Design and Fabrication of Micro-Electromechanical System (MEMS)-Based μ-DMFC (Direct Methanol Fuel Cells) for Portable Applications: An Outlook
Abstract
1. Introduction
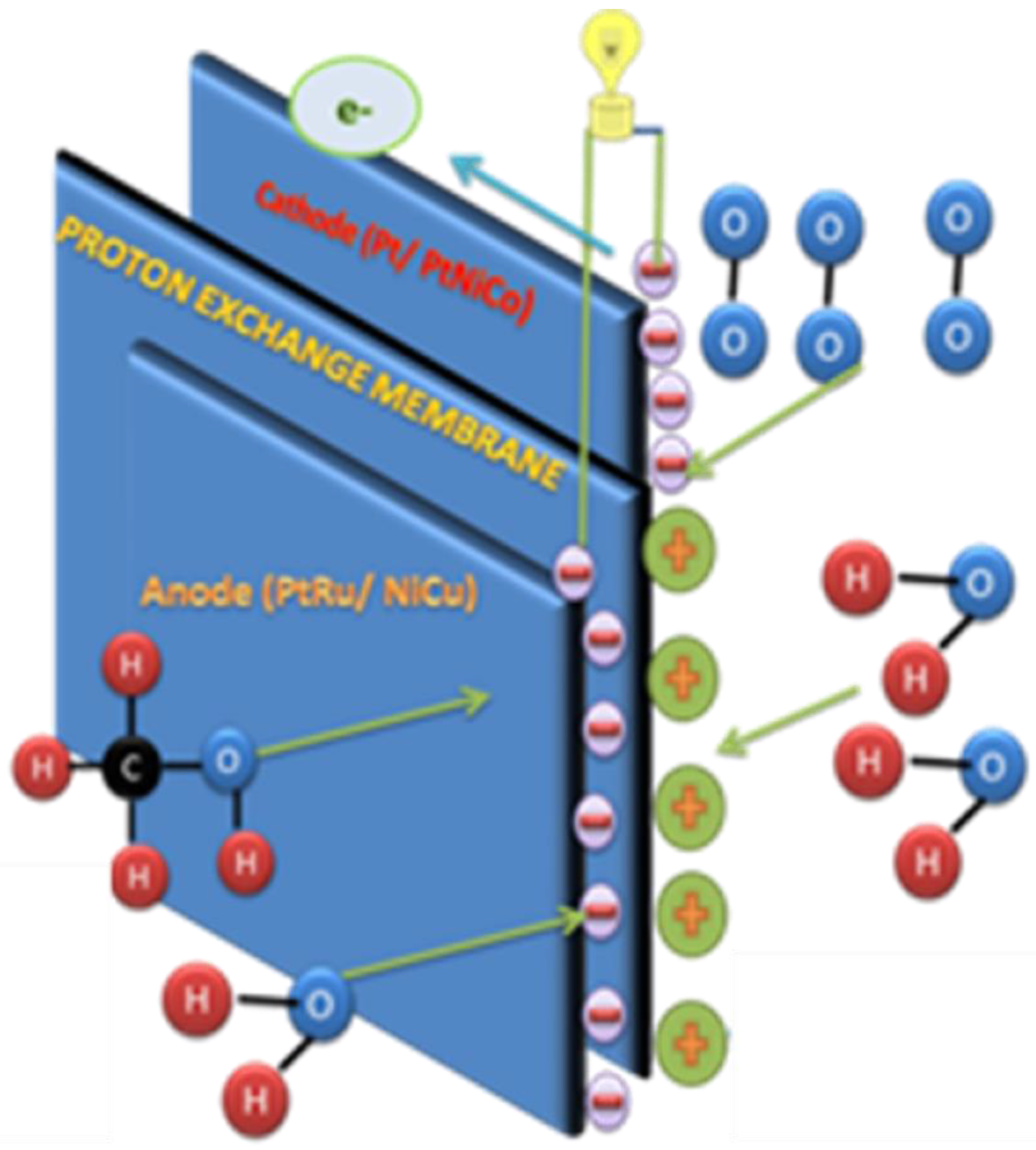
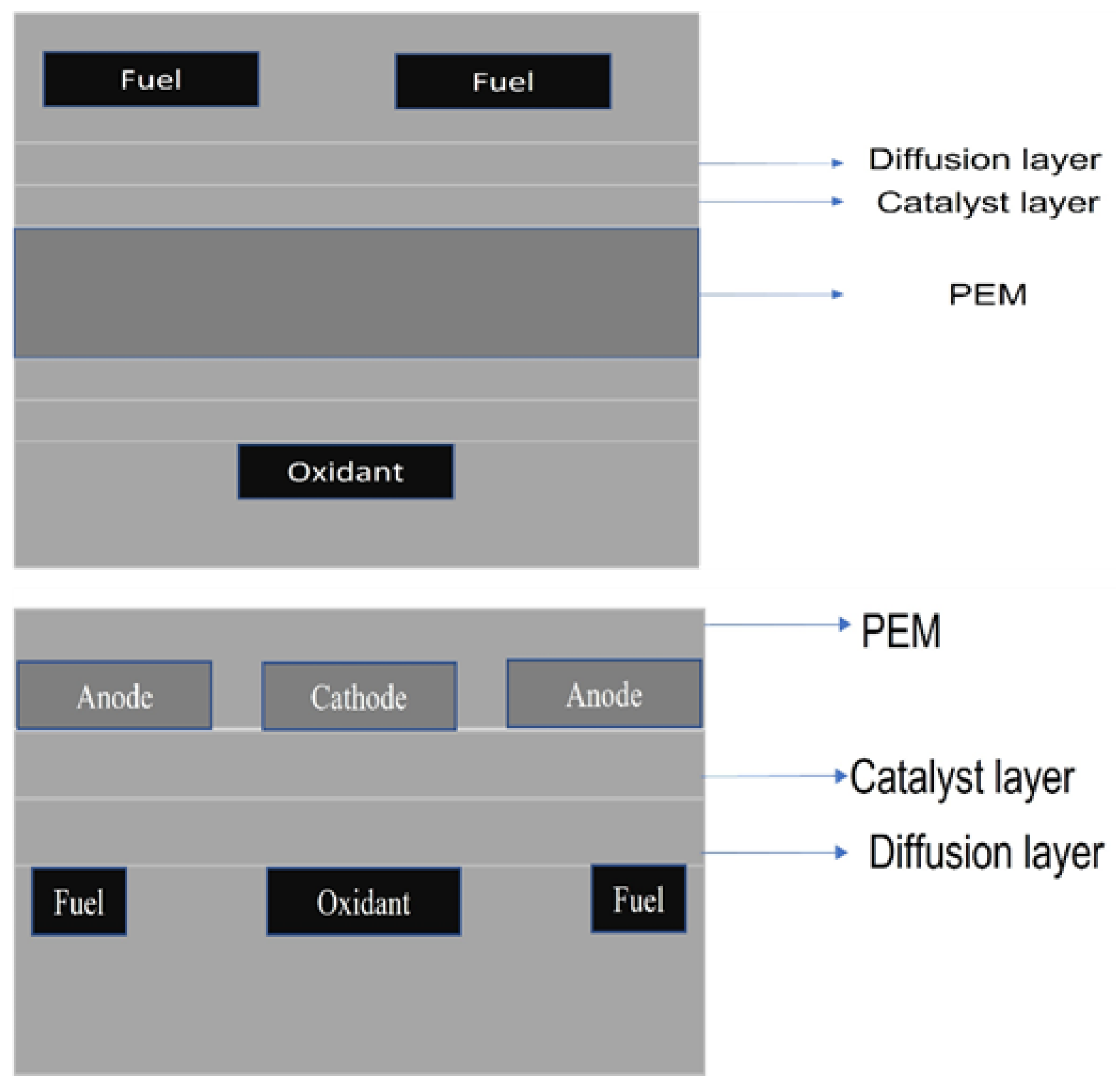
2. Fundamental and Design Consideration
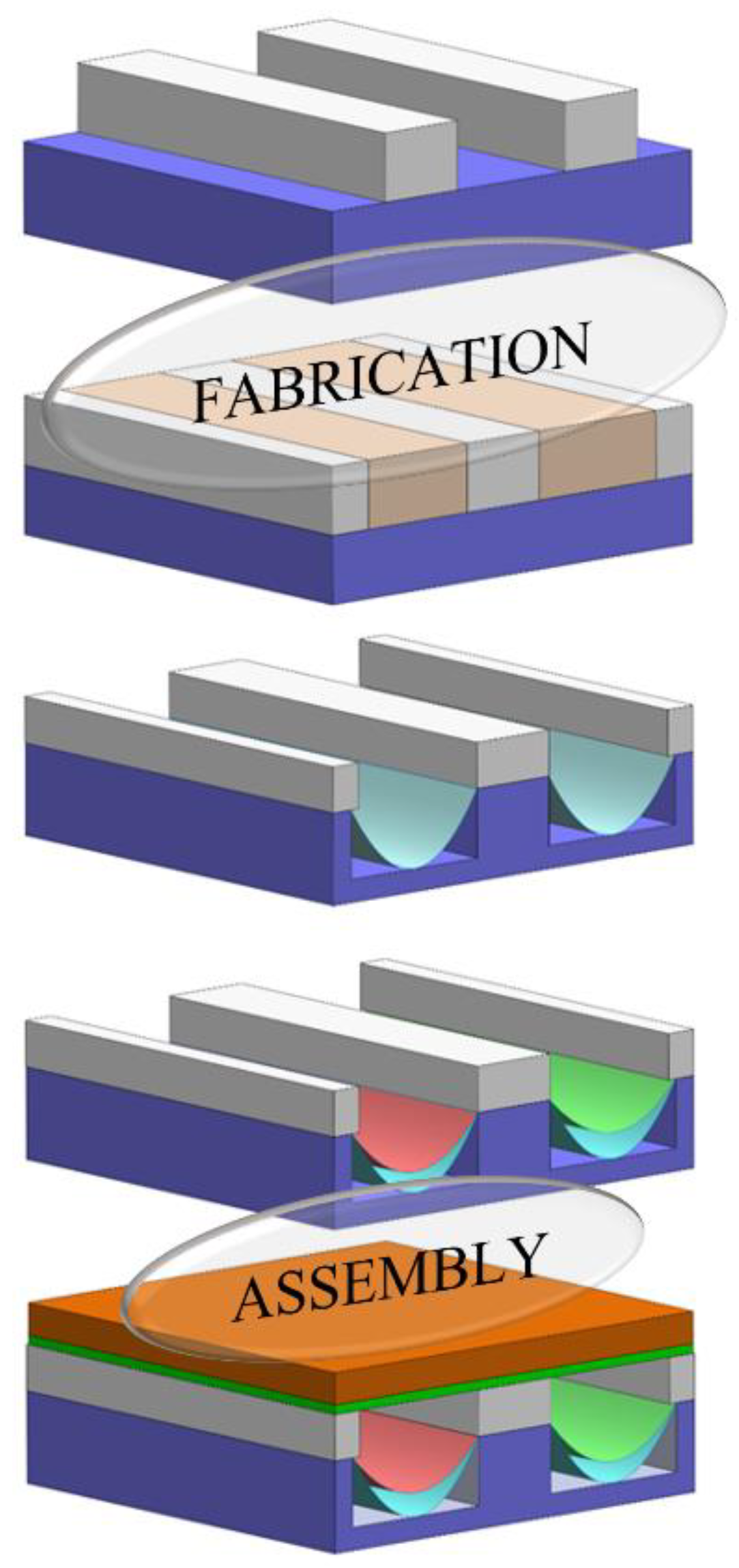
3. Methodology and Fabrication
4. Fuel Delivery System
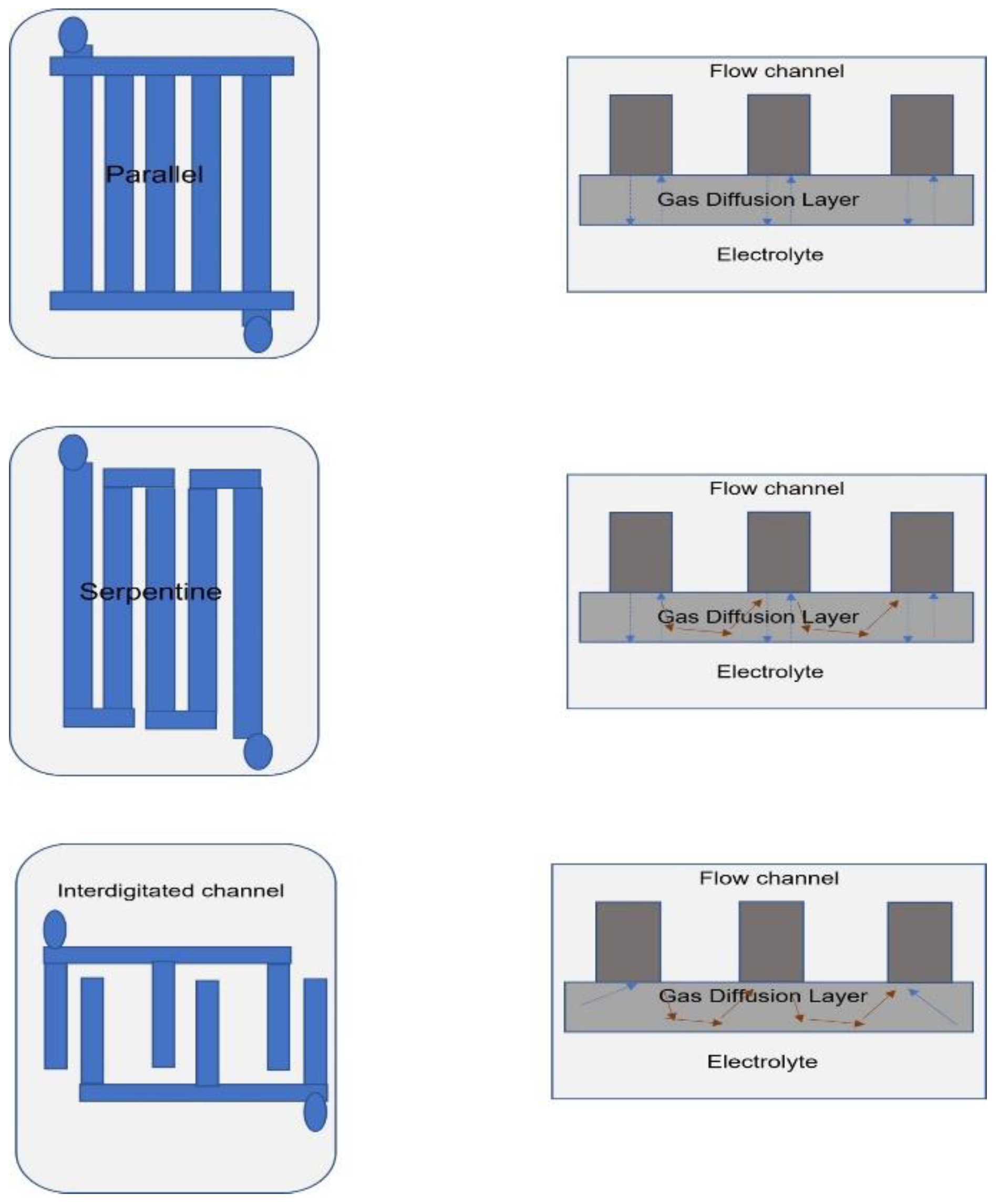
5. Flow Field Designs
5.1. Planar and Bipolar Designs
5.1.1. Concentration Overpotential
5.1.2. Ohmic Resistance
5.2. Bioinspired Flow Fields
6. Parameters for Effective Systems
6.1. Methanol Concentration
6.2. Effect of Temperature
6.3. Effect of Gas Pressure
6.4. Effect of Catalyst Loadings
6.5. Effect of Orientation
7. Performance Analysis
8. Cost Analysis
9. Advanced Applications
10. Numerical Analysis

11. Current Challenges and Future Direction
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choban, E.R.; Markoski, L.J.; Wieckowski, A.; Kenis, P.J. Microfluidic fuel cell based on laminar flow. J. Power Sources 2004, 128, 54–60. [Google Scholar] [CrossRef]
- Jung, D.H.; Lee, C.H.; Kim, C.S.; Shin, D.R. Performance of a direct methanol polymer electrolyte fuel cell. J. Power Sources 1998, 71, 169–173. [Google Scholar] [CrossRef]
- O’Hayre, R.; Fabian, T.; Litster, S.; Prinz, F.B.; Santiago, J.G. Engineering model of a passive planar air breathing fuel cell cathode. J. Power Sources 2007, 167, 118–129. [Google Scholar] [CrossRef]
- Falcão, D.S.; Oliveira, V.B.; Rangel, C.M.; Pinto, A.M.F.R. Review on micro-direct methanol fuel cells. Renew. Sustain. Energy Rev. 2014, 34, 58–70. [Google Scholar] [CrossRef]
- Fachini, E.R.; Diaz-Ayala, R.; Casado-Rivera, E.; File, S.; Cabrera, C.R. Surface coordination of ruthenium clusters on platinum nanoparticles for methanol oxidation catalysts. Langmuir 2003, 19, 8986–8993. [Google Scholar] [CrossRef]
- Chen, T.Y.; Lee, G.W.; Liu, Y.T.; Liao, Y.F.; Huang, C.C.; Lin, D.S.; Lin, T.L. Heterojunction confinement on the atomic structure evolution of near monolayer core–shell nanocatalysts in redox reactions of a direct methanol fuel cell. J. Mater. Chem. A 2015, 3, 1518–1529. [Google Scholar] [CrossRef]
- Yang, W.M.; Chou, S.K.; Shu, C. Effect of current-collector structure on performance of passive micro direct methanol fuel cell. J. Power Sources 2007, 164, 549–554. [Google Scholar] [CrossRef]
- Motokawa, S.; Mohamedi, M.; Momma, T.; Shoji, S.; Osaka, T. MEMS-based design and fabrication of a new concept micro direct methanol fuel cell (μ-DMFC). Electrochem. Commun. 2004, 6, 562–565. [Google Scholar] [CrossRef]
- Baldauf, M.; Preidel, W. Status of the development of a direct methanol fuel cell. J. Power Sources 1999, 84, 161–166. [Google Scholar] [CrossRef]
