Ion-Selective Electrodes in the Food Industry: Development Trends in the Potentiometric Determination of Ionic Pollutants
Abstract
1. Introduction
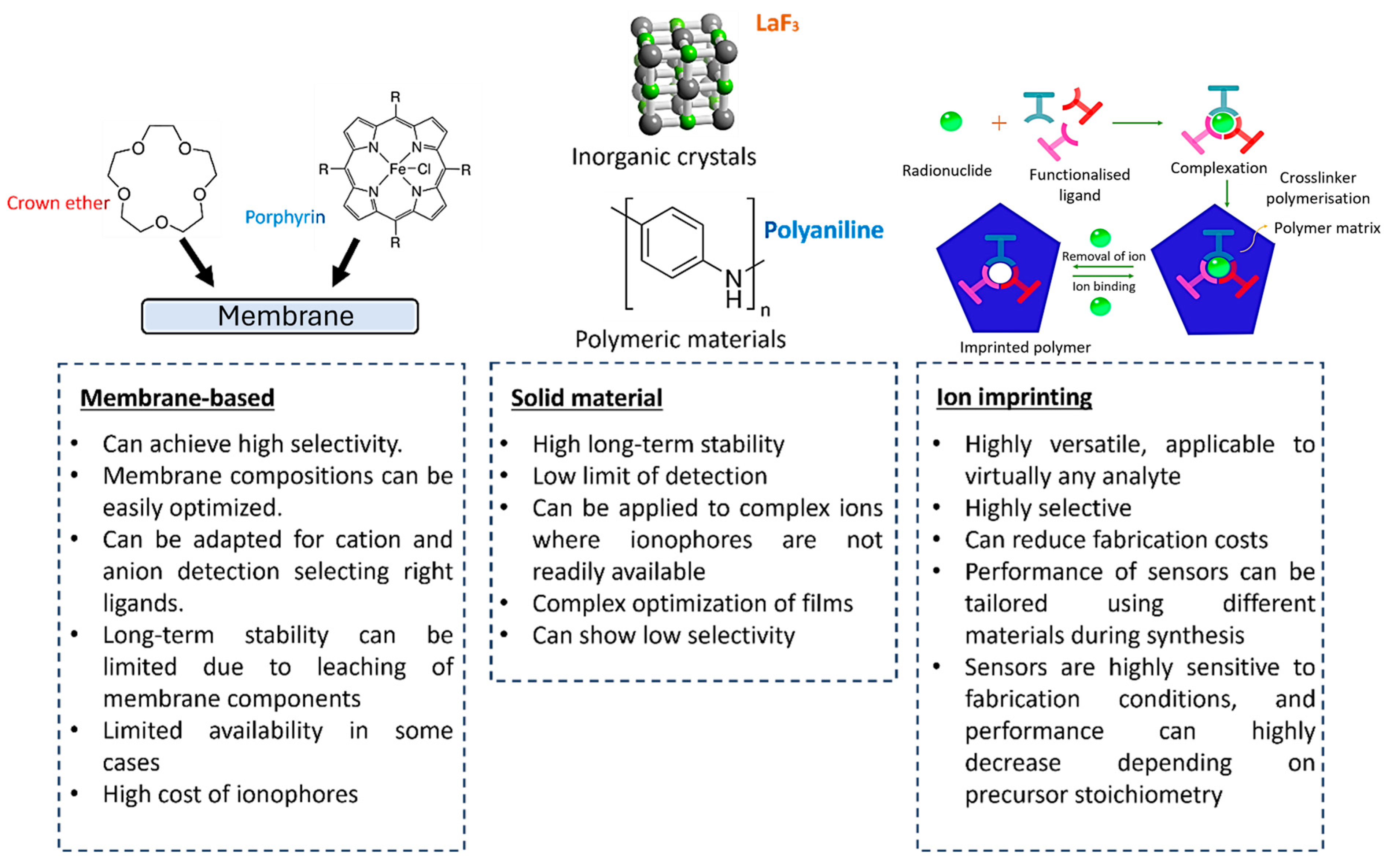
2. Ion-Selective Electrodes
3. Ion-Selective Electrodes for Heavy Metal Measurement
3.1. Lead Detection
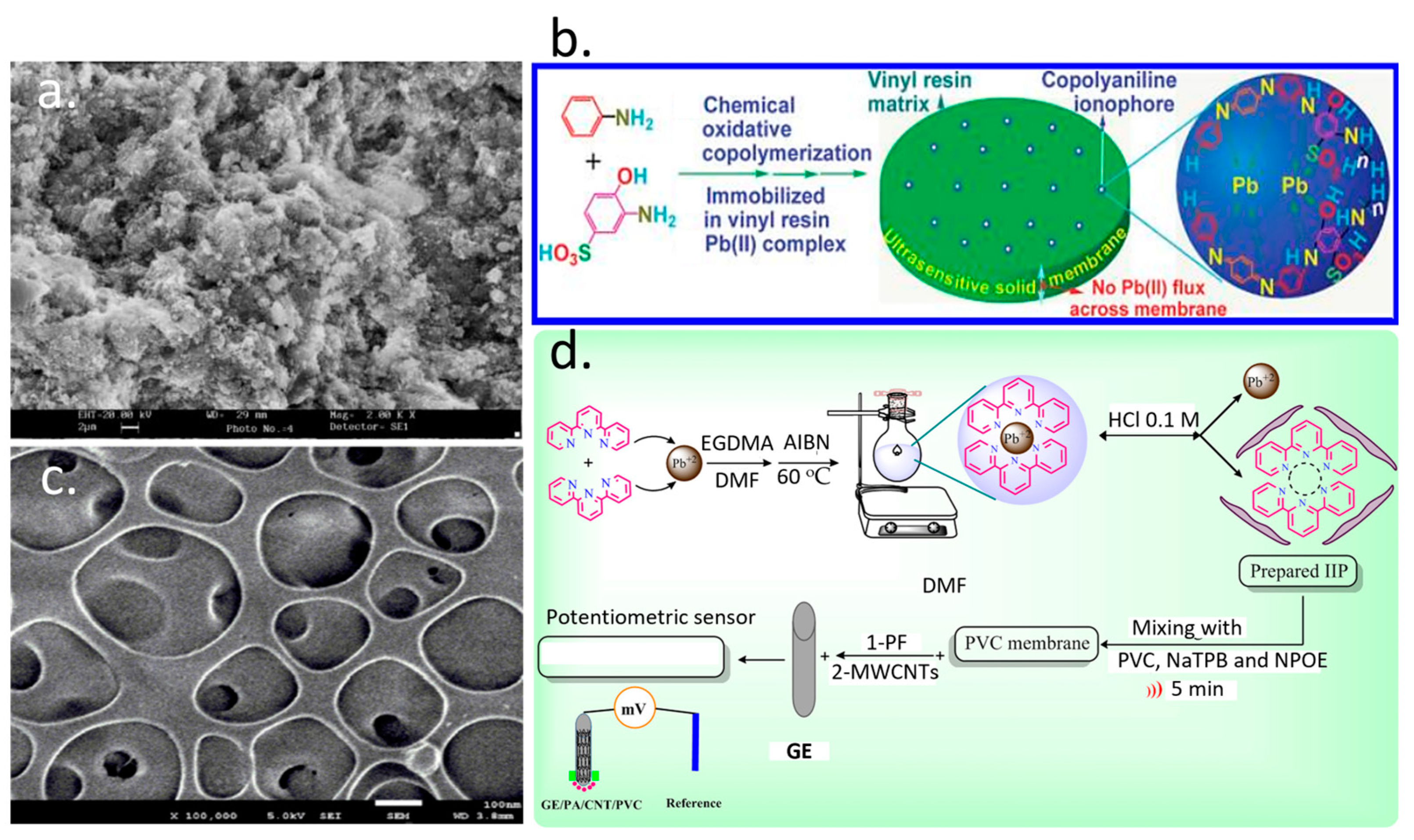
3.2. Arsenic Detection
3.3. Mercury Detection
3.4. Cadmium Detection
4. Anion Detection
4.1. Fluoride Ion Sensors

4.2. Phosphate
4.3. Nitrite and Nitrate
4.4. Sulphide Detection
4.5. Perchlorate Detection
5. Limitations of Ion-Selective Electrodes in Food and Future Trends

6. Conclusions
Funding
Conflicts of Interest
References
- van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- John, Y.; David, V.E.; Mmereki, D. A Comparative Study on Removal of Hazardous Anions from Water by Adsorption: A Review. Int. J. Chem. Eng. 2018, 2018, 3975948. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Sarker, A.; Kim, J.-E.; Islam, A.R.M.T.; Bilal, M.; Rakib, M.R.J.; Nandi, R.; Rahman, M.M.; Islam, T. Heavy metals contamination and associated health risks in food webs—A review focuses on food safety and environmental sustainability in Bangladesh. Environ. Sci. Pollut. Res. 2022, 29, 3230–3245. [Google Scholar] [CrossRef]
- Bawa, U. Heavy metals concentration in food crops irrigated with pesticides and their associated human health risks in Paki, Kaduna State, Nigeria. Cogent Food Agric. 2023, 9, 2191889. [Google Scholar] [CrossRef]
- Zhang, F.; Yan, X.; Zeng, C.; Zhang, M.; Shrestha, S.; Devkota, L.P.; Yao, T. Influence of Traffic Activity on Heavy Metal Concentrations of Roadside Farmland Soil in Mountainous Areas. Int. J. Environ. Res. Public Health 2012, 9, 1715–1731. [Google Scholar] [CrossRef]
- Scharff, R.L. Food Attribution and Economic Cost Estimates for Meat- and Poultry-Related Illnesses. J. Food Prot. 2020, 83, 959–967. [Google Scholar] [CrossRef]
- Wang, B.; Wang, H.; Lu, X.; Zheng, X.; Yang, Z. Recent Advances in Electrochemical Biosensors for the Detection of Foodborne Pathogens: Current Perspective and Challenges. Foods 2023, 12, 2795. [Google Scholar] [CrossRef]
- Guo, W.; Pan, B.; Sakkiah, S.; Yavas, G.; Ge, W.; Zou, W.; Tong, W.; Hong, H. Persistent Organic Pollutants in Food: Contamination Sources, Health Effects and Detection Methods. Int. J. Environ. Res. Public Health 2019, 16, 4361. [Google Scholar] [CrossRef]
- Heluany, C.S.; De Palma, A.; Day, N.J.; Farsky, S.H.P.; Nalesso, G. Hydroquinone, an Environmental Pollutant, Affects Cartilage Homeostasis through the Activation of the Aryl Hydrocarbon Receptor Pathway. Cells 2023, 12, 690. [Google Scholar] [CrossRef] [PubMed]
- Khezerlou, A.; Akhlaghi, A.P.; Alizadeh, A.M.; Dehghan, P.; Maleki, P. Alarming impact of the excessive use of tert-butylhydroquinone in food products: A narrative review. Toxicol. Rep. 2022, 9, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Kumar, P.; Mobin, S.M. A highly sensitive and selective hydroquinone sensor based on a newly designed N-rGO/SrZrO3 composite. Nanoscale Adv. 2020, 2, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Karthika, A.; Ramasamy Raja, V.; Karuppasamy, P.; Suganthi, A.; Rajarajan, M. A novel electrochemical sensor for determination of hydroquinone in water using FeWO4/SnO2 nanocomposite immobilized modified glassy carbon electrode. Arab. J. Chem. 2020, 13, 4065–4081. [Google Scholar] [CrossRef]
- Alrashidi, A.; El-Sherif, A.M.; Ahmed, J.; Faisal, M.; Alsaiari, M.; Algethami, J.S.; Moustafa, M.I.; Abahussain, A.A.M.; Harraz, F.A. A Sensitive Hydroquinone Amperometric Sensor Based on a Novel Palladium Nanoparticle/Porous Silicon/Polypyrrole-Carbon Black Nanocomposite. Biosensors 2023, 13, 178. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Sharma, B.; Chawla, P.; Bhatia, R. Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES): A Powerful Analytical Technique for Elemental Analysis. Food Anal. Methods 2021, 15, 666–688. [Google Scholar] [CrossRef]
- Ghaedi, M.; Shokrollahi, A.; Kianfar, A.H.; Mirsadeghi, A.S.; Pourfarokhi, A.; Soylak, M. The determination of some heavy metals in food samples by flame atomic absorption spectrometry after their separation-preconcentration on bis salicyl aldehyde, 1,3 propan diimine (BSPDI) loaded on activated carbon. J. Hazard. Mater. 2008, 154, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Agatemor, C.; Beauchemin, D. Matrix effects in inductively coupled plasma mass spectrometry: A review. Anal. Chim. Acta 2011, 706, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Meng, R.; Zhu, Q.; Long, T.; He, X.; Luo, Z.; Gu, R.; Wang, W.; Xiang, P. The innovative and accurate detection of heavy metals in foods: A critical review on electrochemical sensors. Food Control 2023, 150, 109743. [Google Scholar] [CrossRef]
- Crespo, G.A.; Cuartero, M.; Bakker, E. Thin Layer Ionophore-Based Membrane for Multianalyte Ion Activity Detection. Anal. Chem. 2015, 87, 7729–7737. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Qi, X.; Zhang, G.; Wang, S.; Li, K.; Wu, J.; Wan, X.; Liu, Y.; Li, Q. Low-cost voltammetric sensors for robust determination of toxic Cd(II) and Pb(II) in environment and food based on shuttle-like α-Fe2O3 nanoparticles decorated β-Bi2O3 microspheres. Microchem. J. 2022, 179, 107515. [Google Scholar] [CrossRef]
- Kim, Y.; Amemiya, S. Stripping Analysis of Nanomolar Perchlorate in Drinking Water with a Voltammetric Ion-Selective Electrode Based on Thin-Layer Liquid Membrane. Anal. Chem. 2008, 80, 6056–6065. [Google Scholar] [CrossRef] [PubMed]
- Soltani, H.; Pardakhty, A.; Ahmadzadeh, S. Determination of hydroquinone in food and pharmaceutical samples using a voltammetric based sensor employing NiO nanoparticle and ionic liquids. J. Mol. Liq. 2016, 219, 63–67. [Google Scholar] [CrossRef]
