified as nanostructures. They are a network of hydrophilic polymers that have been cross-linked to create a larger three-dimensional complex. This structure is naturally porous as the space between the cross-linked polymers allows for material to enter and exit. It is this porosity and inherent hydrophilicity that define the properties of a hydrogel, enabling it to absorb substantial amounts of water (Figure 6), up to 3000 times its own weight, and form a gel that closely mimics the high water content of a biological environment [70]. This characteristic is particularly advantageous in the development of DNA-based hydrogels for biosensors, where maintaining a hydrated, biocompatible matrix is essential for preserving nucleic acid functionality and facilitating molecular recognition. Although hydrogels have been utilized across a wide range of applications since the 1960s [71], their integration into sensor technologies only began in 1992 [72]. Since then, they have become a focal point of innovation in biosensor technology, and their development has been thoroughly reviewed in recent articles, which detail their classification, sensing mechanisms, and device applications [73].
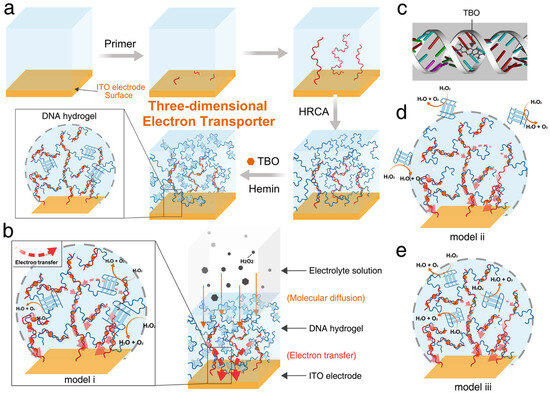
Figure 6.
Schematic representation of a DNA hydrogel-based three-dimensional electron transporter for the Fabrication of the 3D electron transporter on an ITO electrode via hybridization chain reaction amplification. (a) 3D Electron Transporter on the Surface of the ITO Electrode throughHRCA; (b) Catalysis and Electron Transport in the 3D Electron Transporter (Model (i), the DNAzyme is Incorporated at the Node of the Hydrogel Scaffold); (c) Molecular Docking Result of TBO and Double-Stranded DNA; (d) Model (ii), separate DNAzyme is Put in the Electrolyte; and (e) Model (iii), the Separate DNAzyme is encapsulated in the DNA Hydrogel. Image was reprinted with permission from [74] Copyright © 2020, American Chemical Society.
Despite the breadth of research, much of the effort has concentrated on identifying optimal materials for hydrogel construction, with particular attention to four key properties: biocompatibility, rigidity/stability, transduction capacity, and cost-effectiveness [75]. Among the materials currently under exploration for hydrogel construction, the biopolymer DNA has emerged as a promising candidate. First proposed for incorporation into hydrogels in 1996 [76], DNA has since been used in diverse hydrogel-based applications such as hydrophobic drug delivery [77], tissue regeneration [78], bone development [79], and biosensing [14]. Its inherent hydrophilicity and high biocompatibility make DNA particularly well-suited for forming or enhancing hydrogel systems, contributing to its growing adoption over traditional polymers [80,81]. Figure 6 illustrates an example of the development of a three-dimensional DNA hydrogel structure for enhanced electron transport in biosensing. In this system, a highly branched DNA hydrogel is synthesized via hybridization chain reaction amplification (HRCA), forming long double-stranded DNA strands that serve as a scaffold. An electron mediator, toluidine blue O (TBO), intercalates into the DNA scaffold, enabling efficient electron transfer from distant catalytic sites to the electrode surface [74].
DNA hydrogels can generally be categorized into two types: pure and hybridized, reflecting how much DNA is present within the structure. The hybridized form consists of DNA cross-linked with other varieties of hydrophilic polymers, whereas the pure variant is made entirely from DNA. Among the two, the most commonly used in biosensors is the original form, in which DNA pendants are attached to a polymeric (often polyacrylamide) backbone [81,82,83]. This approach is particularly favored by researchers who seek to use the hydrogel both as a scaffold and a transducer, as it combines the molecular recognition and biocompatibility of DNA with the mechanical strength and responsiveness of a polymer such as graphene [14].
4.2. Structure and Assembly
The structural diversity of DNA-based hydrogels is immense. While this versatility is arguably their greatest strength, it also leads to a wide range of architectures, functional features, and fabrication methods. Nevertheless, DNA hydrogels share a set of transferable and consistent traits that, although varying in degree, remain significant across all systems.
DNA hydrogels can be broadly classified into pure DNA and hybrid forms. Hybrid DNA hydrogels exhibit significantly greater structural variability due to the wide range of molecules that can be incorporated. Common configurations include DNA serving as a crosslinker between synthetic polymers like polyacrylamide or nanomaterials such as graphene, with nearly limitless design possibilities [14,84]. Recent studies have also explored the integration of quantum dots and other nanoparticles, although DNA attachment to large polymer backbones remains more prevalent [85].
This structural flexibility allows for the customization of key properties, such as rigidity, transduction compatibility, hydrophilicity, and pore size, tailored to specific sensor applications. In electrochemical biosensors, this modularity is especially valuable, as incorporating nanomaterials into the hydrogel can significantly lower detection limits. For instance, a hybrid hydrogel composed of DNA and a semiconductor like n-doped graphene oxide is highly sensitive to negatively charged molecules such as nucleic acids [14]. Upon target binding to biorecognition elements, an electrical signal is generated. While pure DNA hydrogels lack conductivity, the graphene oxide facilitates efficient charge transfer to the electrode, enhancing signal transduction. The ability to select semiconductors with precise electrical characteristics enables optimized charge transfer from the hybridization event to the electrode.