- Lu, G.Q.; Wang, C.Y.; Yen, T.J.; Zhang, X. Development and characterization of a silicon-based micro direct methanol fuel cell. Electrochim. Acta 2004, 49, 821–828. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Zhong, L.; Zhou, Y.A.; Qiu, X.; Liu, L. Design, optimization and microfabrication of a micro-direct methanol fuel cell with microblocks in anode structure. Sens. Actuators A 2009, 154, 247–254. [Google Scholar] [CrossRef]
- Tominaka, S.; Ohta, S.; Obata, H.; Momma, T.; Osaka, T. On-chip fuel cell: Micro direct methanol fuel cell of an air-breathing, membraneless, and monolithic design. J. Am. Chem. Soc. 2008, 130, 10456–10457. [Google Scholar] [CrossRef] [PubMed]
- Abrego-Martínez, J.C.; Moreno-Zuria, A.; Wang, Y.; Cuevas-Muñiz, F.M.; Arriaga, L.G.; Sun, S.; Mohamedi, M. Fabrication and evaluation of passive alkaline membraneless microfluidic DMFC. Int. J. Hydrogen Energy 2017, 42, 21969–21975. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q.; Zhu, Y.; Liu, L. An air-breathing micro direct methanol fuel cell stack employing a single shared anode using silicon microfabrication technologies. J. Micromech. Microeng. 2009, 19, 094012. [Google Scholar] [CrossRef]
- Zhong, L.; Wang, X.; Jiang, Y.; Zhang, Q.; Qiu, X.; Zhou, Y.A.; Liu, L. A micro-direct methanol fuel cell stack with optimized design and microfabrication. Sens. Actuators A 2008, 143, 70–76. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Zhu, Y.; Zhou, Y.A.; Qiu, X.; Liu, L. Optimized temperature control system integrated into a micro direct methanol fuel cell for extreme environments. J. Power Sources 2009, 192, 494–501. [Google Scholar] [CrossRef]
- Kamitani, A.; Morishita, S.; Kotaki, H.; Arscott, S. Improved fuel use efficiency in microchannel direct methanol fuel cells using a hydrophilic macroporous layer. J. Power Sources 2009, 187, 148–155. [Google Scholar] [CrossRef]
- Zhang, H.; Hsing, I.M. Flexible graphite-based integrated anode plate for direct methanol fuel cells at high methanol feed concentration. J. Power Sources 2007, 167, 450–454. [Google Scholar] [CrossRef]
- Hsu, F.K.; Lee, M.S.; Lin, C.C.; Lin, Y.K.; Hsu, W.T. A flexible portable proton exchange membrane fuel cell. J. Power Sources 2012, 219, 180–187. [Google Scholar] [CrossRef]
- Chang, I.; Lee, M.H.; Lee, J.H.; Kim, Y.S.; Cha, S.W. Air-breathing flexible polydimethylsiloxane (PDMS)-based fuel cell. Int. J. Precis. Eng. Manuf. 2013, 14, 501–504. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, B.; Zhang, Y.; He, H.; Li, J.; Wang, S.; Yuan, Z.; Deng, H. Development and characterization of a novel air-breathing micro direct methanol fuel cell stack for portable applications. J. Micromech. Microeng. 2010, 20, 104008. [Google Scholar] [CrossRef]
- Shen, M.; Walter, S.; Gijs, M.A.M. Monolithic micro-direct methanol fuel cell in polydimethylsiloxane with microfluidic channel-integrated Nafion strip. J. Power Sources 2009, 193, 761–765. [Google Scholar] [CrossRef]