- Keresten, V.; Mikhelson, K. Voltammetric Ion Sensing with Ionophore-Based Ion-Selective Electrodes Containing Internal Aqueous Solution, Improving Lifetime of Sensors. Membranes 2022, 12, 1048. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, Y.; Luo, H.; Zhao, J.; Bi, J.; Wu, G. Review—Ion Interference and Elimination in Electrochemical Detection of Heavy Metals Using Anodic Stripping Voltammetry. J. Electrochem. Soc. 2023, 170, 057507. [Google Scholar] [CrossRef]
- Sridara, T.; Upan, J.; Saianand, G.; Tuantranont, A.; Karuwan, C.; Jakmunee, J. Non-Enzymatic Amperometric Glucose Sensor Based on Carbon Nanodots and Copper Oxide Nanocomposites Electrode. Sensors 2020, 20, 808. [Google Scholar] [CrossRef]
- Revsbech, N.P.; Nielsen, M.; Fapyane, D. Ion Selective Amperometric Biosensors for Environmental Analysis of Nitrate, Nitrite and Sulfate. Sensors 2020, 20, 4326. [Google Scholar] [CrossRef] [PubMed]
- Isildak, Ö.; Özbek, O. Application of Potentiometric Sensors in Real Samples. Crit. Rev. Anal. Chem. 2021, 51, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric ion sensors. Chem. Rev. 2008, 108, 329–351. [Google Scholar] [CrossRef]
- Abedi, M.R.; Zamani, H.A. Construction of Eu3+ Ion-Selective Electrode Based on 1,2-Diaminopropane-N,N,N′,N′-tetraacetic acid. E-J. Chem. 2011, 8, 716406. [Google Scholar] [CrossRef]
- Lerchi, M.; Bakker, E.; Rusterholz, B.; Simon, W. Lead-selective bulk optodes based on neutral ionophores with subnanomolar detection limits. Anal. Chem. 1992, 64, 1534–1540. [Google Scholar] [CrossRef]
- Hu, J.; Stein, A.; Bühlmann, P. Rational design of all-solid-state ion-selective electrodes and reference electrodes. TrAC Trends Anal. Chem. 2016, 76, 102–114. [Google Scholar] [CrossRef]
- Veder, J.P.; De Marco, R.; Clarke, G.; Chester, R.; Nelson, A.; Prince, K.; Pretsch, E.; Bakker, E. Elimination of undesirable water layers in solid-contact polymeric ion-selective electrodes. Anal. Chem. 2008, 80, 6731–6740. [Google Scholar] [CrossRef] [PubMed]
- Okubo, S.; Ozeki, Y.; Yamada, T.; Saito, K.; Ishihara, N.; Yanagida, Y.; Mayanagi, G.; Washio, J.; Takahashi, N. Facile Fabrication of All-solid-state Ion-selective Electrodes by Laminating and Drop-casting for Multi-sensing. Electrochemistry 2022, 90, 077001. [Google Scholar] [CrossRef]
- Ruiz-Gonzalez, A.; Choy, K.-L. Integration of an Aerosol-Assisted Deposition Technique for the Deposition of Functional Biomaterials Applied to the Fabrication of Miniaturised Ion Sensors. Nanomaterials 2021, 11, 938. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Mattinen, U.; Bobacka, J. Improving the Sensitivity of Solid-Contact Ion-Selective Electrodes by Using Coulometric Signal Transduction. ACS Sens. 2019, 4, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zeng, X.; Waterhouse, G.I.N.; Jiang, X.; Zhang, Z.; Yu, L. Potential stability improvement in Pb2+ ion selective electrodes by applying hydrophobic polyaniline as ion-to-electron transducer. Synth. Met. 2021, 281, 116898. [Google Scholar] [CrossRef]
- Wardak, C.; Pietrzak, K.; Morawska, K.; Grabarczyk, M. Ion-Selective Electrodes with Solid Contact Based on Composite Materials: A Review. Sensors 2023, 23, 5839. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, Y.; Zhang, W.; Wang, Y.; Li, G. Solid-Contact Ion-Selective Electrodes for Histamine Determination. Sensors 2021, 21, 6658. [Google Scholar] [CrossRef] [PubMed]
- Elbalkiny, H.T.; Samir, A. Green potentiometric electrode for determination of salbutamol in biological samples. Anal. Biochem. 2022, 659, 114949. [Google Scholar] [CrossRef] [PubMed]
- Pipoyan, D.; Stepanyan, S.; Beglaryan, M.; Stepanyan, S.; Mendelsohn, R.; Deziel, N.C. Health risks of heavy metals in food and their economic burden in Armenia. Environ. Int. 2023, 172, 107794. [Google Scholar] [CrossRef] [PubMed]
- Dasharathy, S.; Arjunan, S.; Maliyur Basavaraju, A.; Murugasen, V.; Ramachandran, S.; Keshav, R.; Murugan, R. Mutagenic, Carcinogenic, and Teratogenic Effect of Heavy Metals. Evid. Based Complement. Altern. Med. 2022, 2022, 8011953. [Google Scholar] [CrossRef] [PubMed]
- Csuros, M.; Csuros, C. Environmental Sampling and Analysis for Metals; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Mawari, G.; Kumar, N.; Sarkar, S.; Daga, M.K.; Singh, M.M.; Joshi, T.K.; Khan, N.A. Heavy Metal Accumulation in Fruits and Vegetables and Human Health Risk Assessment: Findings From Maharashtra, India. Environ. Health Insights 2022, 16, 11786302221119151. [Google Scholar] [CrossRef] [PubMed]
- El Hamiani, O.; El Khalil, H.; Sirguey, C.; Ouhammou, A.; Bitton, G.; Schwartz, C.; Boularbah, A. Metal Concentrations in Plants from Mining Areas in South Morocco: Health Risks Assessment of Consumption of Edible and Aromatic Plants. CLEAN-Soil Air Water 2015, 43, 399–407. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, K.-R.; Kim, W.-I.; Owens, G.; Kim, K.-H. Influence of Road Proximity on the Concentrations of Heavy Metals in Korean Urban Agricultural Soils and Crops. Arch. Environ. Contam. Toxicol. 2017, 72, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-l.; Shi, W.; Jin, Z.-f.; Wu, H.-m.; Sheng, G.D. Excessive uptake of heavy metals by greenhouse vegetables. J. Geochem. Explor. 2017, 173, 76–84. [Google Scholar] [CrossRef]
- Wei, B.; Yu, J.; Cao, Z.; Meng, M.; Yang, L.; Chen, Q. The Availability and Accumulation of Heavy Metals in Greenhouse Soils Associated with Intensive Fertilizer Application. Int. J. Environ. Res. Public Health 2020, 17, 5359. [Google Scholar] [CrossRef] [PubMed]
- Pigłowski, M. Heavy Metals in Notifications of Rapid Alert System for Food and Feed. Int. J. Environ. Res. Public Health 2018, 15, 365. [Google Scholar] [CrossRef] [PubMed]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef] [PubMed]
- No. 1881/2006; Setting Maximum Levels for Certain Contaminants in Foodstuffs. Commission Regulation (EC): Brussels, Belgium, 2006.
