Enhanced Performance of Sodium-Ion Battery Cathodes with Ti and V Co-Doped P2-Type Na0.67Fe0.5Mn0.5O2 Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Synthesis
2.2. Material Characterization
2.3. Electrochemical Characterization
3. Results
3.1. Physicochemical Analysis
3.1.1. XRD and Rietveld Refinement Analysis
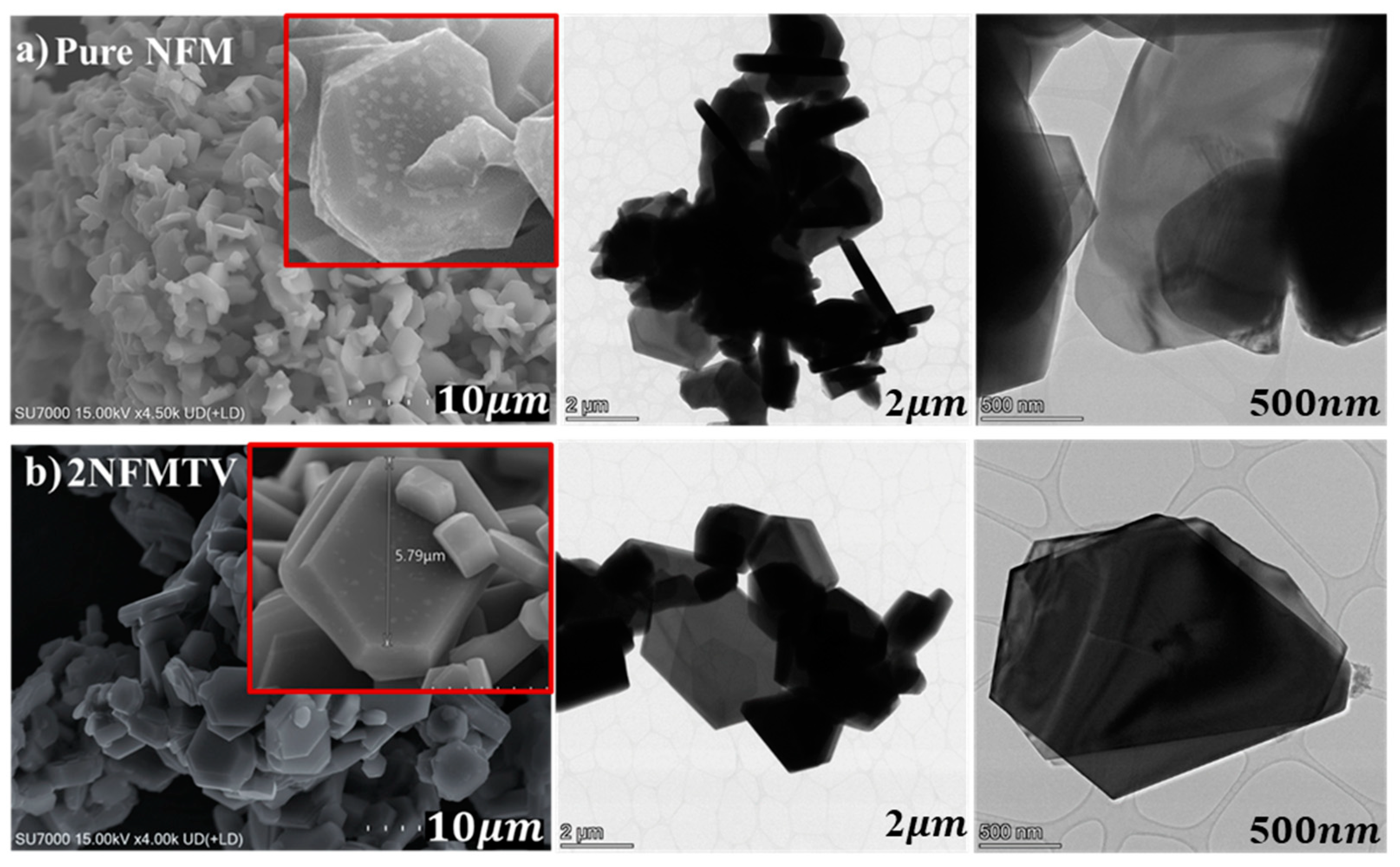
3.1.2. SEM and EDS Analysis
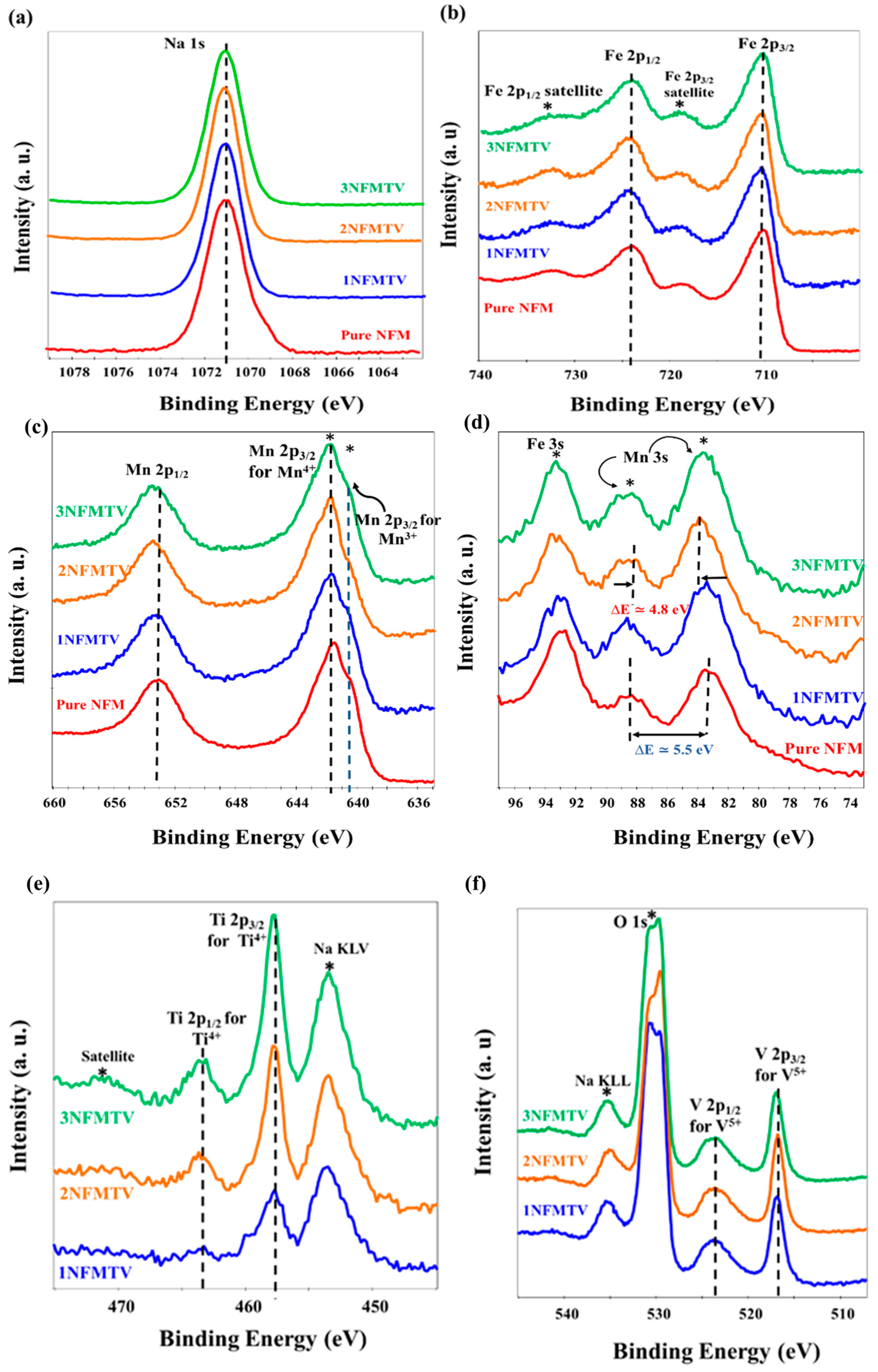
3.1.3. XPS Analysis
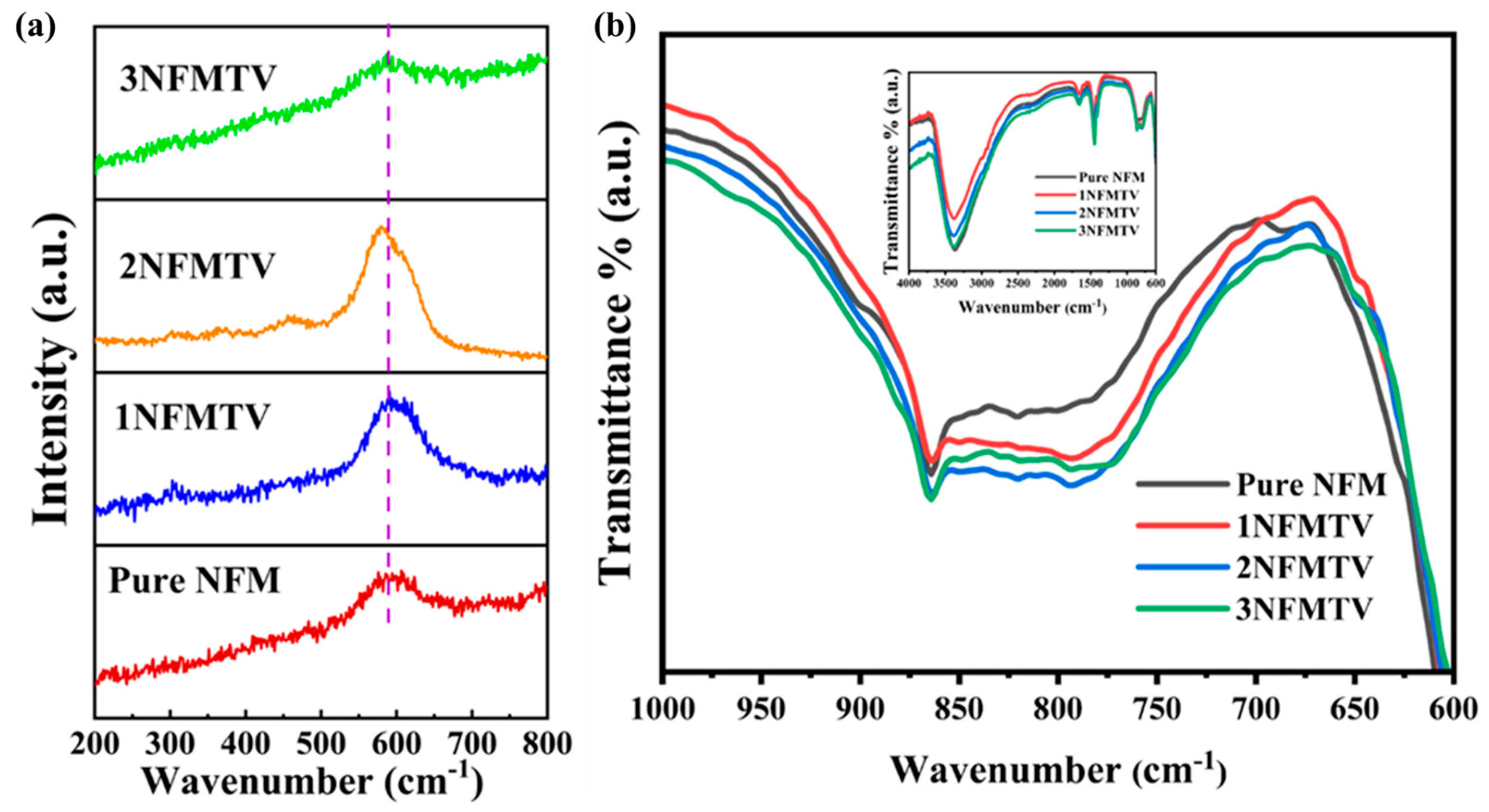
3.1.4. Raman and FTIR Analysis
3.2. Electrochemical Analysis
3.2.1. Galvanostatic Charge/Discharge and Rate Capability Analysis
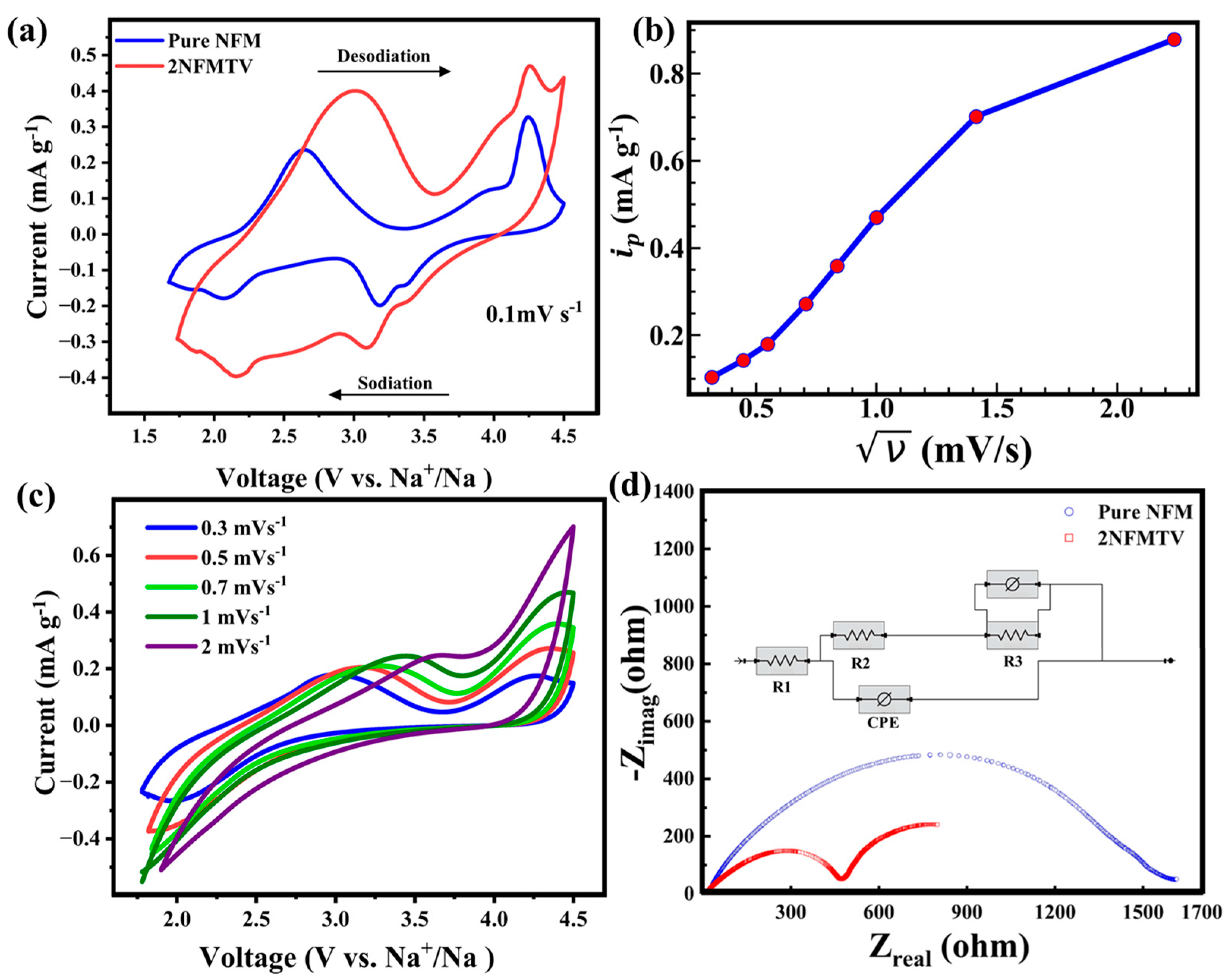
3.2.2. Cyclic Voltammetry and EIS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bloomberg. Lithium-Ion Battery Market Size Worth $182.53 Billion by 2030: Grand View Research, Inc. 2022. Available online: https://www.bloomberg.com/press-releases/2022-06-07/lithium-ion-battery-market-size-worth-182-53-billion-by-2030-grand-view-research-inc (accessed on 3 March 2023).
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From Lithium-Ion to Sodium-Ion Batteries: Advantages, Challenges, and Surprises. Angew. Chemie-Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef]
- Kim, S.W.; Seo, D.H.; Ma, X.; Ceder, G.; Kang, K. Electrode materials for rechargeable sodium-ion batteries: Potential alternatives to current lithium-ion batteries. Adv. Energy Mater. 2012, 2, 710–721. [Google Scholar] [CrossRef]
- Anilkumar, A.; Nair, N.; Nair, S.V.; Baskar, S. Tailoring high Na content in P2-type layered oxide cathodes via Cu–Li dual doping for sodium-ion batteries. J. Energy Storage 2023, 72, 108291. [Google Scholar] [CrossRef]
- Shi, W.J.; Zhang, D.; Meng, X.M.; Bao, C.X.; Xu, S.D.; Chen, L.; Wang, X.M.; Liu, S.B.; Wu, Y.C. Low-Strain Reticular Sodium Manganese Oxide as an Ultrastable Cathode for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 14174–14184. [Google Scholar] [CrossRef]
