The Influence of Thick Cathode Fabrication Processing on Battery Cell Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrode Fabrication
2.3. Electrode Morphology Characterization
2.4. Coin Cell Assembling and Specific Capacity Collection
2.5. Pouch Cell Fabrication and Performance Testing
3. Results and Discussion
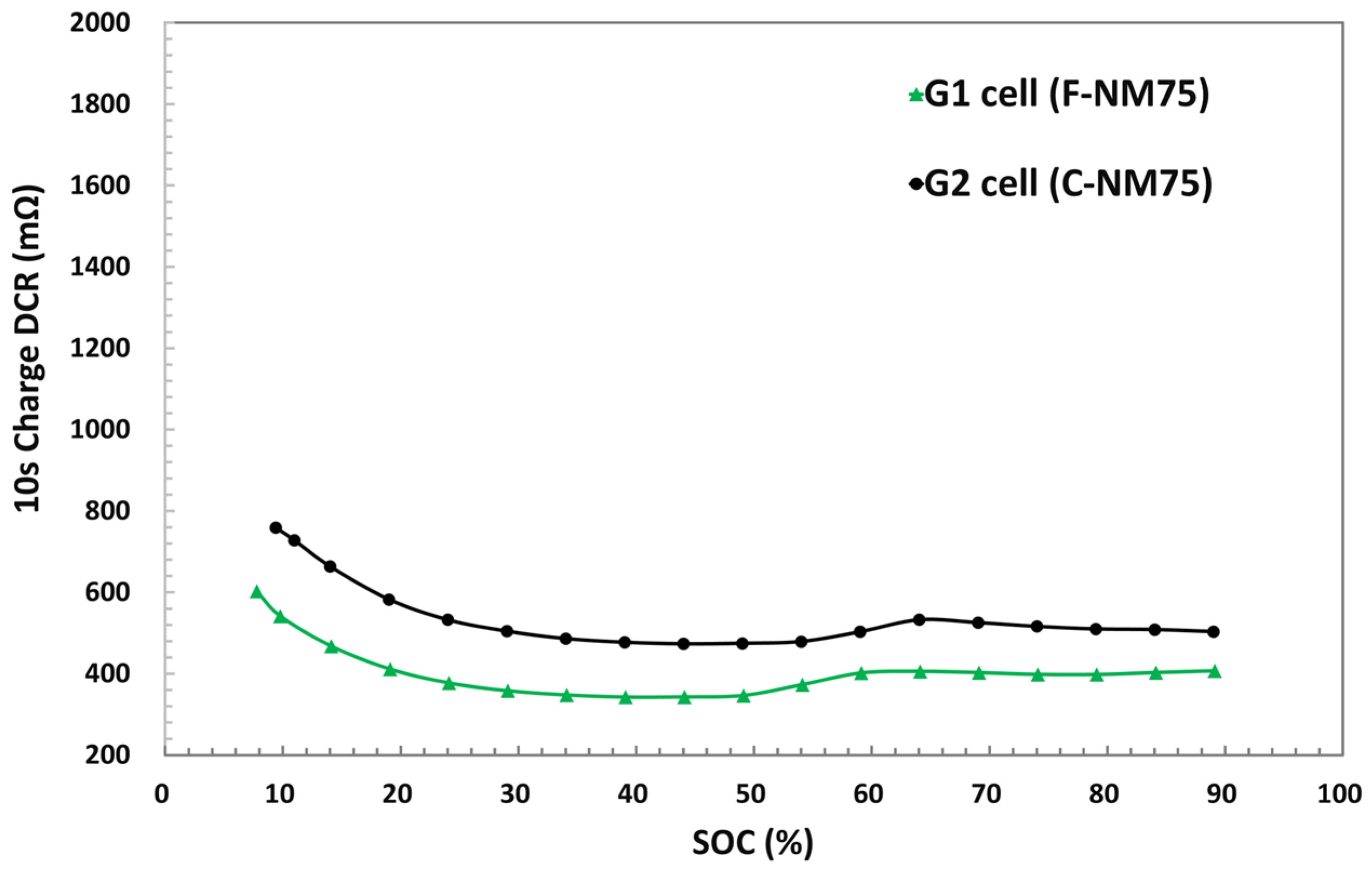
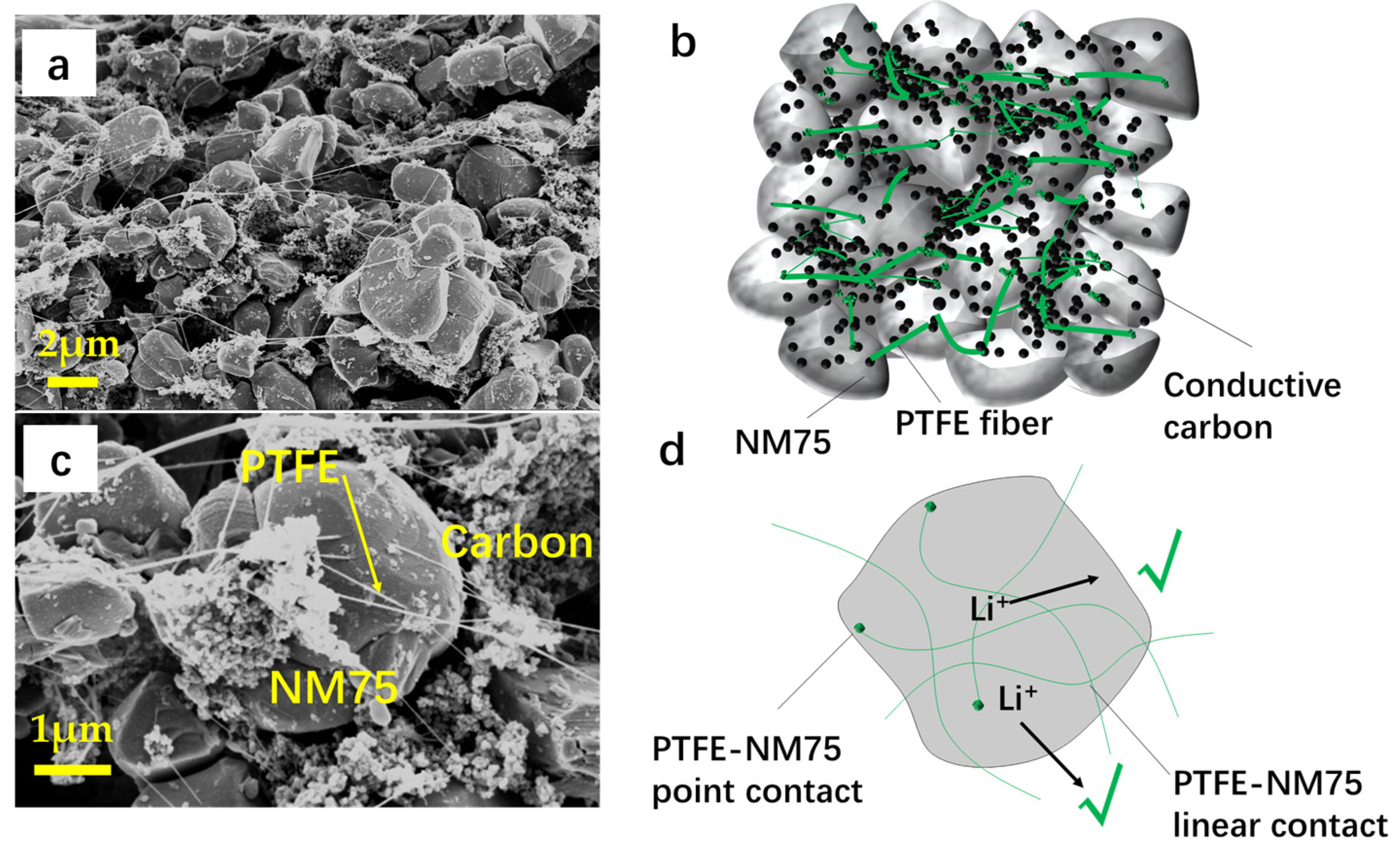
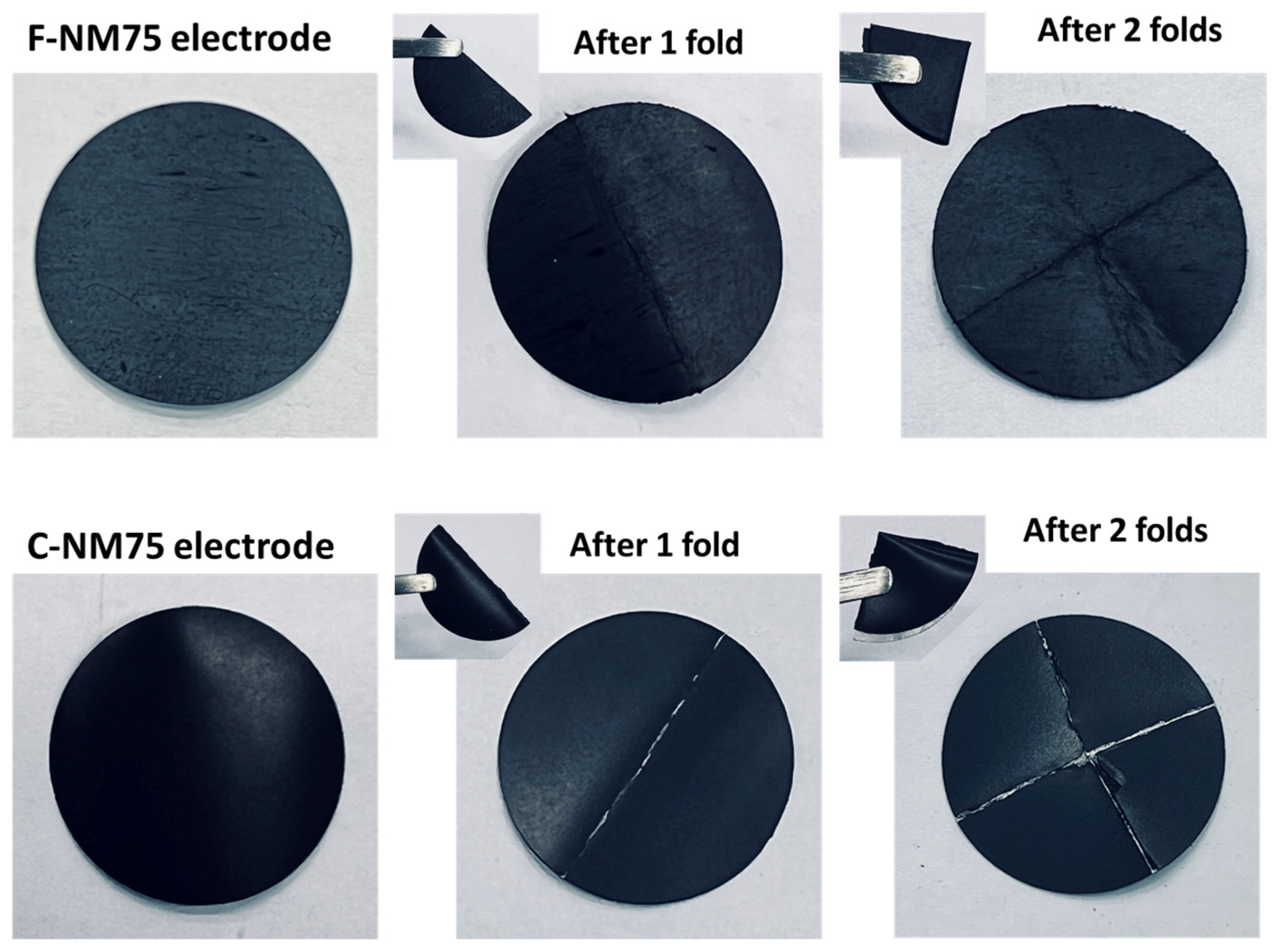
3.1. Electrode Morphology and Coin Cell Evaluation
3.2. Pouch Cell Design Table
3.3. Electrochemical Performance of Pouch Cells
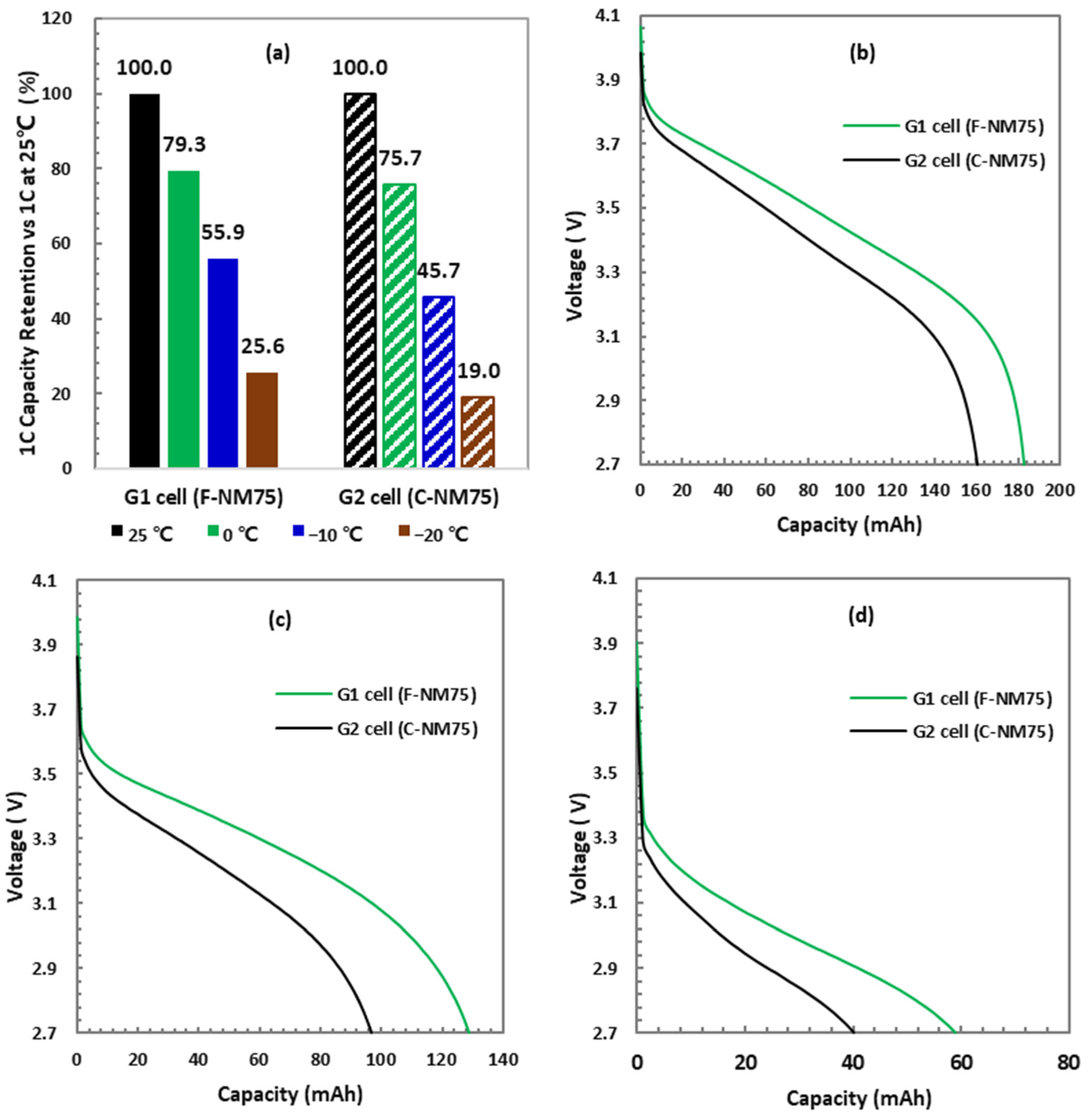
3.3.1. Discharge Rate Test at 25 °C
3.3.2. Charge Rate Test at 25 °C
3.3.3. 1 C Discharge Capacity at Cold Temperatures
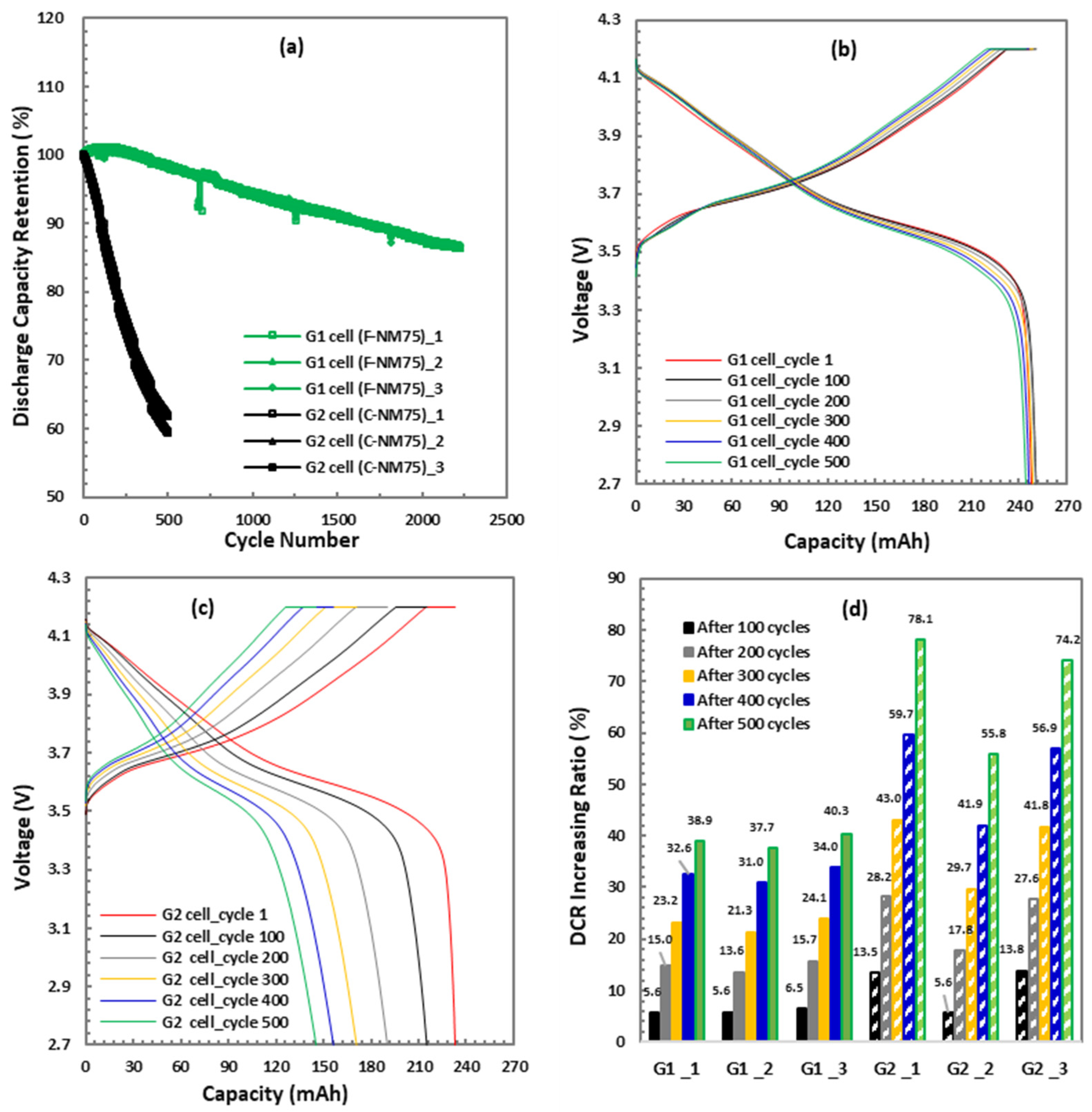
3.3.4. C/3 Cycle Life Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mauro, M.; Biswas, A.; Fiorillo, C.; Wang, H.; Spessa, E.; Miretti, F.; Ahmed, R.; Bonfitto, A.; Emadi, A. Real-Time Implementable Integrated Energy and Cabin Temperature Management for Battery Life Extension in Electric Vehicles. Energies 2024, 17, 3185. [Google Scholar] [CrossRef]
- Un-Noor, F.; Padmanaban, S.; Mihet-Popa, L.; Mollah, M.N.; Hossain, E. A Comprehensive Study of Key Electric Vehicle (EV) Components, Technologies, Challenges, Impacts, and Future Direction of Development. Energies 2017, 10, 1217. [Google Scholar] [CrossRef]
- Nizam Uddin Khan, F.M.; Rasul, M.G.; Sayem, A.S.M.; Mandal, N.K. Design and optimization of lithium-ion battery as an efficient energy storage device for electric vehicles: A comprehensive review. J. Energy Storage 2023, 71, 108033. [Google Scholar] [CrossRef]
- Wei, Z.Q.; Kong, D.W.; Quan, L.J.; He, J.R.; Liu, J.Y.; Tang, Z.Y.; Chen, S.; Cai, Q.Q.; Zhang, R.Q.; Liu, H.J.; et al. Removing electrochemical constraints on polytetrafluoroethylene as dry-process binder for high-loading graphite anodes. Joule 2024, 8, 1350. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359. [Google Scholar] [CrossRef]