In contrast to hybrid forms, pure DNA hydrogels are composed entirely of DNA, offering precise control at the monomeric level. Each nucleotide can be selectively chosen to optimize specific functions, enabling features such as pH-responsive dissociation—achievable by altering just a three-base sequence [85]. DNA is also synthesized base-by-base, allowing for an extremely consistent synthesis [86]. Pure DNA hydrogels were originally produced by crafting and then cross-linking individual monomers [87]. In their first reported use in 2006, three distinct branched DNA monomer shapes were engineered, each with complementary sticky ends that enabled cross-linking through base pairing [87]. The use of these specific and defined monomers creates a consistent framework of pores, in which size could be customized depending on the size of the target molecule.
Though this procedure is still used, there are inherent limitations to its execution. The cost of this precise and repeated replication of an exact sequence of DNA is often too high to be practical for any large-scale use [88]. Because of this, a second category of synthetic methods has been developed in which the hydrogels are produced through techniques capable of producing much larger strands of DNA [88]. This can be achieved through a host of different methods, including rolling circle amplification (RCA), in which much larger pieces of DNA are built before being cross-linked in a manner not dissimilar to the polyacrylamide-backbone strategy. Clamped hybridized chain reaction (C-HCR) takes this even further, as it is capable of replicating a given sequence to produce entire hydrogels as one continuous network of nucleotides [89]. Because the cross-linking between monomers occurs during the synthesis, the chance of an error in this step is greatly reduced compared to the less controlled methods employed by traditional procedures [89].
4.3. Signal Production and Amplification
In hydrogel-based biosensors, signal generation occurs through physical or electrochemical change as a result of the binding of a target molecule to a probe [90]. Due to their high customizability, the mechanism of signal generation can vary widely. Common methods take advantage of changes such as swelling, shrinking, dissolving, or a change in the structural conformation upon target binding [90]. Similarly to other sensor platforms, these changes can be converted into measurable outputs like electrochemical signals, colorimetric shifts, fluorescence, or other optical transmissions [90]. This versatility, rather than specificity, has contributed to the widespread adoption of hydrogel-based biosensors. Since their initial use in surface plasmon resonance (SPR) sensors [72], they have been integrated into a broad range of sensing modalities, including colorimetric, fluorescent, electrochemical, photoelectrochemical, surface-enhanced Raman scattering, Bragg scattering, chemiluminescent, and electrochemiluminescent sensors [91]. In nucleic acid biosensing, analyte concentrations are often extremely low within a given sample, posing a significant detection challenge. This has led to the development of surface engineering strategies that enable both detection and signal amplification. While these strategies often involve incorporating multiple surface components, hydrogels offer a unique advantage: they can function simultaneously as signal amplifiers and transducers [77].
In electrochemical biosensors, hydrogels play a particularly valuable role by facilitating efficient electron transfer and enhancing the interface between the biological recognition element and the electrode surface [14]. At a fundamental level, hydrogels provide a structural support to prevent false signals or steric interference [84]. However, more recent developments have led to the integration of more advanced signal amplification techniques within the overall structure of the hydrogel [92]. In electrochemical biosensors, these systems have included HCR [93], RCA [94], and strand displacement amplification (SDA) [95]. The incorporation of these techniques into the framework of the hydrogel enhances their sensitivity, allowing them to be used for targeting nucleic acids that are present at very low concentrations. Because of this, electrochemical biosensors using DNA hydrogels are becoming increasingly common, as their newfound sensitivity, combined with their structural tunability, has made them incredibly promising for novel diagnostic tools.
One effective strategy to overcome the low analyte concentrations is the use of HCR in a hydrogel to facilitate the production of a stronger signal which harnesses the strong biotin-avidin affinity and the complementarity of related nucleic acid sequences [92]. The process begins with target hybridization to a probe within the hydrogel, followed by ligation with a biotinylated adapter and binding of neutravidin. This initiates a cascade involving hairpin-shaped oligonucleotides, where each hybridization event triggers the unfolding of the next, forming a long nucleic acid chain with multiple biotin sites. These sites bind to fluorescent reporters like streptavidin-phycoerythrin, resulting in a significantly amplified signal [92]. This approach demonstrates how target recognition can initiate a secondary amplification process, enhancing sensitivity by an order of magnitude. Another technique, rolling circle amplification (RCA), achieves similar sensitivity gains by generating multiple copies of the target sequence, increasing the number of binding events [96].
4.4. Biocompatibility
While DNA-based hydrogels are generally biocompatible, thanks in part to their high water content, the specific materials used can significantly influence this property [81,97]. Because DNA hydrogels are inherently tunable, their biocompatibility can similarly fluctuate, as demonstrated by the higher biocompatibility of pure DNA sensors compared to their hybrid counterparts [97]. However, this advantage comes at a cost of reduced resistance to enzymatic degradation. DNA is a naturally occurring biomolecule in all living organisms and is inherently extremely safe [98,99]. Though this safety is an important qualification for in vivo biosensors, its ubiquity also means that the body has mechanisms for recycling these nucleic acids in the form of nucleases. This degradation can be reduced by modifying both the shape and the nucleotide sequence of the monomers [97]. Pure DNA hydrogels, due to their higher proportion of DNA, are more susceptible to enzymatic degradation. While biodegradability helps prevent accumulation and adverse effects in vivo, it also shortens the functional lifespan of the sensor [99]. Therefore, designing DNA hydrogels for biosensing requires a careful balance between biocompatibility, stability, and structural simplicity. Overly complex formulations may reduce synthesis efficiency, increase production costs, and lower overall yield.
4.5. Future Research and Limitations
The use of DNA-based hydrogels is not without limitations. The aforementioned challenges in avoiding interactions with nucleases limit the maximum lifespan of a sensor, often forcing frequent replacements. This issue is further compounded by the inherently single-use design of many hydrogels, particularly those designed to degrade upon interaction with a target molecule to produce a signal [85]. While this design would be suitable for sensors aiming to provide a simple yes/no report on the presence of a characteristic target, it would not be effective for continuous monitoring of biomarkers that fluctuate over time.