- Sun, L.; Liu, C.; Liang, J.; Zhu, X.; Cui, T. A self-pumping and self-breathing micro direct methanol fuel cell with polymer bipolar plates. J. Power Sources 2011, 196, 7533–7540. [Google Scholar] [CrossRef]
- Kamitani, A.; Morishita, S.; Kotaki, H.; Arscott, S. Miniaturized microDMFC using silicon microsystems techniques: Performances at low fuel flow rates. J. Micromech. Microeng. 2008, 18, 125019. [Google Scholar] [CrossRef]
- Torres, N.; Santander, J.; Esquivel, J.P.; Sabaté, N.; Figueras, E.; Ivanov, P.; Fonseca, L.; Gràcia, I.; Cané, C. Performance optimization of a passive silicon-based micro-direct methanol fuel cell. Sens. Actuators B 2008, 132, 540–544. [Google Scholar] [CrossRef]
- Hashim, N.; Kamarudin, S.K.; Daud, W.R.W. Design, fabrication and testing of a PMMA-based passive single-cell and a multi-cell stack micro-DMFC. Int. J. Hydrogen Energy 2009, 34, 8263–8269. [Google Scholar] [CrossRef]
- Meng, D.D.; Kim, C.J. An active micro-direct methanol fuel cell with self-circulation of fuel and built-in removal of CO2 bubbles. J. Power Sources 2009, 194, 445–450. [Google Scholar] [CrossRef]
- Esquivel, J.P.; Sabaté, N.; Santander, J.; Torres, N.; Cané, C. Fabrication and characterization of a passive silicon-based direct methanol fuel cell. J. Microsyst. Technol. 2008, 14, 535–541. [Google Scholar] [CrossRef]
- Faria, L.; Barragán, V. Optimizing Methanol Flow Rate for Enhanced Semi-Passive Mini-Direct Methanol Fuel Cell Performance. Preprints 2025, 2025010827. [Google Scholar] [CrossRef]
- Ghayor, R.; Shakeri, M.; Sedighi, K.; Farhadi, M. Experimental and numerical investigation on passive and active μDMFC. Int. J. Hydrogen Energy 2010, 35, 9329–9337. [Google Scholar] [CrossRef]
- Esquivel, J.P.; Senn, T.; Hernández-Fernández, P.; Santander, J.; Lörgen, M.; Rojas, S.; Löchel, B.; Cané, C.; Sabaté, N. Towards a compact SU-8 micro-direct methanol fuel cell. J. Power Sources 2010, 195, 8110–8115. [Google Scholar] [CrossRef]
- Wang, S.J.; Huo, W.W.; Zou, Z.Q.; Qiao, Y.J.; Yang, H. Computational simulation and experimental evaluation on anodic flow field structures of micro direct methanol fuel cells. Appl. Therm. Eng. 2011, 31, 2877–2884. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, P.; Yuan, Z.; He, H.; Zhao, Y.; Liu, X. A tapered serpentine flow field for the anode of micro direct methanol fuel cells. J. Power Sources 2011, 196, 3255–3259. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, Z.; Li, Y.; Jia, Q.; Chen, S.; Liu, X. Design and fabrication of a silicon-based direct methanol fuel cell with a new cathode spoke structure. J. Power Sources 2011, 196, 3015–3025. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Chan, S.H. Micromachined polymer electrolyte membrane and direct methanol fuel cells—A review. J. Micromech. Microeng. 2006, 16, R1. [Google Scholar] [CrossRef]