- Olivetti, E.A.; Ceder, G.; Gaustad, G.G.; Fu, X. Lithium-ion battery supply chain considerations: Analysis of potential bottlenecks in critical metals. Joule 2017, 1, 229. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Cui, Y. Challenges and opportunities towards fast-charging battery materials. Nat. Energy 2019, 4, 540. [Google Scholar] [CrossRef]
- Li, W.; Erickson, E.M.; Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 2020, 5, 26. [Google Scholar] [CrossRef]
- Yoon, C.S.; Ryu, H.-H.; Park, G.T.; Kim, J.H.; Kim, K.H.; Sun, Y.K. Extracting maximum capacity from Ni-rich Li[Ni0.95Co0.025Mn0.025]O2 cathodes for high-energy-density lithium-ion batteries. J. Mater. Chem. A 2018, 6, 4126. [Google Scholar] [CrossRef]
- Deng, C.; Li, X.; Chen, R.; Ye, K.Q.; Lipton, J.; Maclean, S.A.; Wang, H.; Taylor, A.D.; Weng, G.M. Recent advances in rocking chair batteries and beyond. Energy Storage Mater. 2023, 60, 102820. [Google Scholar] [CrossRef]
- Sandstrom, S.K.; Chen, X.; Ji, X.L. A review of halide charge carriers for rocking-chair and dual-ion batteries. Carbon Energy 2021, 3, 627. [Google Scholar] [CrossRef]
- Hawley, W.B.; Li, J.L. Electrode manufacturing for lithium-ion batteries—Analysis of current and next generation processing. J. Energy Storage 2019, 25, 100862. [Google Scholar] [CrossRef]
- Singh, M.; Kaiser, J.; Hahn, H. Thick electrodes for high energy lithium ion batteries. J. Electrochem. Soc. 2015, 162, A1196. [Google Scholar] [CrossRef]
- Patry, G.; Romagny, A.; Martinet, S.; Froelich, D. Cost modeling of lithium-ion battery cells for automotive applications. Energy Sci. Eng. 2015, 3, 71. [Google Scholar] [CrossRef]