Hydrogels are also often limited to a specific set of operating conditions due to their fragility. The pure DNA hydrogels in particular exhibit poor mechanical strength, which is problematic in wearable biosensors that would need to be capable of withstanding extended exposure to biological conditions and regular movement of the users. Because of this, the hybrid version is perhaps better suited to address this issue due to the more rigid nature of its non-biological backbones. However, this advantage is offset by their generally lower biocompatibility [81]. Additionally, both types would encounter significant limitations in sensitivity. While highly sensitive sensors have been developed using DNA hydrogels, they often rely on non-biological conditions. For example, current HCR protocols require temperatures more than 15 °C above and below the physiological temperature of 37 °C [92]. Further research is needed to optimize these processes for conditions that are safe and practical for use in humans.
Both nuclease activity and the challenges in maintaining structural integrity contribute to a third issue, one that may be even more pressing: financial constraints. The frequent need to replace sensors due to structural failure or the nuclease-induced degradation leads to a bloated cost of operating, making them impractical for clinical use beyond specific single-use applications.
5. Conclusions
Surface modification remains a critical driver in the advancement of nucleic acid biosensors, directly impacting their sensitivity, specificity, and reliability. These techniques are central to the development of nucleic acid biosensing, and each strategy, Tetrahedral DNA Nanostructures (TDNs), Self-Assembled Monolayers (SAMs), and DNA hydrogels, offers unique advantages that contribute to high-performance sensor design. Table 2 summarizes the feature characteristics of these strategies.

Table 2.
Key features of TDN, DNA SAM, and DNA hydrogel strategies in surface engineering of electrochemical biosensors.
TDNs offer a structurally rigid and precisely programmable platform that ensures consistent probe spacing, reduced background signal, and enhanced hybridization efficiency. Their three-point anchoring strategy, combined with defined probe orientation, significantly lowers the limit of detection and improves reproducibility. Additionally, their high biocompatibility and enzymatic resistance make them excellent candidates for wearable or in vivo biosensors. However, widespread adoption is currently limited by synthesis costs and the need for improved scalability. SAMs provide a customizable, chemically robust interface for probe immobilization, enabling precise control over surface chemistry, probe orientation, and density. Their ease of fabrication and compatibility with a wide range of transduction methods have made them broadly applicable, particularly in point-of-care diagnostics. However, challenges such as limited stability, difficulty with multiplexing, and signal saturation remain. DNA hydrogels introduce a soft, three-dimensional matrix for capturing nucleic acids with high sensitivity and adaptability. Their ability to incorporate enzymatic or nanomaterial-based amplification strategies makes them suitable for ultrasensitive detection, particularly in low-resource or portable settings. Despite their promise, further research is needed to improve their stability and+ streamline their clinical usability.
Collectively, these surface modification strategies represent powerful, complementary approaches in the design of next-generation biosensors. Ongoing research aimed at integrating their strengths while overcoming individual limitations will be key to unlocking new possibilities in diagnostics, precision medicine, and environmental monitoring. Despite their limitations, TDNs, SAMs, and DNA Hydrogels have shown exciting applications in cancer detection, cardiovascular diseases, antimicrobial resistance, and many other diseases [3,4,5]. Combining the current approaches with further research possibilities is an exciting route to improving early detection of diseases and overall healthcare.
Author Contributions
Conceptualization, K.P., N.S., S.N.A. and S.A.; investigation, K.P., N.S. and S.N.A.; writing—original draft preparation, K.P., N.S. and S.N.A.; writing—review and editing, K.P., N.S., S.N.A. and S.A.; project administration, S.A.; supervision, S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The Authors are grateful to the undergraduate program at the Department of Chemistry, University of Toronto, for providing the opportunity for this project. During the preparation of this manuscript, the authors used ChatGPT 4.0 and CoPilot for the purposes of English editing. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, J.; Chao, J.; Liu, H.; Su, S.; Wang, L.; Huang, W.; Willner, I.; Fan, C. Clamped Hybridization Chain Reactions for the Self-Assembly of Patterned DNA Hydrogels. Angew. Chem. Int. Ed. Engl. 2017, 56, 2171–2175. [Google Scholar] [CrossRef]
