Full Tailored Metal Content NCM Regeneration from Spent Lithium-Ion Battery Mixture Under Mild Condition
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Metal Recovery from LIB Black Powder
2.2.1. Black Powder Extraction
2.2.2. Hydrometallurgy Driven Metal Recovery
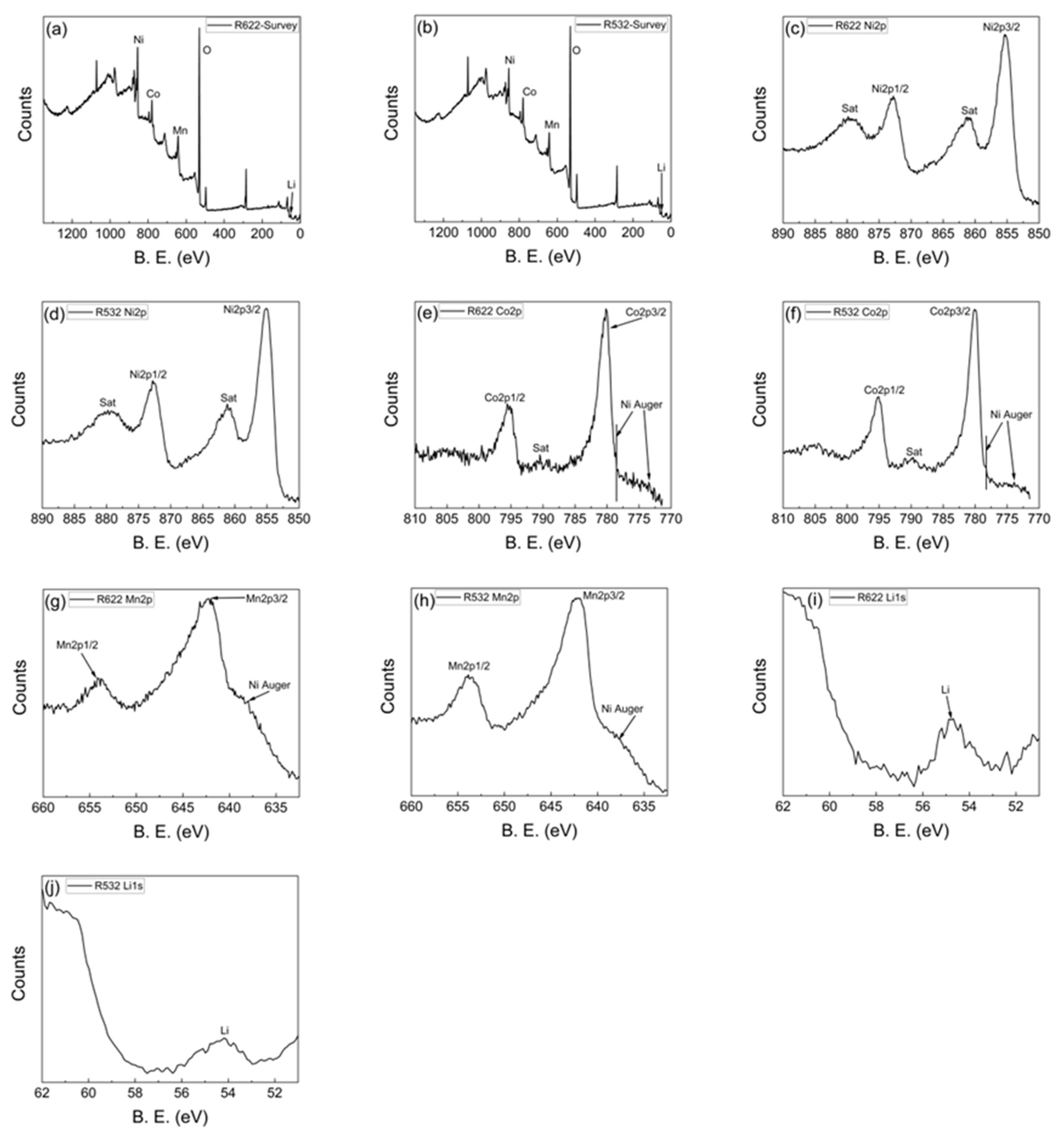
2.2.3. Material Characterisation
2.3. Ternary NCM Black Powder Regeneration and Battery Performace Testing
2.3.1. Ternary NCM Powder Regeneration
2.3.2. Regenerated Battery Assembly
2.3.3. Battery Performance Testing
3. Results and Discussion
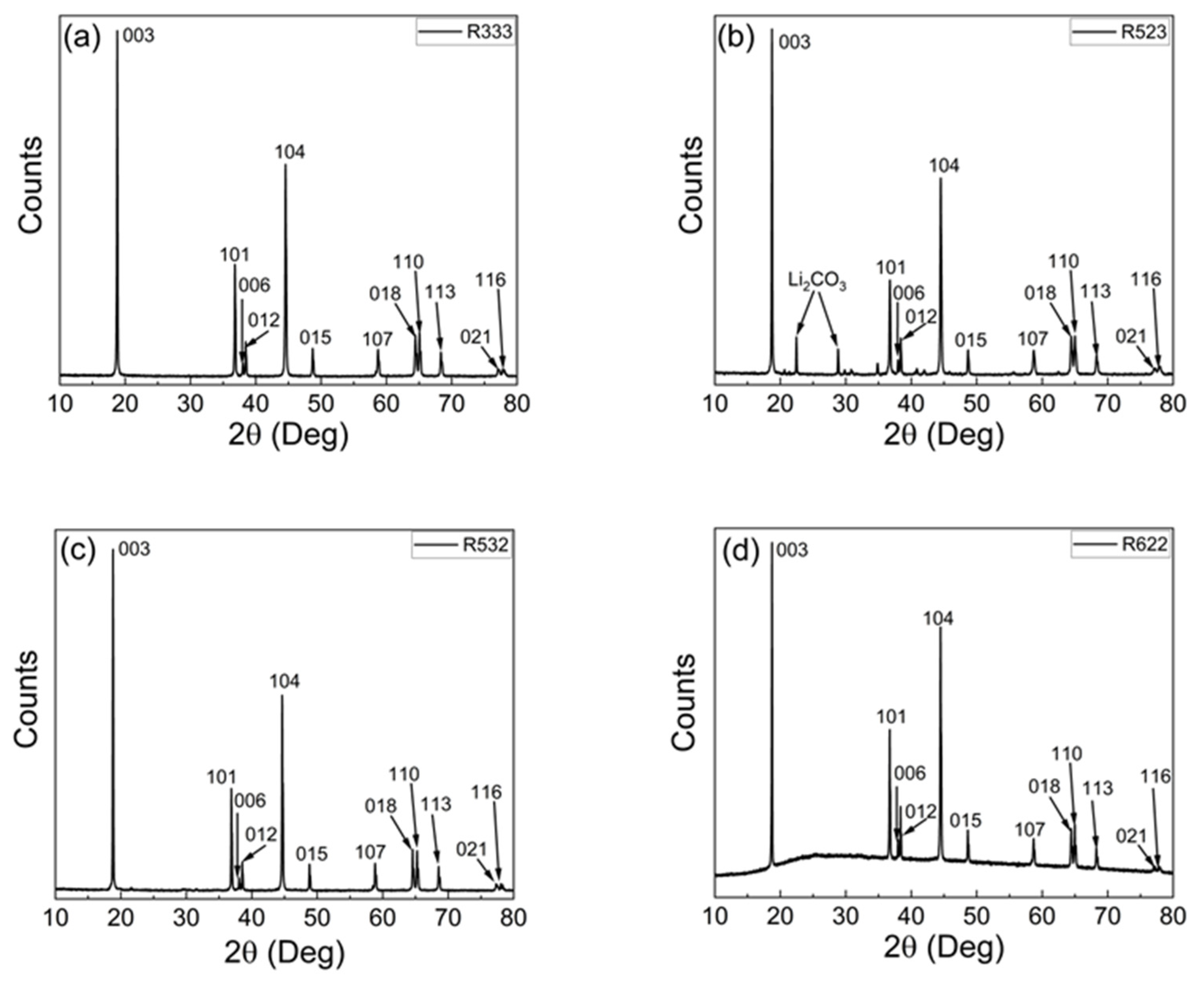
3.1. Material Characteristics of Regenerated NCM
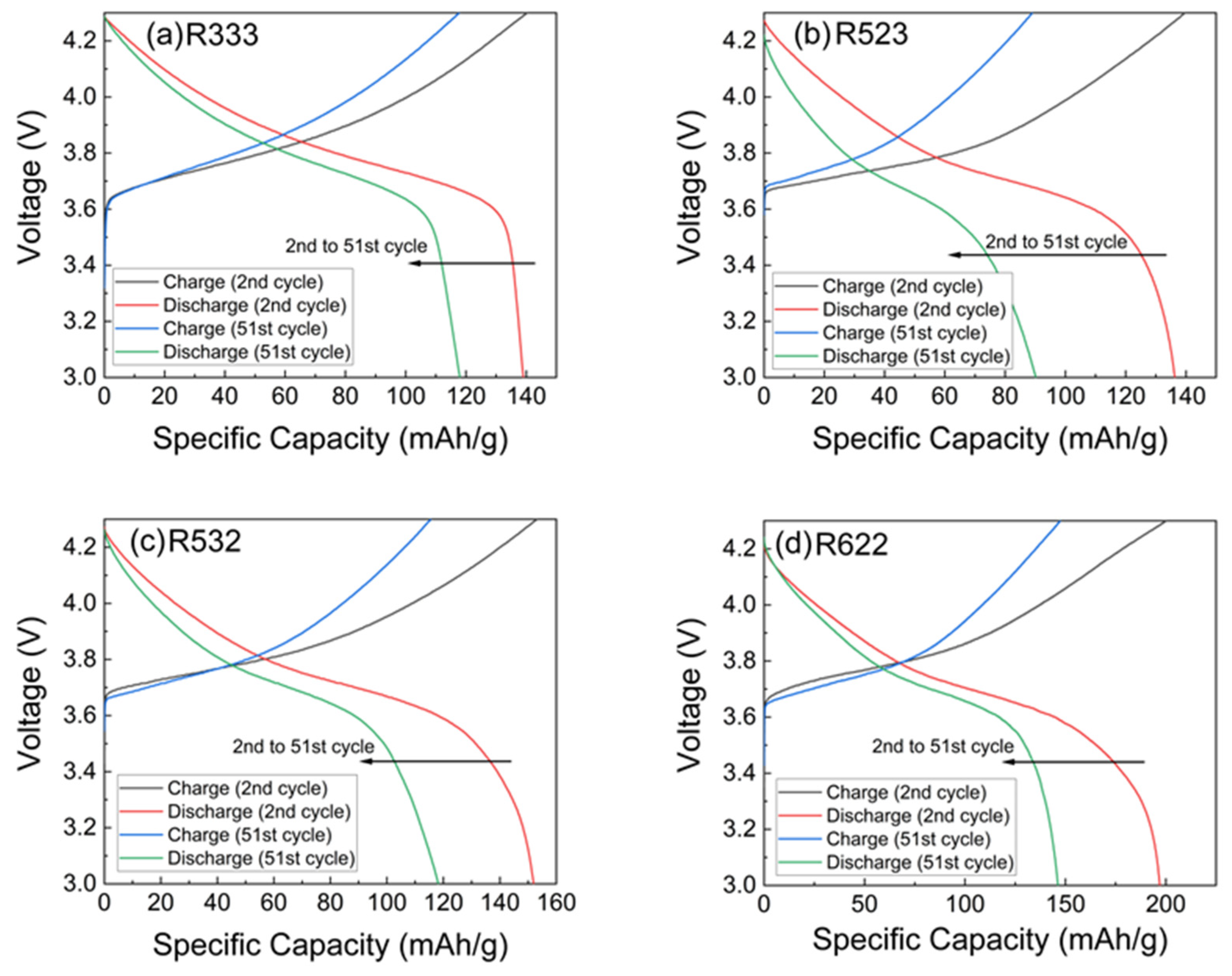
3.2. Battery Performance Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, P.C.; Yang, X.R.; Xiao, L.; Chen, H.; Chen, H. Preparation of Ternary Precursor Derived from Spent LiNixCoyMn1-x-yO2 Materials. JOM 2019, 71, 4492–4499. [Google Scholar] [CrossRef]
- Shang, M.; Peng, L.X. The regeneration and electrochemical performance study of NCM622 cathode materials. Ionics 2021, 27, 527–532. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, G.; Liu, F.; Yue, X.J.; Chen, Z. Resolving the Compositional and Structural Defects of Degraded LiNixCoyMnzO2 Particles to Directly Regenerate High-Performance Lithium-Ion Battery Cathodes. ACS Energy Lett. 2018, 3, 1683–1692. [Google Scholar] [CrossRef]