- Yuan, D.D.; Wang, Y.X.; Cao, Y.L.; Ai, X.P.; Yang, H.X. Improved electrochemical performance of Fe-substituted NaNi0.5Mn0.5O2 cathode materials for sodium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 8585–8591. [Google Scholar] [CrossRef]
- Delmas, C.; Fouassier, C.; Hagenmuller, P. Structural classification and properties of the layered oxides. Physica B+C 1980, 99, 81–85. [Google Scholar] [CrossRef]
- Jia, M.; Qiao, Y.; Li, X.; Qiu, F.; Cao, X.; He, P.; Zhou, H. Identifying Anionic Redox Activity within the Related O3- and P2-Type Cathodes for Sodium-Ion Battery. ACS Appl. Mater. Interfaces 2020, 12, 851–857. [Google Scholar] [CrossRef]
- Bianchini, M.; Gonzalo, E.; Drewett, N.E.; Ortiz-Vitoriano, N.; López Del Amo, J.M.; Bonilla, F.J.; Acebedo, B.; Rojo, T. Layered P2-O3 sodium-ion cathodes derived from earth abundant elements. J. Mater. Chem. A 2018, 6, 3552–3559. [Google Scholar] [CrossRef]
- Toumar, A.J. Phase Transformations in Layered Electrode Materials for Sodium Ion Batteries. Mass. Inst. Technol. 2017, 380, 138156. [Google Scholar]
- Wei, F.; Zhang, Q.; Zhang, P.; Tian, W.; Dai, K.; Zhang, L.; Mao, J.; Shao, G. Review—Research Progress on Layered Transition Metal Oxide Cathode Materials for Sodium Ion Batteries. J. Electrochem. Soc. 2021, 168, 050524. [Google Scholar] [CrossRef]
- Shen, Q.; Zhao, X.; Liu, Y.; Li, Y.; Zhang, J.; Zhang, N.; Yang, C.; Chen, J. Dual-Strategy of Cation-Doping and Nanoengineering Enables Fast and Stable Sodium-Ion Storage in a Novel Fe/Mn-Based Layered Oxide Cathode. Adv. Sci. 2020, 7, 2002199. [Google Scholar] [CrossRef]
- Thorne, J.S.; Dunlap, R.A.; Obrovac, M.N. Structure and Electrochemistry of NaxFexMn1−xO2 (1.0 ≤ x ≤ 0.5) for Na-Ion Battery Positive Electrodes. J. Electrochem. Soc. 2013, 160, A361–A367. [Google Scholar] [CrossRef]