- Chang, H.; Kao, M.-J.; Chen, C.-H.; Cho, K.-C.; Hsu, C.-Y.; Chen, Z.-L. The Fabrication of Flow Field Plates for Direct Methanol Fuel Cell Using Lithography and Radio Frequency Sputtering. J. Nanosci. Nanotechnol. 2015, 15, 6172–6175. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Joh, H.I.; Scibioh, M.A.; Lee, S.Y.; Kim, S.K.; Lee, T.G.; Ha, H.Y. Impact of cathode channel depth on performance of direct methanol fuel cells. J. Power Sources 2008, 183, 223–226. [Google Scholar] [CrossRef]
- Zografos, K.; Barber, R.W.; Emerson, D.R.; Oliveira, M.S. A design rule for constant depth microfluidic networks for power-law fluids. Microfluid. Nanofluidics 2015, 19, 737–749. [Google Scholar] [CrossRef]
- Kloess, J.P.; Wang, X.; Liu, J.; Shi, Z.; Guessous, L. Investigation of bio-inspired flow channel designs for bipolar plates in proton exchange membrane fuel cells. J. Power Sources 2009, 188, 132–140. [Google Scholar] [CrossRef]
- Ghadhban, S.A.; Alawee, W.H.; Dhahad, H.A. Study effects of bio-inspired flow filed design on Polymer Electrolyte Membrane fuel cell performance. Case Stud. Therm. Eng. 2021, 24, 100841. [Google Scholar] [CrossRef]
- Chen, C.Y.; Yang, P. Performance of an air-breathing direct methanol fuel cell. J. Power Sources 2003, 123, 37–42. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, H.; Yang, J. A selective electrocatalyst–based direct methanol fuel cell operated at high concentrations of methanol. Sci. Adv. 2017, 3, e1700580. [Google Scholar] [CrossRef] [PubMed]
- Schulz, T.; Weinmüller, C.; Nabavi, M.; Poulikakos, D. Electrochemical impedance spectroscopy analysis of a thin polymer film-based micro-direct methanol fuel cell. J. Power Sources 2010, 195, 7548–7558. [Google Scholar] [CrossRef]
- Liu, J.G.; Zhao, T.S.; Liang, Z.X.; Chen, R. Effect of membrane thickness on the performance and efficiency of passive direct methanol fuel cells. J. Power Sources 2006, 153, 61–67. [Google Scholar] [CrossRef]
- Hsieh, S.S.; Her, B.S. Heat transfer and pressure drop in serpentine μDMFC flow channels. Int. J. Heat. Mass. Transf. 2007, 50, 5323–5327. [Google Scholar] [CrossRef]
- Tominaka, S.; Momma, T.; Osaka, T. Electrodeposited Pd-Co catalyst for direct methanol fuel cell electrodes: Preparation and characterization. Electrochim. Acta 2008, 53, 4679–4686. [Google Scholar] [CrossRef]
- Wu, Z.; Kuang, X.; Liu, L.; Wang, X. A flexible foldable tubular μDMFC for powering wearable devices. J. Microelectromech. Syst. 2017, 26, 1147–1154. [Google Scholar] [CrossRef]
- Rashidi, R.; Dincer, I.; Naterer, G.F.; Berg, P. Performance evaluation of direct methanol fuel cells for portable applications. J. Power Sources 2009, 187, 509–516. [Google Scholar] [CrossRef]
- Kamarudin, S.K.; Achmad, F.; Daud, W.R.W. Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices. Int. J. Hydrogen Energy 2009, 34, 6902–6916. [Google Scholar] [CrossRef]