- Fan, X.P.; Tan, C.L.; Li, Y.; Chen, Z.Q.; Li, Y.H.; Huang, Y.G.; Pan, Q.C.; Zheng, F.H.; Wang, H.Q.; Li, Q.Y. A green, efficient, closed-loop direct regeneration technology for reconstructing of the LiNi0.5Co0.2Mn0.3O2 cathode material from spent lithium-ion batteries. J. Hazard. Mater. 2021, 410, 124610. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.T.; Chen, M.Y.; Zheng, Z.F.; Bullen, D.; Wang, J.; Harrison, C.; Gratz, E.; Lin, Y.L.; Yang, Z.Z.; Zhang, Y.T.; et al. Recycled cathode materials enabled superior performance for lithium-ion batteries. Joule 2021, 5, 2955–2970. [Google Scholar] [CrossRef]
- Fan, M.; Chang, X.; Meng, Q.; Wan, L.-J.; Guo, Y.-G. Progress in the sustainable recycling of spent lithium-ion batteries. SusMat 2021, 1, 241–254. [Google Scholar] [CrossRef]
- Du, K.D.; Ang, E.H.; Wu, X.L.; Liu, Y.C. Progresses in Sustainable Recycling Technology of Spent Lithium-Ion Batteries. Energy Environ. Mater. 2022, 5, 1012–1036. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, H.; Li, J.; Li, J.; Zhuge, X.; Ren, Y.; Ding, Z. A large volume and low energy consumption recycling strategy for LiNi0.6Co0.2Mn0.2O2 from spent ternary lithium-ion batteries. J. Power Sources 2024, 602, 234407. [Google Scholar] [CrossRef]
