Stability of TiO2–Polypyrrole Heterojunctions for Photoelectrochemical Water Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TiO2 Nanorods
2.3. Polymerization of Pyrrole on TiO2 Nanorods
2.4. Characterizations
3. Results and Discussion
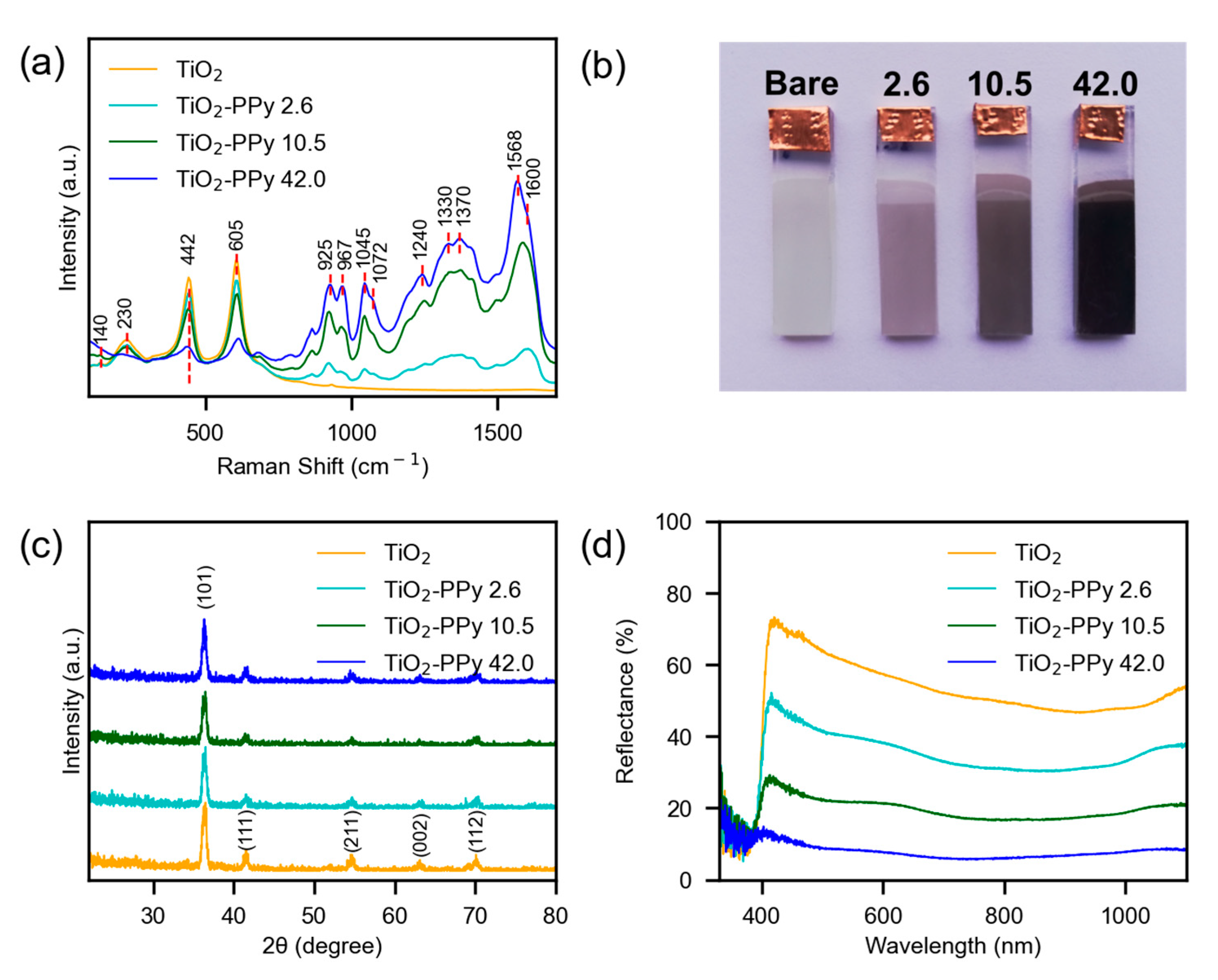
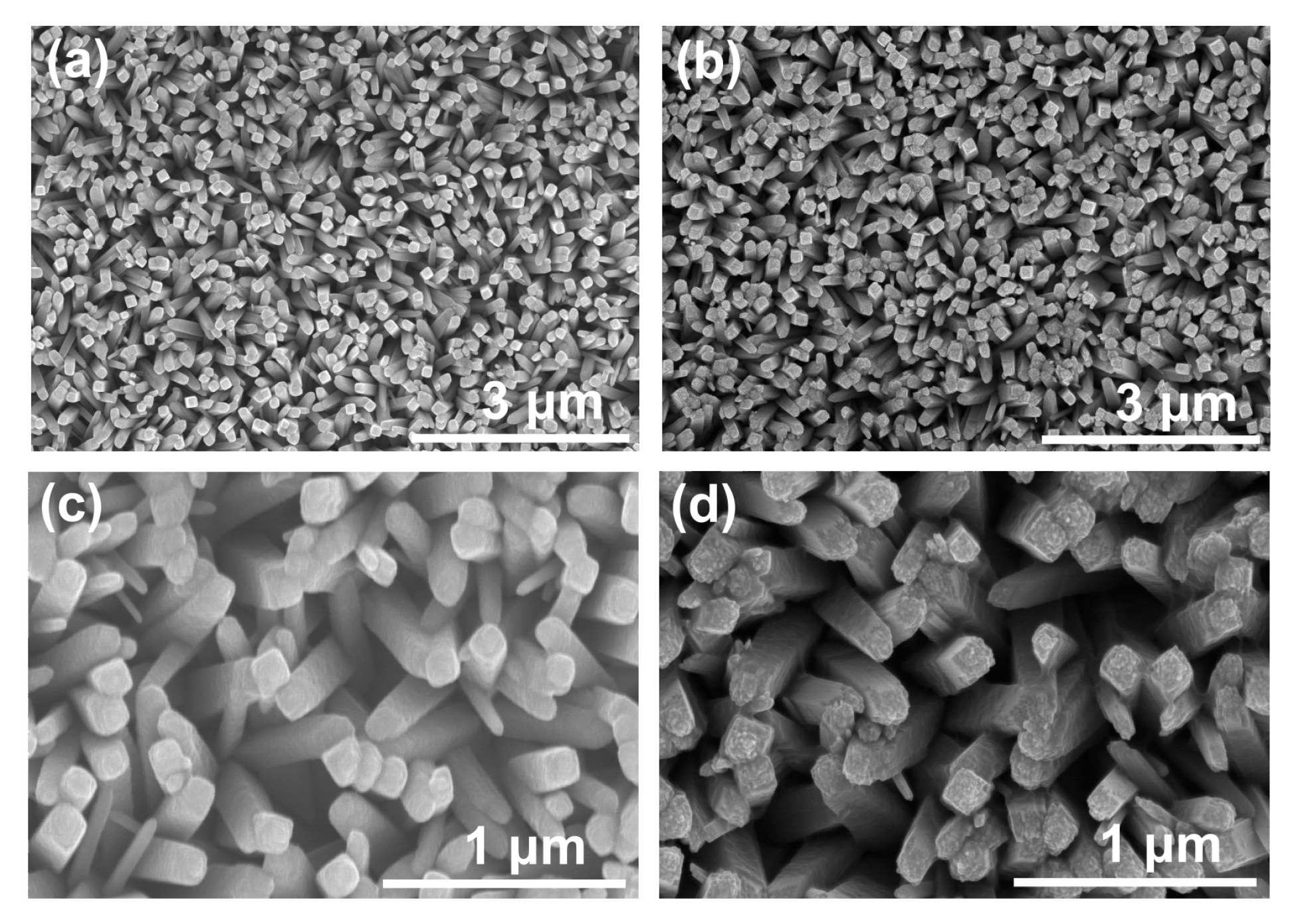
3.1. Elaboration of Photoelectrodes
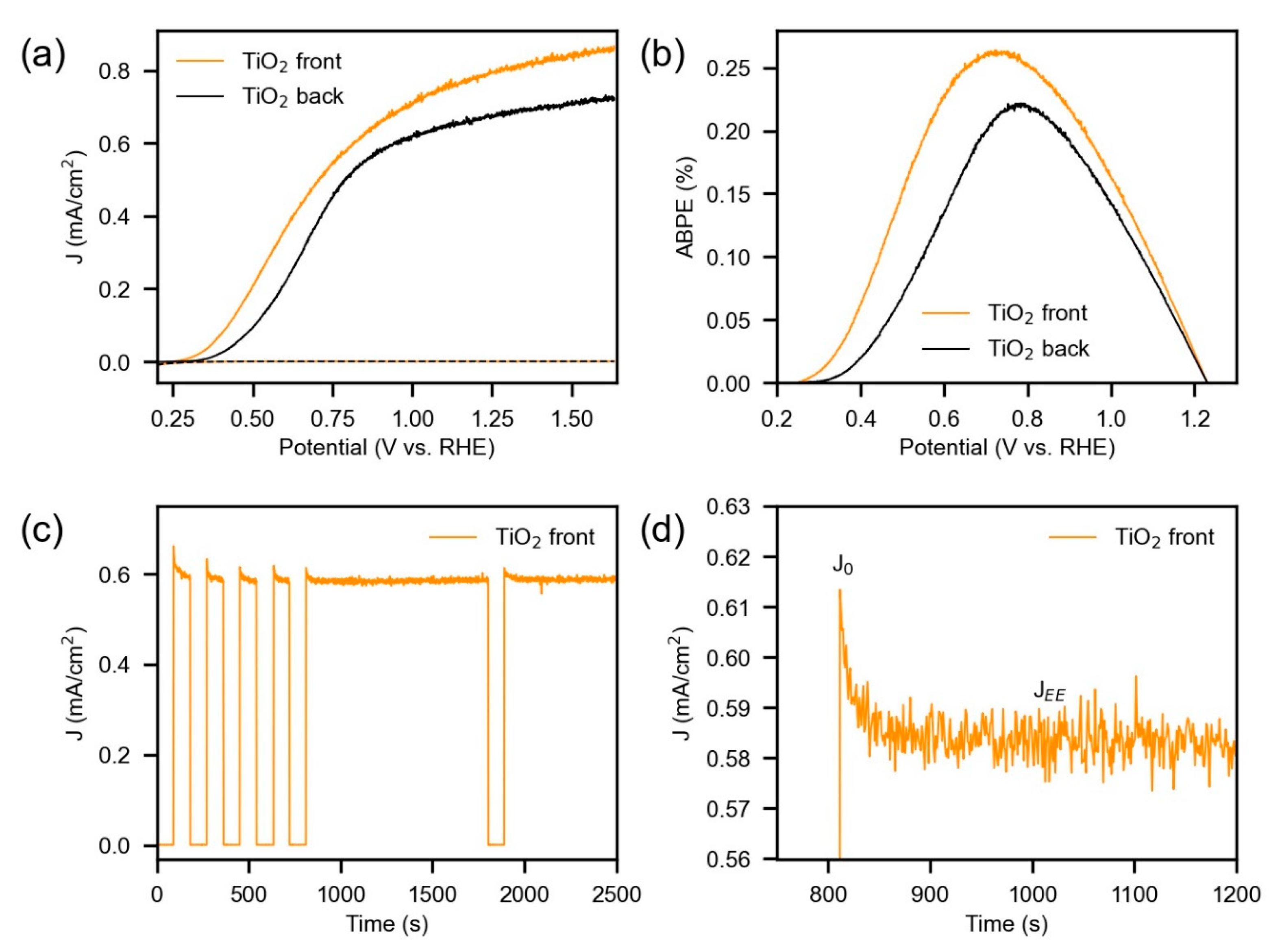
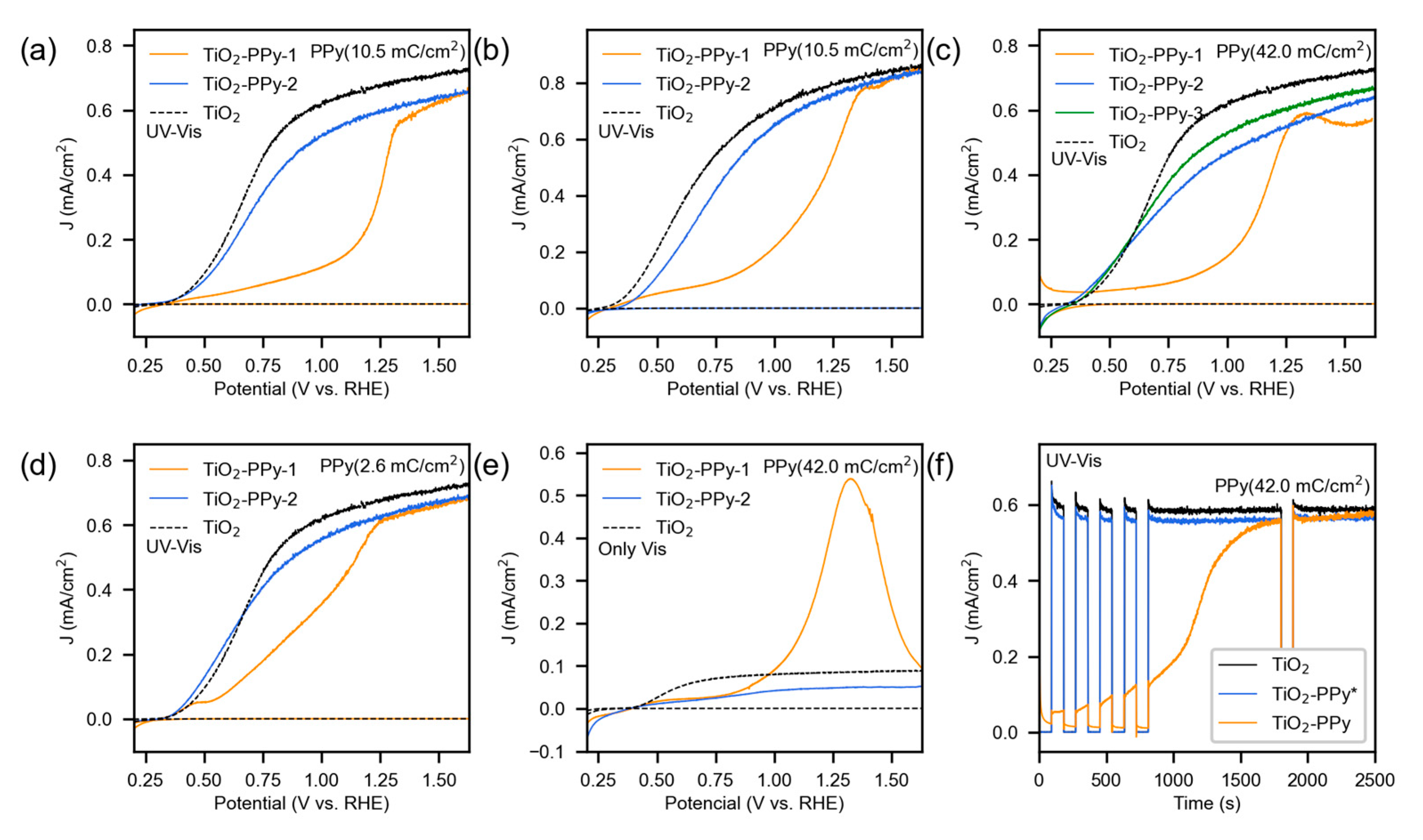
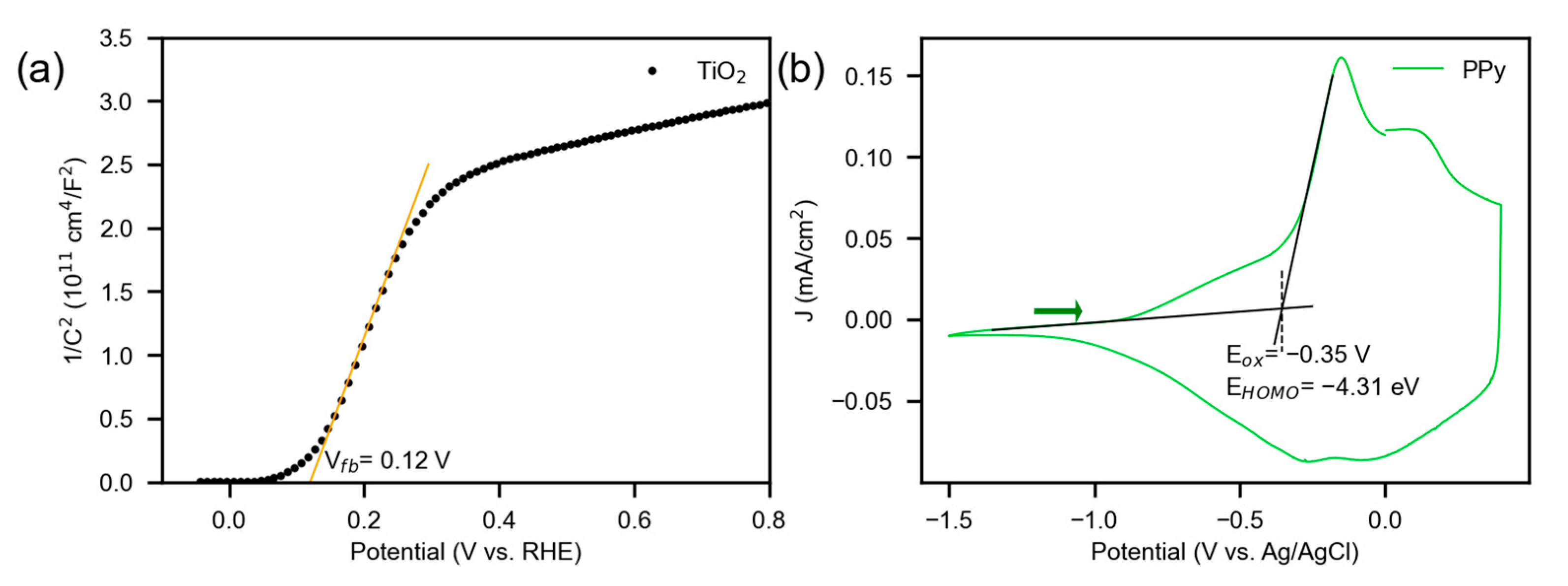
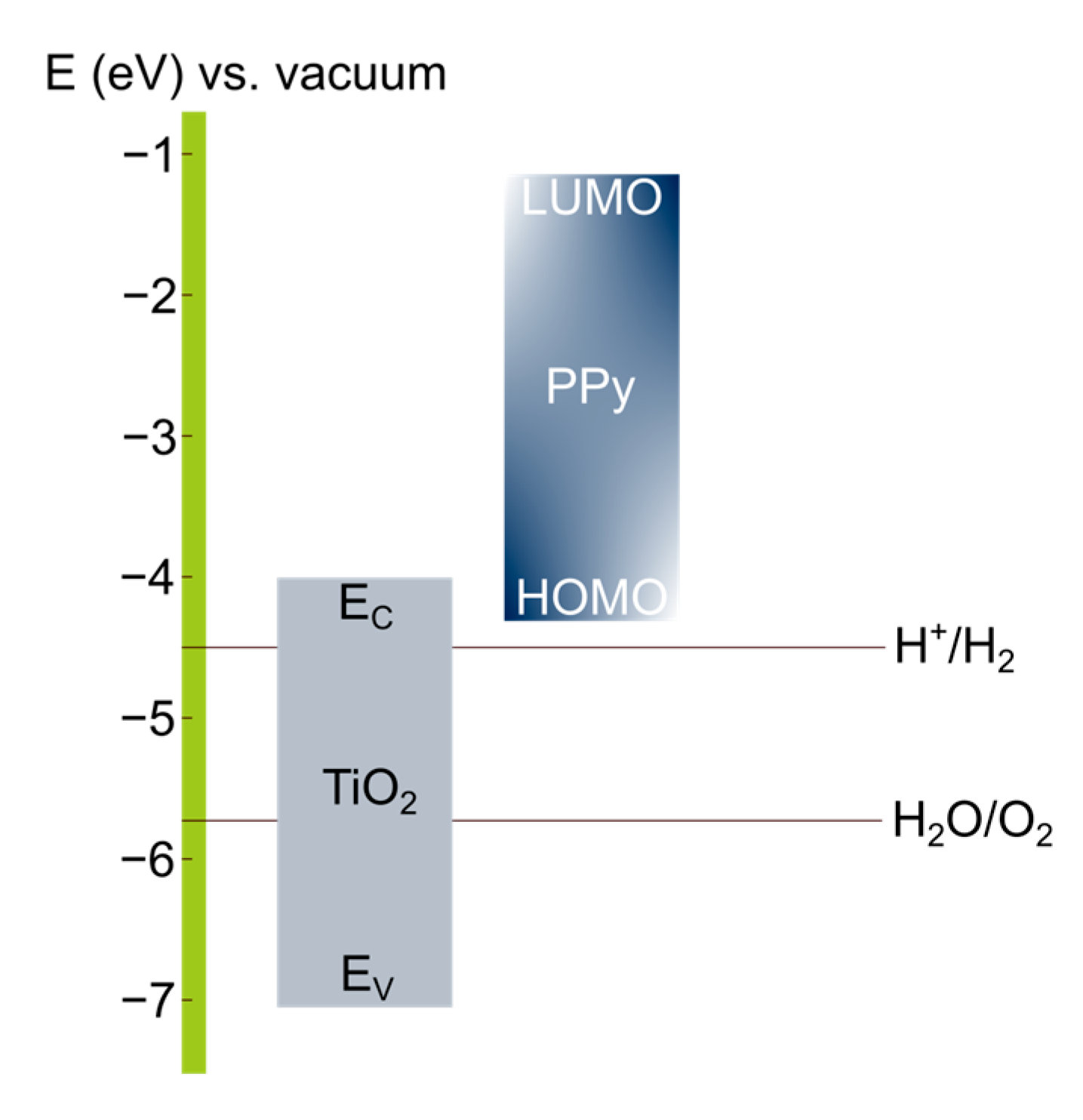
3.2. Photoelectrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FTO | Fluorine-doped tin oxide |
| HOMO | Highest occupied molecular orbital |
| PPy | Polypyrrole |
| OER | Oxygen evolution reaction |
| SEM | Scanning electron microscopy |
| Eg | Band gap energy |
| LSV | Linear sweep voltammetry |
| ABPE | Applied bias photon-to-current efficiency |
| Vfb | Flat band potential |
| LUMO | Lowest unoccupied molecular orbital |
References
- Kang, C.; Song, K.; Ha, S.; Sung, Y.; Kim, Y.; Shin, K.-Y.; Kim, B.H. Influence of Polypyrrole on Phosphorus- and TiO2-Based Anode Nanomaterials for Li-Ion Batteries. Nanomaterials 2024, 14, 1138. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wei, J.; Qiao, X.; Li, X.; Zhang, H.; Xiong, H. Enhanced Adsorption and Conversion of Polysulfides by ZIF-67-Based Co3O4/TiO2 Heterostructure and Attached Polypyrrole 3D Conductive Network for Lithium–Sulfur Batteries with Stable and Extended Cycling. Energy Technol. 2024, 12, 2301166. [Google Scholar] [CrossRef]
- Enailah, A.; Mohamed, F. Growth Mechanism of 2D Heterostructures of Polypyrrole Grown on TiO2 Nanoribbons for High-Performance Supercapacitors. Nanoscale Adv. 2024, 6, 5409–5419. [Google Scholar] [CrossRef]
- Cai, H.; Li, Y.; Liu, D.; Yang, X.; Zhou, D.; Han, E.; Li, X.; Li, Q.; He, Y. Construction of Moiré-like TiO2/Polypyrrole Electrodes for High Performance Photo-Assisted Supercapacitors. Colloids Surfaces A Physicochem. Eng. Asp. 2024, 703, 135386. [Google Scholar] [CrossRef]
- Azizi, E.; Arjomandi, J.; Shi, H.; Kiani, M.A. Flexible Polypyrrole/TiO2/MXene Nanocomposite Supercapacitor: A Promising Energy Storage Device. J. Energy Storage 2024, 75, 109665. [Google Scholar] [CrossRef]
- Barkade, S.S.; Nakate, U.T.; Bansod, P.G.; Deorukhkar, O.A.; Doss, V.R. Ultrasound-Assisted Synthesis of Polypyrrole/TiO2 Nanocomposite for Cu(II) Ions Removal from Aqueous Solution. Synth. Met. 2023, 293, 117253. [Google Scholar] [CrossRef]