- Zhang, K.; Kim, D.; Hu, Z.; Park, M.; Noh, G.; Yang, Y.; Zhang, J.; Lau, V.W.-h.; Chou, S.L.; Cho, M.; et al. Manganese based layered oxides with modulated electronic and thermodynamic properties for sodium ion batteries. Nat. Commun. 2019, 10, 5203. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Kim, J.; Yu, T.Y.; Sun, Y.K. A New P2-Type Layered Oxide Cathode with Extremely High Energy Density for Sodium-Ion Batteries. Adv. Energy Mater. 2019, 9, 1803346. [Google Scholar] [CrossRef]
- Shi, Y.; Li, S.; Gao, A.; Zheng, J.; Zhang, Q.; Lu, X.; Gu, L.; Cao, D. Probing the Structural Transition Kinetics and Charge Compensation of the P2-Na0.78Al0.05Ni0.33Mn0.60O2 Cathode for Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 24122–24131. [Google Scholar] [CrossRef]
- Darbar, D.; Muralidharan, N.; Hermann, R.P.; Nanda, J. Evaluation of Electrochemical Performance and Redox Activity of Fe in Ti doped layered P2-Na0.67Mn0.5Fe0.5O2 cathode for sodium ion batteries. Electrochim. Acta 2021, 380, 138156. [Google Scholar] [CrossRef]
- Han, M.H.; Gonzalo, E.; Sharma, N.; López Del Amo, J.M.; Armand, M.; Avdeev, M.; Saiz Garitaonandia, J.J.; Rojo, T. High-Performance P2-Phase Na2/3Mn0.8Fe0.1Ti0.1O2 Cathode Material for Ambient-Temperature Sodium-Ion Batteries. Chem. Mater. 2016, 28, 106–116. Available online: https://pubs.acs.org/doi/full/10.1021/acs.chemmater.5b03276 (accessed on 5 November 2023). [CrossRef]
- Linnell, S.F.; Manche, A.G.; Liao, Y.; Hirsbrunner, M.; Imada, S.; Naden, A.B.; Irvine, J.T.S.; Duda, L.C.; Armstrong, A.R. Effect of Cu substitution on anion redox behaviour in P3-type sodium manganese oxides. J. Phys. Energy 2022, 4, 044006. [Google Scholar] [CrossRef]
- Ramasamy, H.V.; Kaliyappan, K.; Thangavel, R.; Aravindan, V.; Kang, K.; Kim, D.U.; Park, Y.; Sun, X.; Lee, Y.S. Cu-doped P2-Na0.5Ni0.33Mn0.67O2 encapsulated with MgO as a novel high voltage cathode with enhanced Na-storage properties. J. Mater. Chem. A 2017, 5, 8408–8415. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, C.; Liu, Y.; Ren, M.; Du, J.; Chen, A.; Li, F. Suppressing interlayer-gliding and Jahn-Teller effect in P2-type layered manganese oxide cathode via Mo doping for sodium-ion batteries. Chem. Eng. J. 2021, 426, 130813. [Google Scholar] [CrossRef]