- Xing, L.; Lin, S.; Yu, J.G. Novel Recycling Approach to Regenerate a LiNi0.6Co0.2Mn0.2O2 Cathode Material from Spent Lithium-Ion Batteries. Ind. Eng. Chem. Res. 2021, 60, 10303–10311. [Google Scholar] [CrossRef]
- Song, D.; Yu, J.; Wang, M.; Tan, Q.; Liu, K.; Li, J. Advancing recycling of spent lithium-ion batteries: From green chemistry to circular economy. Energy Storage Mater. 2023, 61, 102870. [Google Scholar] [CrossRef]
- Chen, X.Q.; Yang, C.F.; Yang, Y.B.; Ji, H.M.; Yang, G. Co-precipitation preparation of Ni-Co-Mn ternary cathode materials by using the sources extracting directly from spent lithium-ion batteries. J. Alloy. Compd. 2022, 909, 164691. [Google Scholar] [CrossRef]
- Huang, Z.X.; Liu, X.; Zheng, Y.; Wang, Q.; Liu, J.W.; Xu, S.M. Boosting efficient and low-energy solid phase regeneration for single crystal LiNi0.6Co0.2Mn0.2O2 via highly selective leaching and its industrial application. Chem. Eng. J. 2023, 451, 139039. [Google Scholar] [CrossRef]
- Baars, J.; Domenech, T.; Bleischwitz, R.; Melin, H.E.; Heidrich, O. Circular economy strategies for electric vehicle batteries reduce reliance on raw materials. Nat. Sustain. 2021, 4, 71–79. [Google Scholar] [CrossRef]