- Chen, J.; Yu, M.; Wang, C.; Feng, J.; Yan, W. Insight into the Synergistic Effect on Selective Adsorption for Heavy Metal Ions by a Polypyrrole/TiO2 Composite. Langmuir 2018, 34, 10187–10196. [Google Scholar] [CrossRef]
- Omar, R.A.; Radwan, E.K.; Salih, S.A.; Mohamed, G.G. Synergistic Adsorption–Photocatalytic Degradation of the Emerging Contaminant Hydroxybenzotriazole by a 3D Sponge-like Easy Separation Polypyrrole/TiO2 Composite. Appl. Water Sci. 2024, 14, 229. [Google Scholar] [CrossRef]
- Nazir, M.K.; Javaid, S.; Afzal, H.; Taj, M.B.; Baamer, D.F.; Almasoudi, A.; Aldahiri, R.H.; Ali, O.M.; Khan, M.I.; Ahmed, M.M.; et al. Synthesis, Magnetic, and Photocatalytic Activity of Polypyrrole-Based TiO2–Fe Catalyst for Wastewater Treatment. Catalysts 2024, 14, 692. [Google Scholar] [CrossRef]
- Dhachanamoorthi, N.; Oviya, K.; Sugumaran, S.; Suresh, P.; Parthibavarman, M.; Jeshaa dharshini, K.; Aishwarya, M. Effective Move of Polypyrrole/TiO2 Hybrid Nanocomposites on Removal of Methylene Blue Dye by Photocatalytic Activity. Chem. Phys. Impact 2024, 9, 100723. [Google Scholar] [CrossRef]
- Zhang, J.; Pang, Z.; Sun, Q.; Chen, X.; Zhu, Y.; Li, M.; Wang, J.; Qiu, H.; Li, X.; Li, Y.; et al. TiO2 Nanotube Array Modified with Polypyrrole for Efficient Photoelectrocatalytic Decolorization of Methylene Blue. J. Alloys Compd. 2020, 820, 153128. [Google Scholar] [CrossRef]
- Balu, R.; Devendrapandi, G.; Gnanasekaran, L.; Karthika, P.C.; Abd-Elkader, O.H.; Kim, W.K.; Minnam Reddy, V.R.; Kapoor, M.; Singh, S.; Lavanya, M. Creating an Intermediate Energy Band to Boost the Photoelectrochemical Efficiency of TiO2 for Solar-Driven Hydrogen Production. Sol. Energy Mater. Sol. Cells 2025, 279, 113226. [Google Scholar] [CrossRef]
- Tu, J.; Li, J.; Pan, Z.; Zhu, X.; Ye, D.; Yang, Y.; Wang, H.; An, L.; Chen, R.; Liao, Q. Nitrogen-Doped CdS/TiO2 Nanorods Heterojunction Photoanode for Efficient and Stable Photoelectrochemical Water Splitting. J. Power Sources 2025, 628, 235883. [Google Scholar] [CrossRef]
- Li, A.; Liu, L.; Jiao, Z.; Han, M. Integration of Bi2S3 Nanoflowers into TiO2 Nanorods for Enhanced Photoelectrochemical Water Splitting Performance. Mater. Lett. 2025, 380, 137740. [Google Scholar] [CrossRef]
- Mazumdar, J.; Deb, S. Synthesis of Core-Shell Structured Silver-Polypyrrole Nanocomposite for Cost Effective Photocatalytic Degradation of Methylene Blue under Natural Sunlight. Mater. Lett. 2024, 358, 135755. [Google Scholar] [CrossRef]