- Rabinowitz, M.B. Toxicokinetics of bone lead. Environ. Health Perspect. 1991, 91, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Collin, M.S.; Venkatraman, S.K.; Vijayakumar, N.; Kanimozhi, V.; Arbaaz, S.M.; Stacey, R.G.S.; Anusha, J.; Choudhary, R.; Lvov, V.; Tovar, G.I.; et al. Bioaccumulation of lead (Pb) and its effects on human: A review. J. Hazard. Mater. Adv. 2022, 7, 100094. [Google Scholar] [CrossRef]
- Choudhari, R.; Sathwara, N.G.; Shivgotra, V.K.; Patel, S.; Rathod, R.A.; Shaikh, S.; Shaikh, M.I.; Dodia, S.; Parikh, D.J.; Saiyed, H.N. Study of lead exposure to children residing near a lead-zinc mine. Indian J. Occup. Environ. Med. 2010, 14, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Patterson, C.C.; Settle, D.M. Magnitude of lead flux to the atmosphere from volcanoes. Geochim. Cosmochim. Acta 1987, 51, 675–681. [Google Scholar] [CrossRef]
- Kumar, V.; Chopra, A. Heavy Metals Accumulation in Soil and Agricultural Crops Grown in the Province of Asahi India Glass Ltd., Haridwar (Uttarakhand), India. Adv. Crop Sci. Technol. 2016, 4, 203. [Google Scholar] [CrossRef]
- Chaoua, S.; Boussaa, S.; El Gharmali, A.; Boumezzough, A. Impact of irrigation with wastewater on accumulation of heavy metals in soil and crops in the region of Marrakech in Morocco. J. Saudi Soc. Agric. Sci. 2019, 18, 429–436. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, P.; Yin, L.; Zuo, M.; Chen, Q.; El-Seedi, H.R.; Zou, X. Determination of lead in food by surface-enhanced Raman spectroscopy with aptamer regulating gold nanoparticles reduction. Food Control 2022, 132, 108498. [Google Scholar] [CrossRef]
- Bühlmann, P.; Pretsch, E.; Bakker, E. Carrier-Based Ion-Selective Electrodes and Bulk Optodes. 2. Ionophores for Potentiometric and Optical Sensors. Chem. Rev. 1998, 98, 1593–1688. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Jiang, W.; Waterhouse, G.I.N.; Jiang, X.; Zhang, Z.; Yu, L. Stable Pb(II) ion-selective electrodes with a low detection limit using silver nanoparticles/polyaniline as the solid contact. Microchim. Acta 2021, 188, 393. [Google Scholar] [CrossRef]
- Özbek, O.; Kalay, E.; Berkel, C.; Aslan, O.N.; Tokalı, F.S. Synthesis, characterization and sensor properties of a new sulfonyl hydrazone derivative molecule: Potentiometric determination of Pb(II) ions. Chem. Pap. 2024, 78, 2621–2633. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.; Tahir, H.E.; Zou, X.; Wang, P. Rapid and wide-range determination of Cd(II), Pb(II), Cu(II) and Hg(II) in fish tissues using light addressable potentiometric sensor. Food Chem. 2017, 221, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Özbek, O.; Gezegen, H.; Cetin, A.; Isildak, Ö. A Potentiometric Sensor for the Determination of Pb(II) Ions in Different Environmental Samples. ChemistrySelect 2022, 7, e202202494. [Google Scholar] [CrossRef]
- Arfin, T.; Tarannum, A. Rapid determination of lead ions using polyaniline-zirconium (IV) iodate-based ion selective electrode. J. Environ. Chem. Eng. 2019, 7, 102811. [Google Scholar] [CrossRef]
- Li, X.-G.; Feng, H.; Huang, M.-R.; Gu, G.-L.; Moloney, M.G. Ultrasensitive Pb(II) Potentiometric Sensor Based on Copolyaniline Nanoparticles in a Plasticizer-Free Membrane with a Long Lifetime. Anal. Chem. 2012, 84, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Ardalani, M.; Shamsipur, M.; Besharati-Seidani, A. A new generation of highly sensitive potentiometric sensors based on ion imprinted polymeric nanoparticles/multiwall carbon nanotubes/polyaniline/graphite electrode for sub-nanomolar detection of lead(II) ions. J. Electroanal. Chem. 2020, 879, 114788. [Google Scholar] [CrossRef]
- Abraham, A.A.; Rezayi, M.; Manan, N.S.A.; Narimani, L.; Rosli, A.N.B.; Alias, Y. A Novel Potentiometric Sensor Based on 1,2-Bis(N′-benzoylthioureido)benzene and Reduced Graphene Oxide for Determination of Lead (II) Cation in Raw Milk. Electrochim. Acta 2015, 165, 221–231. [Google Scholar] [CrossRef]
- Yuzhu, S.; Yuan, L.; Chunlei, G.; Yishan, W.; Liping, H.; Monique, T.; Feng, J.H.; Terry, H.; Graham, A.M.; Jingmin, D.; et al. Cross-sectional comparisons of sodium content in processed meat and fish products among five countries: Potential for feasible targets and reformulation. BMJ Open 2021, 11, e046412. [Google Scholar] [CrossRef] [PubMed]
- Branger, C.; Meouche, W.; Margaillan, A. Recent advances on ion-imprinted polymers. React. Funct. Polym. 2013, 73, 859–875. [Google Scholar] [CrossRef]
- Lou, Y.; Chen, W.; Gan, Y.; Zhou, Y.; Ye, H.; Huan, W.; Zhang, Y. Lateral flow analysis of Pb (II) in green tea integrated with ionic imprinted paper-based chip. Microchem. J. 2022, 176, 107235. [Google Scholar] [CrossRef]
- Rahman, F.A.; Allan, D.L.; Rosen, C.J.; Sadowsky, M.J. Arsenic availability from chromated copper arsenate (CCA)-treated wood. J. Environ. Qual. 2004, 33, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Chen, L.; Li, J.; Zhang, Z. Current status and challenges of ion imprinting. J. Mater. Chem. A 2015, 3, 13598–13627. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Buha Djordjevic, A.; Crisponi, G.; Alexander, J.; Bjørklund, G.; Aaseth, J. Arsenic Toxicity: Molecular Targets and Therapeutic Agents. Biomolecules 2020, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Authority, E.F.S.; Arcella, D.; Cascio, C.; Gómez Ruiz, J.Á. Chronic dietary exposure to inorganic arsenic. EFSA J. 2021, 19, e06380. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Rahman, M.M.; Islam, M.R.; Naidu, R. Arsenic accumulation in rice: Consequences of rice genotypes and management practices to reduce human health risk. Environ. Int. 2016, 96, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Linquist, B.A.; Wilson, L.T.; Cassman, K.G.; Stuart, A.M.; Pede, V.; Miro, B.; Saito, K.; Agustiani, N.; Aristya, V.E.; et al. Sustainable intensification for a larger global rice bowl. Nat. Commun. 2021, 12, 7163. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Jiang, Y.; Zhao, Z.; Wang, L.; Chen, J.; Ren, S.; Liu, Z.; Zhang, Y.; Tsang, D.C.W.; Crittenden, J.C. pH Dependence of Arsenic Oxidation by Rice-Husk-Derived Biochar: Roles of Redox-Active Moieties. Environ. Sci. Technol. 2019, 53, 9034–9044. [Google Scholar] [CrossRef] [PubMed]
- Hug, S.J.; Leupin, O. Iron-Catalyzed Oxidation of Arsenic(III) by Oxygen and by Hydrogen Peroxide: pH-Dependent Formation of Oxidants in the Fenton Reaction. Environ. Sci. Technol. 2003, 37, 2734–2742. [Google Scholar] [CrossRef]
- Hasnat, M.A.; Hasan, M.M.; Tanjila, N.; Alam, M.M.; Rahman, M.M. pH dependent kinetic insights of electrocatalytic arsenite oxidation reactions at Pt surface. Electrochim. Acta 2017, 225, 105–113. [Google Scholar] [CrossRef]
- Selig, W. Micro and semimicro determination of arsenate using ion-selective electrodes. Microchim. Acta 1973, 61, 349–359. [Google Scholar] [CrossRef]
- Khan, A.A.; Shaheen, S. Determination of arsenate in water by anion selective membrane electrode using polyurethane–silica gel fibrous anion exchanger composite. J. Hazard. Mater. 2014, 264, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Hamid Kargari, S.; Ahour, F.; Mahmoudian, M. An electrochemical sensor for the detection of arsenic using nanocomposite-modified electrode. Sci. Rep. 2023, 13, 8816. [Google Scholar] [CrossRef] [PubMed]
- Somer, G.; Yilmaz, U.T.; Kalaycı, Ş. Preparation and properties of a new solid state arsenate As(V) ion selective electrode and its application. Talanta 2015, 142, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Singh, A.K.; Bhardwaj, S.; Singhal, D. Electroanalytical Studies of Chromone Based Ionophores for the Selective Determination of Arsenite Ion. Electroanalysis 2015, 27, 1166–1175. [Google Scholar] [CrossRef]
- Alizadeh, T.; Rashedi, M. Synthesis of nano-sized arsenic-imprinted polymer and its use as As3+ selective ionophore in a potentiometric membrane electrode: Part 1. Anal. Chim. Acta 2014, 843, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Ardakani, M.M.; Karimi, M.A.; Mashhadizadeh, M.H.; Pesteh, M.; Azimi, M.S.; Kazemian, H. Potentiometric determination of monohydrogen arsenate by zeolite-modified carbon-paste electrode. Int. J. Environ. Anal. Chem. 2007, 87, 285–294. [Google Scholar] [CrossRef]
- Al-Sulaiti, M.M.; Soubra, L.; Al-Ghouti, M.A. The Causes and Effects of Mercury and Methylmercury Contamination in the Marine Environment: A Review. Curr. Pollut. Rep. 2022, 8, 249–272. [Google Scholar] [CrossRef]