- Liu, Y.T.; Zhang, R.H.; Wang, J.; Wang, Y. Current and future lithium-ion battery manufacturing. iScience 2021, 24, 102332. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, X.B.; Chong, Y.; Yuan, H.; Huang, J.Q.; Zhang, Q. Advanced electrode processing of lithium ion batteries: A review of powder technology in battery fabrication. Particuology 2021, 57, 56. [Google Scholar] [CrossRef]
- Chen, K.B.; Yu, Z.Q.; Deng, S.; Wu, Q.; Zou, J.X.; Zeng, X.Q. Evaluation of the low temperature performance of lithium manganese oxide/lithium titanate lithium-ion batteries for start/stop applications. J. Power Sources 2015, 278, 411. [Google Scholar] [CrossRef]
- Ludwig, B.; Zheng, Z.F.; Wan, S.; Wang, Y.; Pan, H. Solvent-Free Manufacturing of Electrodes for Lithium-ion Batteries. Sci. Rep. 2016, 6, 23150. [Google Scholar] [CrossRef]
- Al-Shroofy, M.; Zhang, Q.L.; Xu, J.G.; Chen, T.; Kaur, A.P.; Cheng, Y.T. Solvent-free dry powder coating process for low-cost manufacturing of LiNi1/3Mn1/3Co1/3O2 cathodes in lithium-ion batteries. J. Power Sources 2017, 352, 187. [Google Scholar] [CrossRef]
- Wood, D.L.; Li, J.L.; Daniel, C. Prospects for reducing the processing cost of lithium ion batteries. J. Power Sources 2015, 275, 234. [Google Scholar] [CrossRef]
- Li, J.L.; Rulison, C.; Kiggans, J.; Daniel, C.; Wood, D.L. Superior Performance of LiFePO4 Aqueous Dispersions via Corona Treatment and Surface Energy Optimization. J. Electrochem. Soc. 2012, 159, A1152. [Google Scholar] [CrossRef]
- Bauer, W.; Nötzel, D.; Wenzel, V.; Nirschl, H. Influence of dry mixing and distribution of conductive additives in cathodes for lithium ion batteries. J. Power Sources 2015, 288, 359. [Google Scholar] [CrossRef]
- Li, Y.X.; Wu, Y.J.; Wang, Z.X.; Xu, J.R.; Ma, T.H.; Chen, L.Q.; Li, H.; Wu, F. Progress in solvent-free dry-film technology for batteries and supercapacitors. Mater. Today 2022, 55, 92. [Google Scholar] [CrossRef]