- Apanel, G.; Johnson, E. Direct methanol fuel cells–ready to go commercial? Fuel Cells Bull. 2004, 2004, 12–17. [Google Scholar] [CrossRef]
- Xie, C.; Bostaph, J.; Pavio, J. Development of a 2 W direct methanol fuel cellpower source. J. Power Sources 2004, 136, 55–65. [Google Scholar] [CrossRef]
- Faghri, A.; Li, X.; Bahrami, H. Recent advances in passive and semi-passive direct methanol fuel cells. Int. J. Therm. Sci. 2012, 62, 12–18. [Google Scholar] [CrossRef]
- Chen, X.; Li, T.; Shen, J.; Hu, Z. From structures, packaging to application: A system-level review for micro direct methanol fuel cell. Renew. Sustain. Energy Rev. 2017, 80, 669–678. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Beillard, M.; Halepoto, D.M.; Kizewski, J.P.; Poynton, S.; Slade, R.C. Membrane and electrode materials for alkaline membrane fuel cells. Ecs Trans. 2008, 16, 1819–1834. [Google Scholar] [CrossRef]
- Qin, Y.; Guo, Q.; Chen, R.; Zhuang, Y.; Wang, Y. Numerical investigation of water droplet impact on PEM fuel cell flow channel surface. Renewable 2021, 45, 29861–29873. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Falcão, D.S.; Oliveira, V.B.; Pinto, A.M.F.R. Numerical simulations of two-phase flow in an anode gas channel of a proton exchange membrane fuel cell. Energy 2015, 82, 619–628. [Google Scholar] [CrossRef]
- Pugal Mani, S.; Kalaiyarasan, M.; Agilan, P.; Ravichandran, K.; Rajendran, N.; Meng, Y. Evaluation of anticorrosion and contact resistance behavior of poly(orthophenylenediamine)-coated 316L SS bipolar plate for proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2022, 47, 41097–41110. [Google Scholar] [CrossRef]
- Guo, Q.; Qin, Y. Numerical investigation of water droplet removal characteristics in novel block channels of PEMFC using dynamic wettability model. Int. J. Hydrogen Energy 2021, 46, 36890–36902. [Google Scholar] [CrossRef]
- Carton, J.G.; Lawlor, V.; Olabi, A.G.; Hochenauer, C.; Zauner, G. Water droplet accumulation and motion in PEM (Proton Exchange Membrane) fuel cell mini-channels. Energy 2012, 39, 63–73. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, X.; Chen, R.; Shangguan, X. Water transport and removal in PEMFC gas flow channel with various water droplet locations and channel surface wettability. Energies 2018, 11, 880. [Google Scholar] [CrossRef]
- He, G.; Yamazaki, Y.; Abudula, A. The effect of wall roughness on the liquid removal in micro-channels related to a proton exchange membrane fuel cell (PEMFC). J. Power Sources 2010, 195, 1561–1568. [Google Scholar] [CrossRef]
- Wen, D.H.; Yin, L.Z.; Piao, Z.Y.; Li, G.; Leng, Q.H. Performance investigation of proton exchange membrane fuel cell with intersectant flow field. Int. J. Heat. Mass. Transf. 2018, 121, 775–787. [Google Scholar] [CrossRef]
- Maslan, N.H.; Rosli, M.I.; Goh, C.W.; Masdar, M.S. Computational fluid dynamics simulation of the flow field of direct methanol fuel cells. Int. J. Mech. Mechatron. Eng. 2015, 15, 114–125. [Google Scholar]