- Bernhoft, R.A. Mercury Toxicity and Treatment: A Review of the Literature. J. Environ. Public Health 2012, 2012, 460508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dutkiewicz, S.; Sunderland, E.M. Impacts of climate change on methylmercury formation and bioaccumulation in the 21st century ocean. One Earth 2021, 4, 279–288. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, P.; Zhang, Y. Climate-driven changes of global marine mercury cycles in 2100. Earth Atmos. Planet. Sci. 2023, 120, e2202488120. [Google Scholar] [CrossRef] [PubMed]
- Skerfving, S.B.; Copplestone, J.F. Poisoning caused by the consumption of organomercury-dressed seed in Iraq. Bull. World Health Organ. 1976, 54, 101–112. [Google Scholar] [PubMed]
- Harada, M. Minamata disease: Methylmercury poisoning in Japan caused by environmental pollution. Crit. Rev. Toxicol. 1995, 25, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Katherine, H.; Taber, P.D.; Robin, A.; Hurley, M.D. Mercury Exposure: Effects Across the Lifespan. J. Neuropsychiatry Clin. Neurosci. 2008, 20, iv-389. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.J.; Kim, B.G.; Jeon, M.J.; Kim, S.Y.; Kim, H.C.; Jang, T.W.; Chae, H.J.; Choi, W.J.; Ha, M.N.; Hong, Y.S. Evaluation of mercury exposure level, clinical diagnosis and treatment for mercury intoxication. Ann. Occup. Environ. Med. 2016, 28, 5. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.L.C.; Lemos, V.A.; Silva, L.O.B.; Queiroz, A.F.S.; Souza, A.S.; da Silva, E.G.P.; dos Santos, W.N.L.; das Virgens, C.F. Analytical strategies of sample preparation for the determination of mercury in food matrices—A review. Microchem. J. 2015, 121, 227–236. [Google Scholar] [CrossRef]
- Mehta, P.K.; Park, H.; Oh, E.-T.; Park, H.J.; Lee, K.-H. Tuning of distinguished fluorescent responses to methylmercury and Hg2+ ions: Selective detection of methylmercury over Hg2+ ions by fluorescent sensor using micelle system. Sens. Actuators B Chem. 2023, 385, 133670. [Google Scholar] [CrossRef]
- Chen, X.; Sun, X.-T.; Cui, M.-S.; Liu, Y.; Cui, K.-P.; Weerasooriya, R. Electrochemical determination of methylmercury via modulating bandgap of sulfur doped graphitic carbon nitride. J. Environ. Chem. Eng. 2021, 9, 105510. [Google Scholar] [CrossRef]
- Mesa, R.; Khan, S.; Sotomayor, M.D.P.T.; Picasso, G. Using Carbon Paste Electrode Modified with Ion Imprinted Polymer and MWCNT for Electrochemical Quantification of Methylmercury in Natural Water Samples. Biosensors 2022, 12, 376. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.-T.; Shih, J.-S. Mercury(II) and silver(I) ion-selective electrodes based on dithia crown ethers. The Analyst 1986, 111, 891–895. [Google Scholar] [CrossRef]
- Mahajan, R.K.; Kaur, R.; Bhalla, V.; Kumar, M.; Hattori, T.; Miyano, S. Mercury(II) sensors based on calix[4]arene derivatives as receptor molecules. Sens. Actuators B Chem. 2008, 130, 290–294. [Google Scholar] [CrossRef]
- Miao, J.; Wang, X.; Fan, Y.; Li, J.; Zhang, L.; Hu, G.; He, C.; Jin, C. Determination of total mercury in seafood by ion-selective electrodes based on a thiol functionalized ionic liquid. J. Food Drug Anal. 2018, 26, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.E.; Abbas, A.A.; Mohamed, G.G. Synthesis and surface characterization of a chemically modified carbon paste electrode and its application in determination of Hg(II) ion in water, food and dental amalgam samples. Microchem. J. 2023, 184, 108178. [Google Scholar] [CrossRef]
- Aglan, R.F.; Saleh, H.M.; Mohamed, G.G. Potentiometric determination of mercury (II) ion in various real samples using novel modified screen-printed electrode. Appl. Water Sci. 2018, 8, 141. [Google Scholar] [CrossRef]
- Shirzadmehr, A.; Afkhami, A.; Madrakian, T. A new nano-composite potentiometric sensor containing an Hg2+-ion imprinted polymer for the trace determination of mercury ions in different matrices. J. Mol. Liq. 2015, 204, 227–235. [Google Scholar] [CrossRef]
- Ramezani, S.; Mashhadizadeh, M.H.; Jahani, R.; Kamali, M. Carbon nanotube grafted pyridinuim compound as a neutral ion-carrier of carbon paste electrode for sub-nanomolar simultaneous monitoring of Cu(II) and Hg(II). Int. J. Environ. Anal. Chem. 2023, 1–24. [Google Scholar] [CrossRef]
- Kim, K.; Melough, M.M.; Vance, T.M.; Noh, H.; Koo, S.I.; Chun, O.K. Dietary Cadmium Intake and Sources in the US. Nutrients 2018, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Snoj Tratnik, J.; Kocman, D.; Horvat, M.; Andersson, A.-M.; Juul, A.; Jacobsen, E.; Ólafsdóttir, K.; Klanova, J.; Andryskova, L.; Janasik, B.; et al. Cadmium exposure in adults across Europe: Results from the HBM4EU Aligned Studies survey 2014–2020. Int. J. Hyg. Environ. Health 2022, 246, 114050. [Google Scholar] [CrossRef] [PubMed]
- Prozialeck, W.C.; Edwards, J.R. Mechanisms of cadmium-induced proximal tubule injury: New insights with implications for biomonitoring and therapeutic interventions. J. Pharmacol. Exp. Ther. 2012, 343, 2–12. [Google Scholar] [CrossRef]
- Akesson, A.; Bjellerup, P.; Lundh, T.; Lidfeldt, J.; Nerbrand, C.; Samsioe, G.; Skerfving, S.; Vahter, M. Cadmium-induced effects on bone in a population-based study of women. Environ. Health Perspect. 2006, 114, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, A. Cadmium and cancer. Met. Ions Life Sci. 2013, 11, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, environmental exposure, and health outcomes. Environ. Health Perspect. 2010, 118, 182–190. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Flavourings (FAF); Younes, M.; Aquilina, G.; Castle, L.; Engel, K.H.; Fowler, P.J.; Frutos Fernandez, M.J.; Fürst, P.; Gundert-Remy, U.; Gürtler, R.; et al. Follow-up of the re-evaluation of sulfur dioxide (E 220), sodium sulfite (E 221), sodium bisulfite (E 222), sodium metabisulfite (E 223), potassium metabisulfite (E 224), calcium sulfite (E 226), calcium bisulfite (E 227) and potassium bisulfite (E 228). EFSA J. 2022, 20, e07594. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Pili, H.B. Potentiometric determination of cadmium using coated platinum and PVC membrane sensors based on N,N′-bis(salicylaldehyde)phenylenediamine (salophen). J. Anal. Chem. 2015, 70, 731–737. [Google Scholar] [CrossRef]
- Rezaei, B.; Meghdadi, S.; Zarandi, R.F. A fast response cadmium-selective polymeric membrane electrode based on N,N′-(4-methyl-1,2-phenylene)diquinoline-2-carboxamide as a new neutral carrier. J. Hazard. Mater. 2008, 153, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Singh, D.; Sarkar, A. PVC Membrane Selective Electrode for Determination of Cadmium(II) Ion in Chocolate Samples. Chin. J. Chem. Eng. 2014, 22, 480–488. [Google Scholar] [CrossRef]
- Rezaei, B.; Meghdadi, S.; Bagherpour, S. Cadmium Selective PVC-Membranes Sensor Based on 1, 2-Bis (Quinoline-2-Carboxamido)-4-Chlorobenzene as a Neutral Carrier. IEEE Sens. J. 2008, 8, 1469–1477. [Google Scholar] [CrossRef]
- Jiang, C.; Li, X.; Yao, Y.; Ying, Y.; Ping, J. Fully Written Flexible Potentiometric Sensor Using Two-Dimensional Nanomaterial-Based Conductive Ink. Anal. Chem. 2018, 90, 13088–13095. [Google Scholar] [CrossRef] [PubMed]
- Özbek, O.; Isildak, Ö.; Gürdere, M.B.; Berkel, C. Cadmium(II)-selective potentiometric sensor based on synthesised (E)-2-benzylidenehydrazinecarbothioamide for the determination of Cd2+ in different environmental samples. Int. J. Environ. Anal. Chem. 2022, 102, 6819–6834. [Google Scholar] [CrossRef]
- Rezvani Ivari, S.A.; Darroudi, A.; Arbab Zavar, M.H.; Zohuri, G.; Ashraf, N. Ion imprinted polymer based potentiometric sensor for the trace determination of Cadmium (II) ions. Arab. J. Chem. 2017, 10, S864–S869. [Google Scholar] [CrossRef]
- Chen, L.; Berry, S.N.; Wu, X.; Howe, E.N.W.; Gale, P.A. Advances in Anion Receptor Chemistry. Chem 2020, 6, 61–141. [Google Scholar] [CrossRef]
- Antonisse, M.M.; Reinhoudt, D.N. Neutral anion receptors: Design and application. Chem. Commun. 1998, 443–448. [Google Scholar] [CrossRef]
- Chandra, S.; Růžička, A.; Švec, P.; Lang, H. Organotin compounds: An ionophore system for fluoride ion recognition. Anal. Chim. Acta 2006, 577, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Mier, E.A.; Cury, J.A.; Heilman, J.R.; Katz, B.P.; Levy, S.M.; Li, Y.; Maguire, A.; Margineda, J.; O’Mullane, D.; Phantumvanit, P.; et al. Zohouri. Development of Gold Standard Ion-Selective Electrode-Based Methods for Fluoride Analysis. Caries Res. 2011, 45, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Saad, H.; Escoube, R.; Babajko, S.; Houari, S. Fluoride Intake Through Dental Care Products: A Systematic Review. Front. Oral Health 2022, 3, 916372. [Google Scholar] [CrossRef] [PubMed]