- Jaiser, S.; Sanchez Salach, N.; Baunach, M.; Scharfer, P.; Schabel, W. Impact of drying conditions and wet film properties on adhesion and film solidification of lithium-ion battery anodes. Dry. Technol. 2017, 35, 1807. [Google Scholar] [CrossRef]
- Baunach, M.; Jaiser, S.; Schmelzle, S.; Nirschl, H.; Scharfer, P.; Schabel, W. Delamination behavior of lithium-ion battery anodes: Influence of drying temperature during electrode processing. Dry. Technol. 2016, 34, 462. [Google Scholar] [CrossRef]
- Jeong, D.; Lee, J. Electrode design optimization of lithium secondary batteries to enhance adhesion and deformation capabilities. Energy 2014, 75, 525. [Google Scholar] [CrossRef]
- Duong, H.; Shin, J.; Yudi, Y. Dry electrode coating technology. In Proceedings of the 48th Power Sources Conference, Denver, CO, USA, 11–14 June 2018; Volume 3, p. 34. [Google Scholar]
- Embleton, T.J.; Choi, J.H.; Won, S.J.; Ali, J.; Saqib, K.S.; Ko, K.; Jo, M.; Hwang, J.; Park, J.; Lee, J.H.; et al. High-energy density ultra-thick drying-free Ni-rich cathode electrodes for application in Lithium-ion batteries. Energy Storage Mater. 2024, 71, 103542. [Google Scholar] [CrossRef]
- Yao, W.L.; Chouchane, M.; Li, W.K.; Bai, S.; Liu, Z.; Li, L.T.; Chen, A.X.; Sayahpour, B.; Shimizu, R.; Raghavendran, G.; et al. A 5 V-class cobalt-free battery cathode with high loading enabled by dry coating. Energy Environ. Sci. 2023, 16, 1620. [Google Scholar] [CrossRef]
- Tao, R.M.; Steinhoff, B.; Sun, X.G.; Sardo, K.; Skelly, B.; Meyer, H.M.; Sawicki, C.; Polizos, G.; Lyu, X.; Du, Z.J.; et al. High-throughput and high-performance lithium-ion batteries via dry processing. Chem. Eng. J. 2023, 471, 144300. [Google Scholar] [CrossRef]