- Puerres, J.; Ortiz, P.; Cortés, M.T. Effect of Electrosynthesis Potential on Nucleation, Growth, Adhesion, and Electronic Properties of Polypyrrole Thin Films on Fluorine-Doped Tin Oxide (FTO). Polymers 2021, 13, 2419. [Google Scholar] [CrossRef]
- Luhakhra, N.; Tiwari, S.K. Polaron and Bipolaron Mediated Photocatalytic Activity of Polypyrrole Nanoparticles under Visible Light. Colloids Surf. A Physicochem. Eng. Asp. 2023, 667, 131380. [Google Scholar] [CrossRef]
- Li, Y.; Yan, S.; Jia, X.; Wu, J.; Yang, J.; Zhao, C.; Wang, S.; Song, H.; Yang, X. Uncovering the Origin of Full-Spectrum Visible-Light-Responsive Polypyrrole Supramolecular Photocatalysts. Appl. Catal. B Environ. 2021, 287, 119926. [Google Scholar] [CrossRef]
- Yuan, X.; Floresyona, D.; Aubert, P.-H.; Bui, T.-T.; Remita, S.; Ghosh, S.; Brisset, F.; Goubard, F.; Remita, H. Photocatalytic Degradation of Organic Pollutant with Polypyrrole Nanostructures under UV and Visible Light. Appl. Catal. B Environ. 2019, 242, 284–292. [Google Scholar] [CrossRef]
- Schichtl, Z.G.; Carvalho, O.Q.; Tan, J.; Saund, S.S.; Ghoshal, D.; Wilder, L.M.; Gish, M.K.; Nielander, A.C.; Stevens, M.B.; Greenaway, A.L. Chemistry of Materials Underpinning Photoelectrochemical Solar Fuel Production. Chem. Rev. 2025, 125, 4768–4839. [Google Scholar] [CrossRef]
- Chen, P.; Kang, B.; Liu, P.; Cheng, X.; Zhong, S.; Wang, X.; Fang, B. Passivation Strategies for Enhanced Photoelectrochemical Water Splitting. J. Power Sources 2025, 628, 235860. [Google Scholar] [CrossRef]
- Abdullah, H.; Shuwanto, H.; Lie, J.; Sillanpää, M. Critical Parameters and Essential Strategies in Designing Photoanodes to Overcome the Sluggish Water Oxidation Reaction. J. Environ. Chem. Eng. 2023, 11, 109356. [Google Scholar] [CrossRef]
- Clarizia, L.; Nadagouda, M.N.; Dionysiou, D.D. Recent Advances and Challenges of Photoelectrochemical Cells for Hydrogen Production. Curr. Opin. Green Sustain. Chem. 2023, 41, 100825. [Google Scholar] [CrossRef]
- He, S.; Meng, Y.; Cao, Y.; Huang, S.; Yang, J.; Tong, S.; Wu, M. Hierarchical Ta-Doped TiO2 Nanorod Arrays with Improved Charge Separation for Photoelectrochemical Water Oxidation under FTO Side Illumination. Nanomaterials 2018, 8, 983. [Google Scholar] [CrossRef]
- Li, T.; Ding, D. Photoelectrochemical Water Splitting with Black Ni/Si-Doped TiO2 Nanostructures. Int. J. Hydrogen Energy 2020, 45, 20983–20992. [Google Scholar] [CrossRef]