- Horst, J.A.; Tanzer, J.M.; Milgrom, P.M. Fluorides and Other Preventive Strategies for Tooth Decay. Dent. Clin. N. Am. 2018, 62, 207–234. [Google Scholar] [CrossRef]
- Authority, E.F.S. Opinion of the Scientific Panel on Dietetic Products, Nutrition and Allergies on a request from the Commission related to the Tolerable Upper Intake Level of Fluoride. EFSA J. 2006, 192, 1–65. [Google Scholar]
- Solanki, Y.S.; Agarwal, M.; Gupta, A.B.; Gupta, S.; Shukla, P. Fluoride occurrences, health problems, detection, and remediation methods for drinking water: A comprehensive review. Sci. Total Environ. 2022, 807, 150601. [Google Scholar] [CrossRef] [PubMed]
- Waugh, D.T.; Potter, W.; Limeback, H.; Godfrey, M. Risk Assessment of Fluoride Intake from Tea in the Republic of Ireland and its Implications for Public Health and Water Fluoridation. Int. J. Environ. Res. Public Health 2016, 13, 259. [Google Scholar] [CrossRef] [PubMed]
- Ganta, S.; Yousuf, A.; Nagaraj, A.; Pareek, S.; Sidiq, M.; Singh, K.; Vishnani, P. Evaluation of Fluoride Retention Due to Most Commonly Consumed Estuarine Fishes Among Fish Consuming Population of Andhra Pradesh as a Contributing Factor to Dental Fluorosis: A Cross-Sectional Study. J. Clin. Diagn. Res. JCDR 2015, 9, Zc11–Zc15. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Samal, A.C.; Banerjee, S.; Pyne, J.; Santra, S.C. Assessment of potential health risk of fluoride consumption through rice, pulses, and vegetables in addition to consumption of fluoride-contaminated drinking water of West Bengal, India. Environ. Sci. Pollut. Res. 2017, 24, 20300–20314. [Google Scholar] [CrossRef] [PubMed]
- Radić, J.; Bralić, M.; Kolar, M.; Genorio, B.; Prkić, A.; Mitar, I. Development of the New Fluoride Ion-Selective Electrode Modified with FexOy Nanoparticles. Molecules 2020, 25, 5213. [Google Scholar] [CrossRef]
- Perdikaki, K.; Tsagkatakis, I.; Chaniotakis, N.A.; Altmann, R.; Jurkschat, K.; Reeske, G. Selective fluoride recognition and potentiometric properties of ion-selective electrodes based on bis(halodiphenylstannyl)alkanes. Anal. Chim. Acta 2002, 467, 197–204. [Google Scholar] [CrossRef]
- Chaniotakis, N.; Jurkschat, K.; Müller, D.; Perdikaki, K.; Reeske, G. Bis[di-n-alkyl(fluoro)stannyl]methanes, (R2FSn)2CH2 (R = n-octyl, n-dodecyl): Stable Fluoride-Selective Carriers. Eur. J. Inorg. Chem. 2004, 2004, 2283–2288. [Google Scholar] [CrossRef]
- Teixeira, B.; Vieira, H.; Lourenço, H.; Gonçalves, S.; Martins, M.F.; Mendes, R. Control of phosphate levels in seafood products from the Portuguese market: Is there a need for concern? J. Food Compos. Anal. 2017, 62, 94–102. [Google Scholar] [CrossRef]
- Morris, E.R.; Hill, A.D. Inositol Phosphate Content of Selected Dry Beans, Peas, and Lentils, Raw and Cooked. J. Food Compos. Anal. 1996, 9, 2–12. [Google Scholar] [CrossRef]
- Calvo, M.S.; Dunford, E.K.; Uribarri, J. Industrial Use of Phosphate Food Additives: A Mechanism Linking Ultra-Processed Food Intake to Cardiorenal Disease Risk? Nutrients 2023, 15, 3510. [Google Scholar] [CrossRef] [PubMed]
- Borah, N.; Kaka, M.N.; Tamuly, C. “AND”-Logic gate-based colorimetric detection of thiocyanate in milk samples using AgNP-EBF as plasmonic nano sensor. Food Chem. 2023, 425, 136522. [Google Scholar] [CrossRef] [PubMed]
- Mitchell-Koch, J.T.; Pietrzak, M.; Malinowska, E.; Meyerhoff, M.E. Aluminum(III) Porphyrins as Ionophores for Fluoride Selective Polymeric Membrane Electrodes. Electroanalysis 2006, 18, 551–557. [Google Scholar] [CrossRef]
- Ali, M.; Singh, J. A novel potentiometric sensor for determination of fluoride ion based on ionophore N,N′-Ethylene-bis(salicylideneaminato) nickel (II). Mater. Today Proc. 2022, 57, 157–163. [Google Scholar] [CrossRef]
- Fein, N.J.; Cerklewski, F.L. Fluoride Content of Foods Made with Mechanically Separated Chicken. J. Agric. Food Chem. 2001, 49, 4284–4286. [Google Scholar] [CrossRef]
- Kjellevold Malde, M.; Bjorvatn, K.; Julshamn, K. Determination of fluoride in food by the use of alkali fusion and fluoride ion-selective electrode. Food Chem. 2001, 73, 373–379. [Google Scholar] [CrossRef]
- McElfresh, P.M. An ion-specific electrode analysis of fluoride in potato tops using an ion-exchange pretreatment. J. Agric. Food Chem. 1978, 26, 276–277. [Google Scholar] [CrossRef]
- Pesselman, R.L.; Loken, R.G.; Hoffman, M.J.; Feit, M.J. Determination of Fluoride in Cocoa Powder by Ion-Selective Electrode. J. Food Sci. 1989, 54, 1650. [Google Scholar] [CrossRef]
- Giljanović, J.; Prkić, A.; Bralić, M.; Brkljača, M. Determination of Fluoride Content in Tea Infusion by Using Fluoride Ion-Selective Electrode. Int. J. Electrochem. Sci. 2012, 7, 2918–2927. [Google Scholar] [CrossRef]
- Fojo, C.; Figueira, M.E.; Almeida, C.M.M. Fluoride content of soft drinks, nectars, juices, juice drinks, concentrates, teas and infusions marketed in Portugal. Food Addit. Contam. Part A 2013, 30, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Y.; Li, Y.; Duan, Y.; Qian, Y.; Zhang, P.; Guo, Q.; Ding, J. Polymeric Membrane Fluoride-Selective Electrodes Using Lewis Acidic Organo-Antimony(V) Compounds as Ionophores. ACS Sens. 2020, 5, 3465–3473. [Google Scholar] [CrossRef]
- Rocha, R.A.; Rojas, D.; Clemente, M.a.J.; Ruiz, A.; Devesa, V.; Vélez, D. Quantification of Fluoride in Food by Microwave Acid Digestion and Fluoride Ion-Selective Electrode. J. Agric. Food Chem. 2013, 61, 10708–10713. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.L.G.; Nascimento, M.S.; Picoloto, R.S.; Flores, E.M.M.; Mello, P.A. A sample preparation method for fluoride detection by potentiometry with ion-selective electrode in medicinal plants. J. Fluor. Chem. 2020, 231, 109459. [Google Scholar] [CrossRef]
- Agustanti, A.; Maharani, D.A.; Gunawan, H.A.; Callea, M. The measurement of fluoride ion in anchovy (Stolephorus insularis) using ion selective electrode. J. Phys. Conf. Ser. 2018, 1073, 022001. [Google Scholar] [CrossRef]
- González, M.A.; Aller, A.J.; Pardo, R.; Barrado, E.; Deban, L. Fluoride and chloride determination in cheese with ion-selective electrodes. Electroanalysis 1991, 3, 439–442. [Google Scholar] [CrossRef]
- Liu, K. Effects of sample size, dry ashing temperature and duration on determination of ash content in algae and other biomass. Algal Res. 2019, 40, 101486. [Google Scholar] [CrossRef]
- Štepec, D.; Ponikvar-Svet, M. Fluoride in Human Health and Nutrition. Acta Chim. Slov. 2019, 66, 255–275. [Google Scholar] [CrossRef] [PubMed]
- Ignacio, M.C.C.D.; Curtzwiler, G.W.; Early, M.R.; Updegraff, K.M.; Vorst, K.L. Ion Selective Electrode (ISE) Method for Determination of Total Fluorine and Total Organic Fluorine in Packaging Substrates. Methods Protoc. 2023, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Ritz, E.; Hahn, K.; Ketteler, M.; Kuhlmann, M.K.; Mann, J. Phosphate additives in food—A health risk. Dtsch. Arztebl. Int. 2012, 109, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J. Phosphorus homeostasis in normal health and in chronic kidney disease patients with special emphasis on dietary phosphorus intake. Semin. Dial. 2007, 20, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, J.; Kestenbaum, B.; Chonchol, M. Phosphate and cardiovascular disease. Adv. Chronic Kidney Dis. 2011, 18, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, G.N.; Uribarri, J. Phosphorus and the kidney: What is known and what is needed. Adv. Nutr. 2014, 5, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.B.; Bigelow, P.; Dubin, J.A.; Mielke, J.G. High Dietary Phosphorus Is Associated with Increased Breast Cancer Risk in a U.S. Cohort of Middle-Aged Women. Nutients 2023, 15, 3735. [Google Scholar] [CrossRef] [PubMed]
- Hruska, K.A.; Mathew, S.; Lund, R.; Qiu, P.; Pratt, R. Hyperphosphatemia of chronic kidney disease. Kidney Int. 2008, 74, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Nagul, E.A.; McKelvie, I.D.; Worsfold, P.; Kolev, S.D. The molybdenum blue reaction for the determination of orthophosphate revisited: Opening the black box. Anal. Chim. Acta 2015, 890, 60–82. [Google Scholar] [CrossRef] [PubMed]
- Doku, G.N.; Haswell, S.J. Further studies into the development of a micro-FIA (μFIA) system based on electroosmotic flow for the determination of phosphate as orthophosphate. Anal. Chim. Acta 1999, 382, 1–13. [Google Scholar] [CrossRef]
- Doku, G.; Agbozo, W.; Haswell, S.; McCreedy, T. Phosphomolybdenum Blue Detection—A Review of Characteristics, Achievements, Challenges and Future Prospects. Ghana J. Sci. 2020, 61, 43–49. [Google Scholar] [CrossRef]