- Wang, F.Q.; Tang, S.; Han, Q.G.; Ji, S.J.; Wang, J.W.; Du, B.H.; Li, X.; Guan, M.Y.; Lou, P.; Zhang, W.X.; et al. Unraveling the impact of the design of current collector on dry-processed lithium-ion battery electrodes. J. Colloid Interface Sci. 2025, 678, 57. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Wei, Y.K.; Jia, R.; Zhang, F.; Tang, Y.B. Rational Design of High-Loading Electrodes with Superior Performances Toward Practical Application for Energy Storage Devices. Small 2024, 20, 2308126. [Google Scholar] [CrossRef]
- Lee, T.; An, J.; Chung, W.J.; Kim, H.; Cho, Y.; Song, H.; Lee, H.; Kang, J.H.; Choi, J.W. Non-Electroconductive Polymer Coating on Graphite Mitigating Electrochemical Degradation of PTFE for a Dry-Processed Lithium-Ion Battery Anode. ACS Appl. Mater. Interfaces 2024, 16, 8930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, D.; Wang, X.; Gao, J.; Shen, H.; Zhang, H.; Rong, C.; Chen, Z. Dry Electrode Processing Technology and Binders. Materials 2024, 17, 2349. [Google Scholar] [CrossRef]
- Lim, C.Y.; Park, G.; Lee, K.J. Effect of carbon conductor dispersion and composition in dry cathode electrode on LiB performances. Carbon Lett. 2024. [Google Scholar] [CrossRef]
- Bouguern, M.D.; Madikere Raghunatha Reddy, A.K.; Li, X.; Deng, S.; Laryea, H.; Zaghib, K. Engineering Dry Electrode Manufacturing for Sustainable Lithium-Ion Batteries. Batteries 2024, 10, 39. [Google Scholar] [CrossRef]
- Liu, J.; Ludwig, B.; Liu, Y.; Zheng, Z.; Wang, F.; Tang, M.; Wang, J.; Pan, H.; Wang, Y. Scalable dry printing manufacturing to enable long-life and high energy lithium-ion batteries. Adv. Mater. Technol. 2017, 2, 1700106. [Google Scholar] [CrossRef]
- Liu, J.; Chen, S.; Kong, D.; Wu, M.; Liu, H. Interaction between LMFP and NCMA and Its Effect on Blending Cathode-Based Cells. Energies 2024, 17, 808. [Google Scholar] [CrossRef]
- GMW16460; General Motors Worldwide Engineering Standards, Static Capacity and Hybrid Pulse Power Characterization Test of Rechargeable Energy Storage Systems. General Motors Worldwide: Detroit, MI, USA, 2021.