- Cho, I.S.; Chen, Z.; Forman, A.J.; Kim, D.R.; Rao, P.M.; Jaramillo, T.F.; Zheng, X. Branched TiO2 Nanorods for Photoelectrochemical Hydrogen Production. Nano Lett. 2011, 11, 4978–4984. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Yu, Y.; Ma, Y.; Yang, W.; Wang, F.; Yin, X.; Wang, X. Surface-Plasmon-Resonance-Enhanced Photoelectrochemical Water Splitting from Au-Nanoparticle-Decorated 3D TiO2 Nanorod Architectures. J. Phys. Chem. C 2017, 121, 12071–12079. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, S.; Su, K.; Jia, K. Graphite Carbon Nitride Coupled S-Doped Hydrogenated TiO2 Nanotube Arrays with Improved Photoelectrochemical Performance. J. Electroanal. Chem. 2020, 862, 114008. [Google Scholar] [CrossRef]
- Yang, J.-S.; Lin, W.-H.; Lin, C.-Y.; Wang, B.-S.; Wu, J.-J. N-Fe2O3 to N+-TiO2 Heterojunction Photoanode for Photoelectrochemical Water Oxidation. ACS Appl. Mater. Interfaces 2015, 7, 13314–13321. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, L.; Su, X.; Chen, S.; Zhang, J.; Yang, H.; Pei, Y. Activating a TiO2/BiVO4 Film for Photoelectrochemical Water Splitting by Constructing a Heterojunction Interface with a Uniform Crystal Plane Orientation. ACS Appl. Mater. Interfaces 2022, 14, 2316–2325. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Ma, Y.; Wang, H.; Chen, J. Preparation of Polypyrrole Sensitized TiO2 Nanotube Arrays Hybrids for Efficient Photoelectrochemical Water Splitting. Electrochim. Acta 2015, 167, 119–125. [Google Scholar] [CrossRef]
- Li, X.; Wang, P.; Huang, B.; Qin, X. Precisely Locate Pd-Polypyrrole on TiO2 for Enhanced Hydrogen Production. Int. J. Hydrogen Energy 2017, 42, 25195–25202. [Google Scholar] [CrossRef]
- Rasouli, H.; Hosseini, M.G.; Yardani sefidi, P.; Kinayyigit, S. Superior Overall Water Splitting Performance in Polypyrrole Photoelectrode by Coupling NrGO and Modifying Electropolymerization Substrate. J. Appl. Polym. Sci. 2021, 138, 50507. [Google Scholar] [CrossRef]
- El-Bery, H.M.; Salah, M.R.; Ahmed, S.M.; Soliman, S.A. Efficient Non-Metal Based Conducting Polymers for Photocatalytic Hydrogen Production: Comparative Study between Polyaniline, Polypyrrole and PEDOT. RSC Adv. 2021, 11, 13229–13244. [Google Scholar] [CrossRef]
- Pal, S.; Das, P.S.; Naskar, M.K.; Ghosh, S. Metal Oxide Nanocrystals Embedded Polypyrrole Nanohybrid: Exploring Role of Interface in Photocatalytic Hydrogen Generation. Mater. Today Sustain. 2024, 25, 100610. [Google Scholar] [CrossRef]