- Zeynaloo, E.; Zahran, E.M.; Fatila, E.M.; Flood, A.H.; Bachas, L.G. Anion-Selective Electrodes Based On a CH-Hydrogen Bonding Bis-macrocyclic Ionophore with a Clamshell Architecture. Anal. Chem. 2021, 93, 5412–5419. [Google Scholar] [CrossRef] [PubMed]
- Kalayci, S. A New Phosphate Selective Electrode and Its Application in Some Foods. Int. J. Electrochem. Sci. 2021, 16, 210949. [Google Scholar] [CrossRef]
- Xu, K.; Li, Y.; Li, M. Potentiometric Phosphate Ion Sensor Based on Electrochemical Modified Tungsten Electrode. ACS Omega 2021, 6, 13795–13801. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Han, C.; Du, H.; Ye, Y.; Tao, C. Potentiometric Phosphate Ion Sensor Based on Electrochemically Modified All-Solid-State Copper Electrode for Phosphate Ions’ Detection in Real Water. Chemosensors 2024, 12, 53. [Google Scholar] [CrossRef]
- Jeong, B.; Oh, J.S.; Kim, D.Y.; Kim, D.G.; Kim, Y.I.; Heo, J.; Lee, H.-K. Ion-Selective Electrode Based on a Novel Biomimetic Nicotinamide Compound for Phosphate Ion Sensor. Polymers 2022, 14, 3392. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, S.; Yokobori, T.; Kato, R.; Yoshimoto, K.; Kamaishi, T.; Teramae, N. Hydrogen-bond forming ionophore for highly efficient transport of phosphate anions across the nitrobenzene-water interface. Analyst 2003, 128, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Li, X.; Xu, Y.; Ye, B. Amide and Acyl-Hydrazine Functionalized Calix[4]arenes as Carriers for Hydrogen Phosphate Selective Electrodes. Electroanalysis 2007, 19, 958–963. [Google Scholar] [CrossRef]
- Ma, L.; Hu, L.; Feng, X.; Wang, S. Nitrate and Nitrite in Health and Disease. Aging Dis. 2018, 9, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Uddin, R.; Thakur, M.U.; Uddin, M.Z.; Islam, G.M.R. Study of nitrate levels in fruits and vegetables to assess the potential health risks in Bangladesh. Sci. Rep. 2021, 11, 4704. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Jeddi, S.; Azizi, F.; Ghasemi, A.; Hadaegh, F. Nitrate and nitrite content of vegetables, fruits, grains, legumes, dairy products, meats and processed meats. J. Food Compos. Anal. 2016, 51, 93–105. [Google Scholar] [CrossRef]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Qi, S.; Wang, Y. Nitrate signaling and use efficiency in crops. Plant Commun. 2022, 3, 100353. [Google Scholar] [CrossRef] [PubMed]
- Shakil, M.H.; Trisha, A.T.; Rahman, M.; Talukdar, S.; Kobun, R.; Huda, N.; Zzaman, W. Nitrites in Cured Meats, Health Risk Issues, Alternatives to Nitrites: A Review. Foods 2022, 11, 3355. [Google Scholar] [CrossRef] [PubMed]
- Karwowska, M.; Stadnik, J.; Wójciak, K. The Effect of Different Levels of Sodium Nitrate on the Physicochemical Parameters and Nutritional Value of Traditionally Produced Fermented Loins. Appl. Sci. 2021, 11, 2983. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Ghasemi, A.; Carlström, M.; Azizi, F.; Hadaegh, F. Association between Dietary Intakes of Nitrate and Nitrite and the Risk of Hypertension and Chronic Kidney Disease: Tehran Lipid and Glucose Study. Nutrients 2016, 8, 811. [Google Scholar] [CrossRef] [PubMed]
- Ramick, M.G.; Kirkman, D.L.; Stock, J.M.; Muth, B.J.; Farquhar, W.B.; Chirinos, J.A.; Doulias, P.T.; Ischiropoulos, H.; Edwards, D.G. The effect of dietary nitrate on exercise capacity in chronic kidney disease: A randomized controlled pilot study. Nitric Oxide Biol. Chem. 2021, 106, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.; Alam, N.; Biswas, P. Chapter 4—Present and Potential Water-Quality Challenges in India. In Separation Science and Technology; Ahuja, S., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 11, pp. 85–112. 525. [Google Scholar]
- Chazelas, E.; Pierre, F.; Druesne-Pecollo, N.; Esseddik, Y.; Szabo de Edelenyi, F.; Agaesse, C.; De Sa, A.; Lutchia, R.; Gigandet, S.; Srour, B.; et al. Nitrites and nitrates from food additives and natural sources and cancer risk: Results from the NutriNet-Santé cohort. Int. J. Epidemiol. 2022, 51, 1106–1119. [Google Scholar] [CrossRef] [PubMed]
- Srour, B.; Chazelas, E.; Druesne-Pecollo, N.; Esseddik, Y.; de Edelenyi, F.S.; Agaësse, C.; De Sa, A.; Lutchia, R.; Debras, C.; Sellem, L.; et al. Dietary exposure to nitrites and nitrates in association with type 2 diabetes risk: Results from the NutriNet-Santé population-based cohort study. PLoS Med. 2023, 20, e1004149. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Statement on nitrites in meat products. EFSA J. 2010, 8, 1538. [Google Scholar] [CrossRef]
- Filgueiras, M.F.; de Jesus, P.C.; Borges, E.M. Quantification of Nitrite in Food and Water Samples Using the Griess Assay and Digital Images Acquired Using a Desktop Scanner. J. Chem. Educ. 2021, 98, 3303–3311. [Google Scholar] [CrossRef]
- Bellavia, L.; Kim-Shapiro, D.B.; King, S.B. Detecting and monitoring NO, SNO and nitrite in vivo. Future Sci. OA 2015, 1, FSO36. [Google Scholar] [CrossRef] [PubMed]
- Yasir Abir, A.; Nizam Uddin, S.M.; Hasan, M.; Abdul Aziz, M.; Shah, S.; Ahmed, J.; Abul Hasnat, M. Cu-electrodeposited gold electrode for the sensitive electrokinetic investigations of nitrate reduction and detection of the nitrate ion in acidic medium. Results Chem. 2023, 5, 100702. [Google Scholar] [CrossRef]
- Cancelliere, R.; Rea, G.; Severini, L.; Cerri, L.; Leo, G.; Paialunga, E.; Mantegazza, P.; Mazzuca, C.; Micheli, L. Expanding the circularity of plastic and biochar materials by developing alternative low environmental footprint sensors. Green Chem. 2023, 25, 6774–6783. [Google Scholar] [CrossRef]
- Bristow, N.; Rengaraj, S.; Chadwick, D.R.; Kettle, J.; Jones, D.L. Development of a LoRaWAN IoT Node with Ion-Selective Electrode Soil Nitrate Sensors for Precision Agriculture. Sensors 2022, 22, 9100. [Google Scholar] [CrossRef] [PubMed]
- Miras, M.; García, M.S.; Martínez, V.; Ortuño, J.Á. Inexpensive ion-selective electrodes for the simultaneous monitoring of potassium and nitrate concentrations in nutrient solutions. Anal. Methods 2021, 13, 3511–3520. [Google Scholar] [CrossRef] [PubMed]
- Consalteri, A.; Rigato, A.; Clamor, L.; Giandon, P. Determination of nitrate in vegetables using an ion-selective electrode. J. Food Compos. Anal. 1992, 5, 252–256. [Google Scholar] [CrossRef]
- Pérez-Olmos, R.; Yoldi, I.; Ruiz, M.P.; Merino, J.M. Potentiometric Determination of Nitrite in Meat Products Using a Nitrite-Selective Electrode. Anal. Sci. 1998, 14, 1001–1003. [Google Scholar] [CrossRef]
- Pankratova, N.; Cuartero, M.; Cherubini, T.; Crespo, G.A.; Bakker, E. In-Line Acidification for Potentiometric Sensing of Nitrite in Natural Waters. Anal. Chem. 2017, 89, 571–575. [Google Scholar] [CrossRef]
- Isildak, O.; Yildiz, I. Highly selective potentiometric determination of nitrate ions using silver bisdiethyldithiocarbamate based membrane electrodes. Electrochim. Acta 2023, 459, 142587. [Google Scholar] [CrossRef]
- Soares, R.R.A.; Hjort, R.G.; Pola, C.C.; Jing, D.; Cecon, V.S.; Claussen, J.C.; Gomes, C.L. Ion-selective electrodes based on laser-induced graphene as an alternative method for nitrite monitoring. Microchim. Acta 2023, 190, 43. [Google Scholar] [CrossRef] [PubMed]
- Capece, A.; Pietrafesa, R.; Siesto, G.; Romano, P. Biotechnological Approach Based on Selected Saccharomyces cerevisiae Starters for Reducing the Use of Sulfur Dioxide in Wine. Microorganisms 2020, 8, 738. [Google Scholar] [CrossRef] [PubMed]
- Mischek, D.; Krapfenbauer-Cermak, C. Exposure assessment of food preservatives (sulphites, benzoic and sorbic acid) in Austria. Food Addit. Contam. Part A 2012, 29, 371–382. [Google Scholar] [CrossRef]
- D’Amore, T.; Di Taranto, A.; Berardi, G.; Vita, V.; Marchesani, G.; Chiaravalle, A.E.; Iammarino, M. Sulfites in meat: Occurrence, activity, toxicity, regulation, and detection. A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2701–2720. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Lively, J.A. Determination of sulfite and antimicrobial residue in imported shrimp to the USA. Aquac. Rep. 2020, 18, 100529. [Google Scholar] [CrossRef]
- Vally, H.; Misso, N.L. Adverse reactions to the sulphite additives. Gastroenterol. Hepatol. Bed Bench 2012, 5, 16–23. [Google Scholar] [PubMed]