- Allart, D.; Montaru, M.; Gualous, H. Model of Lithium Intercalation into Graphite by Potentiometric Analysis with Equilibrium and Entropy Change Curves of Graphite Electrode. J. Electrochem. Soc. 2018, 165, A380. [Google Scholar] [CrossRef]
- Onda, K.; Ohshima, T.; Nakayama, M.; Fukuda, K.; Araki, T. Thermal behavior of small lithium-ion battery during rapid charge and discharge cycles. J. Power Sources 2006, 158, 535. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, M.; Ding, Y.H.; Wu, S.X.; Li, Y.J.; Liang, G.; Li, H.; Pan, H.H. Estimation the internal resistance of lithium-ion-battery using a multi-factor dynamic internal resistance model with an error compensation strategy. Energy Rep. 2021, 7, 3050. [Google Scholar] [CrossRef]
- Belharouak, I.; Johnson, C.; Amine, K. Synthesis and electrochemical analysis of vapor-deposited carbon-coated LiFePO4. Electrochem. Commun. 2005, 7, 983. [Google Scholar] [CrossRef]
- Liu, S.Z.; Chen, J.J.; Zhang, C.; Jin, L.; Yang, Q.X. Experimental study on lithium-ion cell characteristics at different discharge rates. J. Energy Storage 2022, 45, 103418. [Google Scholar] [CrossRef]
- Guo, R.; Shen, W. A Review of Equivalent Circuit Model Based Online State of Power Estimation for Lithium-Ion Batteries in Electric Vehicles. Vehicles 2022, 4, 1. [Google Scholar] [CrossRef]
- Wang, S.; Verbrugge, M.; Wang, J.S.; Liu, P. Multi-Parameter Battery State Estimator Based on the Adaptive and Direct Solution of the Governing Differential Equations. J. Power Sources 2011, 196, 8735. [Google Scholar] [CrossRef]