- Puerres, J.; Díaz, M.; Hurtado, J.; Ortiz, P.; Cortés, M.T. Photoelectrochemical Stability under Anodic and Cathodic Conditions of Meso-Tetra-(4-Sulfonatophenyl)-Porphyrinato Cobalt (II) Immobilized in Polypyrrole Thin Films. Polymers 2021, 13, 657. [Google Scholar] [CrossRef] [PubMed]
- Puerres, J.; Polanía, S.; Pérez-Torres, A.F.; Erazo, E.A.; Cortés, M.T.; Ortiz, P. Reduced TiO2 Nanorods Decorated with Carbon Nanodots for Photoelectrochemical Water Oxidation. ACS Appl. Nano Mater. 2023, 6, 14029–14039. [Google Scholar] [CrossRef]
- Luo, Z.; Li, Z.; Xie, Z.; Sokolova, I.M.; Song, L.; Peijnenburg, W.J.G.M.; Hu, M.; Wang, Y. Rethinking Nano-TiO2 Safety: Overview of Toxic Effects in Humans and Aquatic Animals. Small 2020, 16, 14029–14039. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, G.G.; Perfetti-Bolaño, A.; Meléndrez, M.; Pozo, K.; Corsi, I.; Barra, R.O.; Urrutia, R. First Evidence of Anthropogenic TiO2 Nanoparticles Occurrence in Chilean Rivers. Environ. Adv. 2024, 16, 100536. [Google Scholar] [CrossRef]
- Patois, T.; Lakard, B.; Monney, S.; Roizard, X.; Fievet, P. Characterization of the Surface Properties of Polypyrrole Films: Influence of Electrodeposition Parameters. Synth. Met. 2011, 161, 2498–2505. [Google Scholar] [CrossRef]
- Tamilselvan, V.; Yuvaraj, D.; Rakesh Kumar, R.; Narasimha Rao, K. Growth of Rutile TiO2 Nanorods on TiO2 Seed Layer Deposited by Electron Beam Evaporation. Appl. Surf. Sci. 2012, 258, 4283–4287. [Google Scholar] [CrossRef]
- Challagulla, S.; Tarafder, K.; Ganesan, R.; Roy, S. Structure Sensitive Photocatalytic Reduction of Nitroarenes over TiO2. Sci. Rep. 2017, 7, 8783. [Google Scholar] [CrossRef]
- Vásquez, G.C.; Maestre, D.; Cremades, A.; Piqueras, J. Assessment of the Cr Doping and Size Effects on the Raman-Active Modes of Rutile TiO2 by UV/Visible Polarized Raman Spectroscopy. J. Raman Spectrosc. 2017, 48, 847–854. [Google Scholar] [CrossRef]
- Yan, J.; Wu, G.; Guan, N.; Li, L.; Li, Z.; Cao, X. Understanding the Effect of Surface/Bulk Defects on the Photocatalytic Activity of TiO2: Anatase versus Rutile. Phys. Chem. Chem. Phys. 2013, 15, 10978. [Google Scholar] [CrossRef] [PubMed]
- Varade, V.; Honnavar, G.V.; Anjaneyulu, P.; Ramesh, K.P.; Menon, R. Probing Disorder and Transport Properties in Polypyrrole Thin-Film Devices by Impedance and Raman Spectroscopy. J. Phys. D. Appl. Phys. 2013, 46, 365306. [Google Scholar] [CrossRef]