- Wang, G.G.; Wu, T.; Liu, B.R.; Gong, S.S.; Huang, Q.; Su, Y.F.; Wu, F.; Kelly, R.M. Gradient-Regeneration of Li(Ni0.9Co0.05Mn0.05)O2 from Spent LiCoO2 lithium-Ion Battery. J. Electrochem. Soc. 2020, 167, 160557. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, M.H.; Meng, Y.S.; Chen, Z. Ambient-Pressure Relithiation of Degraded LixNi0.5Co0.2Mn0.3O2 (0<x<1) via Eutectic Solutions for Direct Regeneration of Lithium-Ion Battery Cathodes. Adv. Energy Mater. 2019, 9, 1900454. [Google Scholar]
- Zhou, H.M.; Zhao, X.X.; Yin, C.J.; Li, J. Regeneration of LiNi0.5Co0.2Mn0.3O2 cathode material from spent lithium-ion batteries. Electrochim. Acta 2018, 291, 142–150. [Google Scholar] [CrossRef]
- Jiang, G.H.; Zhang, Y.N.; Meng, Q.; Zhang, Y.J.; Dong, P.; Zhang, M.Y.; Yang, X. Direct Regeneration of LiNi0.5Co0.2Mn0.3O2 Cathode from Spent Lithium-Ion Batteries by the Molten Salts Method. ACS Sustain. Chem. Eng. 2020, 8, 18138–18147. [Google Scholar] [CrossRef]
- Chen, X.Q.; Feng, Y.Y.; Zhang, S.; Kou, W.Z.; Ji, H.M.; Yang, G. Comparison study on regeneration of spent ternary materials by molten salt solid-liquid method and traditional solid-solid method. J. Alloy. Compd. 2022, 900, 163308. [Google Scholar] [CrossRef]