- Sui, Y.; Hao, Y.; Zhang, X.; Li, J.; Wen, G.; Zhong, S.; Zhang, Z.; Wu, L. Improved electrochemical properties of vanadium substituted Na0·67Fe0·5Mn0·5O2 cathode material for sodium-ion batteries. Ceram. Int. 2021, 47, 5227–5234. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, Y.B.; Xu, D.; Ma, D.L.; Wang, S.; Yang, X.H.; Cao, Z.Y.; Zhang, X.B. Pure Single-Crystalline Na1.1V3O7.9 Nanobelts as Superior Cathode Materials for Rechargeable Sodium-Ion Batteries. Adv. Sci. 2015, 2, 1400018. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, R.; Hu, Y.S.; Avdeev, M.; Chen, L. P2-Na0.6[Cr0.6Ti0.4]O2 cation-disordered electrode for high-rate symmetric rechargeable sodium-ion batteries. Nat. Commun. 2015, 6, 6954. [Google Scholar] [CrossRef]
- Do, J.; Kim, I.; Kim, H.; Jung, Y. Towards stable Na-rich layered transition metal oxides for high energy density sodium-ion batteries. Energy Storage Mater. 2020, 25, 62–69. [Google Scholar] [CrossRef]
- Ramasamy, H.V.; Kaliyappan, K.; Thangavel, R.; Seong, W.M.; Kang, K.; Chen, Z.; Lee, Y.S. Efficient Method of Designing Stable Layered Cathode Material for Sodium Ion Batteries Using Aluminum Doping. J. Phys. Chem. Lett. 2017, 8, 5021–5030. [Google Scholar] [CrossRef]
- Wang, H.; Gao, R.; Li, Z.; Sun, L.; Hu, Z.; Liu, X. Different Effects of Al Substitution for Mn or Fe on the Structure and Electrochemical Properties of Na0.67Mn0.5Fe0.5O2 as a Sodium Ion Battery Cathode Material. Inorg. Chem. 2018, 57, 5249–5257. Available online: https://pubs.acs.org/doi/full/10.1021/acs.inorgchem.8b00284 (accessed on 1 February 2024). [CrossRef]