- Mani-López, E.; Palou, E.; López-Malo, A. Preservatives: Classifications and Analysis. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 497–504. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Marei, S.A.; Badr, I.H.; Arida, H.A. Flow injection analysis of sulfite ion with a potentiometric titanium phosphate–epoxy based membrane sensor. Talanta 2001, 54, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Nezamzadeh-Ejhieh, A.; Mirzaeyan, E. Hexadecylpyridinium surfactant modified zeolite A as an active component of a polymeric membrane sulfite selective electrode. Mater. Sci. Eng. C 2013, 33, 4751–4758. [Google Scholar] [CrossRef] [PubMed]
- Isaac, A.; Davis, J.; Livingstone, C.; Wain, A.J.; Compton, R.G. Electroanalytical methods for the determination of sulfite in food and beverages. TrAC Trends Anal. Chem. 2006, 25, 589–598. [Google Scholar] [CrossRef]
- Jeon, S.; Yeo, H.; Jeong, H.; Min Oh, J.; Chun Nam, K. Polymeric ISE for Hydrogen Sulfite Based on Bis-Urea Calix[4]diquinones as Neutral Lipophilic Ionophores. Electroanalysis 2003, 15, 872–877. [Google Scholar] [CrossRef]
- Abd-Rabboh, H.S.M.; Amr, A.E.-G.E.; Kamel, A.H.; Al-Omar, M.A.; Sayed, A.Y.A. Integrated all-solid-state sulfite sensors modified with two different ion-to-electron transducers: Rapid assessment of sulfite in beverages. RSC Adv. 2021, 11, 3783–3791. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.M.; H. Kamel, A.; Amr, A.E.-G.E.; Abd-Rabboh, H.S.M.; Al-Omar, M.A.; Elsayed, E.A. A New Validated Potentiometric Method for Sulfite Assay in Beverages Using Cobalt(II) Phthalocyanine as a Sensory Recognition Element. Molecules 2020, 25, 3076. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, V.; Ghaznavi, S.A.; Paschke, R. Environmental Goitrogens. In Encyclopedia of Endocrine Diseases, 2nd ed.; Huhtaniemi, I., Martini, L., Eds.; Academic Press: Oxford, UK, 2018; pp. 506–511. [Google Scholar] [CrossRef]
- Cuartero, M.; Amorim, C.G.; Araújo, A.N.; Ortuño, J.A.; Montenegro, M.C. A SO2-selective electrode based on a Zn-porphyrin for wine analysis. Anal. Chim. Acta 2013, 787, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Rashitova, K.; Kirsanov, D.; Voznesenskiy, M.; Osmolovskaya, O. PVC plasticized membranes modified with Fe3O4 nanoparticles for potentiometric sensing of sulfate. Surf. Interfaces 2024, 48, 104326. [Google Scholar] [CrossRef]
- Brown, G.M.; Gu, B. The Chemistry of Perchlorate in the Environment. In Perchlorate: Environmental Occurrence, Interactions and Treatment; Gu, B., Coates, J.D., Eds.; Springer: Boston, MA, USA, 2006; pp. 17–47. [Google Scholar] [CrossRef]
- Srinivasan, A.; Viraraghavan, T. Perchlorate: Health effects and technologies for its removal from water resources. Int. J. Environ. Res. Public Health 2009, 6, 1418–1442. [Google Scholar] [CrossRef]
- Kumarathilaka, P.; Oze, C.; Indraratne, S.P.; Vithanage, M. Perchlorate as an emerging contaminant in soil, water and food. Chemosphere 2016, 150, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, P.K.; Martinelango, P.K.; Jackson, W.A.; Anderson, T.A.; Tian, K.; Tock, R.W.; Rajagopalan, S. The Origin of Naturally Occurring Perchlorate: The Role of Atmospheric Processes. Environ. Sci. Technol. 2005, 39, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafiz, M.; Yehia, M.; Mostafa, H.E.; Wafy, T. Self-catalyzed nanoscale ammonium perchlorate for advanced composite solid rocket propellant. Nano Express 2021, 2, 030008. [Google Scholar] [CrossRef]
- Urbansky, E.T.; Brown, S.K.; Magnuson, M.L.; Kelty, C.A. Perchlorate levels in samples of sodium nitrate fertilizer derived from Chilean caliche. Environ. Pollut. 2001, 112, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, Z.; Qiu, H.; Lu, S. Occurrence of perchlorate, chlorate and bromate in drinking water in Shenzhen and related human exposure risks. Environ. Adv. 2022, 8, 100205. [Google Scholar] [CrossRef]
- Asami, M.; Yoshida, N.; Kosaka, K.; Ohno, K.; Matsui, Y. Contribution of tap water to chlorate and perchlorate intake: A market basket study. Sci. Total Environ. 2013, 463–464, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Maffini, M.V.; Trasande, L.; Neltner, T.G. Perchlorate and Diet: Human Exposures, Risks, and Mitigation Strategies. Curr. Environ. Health Rep. 2016, 3, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Soldin, O.P.; Braverman, L.E.; Lamm, S.H. Perchlorate clinical pharmacology and human health: A review. Ther. Drug Monit. 2001, 23, 316–331. [Google Scholar] [CrossRef] [PubMed]
- Brent, G.A. The impact of perchlorate exposure in early pregnancy: Is it safe to drink the water? J. Clin. Endocrinol. Metab. 2010, 95, 3154–3157. [Google Scholar] [CrossRef] [PubMed]
- COMMISSION REGULATION (EU) No 10/2011; Plastic Materials and Articles Intended to Come into Contact with Food. Official Journal of the European Union: Luxembourg, 2011.
- Calderón, R.; Godoy, F.; Escudey, M.; Palma, P. A review of perchlorate (ClO4−) occurrence in fruits and vegetables. Environ. Monit. Assess. 2017, 189, 82. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xiao, M.; Xiao, Q.; Chen, Y.; Guo, Y.; Sun, J.; Li, R.; Li, C.; Zhu, Z.; Qiu, H.; et al. Perchlorate and chlorate in breast milk, infant formulas, baby supplementary food and the implications for infant exposure. Environ. Int. 2022, 158, 106939. [Google Scholar] [CrossRef] [PubMed]
- Huertas-Pérez, J.F.; Mottier, P.; Baslé, Q.; Tan, S.Y.; Kopeć-Durska, M.; Zawada, P.; Burton, A.; Griffin, A.; Sánchez-Calderón, M.G. Chlorate and perchlorate—LC-MS/MS analytical method validation in a broad range of food commodities. Microchem. J. 2022, 177, 107281. [Google Scholar] [CrossRef]
- Sánchez-Pedreño, C.; Ortuño, J.n.A.; Hernández, J. Perchlorate-selective polymeric membrane electrode based on a gold(I) complex: Application to water and urine analysis. Anal. Chim. Acta 2000, 415, 159–164. [Google Scholar] [CrossRef]
- Rezaei, B.; Meghdadi, S.; Bagherpour, S. Perchlorate-selective polymeric membrane electrode based on bis(dibenzoylmethanato)cobalt(II) complex as a neutral carrier. J. Hazard. Mater. 2009, 161, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Itterheimová, P.; Bobacka, J.; Šindelář, V.; Lubal, P. Perchlorate Solid-Contact Ion-Selective Electrode Based on Dodecabenzylbambus [6] uril. Chemosensors 2022, 10, 115. [Google Scholar] [CrossRef]
- Ertürün, H.E.K.; Özel, A.D.; Ayanoğlu, M.N.; Şahin, Ö.; Yılmaz, M. A calix [4]arene derivative-doped perchlorate-selective membrane electrodes with/without multi-walled carbon nanotubes. Ionics 2017, 23, 917–927. [Google Scholar] [CrossRef]
- Onder, A.; Topcu, C.; Coldur, F.J.C. Construction of a novel highly selective potentiometric perchlorate sensor based on neocuproine–Cu(II) complex formed in situ during the conditioning period. Chemija 2018, 29. [Google Scholar] [CrossRef]
- Wang, P.-Y.; Shah, S.I.; Huang, C.P. A polymeric membrane electrode for the detection of perchlorate in water at the sub-micro-molar level. Anal. Methods 2013, 5, 3530–3537. [Google Scholar] [CrossRef]
- Leoterio, D.M.S.; Paim, A.P.S.; Belian, M.F.; Galembeck, A.; Lavorante, A.F.; Pinto, E.; Amorim, C.G.; Araújo, A.N.; Montenegro, M.C.B.S.M. Potentiometric perchlorate determination at nanomolar concentrations in vegetables. Food Chem. 2017, 227, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, İ.B.; Chłodowska, A.; Olejnik, M. Ionophore Toxicity in Animals: A Review of Clinical and Molecular Aspects. Int. J. Mol. Sci. 2023, 24, 1696. [Google Scholar] [CrossRef] [PubMed]
- Bühlmann, P.; Umezawa, Y.; Rondinini, S.; Vertova, A.; Pigliucci, A.; Bertesago, L. Lifetime of Ion-Selective Electrodes Based on Charged Ionophores. Anal. Chem. 2000, 72, 1843–1852. [Google Scholar] [CrossRef]
- Ruiz-Gonzalez, A.; Huang, J.; Xun, C.; Chhabra, R.; Lee, R.; Yizhong, H.; Davenport, A.; Li, B.; Palgrave, R.; Choy, K.L. Ultrasensitive and miniaturized ion sensors using ionically imprinted nanostructured films. Appl. Mater. Today 2022, 29, 101600. [Google Scholar] [CrossRef]
- Kusumkar, V.V.; Galamboš, M.; Viglašová, E.; Daňo, M.; Šmelková, J. Ion-Imprinted Polymers: Synthesis, Characterization, and Adsorption of Radionuclides. Materials 2021, 14, 1083. [Google Scholar] [CrossRef]
- Simões da Costa, A.M.; Delgadillo, I.; Rudnitskaya, A. Detection of copper, lead, cadmium and iron in wine using electronic tongue sensor system. Talanta 2014, 129, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Abbas, M.N.; Radwan, A.L.A.; Valle, M.d. Potentiometric Electronic Tongue to Resolve Mixtures of Sulfide and Perchlorate Anions. Sensors 2011, 11, 3214–3226. [Google Scholar] [CrossRef]
- Molinara, M.; Cancelliere, R.; Di Tinno, A.; Ferrigno, L.; Shuba, M.; Kuzhir, P.; Maffucci, A.; Micheli, L. A Deep Learning Approach to Organic Pollutants Classification Using Voltammetry. Sensors 2022, 22, 8032. [Google Scholar] [CrossRef] [PubMed]