- Steinstraeter, M.; Heinrich, T.; Lienkamp, M. Effect of Low Temperature on Electric Vehicle Range. World Electr. Veh. J. 2021, 12, 115. [Google Scholar] [CrossRef]
- Zhang, X.; Hui, Z.Y.; King, S.; Wang, L.; Ju, Z.Y.; Wu, J.Y.; Takeuchi, K.J.; Marschilok, A.C.; West, A.C.; Takeuchi, E.S.; et al. Tunable Porous Electrode Architectures for Enhanced Li-Ion Storage Kinetics in Thick Electrodes. Nano Lett. 2021, 21, 5896. [Google Scholar] [CrossRef]
- Jung, R.; Metzger, M.; Maglia, F.; Stinner, C.; Gasteiger, H.A. Oxygen Release and Its Effect on the Cycling Stability of LiNixMnyCozO2 (NMC) Cathode Materials for Li-Ion Batteries. J. Electrochem. Soc. 2017, 164, A1361. [Google Scholar] [CrossRef]
- Geldasa, F.T.; Kebede, M.A.; Shura, M.W.; Hone, F.G. Identifying surface degradation, mechanical failure, and thermal instability phenomena of high energy density Ni-rich NCM cathode materials for lithium-ion batteries: A review. RSC Adv. 2022, 12, 5891–5909. [Google Scholar] [CrossRef]
- Ryu, M.; Hong, Y.K.; Lee, S.Y. Ultrahigh loading dry-process for solvent-free lithium-ion battery electrode fabrication. Nat Commun. 2023, 14, 1316. [Google Scholar] [CrossRef]
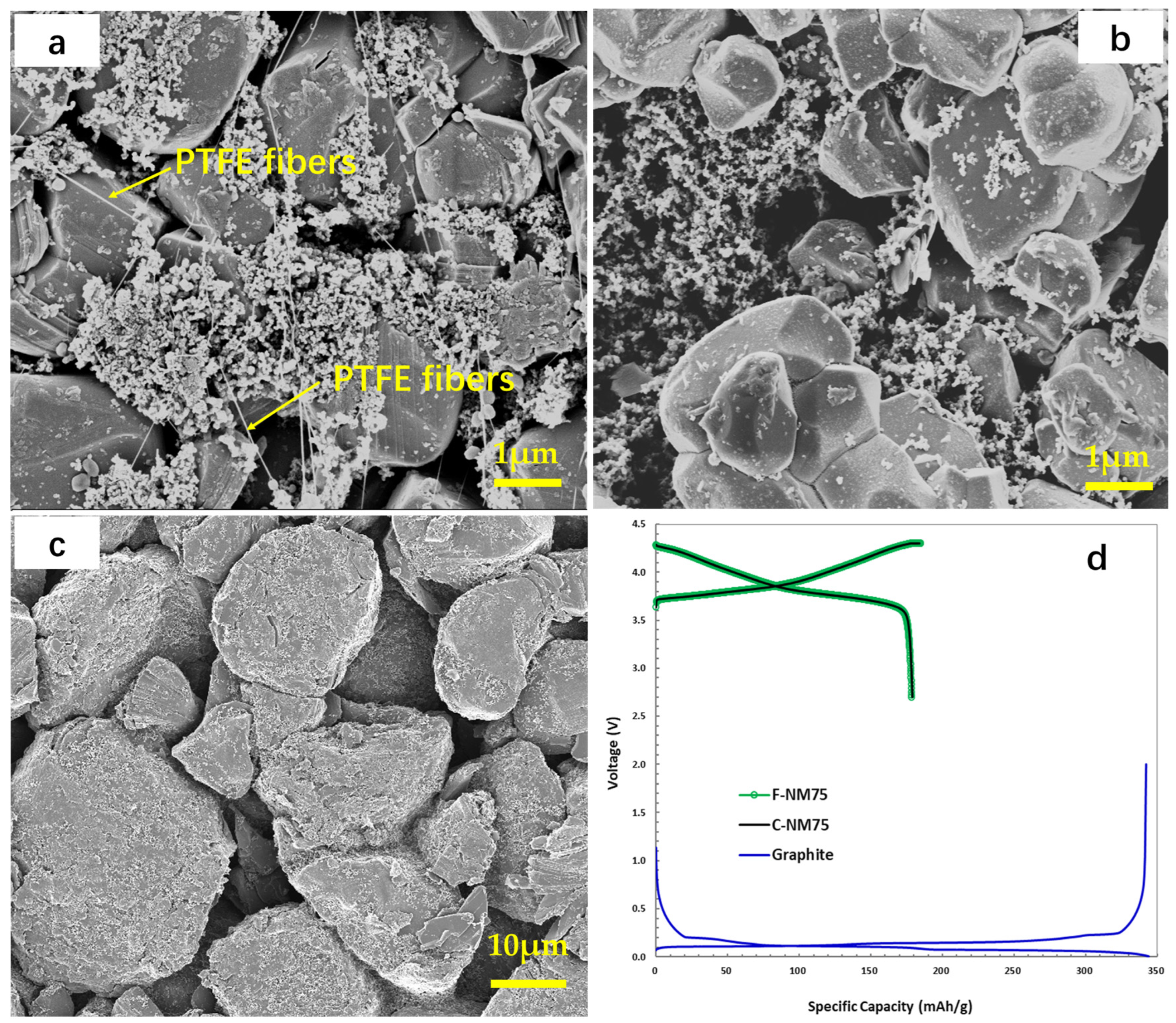
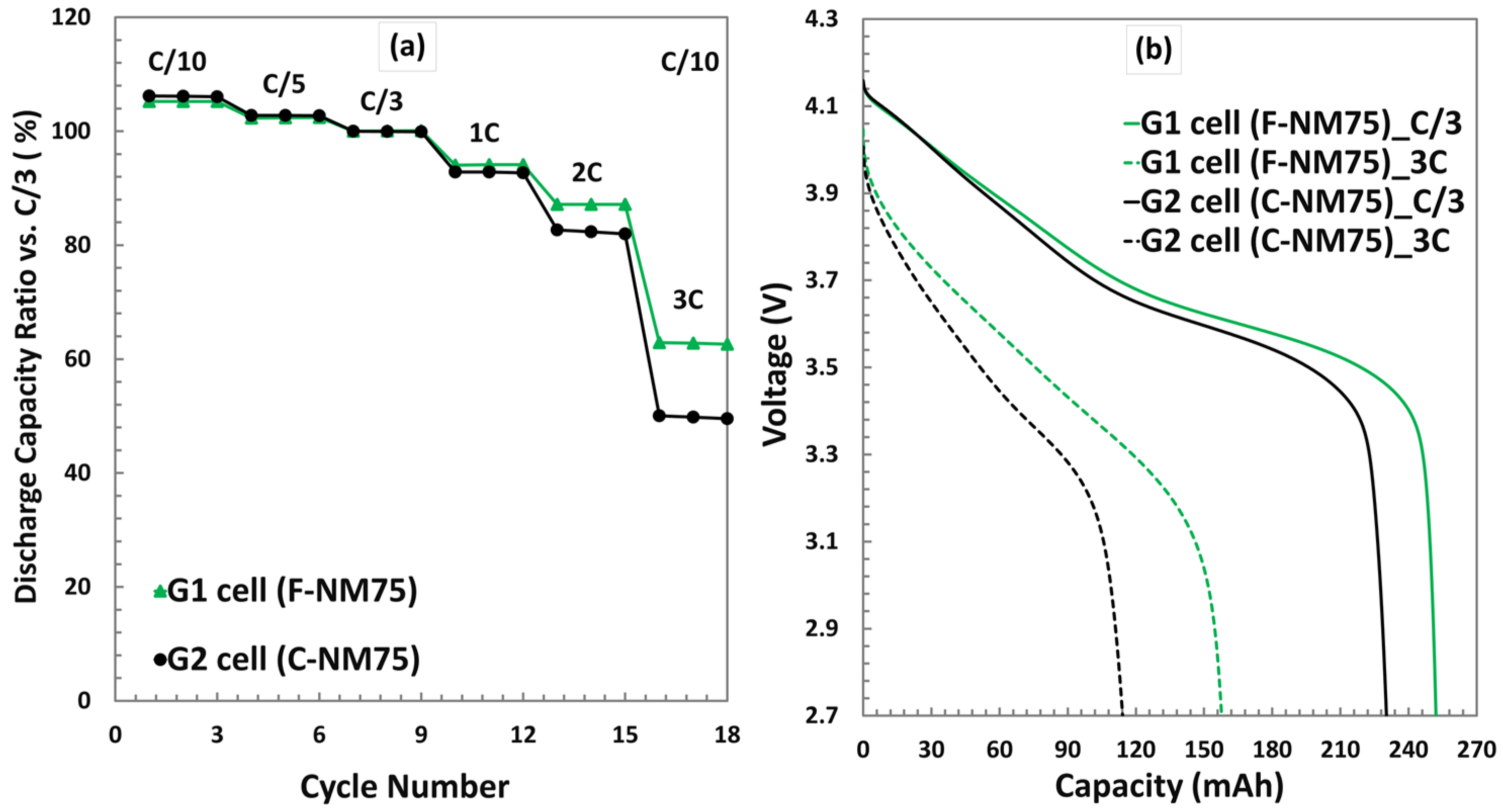
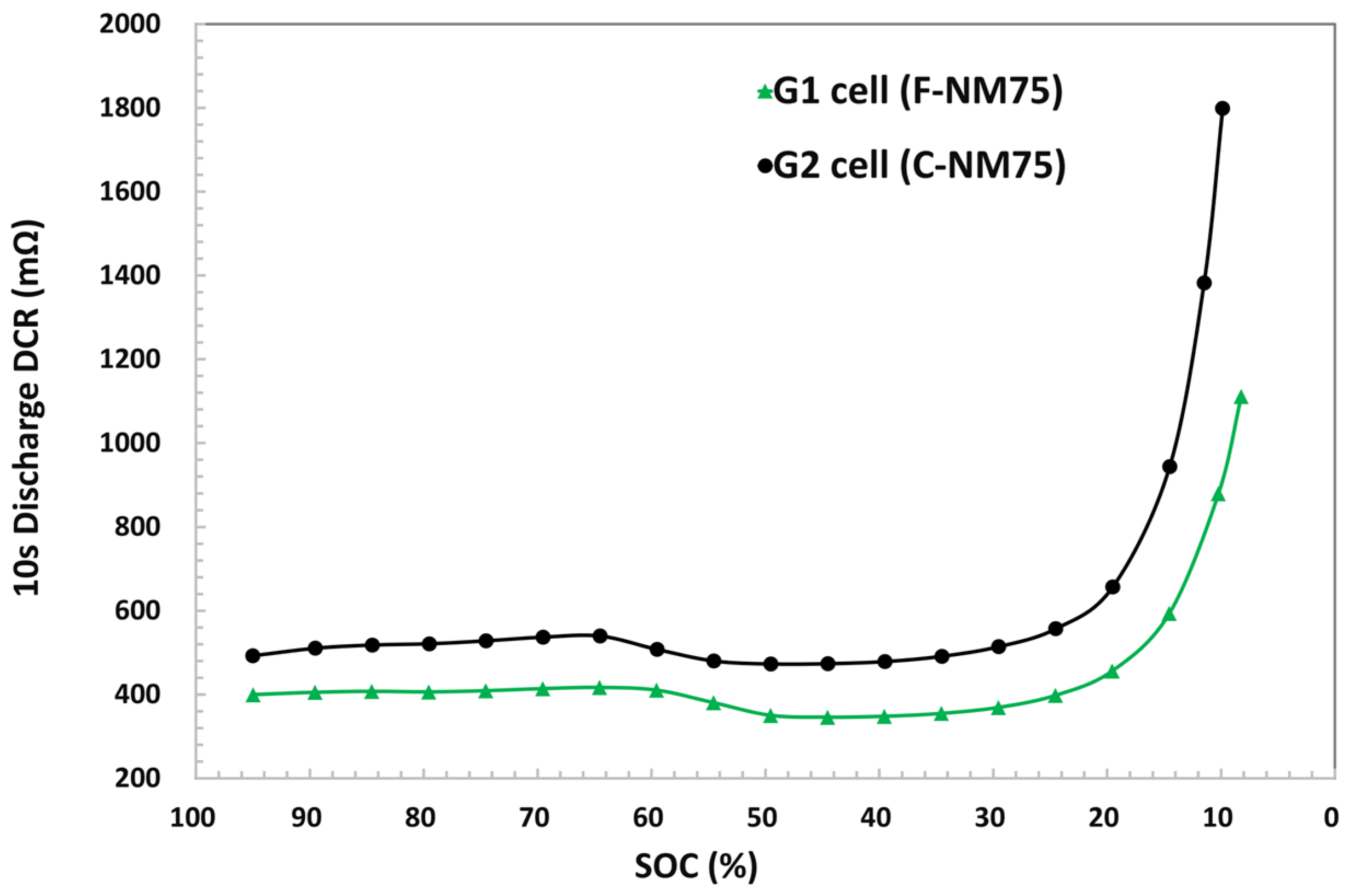
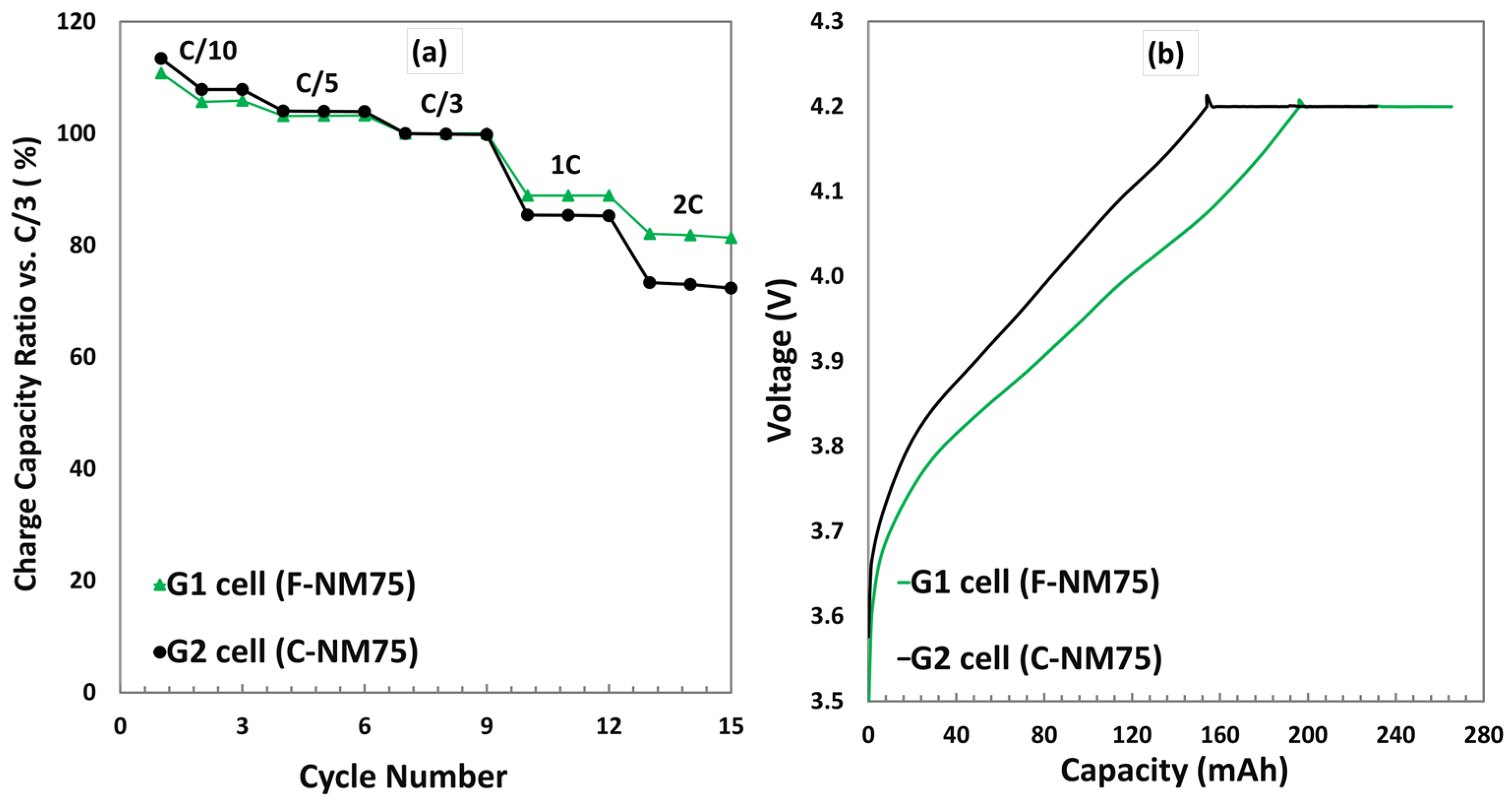

| Electrode | Formulation at Mass Ratio, % | 1 Mass Loading (mg/cm2) | Press Density (g/cm3) | Specific Capacity (mAh/g) |