- Lei, H.; Huang, H.; Li, C.; Pan, M.; Guo, X.; Chen, Y.; Liu, M.; Wang, T. Numerical simulation of water droplet transport characteristics in cathode channel of proton exchange membrane fuel cell with tapered slope structures. Int. J. Hydrogen Energy 2020, 45, 29331–29344. [Google Scholar] [CrossRef]
- Selvaraj, A.S.; Rajagopal, T.K.R. Numerical investigation on the effect of flow field and landing to channel ratio on the performance of PEMFC. Int. J. Energy Res. 2020, 44, 171–191. [Google Scholar] [CrossRef]
- Liu, S.; Chen, T.; Xie, Y.; Zhang, J.; Wu, C. Numerical simulation and experimental study on the effect of symmetric and asymmetric bionic flow channels on PEMFC performance under gravity. Int. J. Hydrogen Energy 2019, 44, 29618–29630. [Google Scholar] [CrossRef]
- Uzundurukan, A.; Bilgili, M.; Devrim, Y. Examination of compression effects on PEMFC performance by numerical and experimental analyses. Int. J. Hydrogen Energy 2020, 45, 35085–35096. [Google Scholar] [CrossRef]
- Vasile, N.S.; Videla, A.H.M.; Specchia, S. Effects of the current density distribution on a single-cell DMFC by tuning the anode catalyst in layers of gradual loadings: Modelling and experimental approach. Chem. Eng. J. 2017, 322, 722–741. [Google Scholar]
- Shyu, J.-C.; Hung, S.-H. Flow field effect on the performance of direct formic acid membraneless fuel cells: A numerical study. Processes 2021, 9, 746. [Google Scholar] [CrossRef]
- Sharifi, S.; Rahimi, R.; Mohebbi-Kalhori, D.; Colpan, C.O. Numerical investigation of methanol crossover through the membrane in a direct methanol fuel cell. Hydrog. Fuel Cell Energy Storage 2018, 5, 21–33. [Google Scholar]
- Jung, G.B.; Su, A.; Tu, C.H.; Lin, Y.T.; Weng, F.B.; Chan, S.H. Effects of cathode flow fields on direct methanol fuel cell-simulation study. J. Power Sources 2007, 171, 212–217. [Google Scholar] [CrossRef]
- Tang, Y.; Yuan, W.; Pan, M.; Wan, Z. Feasibility study of porous copper fiber sintered felt: A novel porous flow field in proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2010, 35, 9661–9677. [Google Scholar] [CrossRef]
- Shang, K.; Han, C.; Jiang, T.; Chen, Z. Numerical study of PEMFC heat and mass transfer characteristics based on roughness interface thermal resistance model. Int. J. Hydrogen Energy 2023, 48, 7460–7475. [Google Scholar] [CrossRef]
- Tan, Q.; Lei, H.; Liu, Z. Numerical simulation analysis of the performance on the PEMFC with a new flow field designed based on constructal-theory. Int. J. Hydrogen Energy 2022, 47, 11975–11990. [Google Scholar] [CrossRef]
- Dang, D.K.; Zhou, B. Effects of pin shapes on gas-liquid transport behaviors in PEMFC cathode. J. Power Sources 2023, 557, 232584. [Google Scholar] [CrossRef]