- Sun, X.; Agate, S.; Salem, K.S.; Lucia, L.; Pal, L. Hydrogel-Based Sensor Networks: Compositions, Properties, and Applications-A Review. ACS Appl. Bio Mater. 2021, 4, 140–162. [Google Scholar] [CrossRef]
- Yan, C.; Hua, Y.; Guo, J.; Miao, P. Programmable DNA Hydrogels Construction with Functional Regulations for Biosensing Applications. TrAC Trends Anal. Chem. 2024, 173, 117628. [Google Scholar] [CrossRef]
- Kim, J.; Shim, J.S.; Han, B.H.; Kim, H.J.; Park, J.; Cho, I.-J.; Kang, S.G.; Kang, J.Y.; Bong, K.W.; Choi, N. Hydrogel-Based Hybridization Chain Reaction (HCR) for Detection of Urinary Exosomal miRNAs as a Diagnostic Tool of Prostate Cancer. Biosens. Bioelectron. 2021, 192, 113504. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Xie, S.; Zhang, J.; Tang, D.; Tang, Y. An Electrochemical Impedance Biosensor for Hg2+ Detection Based on DNA Hydrogel by Coupling with DNAzyme-Assisted Target Recycling and Hybridization Chain Reaction. Biosens. Bioelectron. 2017, 98, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, W.; Zhou, X.; Lin, H.; Zhu, X.; Lou, Y.; Zheng, L. CRISPR-Responsive RCA-Based DNA Hydrogel Biosensing Platform with Customizable Signal Output for Rapid and Sensitive Nucleic Acid Detection. Anal. Chem. 2024, 96, 15998–16006. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Liu, R.; Xie, L.; Yang, H.; Ge, S.; Yu, J. Strand Displacement Amplification Triggered 3D DNA Roller Assisted CRISPR/Cas12a Electrochemiluminescence Cascaded Signal Amplification for Sensitive Detection of Ec-16S rDNA. Anal. Chim. Acta 2024, 1291, 342213. [Google Scholar] [CrossRef]
- Zhang, T.; Tao, Q.; Bian, X.-J.; Chen, Q.; Yan, J. Rapid Visualized Detection of Escherichia Coli O157:H7 by DNA Hydrogel Based on Rolling Circle Amplification. Chin. J. Anal. Chem. 2021, 49, 377–386. [Google Scholar] [CrossRef]
- Lattuada, E.; Leo, M.; Caprara, D.; Salvatori, L.; Stoppacciaro, A.; Sciortino, F.; Filetici, P. DNA-GEL, Novel Nanomaterial for Biomedical Applications and Delivery of Bioactive Molecules. Front. Pharmacol. 2020, 11, 01345. [Google Scholar] [CrossRef]
- Acharya, R.; Dutta, S.D.; Mallik, H.; Patil, T.V.; Ganguly, K.; Randhawa, A.; Kim, H.; Lee, J.; Park, H.; Mo, C.; et al. Physical Stimuli-Responsive DNA Hydrogels: Design, Fabrication Strategies, and Biomedical Applications. J. Nanobiotechnol. 2025, 23, 233. [Google Scholar] [CrossRef]
- Nishikawa, M.; Ogawa, K.; Umeki, Y.; Mohri, K.; Kawasaki, Y.; Watanabe, H.; Takahashi, N.; Kusuki, E.; Takahashi, R.; Takahashi, Y.; et al. Injectable, Self-Gelling, Biodegradable, and Immunomodulatory DNA Hydrogel for Antigen Delivery. J. Control Release 2014, 180, 25–32. [Google Scholar] [CrossRef]
- Bush, J.; Hu, C.-H.; Veneziano, R. Mechanical Properties of DNA Hydrogels: Towards Highly Programmable Biomaterials. Appl. Sci. 2021, 11, 1885. [Google Scholar] [CrossRef]
- Lin, M.; Song, P.; Zhou, G.; Zuo, X.; Aldalbahi, A.; Lou, X.; Shi, J.; Fan, C. Electrochemical Detection of Nucleic Acids, Proteins, Small Molecules and Cells Using a DNA-Nanostructure-Based Universal Biosensing Platform. Nat. Protoc. 2016, 11, 1244–1263. [Google Scholar] [CrossRef]
- Bai, H.; Wang, Y.; Li, X.; Guo, J. Electrochemical Nucleic Acid Sensors: Competent Pathways for Mobile Molecular Diagnostics. Biosens. Bioelectron. 2023, 237, 115407. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, Y.; Li, H.; Gao, Y.; Xiong, X.; Zhang, T.; Zhu, L.; Yang, X. Electrochemical Biosensor Based on a Tetrahedral DNA Nanostructure and an “AND” Logic Gate-Regulated Cascade Amplification System for Parallel Detection of Dual Disease Biomarkers. ACS Sens. 2025, 10, 7744–7756. [Google Scholar] [CrossRef] [PubMed]
- Sandhyarani, N. Surface Modification Methods for Electrochemical Biosensors. In Electrochemical Biosensors; Elsevier: Amsterdam, The Netherlands, 2019; pp. 45–75. ISBN 978-0-12-816491-4. [Google Scholar]
- Hashkavayi, A.B.; Raoof, J.B. Nucleic Acid–Based Electrochemical Biosensors. In Electrochemical Biosensors; Elsevier: Amsterdam, The Netherlands, 2019; pp. 253–276. ISBN 978-0-12-816491-4. [Google Scholar]
- Nascimento, G.A.; Souza, E.V.M.; Campos-Ferreira, D.S.; Arruda, M.S.; Castelletti, C.H.M.; Wanderley, M.S.O.; Ekert, M.H.F.; Bruneska, D.; Lima-Filho, J.L. Electrochemical DNA Biosensor for Bovine Papillomavirus Detection Using Polymeric Film on Screen-Printed Electrode. Biosens. Bioelectron. 2012, 38, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Sumana, G.; Verma, R.; Sood, S.; Sood, K.N.; Gupta, R.K.; Malhotra, B.D. Fabrication of Neisseria Gonorrhoeae Biosensor Based on Chitosan–MWCNT Platform. Thin Solid Film. 2010, 519, 1135–1140. [Google Scholar] [CrossRef]