- Ding, Z.; Liu, Y.; Tang, Q.; Jiang, Q.; Lu, J.; Xiao, Z.; Yao, P.; Monasterio, M.; Wu, J.; Liu, X. Enhanced electrochemical performance of iron-manganese based cathode by Li doping for sodium-ion batteries. Electrochim. Acta 2018, 292, 871–878. [Google Scholar] [CrossRef]
- Park, J.K.; Park, G.G.; Kwak, H.H.; Hong, S.T.; Lee, J.W. Enhanced Rate Capability and Cycle Performance of Titanium-Substituted P2-Type Na0.67Fe0.5Mn0.5O2 as a Cathode for Sodium-Ion Batteries. ACS Omega 2017, 3, 361–368. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kajiyama, M.; Iwatate, J.; Nishikawa, H.; Hitomi, S.; Okuyama, R.; Usui, R.; Yamada, Y.; Komaba, S. P2-type Nax[Fe1/2Mn1/2]O2 made from earth-abundant elements for rechargeable Na batteries. Nat. Mater. 2012, 11, 512–517. [Google Scholar] [CrossRef]
- Zhou, D.; Huang, W.; Zhao, F.; Lv, X. The effect of Na content on the electrochemical performance of the O3-type NaxFe0.5Mn0.5O2 for sodium-ion batteries. J. Mater. Sci. 2019, 54, 7156–7164. [Google Scholar] [CrossRef]
- Nakrela, A.; Benramdane, N.; Bouzidi, A.; Kebbab, Z.; Medles, M.; Mathieu, C. Site location of Al-dopant in ZnO lattice by exploiting the structural and optical characterisation of ZnO: Al thin films. Results Phys. 2016, 6, 133–138. [Google Scholar] [CrossRef]
- Mao, D.; Tan, X.; Guo, L.; Zhao, T.; Fan, Z.; Song, L.; Zhang, Y.; Liu, G.; Wang, H.; Chu, W. Lithium Antievaporation-Loss Engineering via Sodium/Potassium Doping Enables Superior Electrochemical Performance of High-Nickel Li-Rich Layered Oxide Cathodes; American Chemical Society: Washington, WA, USA, 2022; Volume 14, pp. 19594–19603. [Google Scholar]
- Billaud, J.; Singh, G.; Armstrong, A.R.; Gonzalo, E.; Roddatis, V.; Armand, M.; Rojo, T.; Bruce, P.G. Na0.67Mn1−xMgxO2 (0 ≤ x ≤ 0.2): A high capacity cathode for sodium-ion batteries. Energy Environ. Sci. 2014, 7, 1387–1391. [Google Scholar] [CrossRef]
- Widiyandari, H.; Sukmawati, A.N.; Sutanto, H.; Yudha, C.; Purwanto, A. Synthesis of LiNi0.8Mn0.1Co0.1O2 cathode material by hydrothermal method for high energy density lithium ion battery. J. Phys. Conf. Ser. 2019, 1153, 012074. [Google Scholar] [CrossRef]