- Trchová, M.; Stejskal, J. Resonance Raman Spectroscopy of Conducting Polypyrrole Nanotubes: Disordered Surface versus Ordered Body. J. Phys. Chem. A 2018, 122, 9298–9306. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Du, Y.; Bai, Y.; An, J.; Cai, X.; Chen, Y.; Wang, P.; Yang, X.; Feng, Q. Facile Formation of Anatase/Rutile TiO2 Nanocomposites with Enhanced Photocatalytic Activity. Molecules 2019, 24, 2996. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Chen, Y.-H.; Chen, J.-W.; Cao, F.; Li, L.; Luo, Z.-M.; Leu, I.-C.; Pu, Y.-C. New Insights into the Electron-Collection Efficiency Improvement of CdS-Sensitized TiO2 Nanorod Photoelectrodes by Interfacial Seed-Layer Mediation. ACS Appl. Mater. Interfaces 2019, 11, 8126–8137. [Google Scholar] [CrossRef] [PubMed]
- Yoo, I.; Kalanur, S.S.; Seo, H. A Nanoscale p–n Junction Photoelectrode Consisting of an NiOx Layer on a TiO2/CdS Nanorod Core-Shell Structure for Highly Efficient Solar Water Splitting. Appl. Catal. B Environ. 2019, 250, 200–212. [Google Scholar] [CrossRef]
- Tong, R.; Wang, X.; Zhou, X.; Liu, Q.; Wang, H.; Peng, X.; Liu, X.; Zhang, Z.; Wang, H.; Lund, P.D. Cobalt-Phosphate Modified TiO2/BiVO4 Nanoarrays Photoanode for Efficient Water Splitting. Int. J. Hydrogen Energy 2017, 42, 5496–5504. [Google Scholar] [CrossRef]
- Diby, N.D.; Duan, Y.; Grah, P.A.; Cai, F.; Yuan, Z. Enhanced Photoelectrochemical Water-Splitting Performance of TiO2 Nanorods Sensitized with CdS via Hydrothermal Approach. J. Alloys Compd. 2019, 803, 456–465. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Carquigny, S.; Segut, O.; Lakard, B.; Lallemand, F.; Fievet, P. Effect of Electrolyte Solvent on the Morphology of Polypyrrole Films: Application to the Use of Polypyrrole in PH Sensors. Synth. Met. 2008, 158, 453–461. [Google Scholar] [CrossRef]
- Debiemme-Chouvy, C.; Cachet, H.; Deslouis, C. Investigation by EQCM of the Electrosynthesis and the Properties of Polypyrrole Films Doped with Sulphate Ions and/or a Keggin-Type Heteropolyanion, SiMo12O404−. Electrochim. Acta 2006, 51, 3622–3631. [Google Scholar] [CrossRef]
- van de Krol, R.; Grätzel, M. (Eds.) Photoelectrochemical Hydrogen Production; Electronic Materials: Science & Technology; Springer: Boston, MA, USA, 2012; Volume 102, ISBN 978-1-4614-1379-0. [Google Scholar]
- Polo, A.; Lhermitte, C.R.; Dozzi, M.V.; Selli, E.; Sivula, K. Hydrogenation of ZnFe2O4 Flat Films: Effects of the Pre-Annealing Temperature on the Photoanodes Efficiency for Water Oxidation. Surfaces 2020, 3, 93–104. [Google Scholar] [CrossRef]
- Kong, W.; Zhang, X.; Chang, B.; Guo, Y.; Li, Y.; Zhang, S.; Yang, B. TiO2 Nanorods Co-decorated with Metal-Free Carbon Materials for Boosted Photoelectrochemical Water Oxidation. ChemElectroChem 2020, 7, 792–799. [Google Scholar] [CrossRef]