- Moustakim, H.; Mohammadi, H.; Amine, A. Electrochemical DNA Biosensor Based on Immobilization of a Non-Modified ssDNA Using Phosphoramidate-Bonding Strategy and Pencil Graphite Electrode Modified with AuNPs/CB and Self-Assembled Cysteamine Monolayer. Sensors 2022, 22, 9420. [Google Scholar] [CrossRef]
- Ge, Z.; Lin, M.; Wang, P.; Pei, H.; Yan, J.; Shi, J.; Huang, Q.; He, D.; Fan, C.; Zuo, X. Hybridization Chain Reaction Amplification of MicroRNA Detection with a Tetrahedral DNA Nanostructure-Based Electrochemical Biosensor. Anal. Chem. 2014, 86, 2124–2130. [Google Scholar] [CrossRef]
- Santos, A.; Piccoli, J.P.; Santos-Filho, N.A.; Cilli, E.M.; Bueno, P.R. Redox-Tagged Peptide for Capacitive Diagnostic Assays. Biosens. Bioelectron. 2015, 68, 281–287. [Google Scholar] [CrossRef]
- Sun, L.; Hu, N.; Peng, J.; Chen, L.; Weng, J. DNA Mutation: Ultrasensitive Detection of Mitochondrial DNA Mutation by Graphene Oxide/DNA Hydrogel Electrode (Adv. Funct. Mater. 44/2014). Adv. Funct. Mater. 2014, 24, 6897. [Google Scholar] [CrossRef][Green Version]
- Carr, O.; Raymundo-Pereira, P.A.; Shimizu, F.M.; Sorroche, B.P.; Melendez, M.E.; de Oliveira Pedro, R.; Miranda, P.B.; Carvalho, A.L.; Reis, R.M.; Arantes, L.M.R.B.; et al. Genosensor Made with a Self-Assembled Monolayer Matrix to Detect MGMT Gene Methylation in Head and Neck Cancer Cell Lines. Talanta 2020, 210, 120609. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, J.; Peng, Y.; Bai, J.; Li, S.; Han, D.; Ren, S.; Qin, K.; Zhou, H.; Han, T.; et al. Design and Synthesis of DNA Hydrogel Based on EXPAR and CRISPR/Cas14a for Ultrasensitive Detection of Creatine Kinase MB. Biosens. Bioelectron. 2022, 218, 114792. [Google Scholar] [CrossRef]
- Loughrey, C.M.; Young, I.S. Clinical Biochemistry of the Cardiovascular System. In Clinical Biochemistry: Metabolic and Clinical Aspects; Elsevier: Amsterdam, The Netherlands, 2014; pp. 737–766. ISBN 978-0-7020-5140-1. [Google Scholar]
- Ranallo, S.; Porchetta, A.; Ricci, F. DNA-Based Scaffolds for Sensing Applications. Anal. Chem. 2019, 91, 44–59. [Google Scholar] [CrossRef]
- Goodman, R.P.; Schaap, I.a.T.; Tardin, C.F.; Erben, C.M.; Berry, R.M.; Schmidt, C.F.; Turberfield, A.J. Rapid Chiral Assembly of Rigid DNA Building Blocks for Molecular Nanofabrication. Science 2005, 310, 1661–1665. [Google Scholar] [CrossRef]
- Zhang, T.; Tian, T.; Zhou, R.; Li, S.; Ma, W.; Zhang, Y.; Liu, N.; Shi, S.; Li, Q.; Xie, X.; et al. Design, Fabrication and Applications of Tetrahedral DNA Nanostructure-Based Multifunctional Complexes in Drug Delivery and Biomedical Treatment. Nat. Protoc. 2020, 15, 2728–2757. [Google Scholar] [CrossRef]
- Qiu, Y.; Qiu, Y.; Zhou, W.; Lu, D.; Wang, H.; Li, B.; Liu, B.; Wang, W. Advancements in Functional Tetrahedral DNA Nanostructures for Multi-Biomarker Biosensing: Applications in Disease Diagnosis, Food Safety, and Environmental Monitoring. Mater. Today Bio 2025, 31, 101486. [Google Scholar] [CrossRef]
- Yang, B.; Wang, H.; Kong, J.; Fang, X. Long-Term Monitoring of Ultratrace Nucleic Acids Using Tetrahedral Nanostructure-Based NgAgo on Wearable Microneedles. Nat. Commun. 2024, 15, 1936. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, J.; Mao, W.; He, X.; Ruan, L.; Zhu, J.; Shu, P.; Zhang, Z.; Jiang, B.; Zhang, X. A Tetrahedral DNA Nanostructure-Decorated Electrochemical Platform for Simple and Ultrasensitive EGFR Genotyping of Plasma ctDNA. Analyst 2020, 145, 4671–4679. [Google Scholar] [CrossRef]
- Zeng, D.; Wang, Z.; Meng, Z.; Wang, P.; San, L.; Wang, W.; Aldalbahi, A.; Li, L.; Shen, J.; Mi, X. DNA Tetrahedral Nanostructure-Based Electrochemical miRNA Biosensor for Simultaneous Detection of Multiple miRNAs in Pancreatic Carcinoma. ACS Appl. Mater. Interfaces 2017, 9, 24118–24125. [Google Scholar] [CrossRef]
- Dong, S.; Zhao, R.; Zhu, J.; Lu, X.; Li, Y.; Qiu, S.; Jia, L.; Jiao, X.; Song, S.; Fan, C.; et al. Electrochemical DNA Biosensor Based on a Tetrahedral Nanostructure Probe for the Detection of Avian Influenza A (H7N9) Virus. ACS Appl. Mater. Interfaces 2015, 7, 8834–8842. [Google Scholar] [CrossRef]
- Zhou, Z.; Han, B.; Wang, Y.; Lin, N.; Zhou, Z.; Zhang, Y.; Bai, Y.; Shen, L.; Shen, Y.; Zhang, Y.; et al. Fast and Sensitive Multivalent Spatial Pattern-Recognition for Circular RNA Detection. Nat. Commun. 2024, 15, 10900. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Chen, Y.; Tan, X.; Ge, S.; Zhang, L.; Li, L.; Yu, J.; Li, L. Tetrahedral DNA Nanostructure-Engineered Paper-Based Sensor with an Enhanced Antifouling Ability for Photoelectrochemical Sensing. Anal. Chem. 2023, 95, 4760–4767. [Google Scholar] [CrossRef]
- Lichtenberg, J.Y.; Ling, Y.; Kim, S. Non-Specific Adsorption Reduction Methods in Biosensing. Sensors 2019, 19, 2488. [Google Scholar] [CrossRef]