- Hasan, F.; Kim, J.; Song, H.; Lee, S.H.; Sung, J.H.; Kim, J.; Yoo, H.D. Effect of particle size and doping on the electrochemical characteristics of Ca-doped LiCoO2 cathodes. J. Electrochem. Sci. Technol. 2020, 11, 352–360. [Google Scholar] [CrossRef]
- Julien, C.; Mauger, A.; Zaghib, K.; Groult, H. Optimization of layered cathode materials for lithium-ion batteries. Materials 2016, 9, 595. [Google Scholar] [CrossRef]
- Stoyanova, R.; Carlier, D.; Sendova-Vassileva, M.; Yoncheva, M.; Zhecheva, E.; Nihtianova, D.; Delmas, C. Stabilization of over-stoichiometric Mn4+ in layered Na2/3MnO2. J. Solid State Chem. 2010, 183, 1372–1379. [Google Scholar] [CrossRef]
- Dang, R.; Chen, M.; Li, Q.; Wu, K.; Lee, Y.L.; Hu, Z.; Xiao, X. Na+-Conductive Na2Ti3O7-Modified P2-type Na2/3Ni1/3Mn2/3O2 via a Smart in Situ Coating Approach: Suppressing Na+/Vacancy Ordering and P2-O2 Phase Transition. ACS Appl. Mater. Interfaces 2019, 11, 856–864. [Google Scholar] [CrossRef]
- Pang, W.L.; Zhang, X.H.; Guo, J.Z.; Li, J.Y.; Yan, X.; Hou, B.H.; Guan, H.Y.; Wu, X.L. P2-type Na2/3Mn1−xAlxO2 cathode material for sodium-ion batteries: Al-doped enhanced electrochemical properties and studies on the electrode kinetics. J. Power Sources 2017, 356, 80–88. [Google Scholar] [CrossRef]
- Hirsh, H.; Olguin, M.; Chung, H.; Li, Y.; Bai, S.; Feng, D.; Wang, D.; Zhang, M.; Meng, Y.S. Meso-Structure Controlled Synthesis of Sodium Iron-Manganese Oxides Cathode for Low-Cost Na-Ion Batteries. J. Electrochem. Soc. 2019, 166, A2528–A2535. [Google Scholar] [CrossRef]
- Zhao, W.; Kirie, H.; Tanaka, A.; Unno, M.; Yamamoto, S.; Noguchi, H. Synthesis of metal ion substituted P2-Na2/3Ni1/3Mn2/3O2 cathode material with enhanced performance for Na ion batteries. Mater. Lett. 2014, 135, 131–134. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, M.; Shao, L.; Wang, W.; Fan, K.; Qin, Q. Reactions of Fe with H2O and Feo with H2. A combined matrix isolation FTIR and theoretical study. J. Phys. Chem. A 2001, 105, 6998–7003. [Google Scholar] [CrossRef]