- Tang, X.D.; Guo, Q.K.; Zhou, M.M.; Zhong, S.W. Direct regeneration of LiNi0.5Co0.2Mn0.3O2 cathode material from spent lithium-ion batteries. Chin. J. Chem. Eng. 2021, 40, 278–286. [Google Scholar] [CrossRef]
- Wang, B.; Lin, X.Y.; Tang, Y.Y.; Wang, Q.; Leung, M.K.H.; Lu, X.Y. Recycling LiCoO2 with methanesulfonic acid for regeneration of lithium-ion battery electrode materials. J. Power Sources 2019, 436, 226828. [Google Scholar] [CrossRef]
- Mugumya, J.H.; Rasche, M.L.; Rafferty, R.F.; Patel, A.; Mallick, S.; Mou, M.Y.; Bobb, J.A.; Gupta, R.B.; Jiang, M. Synthesis and Theoretical Modeling of Suitable Co-precipitation Conditions for Producing NMC111 Cathode Material for Lithium-Ion Batteries. Energy Fuels 2022, 36, 12261–12270. [Google Scholar] [CrossRef]
- Wood, K.N.; Teeter, G. XPS on Li-Battery-Related Compounds: Analysis of Inorganic SEI Phases and a Methodology for Charge Correction. ACS Appl. Energ. Mater. 2018, 1, 4493–4504. [Google Scholar] [CrossRef]
- Wang, L.Z.; Zhang, J.H.; Fang, H.; Gao, K.Z.; Yan, J. LiNi0.8Co0.1Mn0.1O2/LixCoO2 hybrid cathode and Its Enhanced Electrochemical Properties for Lithium Ion Batteries Lithium Ion Batteries. Int. J. Electrochem. Sci. 2020, 15, 11684–11699. [Google Scholar] [CrossRef]