- Vilcapoma, J.; Patel, A.; Chandrasekaran, A.R.; Halvorsen, K. The Role of Size in Biostability of DNA Tetrahedra. Chem. Commun. 2023, 59, 5083–5085. [Google Scholar] [CrossRef]
- Sadowski, J.P.; Calvert, C.R.; Zhang, D.Y.; Pierce, N.A.; Yin, P. Developmental Self-Assembly of a DNA Tetrahedron. ACS Nano 2014, 8, 3251–3259. [Google Scholar] [CrossRef]
- Pei, H.; Zuo, X.; Pan, D.; Shi, J.; Huang, Q.; Fan, C. Scaffolded Biosensors with Designed DNA Nanostructures. NPG Asia Mater. 2013, 5, e51. [Google Scholar] [CrossRef]
- Ma, S.; Ren, Q.; Jiang, L.; Liu, Z.; Zhu, Y.; Zhu, J.; Zhang, Y.; Zhang, M. A Triple-Aptamer Tetrahedral DNA Nanostructures Based Carbon-Nanotube-Array Transistor Biosensor for Rapid Virus Detection. Talanta 2024, 266, 124973. [Google Scholar] [CrossRef]
- Li, M.; Song, L.; Liu, M.; Guo, R.; Lin, M.; Zuo, X. Framework Nucleic Acid-Programmed Sensing Interface with Densely Monodispersed Probes. ACS Nano 2025, 19, 23142–23150. [Google Scholar] [CrossRef]
- Hui, X.; Yang, C.; Li, D.; He, X.; Huang, H.; Zhou, H.; Chen, M.; Lee, C.; Mu, X. Infrared Plasmonic Biosensor with Tetrahedral DNA Nanostructure as Carriers for Label-Free and Ultrasensitive Detection of miR-155. Adv. Sci. 2021, 8, 2100583. [Google Scholar] [CrossRef]
- Koshiol, J.; Wang, E.; Zhao, Y.; Marincola, F.; Landi, M.T. Strengths and Limitations of Laboratory Procedures for microRNA Detection. Cancer Epidemiol. Biomark. Prev. 2010, 19, 907–911. [Google Scholar] [CrossRef]
- Ji, X.; Takahashi, R.; Hiura, Y.; Hirokawa, G.; Fukushima, Y.; Iwai, N. Plasma miR-208 as a Biomarker of Myocardial Injury. Clin. Chem. 2009, 55, 1944–1949. [Google Scholar] [CrossRef]
- Chen, Y.; Gelfond, J.A.L.; McManus, L.M.; Shireman, P.K. Reproducibility of Quantitative RT-PCR Array in miRNA Expression Profiling and Comparison with Microarray Analysis. BMC Genom. 2009, 10, 407. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xie, Z.; Chen, S.; Chen, M.; Wang, X.; Yi, G. A Novel Biosensor Based on Tetrahedral DNA Nanostructure and Terminal Deoxynucleotidyl Transferase-Assisted Amplification Strategy for Fluorescence Analysis of Uracil-DNA Glycosylase Activity. Anal. Chim. Acta 2023, 1271, 341432. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, M.; Weng, X.; Zhang, Y.; Li, J. DNA-Tetrahedral-Nanostructure-Based Entropy-Driven Amplifier for High-Performance Photoelectrochemical Biosensing. ACS Nano 2021, 15, 1710–1717. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Zeng, D.; Sun, W.; Zhang, H.; Aldalbahi, A.; Wang, Y.; San, L.; Fan, C.; Zuo, X.; et al. Elaborately Designed Diblock Nanoprobes for Simultaneous Multicolor Detection of microRNAs. Nanoscale 2015, 7, 15822–15829. [Google Scholar] [CrossRef]
- Lu, J.; Wang, J.; Hu, X.; Gyimah, E.; Yakubu, S.; Wang, K.; Wu, X.; Zhang, Z. Electrochemical Biosensor Based on Tetrahedral DNA Nanostructures and G-Quadruplex–Hemin Conformation for the Ultrasensitive Detection of MicroRNA-21 in Serum. Anal. Chem. 2019, 91, 7353–7359. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Q.; Yang, X.; Wang, K.; Li, Q.; Li, Z.; Gao, L.; Nie, W.; Zheng, Y. An Isothermal Electrochemical Biosensor for the Sensitive Detection of microRNA Based on a Catalytic Hairpin Assembly and Supersandwich Amplification. Analyst 2017, 142, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Z.; Hong, L.; Wang, X.; Cao, J. Tetrahedral DNA Nanostructure-Engineered Paper-Based Electrochemical Aptasensor for Fumonisin B1 Detection Coupled with Au@Pt Nanocrystals as an Amplification Label. J. Agric. Food Chem. 2023, 71, 19121–19128. [Google Scholar] [CrossRef]
- Langlois, N.I.; Clark, H.A. Characterization of DNA Nanostructure Stability by Size Exclusion Chromatography. Anal. Methods 2022, 14, 1006–1014. [Google Scholar] [CrossRef]
- Hisano, O.; Ito, T.; Miura, F. Short Single-Stranded DNAs with Putative Non-Canonical Structures Comprise a New Class of Plasma Cell-Free DNA. BMC Biol. 2021, 19, 225. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, S.; Shi, S.; Zhang, T.; Ma, Q.; Tian, T.; Zhou, T.; Cai, X.; Lin, Y. Anti-Inflammatory and Antioxidative Effects of Tetrahedral DNA Nanostructures via the Modulation of Macrophage Responses. ACS Appl. Mater. Interfaces 2018, 10, 3421–3430. [Google Scholar] [CrossRef] [PubMed]
- Praetorius, F.; Kick, B.; Behler, K.L.; Honemann, M.N.; Weuster-Botz, D.; Dietz, H. Biotechnological Mass Production of DNA Origami. Nature 2017, 552, 84–87. [Google Scholar] [CrossRef]
- Nguyen, M.-K.; Nguyen, V.H.; Natarajan, A.K.; Huang, Y.; Ryssy, J.; Shen, B.; Kuzyk, A. Ultrathin Silica Coating of DNA Origami Nanostructures. Chem. Mater. 2020, 32, 6657–6665. [Google Scholar] [CrossRef]