- Altin, E.; Altundag, S.; Altin, S.; Bayri, A. Fabrication of Cr doped Na0.67Fe0.5Mn0.5O2 compounds and investigation of their structural, electrical, magnetic and electrochemical properties. J. Mater. Sci. Mater. Electron. 2019, 30, 17848–17855. [Google Scholar] [CrossRef]
- Uchaker, E.; Jin, H.; Yi, P.; Cao, G. Elucidating the Role of Defects for Electrochemical Intercalation in Sodium Vanadium Oxide. Chem. Mater. 2015, 27, 7082–7090. [Google Scholar] [CrossRef]
- Wan, D.Y.; Fan, Z.Y.; Dong, Y.X.; Baasanjav, E.; Jun, H.B.; Jin, B.; Jin, E.M.; Jeong, S.M. Effect of Metal (Mn, Ti) Doping on NCA Cathode Materials for Lithium Ion Batteries. J. Nanomater. 2018, 2018, 8082502. [Google Scholar] [CrossRef]
- Mortemard de Boisse, B.; Carlier, D.; Guignard, M.; Delmas, C. Structural and Electrochemical Characterizations of P2 and New O3-NaxMn1−yFeyO2 Phases Prepared by Auto-Combustion Synthesis for Na-Ion Batteries. J. Electrochem. Soc. 2013, 160, A569–A574. [Google Scholar] [CrossRef]
- Zhu, Q.; Cheng, H.; Zhang, X.; He, L.; Hu, L.; Yang, J.; Chen, Q.; Lu, Z. Improvement in electrochemical performance of Na3V2(PO4)3/C cathode material for sodium-ion batteries by K-Ca co-doping. Electrochim. Acta 2018, 281, 208–217. [Google Scholar] [CrossRef]
- Sim, S.; Lee, S.; Jin, B.; Kim, H. Improving the electrochemical performances using a V-doped Ni- rich NCM cathode. Sci. Rep. 2019, 9, 8952. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Wu, C.Y.; Duh, J.G. Synthesis of Ni-rich NMC cathode material by redox-assisted deposition method for lithium ion batteries. Electrochim. Acta 2021, 381, 138244. [Google Scholar] [CrossRef]




| Samples | Lattice Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pure NFM | 2.9176 | 11.2885 | 3.869 | 83.218 | 5.6441 | 3.713 | 1.931 | 7.8 | 5.45 |