- Pang, Y.; Zang, W.; Kou, Z.; Zhang, L.; Xu, G.; Lv, J.; Gao, X.; Pan, Z.; Wang, J.; Wu, Y. Assembling of Bi Atoms on TiO2 Nanorods Boosts Photoelectrochemical Water Splitting of Semiconductors. Nanoscale 2020, 12, 4302–4308. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Xu, D.; Ding, J.; Fan, W.; Zhang, X.; Li, Y.; Shi, W. ZIF-8 Derived ZnO/TiO2 Heterostructure with Rich Oxygen Vacancies for Promoting Photoelectrochemical Water Splitting. J. Colloid Interface Sci. 2021, 603, 120–130. [Google Scholar] [CrossRef]
- Wannapop, S.; Somdee, A. Enhanced Visible Light Absorption of TiO2 Nanorod Photoanode by NiTiO3 Decoration for High-Performance Photoelectrochemical Cells. Ceram. Int. 2020, 46, 25758–25765. [Google Scholar] [CrossRef]
- Cosnier, S.; Karyakin, A. (Eds.) Electropolymerization; Wiley: Hoboken, NJ, USA, 2010; ISBN 9783527324149. [Google Scholar]
- Debiemme-Chouvy, C.; Tran, T.T.M. An Insight into the Overoxidation of Polypyrrole Materials. Electrochem. Commun. 2008, 10, 947–950. [Google Scholar] [CrossRef]
- Holze, R. Overoxidation of Intrinsically Conducting Polymers. Polymers 2022, 14, 1584. [Google Scholar] [CrossRef]
- Ghosh, S.; Bowmaker, G.A.; Cooney, R.P.; Seakins, J.M. Infrared and Raman Spectroscopic Studies of the Electrochemical Oxidative Degradation of Polypyrrole. Synth. Met. 1998, 95, 63–67. [Google Scholar] [CrossRef]
- Mazeikiene, R.; Malinauskas, A. Kinetics of the Electrochemical Degradation of Polypyrrole. Polym. Degrad. Stab. 2002, 75, 255–258. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, J.; Wang, F.; Shi, G. Raman Spectroscopic Studies on the Structural Changes of Electrosynthesized Polypyrrole Films during Heating and Cooling Processes. J. Appl. Polym. Sci. 2003, 89, 3390–3395. [Google Scholar] [CrossRef]
- Ghobadi, A.; Ghobadi, T.G.U.; Karadas, F.; Ozbay, E. Angstrom Thick ZnO Passivation Layer to Improve the Photoelectrochemical Water Splitting Performance of a TiO2 Nanowire Photoanode: The Role of Deposition Temperature. Sci. Rep. 2018, 8, 16322. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, Y.; Nosaka, A.Y. Reconsideration of Intrinsic Band Alignments within Anatase and Rutile TiO2. J. Phys. Chem. Lett. 2016, 7, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Resasco, J.; Zhang, H.; Kornienko, N.; Becknell, N.; Lee, H.; Guo, J.; Briseno, A.L.; Yang, P. TiO2/BiVO4 Nanowire Heterostructure Photoanodes Based on Type II Band Alignment. ACS Cent. Sci. 2016, 2, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical Considerations for Determining Absolute Frontier Orbital Energy Levels of Conjugated Polymers for Solar Cell Applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef]