|---|---|---|---|---|
| F-NM75 | AM/SP/KB/PTFE = 96/1/ 1/2 | 28.0 | 3.0 | 2 178.6 |
| C-NM75 | AM/SP/KB/PVDF = 94.5/ 2/1/2.5 | 26.7 | 3.0 | 2 178.9 |
| Graphite | AM: SP: CMC:SBR = 94.5:1.8:1.4:2.3 | 16.3 | 1.5 | 3 342.2 |
| Electrode | Group1 (G1) | Group2 (G2) |
|---|---|---|
| Cathode | F-NM75 | C-NM75 |
| Cathode areal capacity (mAh/cm2) | 4.8 | 4.5 |
| Anode | Slurry casting graphite | |
| Anode areal capacity (mAh/cm2) | 5.3 | |
| N/P ratio | 1.10 | 1.18 |
| Separator | Celgard EH2010 (20 µm) | |
| 1 Nominal capacity | 250 mAh | 230 mAh |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, D.; Liu, H.; Chen, S.; Wu, M. The Influence of Thick Cathode Fabrication Processing on Battery Cell Performance. Electrochem 2024, 5, 421-436. https://doi.org/10.3390/electrochem5040028
Kong D, Liu H, Chen S, Wu M. The Influence of Thick Cathode Fabrication Processing on Battery Cell Performance. Electrochem. 2024; 5(4):421-436. https://doi.org/10.3390/electrochem5040028
Chicago/Turabian StyleKong, Dewen, Haijing Liu, Si Chen, and Meiyuan Wu. 2024. "The Influence of Thick Cathode Fabrication Processing on Battery Cell Performance" Electrochem 5, no. 4: 421-436. https://doi.org/10.3390/electrochem5040028
APA StyleKong, D., Liu, H., Chen, S., & Wu, M. (2024). The Influence of Thick Cathode Fabrication Processing on Battery Cell Performance. Electrochem, 5(4), 421-436. https://doi.org/10.3390/electrochem5040028