- Rodriguez, D.; Marquez, M.D.; Zenasni, O.; Han, L.T.; Baldelli, S.; Lee, T.R. Surface Dipoles Induce Uniform Orientation in Contacting Polar Liquids. Chem. Mater. 2020, 32, 7832–7841. [Google Scholar] [CrossRef]
- Pathak, P.; Cho, H.J. Self-Assembled 1-Octadecanethiol Membrane on Pd/ZnO for a Selective Room Temperature Flexible Hydrogen Sensor. Micromachines 2021, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Sakunkaewkasem, S.; Gonzalez, M.A.; Marquez, M.D.; Lee, T.R. Olefin-Bridged Bidentate Adsorbates for Generating Self-Assembled Monolayers on Gold. Langmuir 2020, 36, 10699–10707. [Google Scholar] [CrossRef]
- Hoang, J.; Park, C.S.; Marquez, M.D.; Gunaratne, P.H.; Lee, T.R. DNA Binding on Self-Assembled Monolayers Terminated with Mixtures of Ammonium and Trimethylammonium Groups: Toward a Gene-Delivery Platform. ACS Appl. Nano Mater. 2020, 3, 6621–6628. [Google Scholar] [CrossRef]
- Yu, T.; Marquez, M.D.; Tran, H.-V.; Lee, T.R. Crosslinked Organosulfur-Based Self-Assembled Monolayers: Formation and Applications. Soft Sci. 2022, 2, 5. [Google Scholar] [CrossRef]
- Hoang, J.; Tajalli, P.; Omidiyan, M.; Marquez, M.D.; Khantamat, O.; Tuntiwechapikul, W.; Li, C.-H.; Kohlhatkar, A.; Tran, H.-V.; Gunaratne, P.H.; et al. Self-Assembled Monolayers Derived from Positively Charged Adsorbates on Plasmonic Substrates for MicroRNA Delivery: A Review. J. Nanotheranostics 2023, 4, 171–200. [Google Scholar] [CrossRef]
- Lee, L.; Gunby, N.R.; Crittenden, D.L.; Downard, A.J. Multifunctional and Stable Monolayers on Carbon: A Simple and Reliable Method for Backfilling Sparse Layers Grafted from Protected Aryldiazonium Ions. Langmuir 2016, 32, 2626–2637. [Google Scholar] [CrossRef]
- Peeters, S.; Stakenborg, T.; Reekmans, G.; Laureyn, W.; Lagae, L.; Van Aerschot, A.; Van Ranst, M. Impact of Spacers on the Hybridization Efficiency of Mixed Self-Assembled DNA/Alkanethiol Films. Biosens. Bioelectron. 2008, 24, 72–77. [Google Scholar] [CrossRef]
- Szymczyk-Drozd, A.; Baran, D.; Skiba, A.; Zasada, A.; Mosiej, E.; Krzemiński, J.; Pepłowski, A.; Malinowska, E.; Ziółkowski, R. Practical Aspects of SAM-Based Electrochemical DNA Biosensors on Miniaturized Planar Gold Transducers: A Case Study with SARS-CoV-2. Measurement 2025, 250, 117212. [Google Scholar] [CrossRef]
- Williamson, P.; Piskunen, P.; Ijäs, H.; Butterworth, A.; Linko, V.; Corrigan, D.K. Signal Amplification in Electrochemical DNA Biosensors Using Target-Capturing DNA Origami Tiles. ACS Sens. 2023, 8, 1471–1480. [Google Scholar] [CrossRef]
- Cortés, E.; Rubert, A.A.; Benitez, G.; Carro, P.; Vela, M.E.; Salvarezza, R.C. Enhanced Stability of Thiolate Self-Assembled Monolayers (SAMs) on Nanostructured Gold Substrates. Langmuir 2009, 25, 5661–5666. [Google Scholar] [CrossRef]
- Liu, N.; Huang, F.; Lou, X.; Xia, F. DNA Hybridization Chain Reaction and DNA Supersandwich Self-Assembly for Ultrasensitive Detection. Sci. China Chem. 2017, 60, 311–318. [Google Scholar] [CrossRef]
- Cajigas, S.; Alzate, D.; Fernández, M.; Muskus, C.; Orozco, J. Electrochemical Genosensor for the Specific Detection of SARS-CoV-2. Talanta 2022, 245, 123482. [Google Scholar] [CrossRef]
- Oberhaus, F.V.; Frense, D.; Beckmann, D. Immobilization Techniques for Aptamers on Gold Electrodes for the Electrochemical Detection of Proteins: A Review. Biosensors 2020, 10, 45. [Google Scholar] [CrossRef]
- Veselinovic, J.; Li, Z.; Daggumati, P.; Seker, E. Electrically Guided DNA Immobilization and Multiplexed DNA Detection with Nanoporous Gold Electrodes. Nanomaterials 2018, 8, 351. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Y.; Yang, Q.; Yuan, N.; Zhang, W. Review of Electrochemical DNA Biosensors for Detecting Food Borne Pathogens. Sensors 2019, 19, 4916. [Google Scholar] [CrossRef]
- Czarnomska, M.; Lewkowicz, A.; Gruszczyńska, E.; Walczewska-Szewc, K.; Gryczyński, Z.; Bojarski, P.; Steinborn, S. Preliminary Investigation of a Potential Optical Biosensor Using the DiamondTM Nucleic Acid Dye Applied to DNA and Friction Ridge Analysis from Fingerprint Traces. Biosensors 2024, 14, 546. [Google Scholar] [CrossRef]
- Karadurmus, L.; Dogan-Topal, B.; Kurbanoglu, S.; Shah, A.; Ozkan, S.A. The Interaction between DNA and Three Intercalating Anthracyclines Using Electrochemical DNA Nanobiosensor Based on Metal Nanoparticles Modified Screen-Printed Electrode. Micromachines 2021, 12, 1337. [Google Scholar] [CrossRef]
- Srisombat, L.; Jamison, A.C.; Lee, T.R. Stability: A Key Issue for Self-Assembled Monolayers on Gold as Thin-Film Coatings and Nanoparticle Protectants. Colloids Surf. A Physicochem. Eng. Asp. 2011, 390, 1–19. [Google Scholar] [CrossRef]
- Yang, Z.; Pujari, S.P.; Armstrong, R.; Mathwig, K.; Rutjes, F.P.J.T.; Smulders, M.M.J.; Zuilhof, H. Hydrolytic, Thermal, and Electrochemical Stability of Thiol- and Terminal Alkyne-Based Monolayers on Gold: A Comparative Study. Langmuir 2025, 41, 6197–6207. [Google Scholar] [CrossRef]