- Zhang, L.L.; Wang, J.Q.; Yang, X.L.; Liang, G.; Li, T.; Yu, P.L.; Ma, D. Enhanced Electrochemical Performance of Fast Ionic Conductor LiTi2(PO4)3-Coated LiNi1/3Co1/3Mn1/3O2 Cathode Material. ACS Appl. Mater. Interfaces 2018, 10, 11663–11670. [Google Scholar] [CrossRef]
- Bondarchuk, O.; LaGrow, A.P.; Kvasha, A.; Thieu, T.; Ayerbe, E.; Urdampilleta, I. On the X-ray photoelectron spectroscopy analysis of LiNixMnyCozO2 material and electrodes. Appl. Surf. Sci. 2021, 535, 147699. [Google Scholar] [CrossRef]
- Su, X.; Wang, X.P.; Bareno, J.; Qin, Y.; Aguesse, F.; Lu, W.Q. Enable High-Energy LiNi0.5Co0.2Mn0.3O2 by Ultra-Thin Coating through Wet Impregnation. Batteries 2022, 8, 136. [Google Scholar] [CrossRef]
- Liu, J.; Mak, T.Y.; Meng, Z.; Wang, X.; Cao, Y.; Lu, Z.; Suen, D.W.-S.; Lu, X.-Y.; Tang, Y. Efficient recovery of lithium as Li2CO3 and cobalt as Co3O4 from spent lithium-ion batteries after leaching with p-toluene sulfonic acid. Hydrometallurgy 2023, 216, 106012. [Google Scholar] [CrossRef]


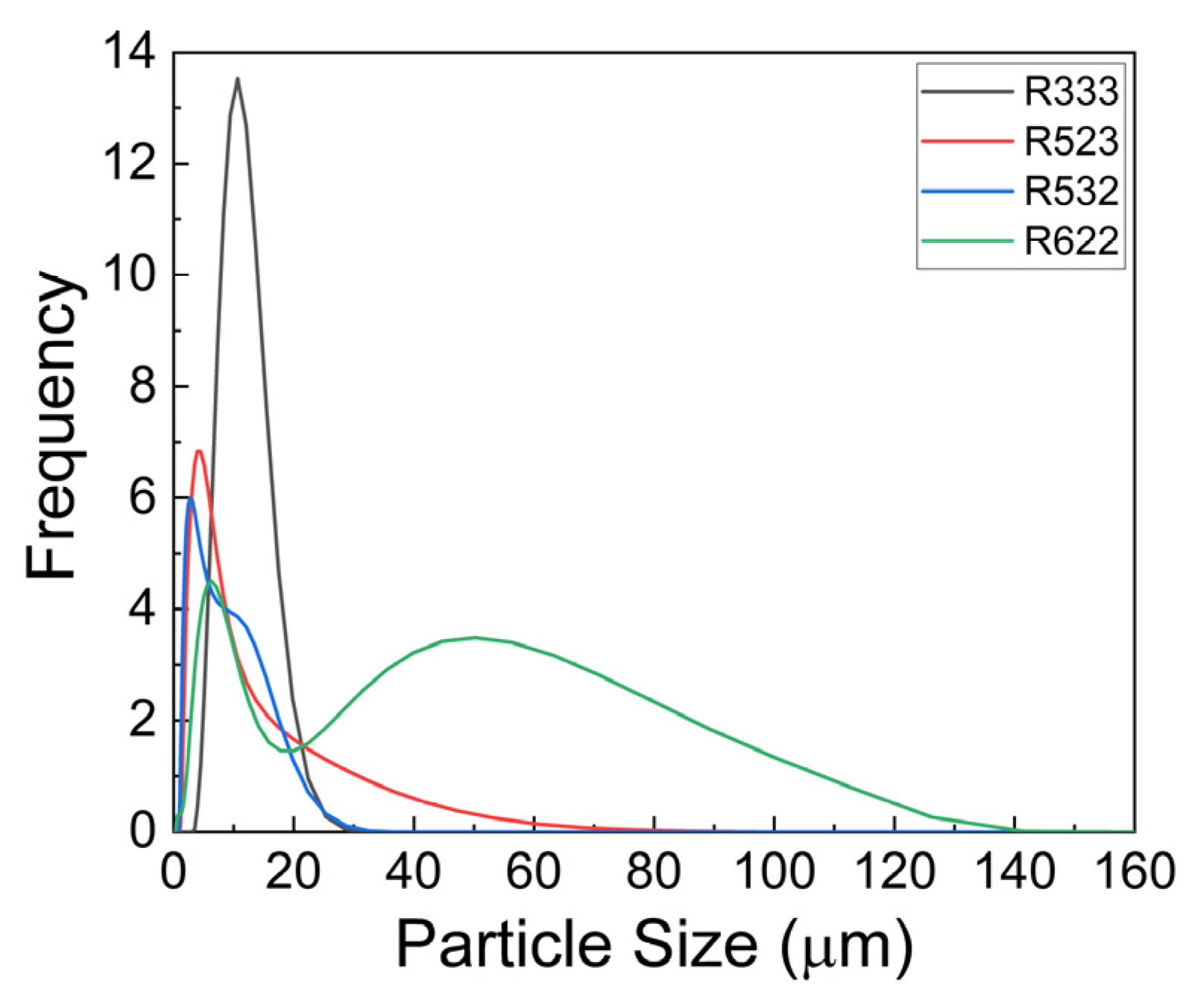

| Sample | Ni (at%) | Co (at%) | Mn (at%) | NCM Composition | Particle Size (D90) (µm) |
|---|---|---|---|---|---|
| R333 | 20.0 | 20.2 | 18.2 | Ni1.10Co1.11Mn1 | 14.8 |