- Huang, H.; Li, X.; Li, S.; Guo, X.; Liu, M.; Wang, T.; Lei, H. Evaluating the effect of refined flow channels in a developed biomimetic flow field on PEMFC performance. Energy 2023, 266, 126442. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, P.; Ding, H.; Xin, D.; Sun, S. Investigation of the three-dimensional flow field for proton exchange membrane fuel cell with additive manufactured stainless steel bipolar plates: Numerical simulation and experiments. Energy 2023, 269, 126709. [Google Scholar] [CrossRef]
- Hamrang, A.; Abdollahzadeh, M.; Kermani, M.J.; Rahgoshay, S.M. Numerical simulation of the PEM fuel cell performance enhancement by various blockage arrangement of the cathode serpentine gas flow channel outlets/inlets. Int. J. Heat. Mass. Transf. 2022, 186, 122475. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Fan, Y.; He, W.; Guan, J.; Wang, X. Numerical investigation of tapered flow field configurations for enhanced polymer electrolyte membrane fuel cell performance. Appl. Energy 2022, 306, 118021. [Google Scholar] [CrossRef]
- Bao, Z.; Niu, Z.; Jiao, K. Analysis of single-and two-phase flow characteristics of 3-D fine mesh flow field of proton exchange membrane fuel cells. J. Power Sources 2019, 438, 226995. [Google Scholar] [CrossRef]
- Ramesh, P.; Duttagupa, S.P. Effect of channel dimensions on micro-PEM fuel cell performance using 3D modeling. Int. J. Renew. Energy Res. 2013, 3, 353–358. [Google Scholar]
- Vasile, N.S.; Videla, A.H.M.; Simari, C.; Nicotera, I.; Specchia, S. Influence of membrane-type and flow field design on methanol crossover on a single-cell DMFC: An experimental and multi-physics modeling study. Int. J. Hydrogen Energy 2017, 42, 27995–28010. [Google Scholar] [CrossRef]
- El-Zoheiry, R.M.; Ookawara, S.; Ahmed, M. Efficient fuel utilization by enhancing the under-rib mass transport using new serpentine flow field designs of direct methanol fuel cells. Energy Convers. Manag. 2017, 144, 88–103. [Google Scholar] [CrossRef]
- Govindarasu, R.; Somasundaram, S. Studies on influence of cell temperature in direct methanol fuel cell operation. Processes 2020, 8, 353. [Google Scholar] [CrossRef]
- Binyamin, B.; Lim, O. Analyzing Temperature Distribution, Mass Transport, and Cell Performance in PEM Fuel Cells with Emphasis on GDL Face Permeability and Thermal Contact Resistance Parameters. ACS Omega 2023, 9, 1516–1534. [Google Scholar] [CrossRef] [PubMed]
- Kishore, S.C.; Perumal, S.; Atchudan, R.; Alagan, M.; Sundramoorthy, A.K.; Lee, Y.R. A Critical Review on Artificial Intelligence for Fuel Cell Diagnosis. Catalysts 2022, 12, 743. [Google Scholar] [CrossRef]
- Talukdar, A.; Chakrovorty, A.; Sarmah, P.; Paramasivam, P.; Kumar, V.; Yadav, S.K.; Manickkam, S. A Review on Solid Oxide Fuel Cell Technology: An Efficient Energy Conversion System. Int. J. Energy Res. 2024, 2024, 6443247. [Google Scholar] [CrossRef]