- Willey, T.M.; Vance, A.L.; Van Buuren, T.; Bostedt, C.; Terminello, L.J.; Fadley, C.S. Rapid Degradation of Alkanethiol-Based Self-Assembled Monolayers on Gold in Ambient Laboratory Conditions. Surf. Sci. 2005, 576, 188–196. [Google Scholar] [CrossRef]
- Gannon, G.; Greer, J.C.; Larsson, J.A.; Thompson, D. Molecular Dynamics Study of Naturally Occurring Defects in Self-Assembled Monolayer Formation. ACS Nano 2010, 4, 921–932. [Google Scholar] [CrossRef]
- Nimse, S.B.; Song, K.; Sonawane, M.D.; Sayyed, D.R.; Kim, T. Immobilization Techniques for Microarray: Challenges and Applications. Sensors 2014, 14, 22208–22229. [Google Scholar] [CrossRef]
- Biagiotti, V.; Porchetta, A.; Desiderati, S.; Plaxco, K.W.; Palleschi, G.; Ricci, F. Probe Accessibility Effects on the Performance of Electrochemical Biosensors Employing DNA Monolayers. Anal. Bioanal. Chem. 2012, 402, 413–421. [Google Scholar] [CrossRef]
- Cipriano, B.H.; Banik, S.J.; Sharma, R.; Rumore, D.; Hwang, W.; Briber, R.M.; Raghavan, S.R. Superabsorbent Hydrogels That Are Robust and Highly Stretchable. Macromolecules 2014, 47, 4445–4452. [Google Scholar] [CrossRef]
- Wichterle, O.; Lim, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Fägerstam, L.G.; Frostell-Karlsson, A.; Karlsson, R.; Persson, B.; Rönnberg, I. Biospecific Interaction Analysis Using Surface Plasmon Resonance Detection Applied to Kinetic, Binding Site and Concentration Analysis. J. Chromatogr. 1992, 597, 397–410. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Zhang, J.; Peng, Y.; Li, S.; Han, D.; Ren, S.; Qin, K.; Li, S.; Gao, Z. Stimuli-Responsive DNA-Based Hydrogels for Biosensing Applications. J. Nanobiotechnol. 2022, 20, 40. [Google Scholar] [CrossRef]
- Mao, X.; Mao, D.; Chen, T.; Jalalah, M.; Al-Assiri, M.S.; Harraz, F.A.; Zhu, X.; Li, G. DNA Hydrogel-Based Three-Dimensional Electron Transporter and Its Application in Electrochemical Biosensing. ACS Appl. Mater. Interfaces 2020, 12, 36851–36859. [Google Scholar] [CrossRef]
- Muya, F.N.; Sunday, C.E.; Baker, P.; Iwuoha, E. Environmental Remediation of Heavy Metal Ions from Aqueous Solution through Hydrogel Adsorption: A Critical Review. Water Sci. Technol. 2016, 73, 983–992. [Google Scholar] [CrossRef]
- Nagahara, S.; Matsuda, T. Hydrogel Formation via Hybridization of Oligonucleotides Derivatized in Water-Soluble Vinyl Polymers. Polym. Gels Netw. 1996, 4, 111–127. [Google Scholar] [CrossRef]
- Mo, F.; Jiang, K.; Zhao, D.; Wang, Y.; Song, J.; Tan, W. DNA Hydrogel-Based Gene Editing and Drug Delivery Systems. Adv. Drug Deliv. Rev. 2021, 168, 79–98. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, Y.; Jiang, Y.; Gu, T.; Siow, L.; Gao, Y.; Zheng, Y.; Xing, K.; Zhou, S.; Zhang, C.; et al. DNA Hydrogels in Tissue Engineering: From Molecular Design to Next-Generation Biomedical Applications. Adv. Healthc. Mater. 2025, 14, e2500192. [Google Scholar] [CrossRef]
- Stancu, I.C.; Lungu, A.; Iovu, H. Hydrogels for Bone Regeneration. In Biomaterials for Bone Regeneration; Elsevier: Amsterdam, The Netherlands, 2014; pp. 62–86. ISBN 978-0-85709-804-7. [Google Scholar]
- Gačanin, J.; Synatschke, C.V.; Weil, T. Biomedical Applications of DNA-Based Hydrogels. Adv. Funct. Mater. 2020, 30, 1906253. [Google Scholar] [CrossRef]
- Liang, Q.; Lu, Y.; Zhang, Q. Hydrogels-Based Electronic Devices for Biosensing Applications. In Smart Stimuli—Responsive Polymers, Films, and Gels; Serpe, M.J., Hu, L., Gao, Y., Eds.; Wiley: Hoboken, NJ, USA, 2022; pp. 339–373. ISBN 978-3-527-34901-2. [Google Scholar]
- Si, Y.; Li, L.; Wang, N.; Zheng, J.; Yang, R.; Li, J. Oligonucleotide Cross-Linked Hydrogel for Recognition and Quantitation of MicroRNAs Based on a Portable Glucometer Readout. ACS Appl. Mater. Interfaces 2019, 11, 7792–7799. [Google Scholar] [CrossRef]
- Liu, S.; Su, W.; Li, Y.; Zhang, L.; Ding, X. Manufacturing of an Electrochemical Biosensing Platform Based on Hybrid DNA Hydrogel: Taking Lung Cancer-Specific miR-21 as an Example. Biosens. Bioelectron. 2018, 103, 1–5. [Google Scholar] [CrossRef]
- Tang, J.; Xiao, P. Polymerizing Immobilization of Acrylamide-Modified Nucleic Acids and Its Application. Biosens. Bioelectron. 2009, 24, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jean, S.R.; Ahmed, S.; Aldridge, P.M.; Li, X.; Fan, F.; Sargent, E.H.; Kelley, S.O. Multifunctional Quantum Dot DNA Hydrogels. Nat. Commun. 2017, 8, 381. [Google Scholar] [CrossRef]
- Sontakke, V.A.; Yokobayashi, Y. Programmable Macroscopic Self-Assembly of DNA-Decorated Hydrogels. J. Am. Chem. Soc. 2022, 144, 2149–2155. [Google Scholar] [CrossRef] [PubMed]
- Um, S.H.; Lee, J.B.; Park, N.; Kwon, S.Y.; Umbach, C.C.; Luo, D. Enzyme-Catalysed Assembly of DNA Hydrogel. Nat. Mater. 2006, 5, 797–801. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).