| 1NFMTV | 2.9216 | 11.2964 | 3.8665 | 83.5048 | 5.6489 | 3.2483 | 2.0695 | 7.28 | 4.89 |
| 2NFMTV | 2.9182 | 11.2975 | 3.8714 | 83.3158 | 5.6493 | 3.4747 | 1.994 | 8.59 | 5.71 |
| 3NFMTV | 2.9222 | 11.2951 | 3.8653 | 83.5243 | 5.6476 | 3.5594 | 1.97514 | 6.29 | 4.12 |
| 5NFMTV | 2.9215 | 11.2909 | 3.8654 | 83.4293 | 5.6458 | 3.5434 | 1.931 | 5.46 | 3.96 |
| 10NFMTV | 2.9947 | 11.3154 | 3.7784 | 87.885 | 5.6577 | 3.516 | 1.925 | 9.93 | 7.73 |
| Sample | ) | Warburg Co-Efficient | Diffusion Co-Efficient |
|---|---|---|---|
| Pure NFM | 1510 | 103.4718 | |
| 1NFMTV | 2630 | 453.3639 | |
| 2NFMTV | 498 | 81.3622 | |
| 3NFMTV | 2723 | 1469.8327 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banik, T.; Bhattacharya, I.; Savunthari, K.V.; Mukerjee, S.; Adepoju, W.; Olatunji, A. Enhanced Performance of Sodium-Ion Battery Cathodes with Ti and V Co-Doped P2-Type Na0.67Fe0.5Mn0.5O2 Materials. Electrochem 2024, 5, 437-454. https://doi.org/10.3390/electrochem5040029
Banik T, Bhattacharya I, Savunthari KV, Mukerjee S, Adepoju W, Olatunji A. Enhanced Performance of Sodium-Ion Battery Cathodes with Ti and V Co-Doped P2-Type Na0.67Fe0.5Mn0.5O2 Materials. Electrochem. 2024; 5(4):437-454. https://doi.org/10.3390/electrochem5040029
Chicago/Turabian StyleBanik, Trapa, Indranil Bhattacharya, Kirankumar Venkatesan Savunthari, Sanjeev Mukerjee, Webster Adepoju, and Abiodun Olatunji. 2024. "Enhanced Performance of Sodium-Ion Battery Cathodes with Ti and V Co-Doped P2-Type Na0.67Fe0.5Mn0.5O2 Materials" Electrochem 5, no. 4: 437-454. https://doi.org/10.3390/electrochem5040029
APA StyleBanik, T., Bhattacharya, I., Savunthari, K. V., Mukerjee, S., Adepoju, W., & Olatunji, A. (2024). Enhanced Performance of Sodium-Ion Battery Cathodes with Ti and V Co-Doped P2-Type Na0.67Fe0.5Mn0.5O2 Materials. Electrochem, 5(4), 437-454. https://doi.org/10.3390/electrochem5040029