| Substrate Types | Fabrication Techniques | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Silicon | DRIE (deep reactive ion etching), CVD (chemical vapor deposition), and PVD (physical vapor deposition) | High temperature resistance | Fragility | [14] |
| Stainless steel | Etching and laser machining | High conductivity | Possibility of corrosion | [21] |
| PDMS | Hot embossing and soft lithography | Low cost and good chemical stability | Low power density | [22] |
| Acrylonitrile Butadiene Styrene (ABS) | Double-side hot embossing | Low cost and highly accurate batch process | Low power density | [23] |
| Type | Active Area (cm2) | Flow Field | Methanol Concentration | Power Density (mW/cm2) | T (°C) | References |
|---|---|---|---|---|---|---|
| Active | 0.3 | serpentine | 3 M | 12.4 | 20 | [24] |
| Active | 2.5 | - | 2 M | 17 | 20 | [27] |
| Active | - | planar | 1 M | 3 | 20 | [22] |
| Active | 0.47 | serpentine | 2 M | 8.8 | 20 | [11] |
| Active | 0.47 | serpentine | 2 M | 5.55 | 56 | [16] |
| Passive | 0.28 | - | 4 M | 10 | 25 | [25] |
| Passive | 0.28 | - | 5 M | 11.4 | 25 | [28] |
| Semi passive | 1.56 | - | - | 5 | 25 | [29] |
| Passive | - | - | 2 M | 85 | 60 | [30] |
| Passive | 0.25 | - | 4 M | 4.15 | 40 | [31] |
| Self-breathing | 0.64 | parallel | 1 M | 24.75 | 20 | [32] |
| Self-breathing | 0.64 | parallel | 1 M | 27.11 | 20 | [33] |
| Self-breathing | 1.44 | double serpentine | 2 M | 34.2 | 25 | [34] |
| Self-breathing | 0.64 | tapered serpentine | 1 M | 15.4 | 20 | [35] |
| Self-breathing | 0.64 | serpentine | 1 M | 14.79 | 20 | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sesu, D.C.; Narendran, G.; Ramakrishnan, S.; Vediappan, K.; Esakki Muthu, S.; Shanmugan, S.; Kannan, K. Design and Fabrication of Micro-Electromechanical System (MEMS)-Based μ-DMFC (Direct Methanol Fuel Cells) for Portable Applications: An Outlook. Electrochem 2025, 6, 11. https://doi.org/10.3390/electrochem6020011
Sesu DC, Narendran G, Ramakrishnan S, Vediappan K, Esakki Muthu S, Shanmugan S, Kannan K. Design and Fabrication of Micro-Electromechanical System (MEMS)-Based μ-DMFC (Direct Methanol Fuel Cells) for Portable Applications: An Outlook. Electrochem. 2025; 6(2):11. https://doi.org/10.3390/electrochem6020011
Chicago/Turabian StyleSesu, Divya Catherin, Ganesan Narendran, Saraswathi Ramakrishnan, Kumaran Vediappan, Sankaran Esakki Muthu, Sengottaiyan Shanmugan, and Karthik Kannan. 2025. "Design and Fabrication of Micro-Electromechanical System (MEMS)-Based μ-DMFC (Direct Methanol Fuel Cells) for Portable Applications: An Outlook" Electrochem 6, no. 2: 11. https://doi.org/10.3390/electrochem6020011
APA StyleSesu, D. C., Narendran, G., Ramakrishnan, S., Vediappan, K., Esakki Muthu, S., Shanmugan, S., & Kannan, K. (2025). Design and Fabrication of Micro-Electromechanical System (MEMS)-Based μ-DMFC (Direct Methanol Fuel Cells) for Portable Applications: An Outlook. Electrochem, 6(2), 11. https://doi.org/10.3390/electrochem6020011