| R523 | 28.9 | 10.9 | 14.4 | Ni5.30Co2Mn2.64 | 18.3 |
| R532 | 22.5 | 15.1 | 9.5 | Ni4.74Co3.18Mn2 | 14.7 |
| R622 | 43.5 | 14.9 | 10.3 | Ni6.12Co2Mn1.46 | 61.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsang, A.C.H.; Ouyang, S.; Lv, Y.; Lee, C.C.; Tsang, C.-W.; Lu, X.-Y. Full Tailored Metal Content NCM Regeneration from Spent Lithium-Ion Battery Mixture Under Mild Condition. Electrochem 2024, 5, 546-559. https://doi.org/10.3390/electrochem5040035
Tsang ACH, Ouyang S, Lv Y, Lee CC, Tsang C-W, Lu X-Y. Full Tailored Metal Content NCM Regeneration from Spent Lithium-Ion Battery Mixture Under Mild Condition. Electrochem. 2024; 5(4):546-559. https://doi.org/10.3390/electrochem5040035
Chicago/Turabian StyleTsang, Alpha Chi Him, Shaobo Ouyang, Yang Lv, Chi Chung Lee, Chi-Wing Tsang, and Xiao-Ying Lu. 2024. "Full Tailored Metal Content NCM Regeneration from Spent Lithium-Ion Battery Mixture Under Mild Condition" Electrochem 5, no. 4: 546-559. https://doi.org/10.3390/electrochem5040035
APA StyleTsang, A. C. H., Ouyang, S., Lv, Y., Lee, C. C., Tsang, C.-W., & Lu, X.-Y. (2024). Full Tailored Metal Content NCM Regeneration from Spent Lithium-Ion Battery Mixture Under Mild Condition. Electrochem, 5(4), 546-559. https://doi.org/10.3390/electrochem5040035