- Bruchlos, K.; Trefz, D.; Hamidi-Sakr, A.; Brinkmann, M.; Heinze, J.; Ruff, A.; Ludwigs, S. Poly(3-Hexylthiophene) Revisited—Influence of Film Deposition on the Electrochemical Behaviour and Energy Levels. Electrochim. Acta 2018, 269, 299–311. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting Polymers: A Comprehensive Review on Recent Advances in Synthesis, Properties and Applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar] [CrossRef]
- Milojković, N.; Simović, B.; Žunić, M.; Radovanović, L.; Prekajski-Đorđević, M.; Dapčević, A. Modified Z-scheme Heterojunction of TiO2/Polypyrrole Recyclable Photocatalyst. J. Am. Ceram. Soc. 2025, 108, e20431. [Google Scholar] [CrossRef]
- Thakur, A.; Pal, S.; Sharma, U.; Sharma, A.; Choudhary, M.; Joshi, M.C.; Setiabudi, H.D.; Singh, P.P.; Singh, A.; Shukla, S.K. Advanced TiO2-Polypyrrole Nanostructures Enhance Glucose Detection Accuracy with Cutting-Edge Non-Enzymatic Electrochemical Capabilities. Chem. Phys. Impact 2025, 10, 100818. [Google Scholar] [CrossRef]
- Bassaid, S.; Dehbi, A.; Aissa, A.Y.B.; Menad, D.; Alsalme, A.; Colucci, G.; Darjazi, H.; Piovano, A.; Gerbaldi, C.; Messori, M. Synthesis and Characterization of Polypyrrole/TiO2 Hybrid Composite: Structural and Electrical Insights. Polym. Sci. Ser. B 2024, 66, 662–672. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puerres, J.; Ortiz, P.; Cortés, M.T. Stability of TiO2–Polypyrrole Heterojunctions for Photoelectrochemical Water Oxidation. Electrochem 2025, 6, 31. https://doi.org/10.3390/electrochem6030031
Puerres J, Ortiz P, Cortés MT. Stability of TiO2–Polypyrrole Heterojunctions for Photoelectrochemical Water Oxidation. Electrochem. 2025; 6(3):31. https://doi.org/10.3390/electrochem6030031
Chicago/Turabian StylePuerres, Jhon, Pablo Ortiz, and María T. Cortés. 2025. "Stability of TiO2–Polypyrrole Heterojunctions for Photoelectrochemical Water Oxidation" Electrochem 6, no. 3: 31. https://doi.org/10.3390/electrochem6030031
APA StylePuerres, J., Ortiz, P., & Cortés, M. T. (2025). Stability of TiO2–Polypyrrole Heterojunctions for Photoelectrochemical Water Oxidation. Electrochem, 6(3), 31. https://doi.org/10.3390/electrochem6030031