- Popescu, S.M.; Mansoor, S.; Wani, O.A.; Kumar, S.S.; Sharma, V.; Sharma, A.; Arya, V.M.; Kirkham, M.B.; Hou, D.; Bolan, N.; et al. Artificial intelligence and IoT driven technologies for environmental pollution monitoring and management. Front. Environ. Sci. 2024, 12, 1336088. [Google Scholar] [CrossRef]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Mu, R.; Bu, N.; Pang, J.; Wang, L.; Zhang, Y. Recent Trends of Microfluidics in Food Science and Technology: Fabrications and Applications. Foods 2022, 11, 3727. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gonzalez, A.; Kempson, H.; Haseloff, J. A Simple Reversed Iontophoresis-Based Sensor to Enable In Vivo Multiplexed Measurement of Plant Biomarkers Using Screen-Printed Electrodes. Sensors 2023, 23, 780. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.I.G.; McCullagh, J.; Guy, R.H.; Compton, R.G. Reverse Iontophoretic Extraction of Metabolites from Living Plants and their Identification by Ion-chromatography Coupled to High Resolution Mass Spectrometry. Phytochem. Anal. 2017, 28, 195–201. [Google Scholar] [CrossRef] [PubMed]
- El Hosry, L.; Sok, N.; Richa, R.; Al Mashtoub, L.; Cayot, P.; Bou-Maroun, E. Sample Preparation and Analytical Techniques in the Determination of Trace Elements in Food: A Review. Foods 2023, 12, 895. [Google Scholar] [CrossRef]


| Element | Maximum Concentration (mg/kg) | ||
|---|---|---|---|
| Vegetables | Fish | Meat | |
| Cadmium | 0.05 | 0.05 | 0.05 |
| Lead | 0.1 | 0.3 | 0.1 |
| Mercury | 0.1 | 0.5 | - |
| Arsenic | 0.3 | 0.3 | 0.3 |
| Material | Ion | Sensitivity (mV/Log[Pb2+]) | LOD | pH Range | Recovery (%) | Selectivity | Sample | Ref. |
|---|---|---|---|---|---|---|---|---|
| Lead ionophore IV, /Ag@PANI solid contact | Pb2+ | 29.1 | 0.6 nM | 3.0–9.0 | 97–109 | Drinking water | [61] | |
| Synthesis of 2-(2-formylphenoxy)acetic acid | Pb2+ | 27.7 | 2.9 µM | 2.0–12.0 | 97–98 | Drinking water | [62] | |
| N,N′-tetrabutyldipicolinamide | Pb2+, Hg2+, Cd2+ | 25.6 | 70 nM | - | - | Fish | [63] | |
| (E)-2-(1-(4-(3-(4-chlorophenyl)ureido)phenyl)ethylidene)hydrazinecarbothioamide | Pb2+ | 28.0 | 1.7 µM | 5.0–11.0 | 94–98.5 | 1.2 2.0 | Drinking water | [64] |
| Polyaniline-zirconium (IV) iodate | Pb2+ | 29.4 | 3.3 nM | 2.5–6.5 | 90–97 | Drinking water | [65] | |
| Poly(aniline-co-2-hydroxy-5-sulfonic aniline) | Pb2+ | 29.3 | 16 pM | 3.5–7.0 | - | Drinking water (pH = 7.3), green gram (pH = 4.1) | [66] | |
| 2,2′:6′,6″- terpyridine (terpy)-based Pb2+ -imprinted polymer | Pb2+ | 28.6 | 0.3 nM | 4.5–8.0 | 98–102 | Drinking water | [67] | |
| 1,2-Bis(N’- Benzoylthioureido)benzene/reduced graphene oxide | Pb2+ | 30.4 | 25 nM | 4.0–8.0 | 84–101 | Milk | [68] |
| Material | Ion | Sensitivity (mV/Log[As]) | LOD | pH Range | Recovery (%) | Selectivity | Sample | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ag3AsO4 | AsO43− | 19.0 | 1 µM | 6.0–10.0 | - | Beer (pH = 10.2 in buffer) | [84] | |
| 4H-1-benzopyran-3-carboxaldehyde, 4-oxo-, 3-[2-(2,4-dinitrophenyl)hydrazone] | AsO2− | 61.6 | 80 nM | 3.0–9.0 | 96–102 | Drinking water (pH = 5.5 in buffer) | [85] | |
| Methacrylic acid | As3+ | 20.5 | 0.5 µM | 4.0–8.0 | 96–107 | Drinking water (pH = 6.5 in buffer) | [86] | |
| Clinoptilolite | HAsO42− | −28.3 | 30 nM | 7.0–11.0 | 101–105 | Drinking water | [87] |
| Material | Ion | Sensitivity (mV Log[Hg2+]) | LOD | pH Range | Recovery (%) | Selectivity | Sample | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1-methyl-2-butylthioimidazolium bis(trifluoromethane sulphonyl)imide | Hg2+ | 6.7 | 41 pM | 2.0–3.0 | - | Seafood (pH = 3 using NaOH) | [102] | |
| 1,3-bis [2-(N-morpholino)acetamidothiophenoxy]propane | Hg2+ | 30.0 | 5 nM | 2.2–4.5 | 96–104 | Drinking water | [103] | |
| Zirconium antimonate | Hg2+ | 30.0 | 50 nM | 2.5–8.5 | 98–99 | Tap water, fish | [104] | |
| 4-(2- Thiazolylazo) resorcinol | Hg2+ | 29.7 | 2 nM | 3.0–4.5 | 98–101 | Fish, shrimp (pH adjusted to 3) | [105] | |
| Multi-walled carbon nanotube (MWCNT)-grafted 2, 6-bis [2-(amino methyl)phenol]pyridine | Hg2+ | 29.8 | 0.8 nM | 3.0–4.5 | 88–112 | Drinking water (pH = 4.2) | [106] |
| Material | Ion | Sensitivity (mV Log[Cd2+]) | LOD | pH Range | Recovery (%) | Selectivity | Sample | Ref. |
|---|---|---|---|---|---|---|---|---|
| Salophen | Cd2+ | 29.2 | 16 nM | 2.5–7.5 | 99–102 | 1.9 | Drinking water | [114] |
| N,N-(4-methyl-1,2-phenylene)diquinoline-2-carboxamide | Cd2+ | 0.8 µM | 4.0–9.0 | 97–101 | Drinking water | [115] | ||
| Benzyl bis(carbohydrazone) | Cd2+ | 29.7 | 32 nM | 2.0–9.0 | - | 2.1 | Chocolate | [116] |
| 1, 2-bis(quinoline-2-Carboxamido)-4-chlorobenzene | Cd2+ | 30.3 | 0.8 µM | 2.4–9.0 | 98–103 | 2 | Drinking water | [117] |
| N,N,N′,N′-Tetrabutyl-3,6-dioxaoctanedi(thioamide) | Cd2+ | 28.6 | 1 µM | - | 94–100 | 2.5 3.7 4.1 4.1 | Drinking water | [118] |
| (E)-2-benzylidenehydrazinecarbothioamide | Cd2+ | 22.9 | 1.8 µM | 5.0–10.0 | 95–97 | 2.5 2.6 | Drinking water | [119] |
| Methyl methacrylate | Cd2+ | 29.9 | 100 nM | 4.0–7.0 | 92 | 2 | Drinking water | [120] |
| Material | Ion | Sensitivity (mV Log[F−]) | LOD | pH Range | Recovery (%) | Selectivity | Sample | Ref. |
|---|---|---|---|---|---|---|---|---|
| N, N’-Ethylene-bis(salicylideneaminato) nickel(II) | F− | −57.2 | 3.6 µM | 2.1–9.1 | - | Drinking water, tea | [140] | |
| ThermoOrion model 96-09 | F− | - | - | - | - | - | Chicken (pH = 5.5 in buffer) | [141] |
| ThermoOrion model 96-09 | F− | −60.0 | - | - | 81–105 | - | Seafood (pH = 7.2 using HCl) | [142] |
| ThermoOrion model 96-09 | F− | −57.8 | - | - | 99–103 | - | Potato chips | [143] |
| ThermoOrion model 96-09 | F− | - | 27 µM | - | 81–89 | - | Cocoa powder (pH = 5.2 in buffer) | [144] |
| Fluoride ion-selective electrode (FISE) DC219 from Mettler Toledo | F− | −57.5 | 500 nM | - | - | Pomegranate, Mint, Green tea (pH = 5.3 using buffer) | [145] | |
| Thermo Scientific, model 9609BNWP | F− | −54.7 | 160 nM | 5–7 | 86–110 | - | Soft drinks, juice, tea (pH ~6 using buffer) | [146] |
| Tetrachloro-substituted organoantimony(V) compound | F− | −59.5 | 5 µM | 3 | - | Tap water | [147] | |
| Fluoride ion-selective electrode (FISE) DC219 from Mettler Toledo | F− | −57.9 | 100 µM | - | 84–101 | - | Fish, seafood, vegetables (pH = 7.0 using NaOH) | [148] |
| Ion-selective electrode (Model 6.0502.150, Metrohm) | F− | - | 40 nmol g−1 | - | 92 | - | Medicinal plants (pH = 5.5 using NaOH) | [149] |
| pH/ion meter devices (Metrohm types 713 and 744) | F− | - | - | - | - | - | Anchovies (pH = 5 using NaOH) | [150] |
| Digital Ion-meter (Philips PW 9414) | F− | −55.0 | 5.6 µM | 3.5–8.0 | - | - | Cheese | [151] |
| Material | Ion | Sensitivity (mV Log[HPO4−]) | LOD | pH Range | Recovery (%) | Selectivity | Sample | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ba3PO4, Cu2S and Ag2S | HPO4− | −57.0 | 1 µM | 7.0–9.0 | - | Beef, beans, garlic, dried apricots | [165] | |
| Surface-modified tungsten | HPO42− | −36.9 | 1 µM | 7.0–10.0 | 95–98 | Juice, Coca-Cola, milk, tap water | [166] | |
| Copper nanoparticles | HPO42− | −27.8 | 1 µM | 6.0–11.0 | 97–102 | Drinking water (pH = 7.6) | [167] | |
| Nicotinamide | HPO4− | −53.3 | 0.9 µM | - | - | Water | [168] | |
| Acyl-Hydrazine Functionalized Calix [4]arene | HPO42− | −29.3 | 10 µM | 6.5–9.5 | - | Water | [170] |
| Material | Ion | Sensitivity (mV Log[NOx−]) | LOD | pH Range | Recovery (%) | Selectivity | Sample | Ref. |
|---|---|---|---|---|---|---|---|---|
| Orion 93-07 nitrate ion-selective electrode | NO3− | - | - | - | 100–110 | - | Carrot, wild endive, chicory, spinach, parsley, and celery | [190] |
| Orion 93-46 nitrite ion-selective electrode | NO2− | −54.7 | 10 µM | 4.0–5.5 | - | Meat products (pH = 5.5 in buffer) | [191] | |
| FKL cation exchange membrane | NO2− | −57.1 | 0.55 µM | 4.0–6.0 | - | Drinking water (pH = 5.0 by exchange matrix) | [192] | |
| Silver (I) bisdiethyldithiocarbamate | NO2− | −56.3 | 6.7 µM | 4.0–9.0 | 92–97 | Cherry, apricot, drinking water | [193] | |
| Laser-induced graphene | NO2− | −59.5 | 7.2 µM | - | - | Meat products | [194] |
| Material | Ion | Sensitivity (mV Log[SOx−]) | LOD | pH Range | Recovery (%) | Selectivity | Sample | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cobalt(II) Phthalocyanine | SO32− | −29.8 | 1.1 µM | 5.0–7.2 | - | Malt beverage, juice (pH = 6.0 in buffer) | [205] | |
| Cobalt(II) Phthalocyanine | SO32− | −27.4 | 1 µM | 5.0–7.0 | - | - | Beer, malt beverage, vinegar, sugar lumps, grape drink (pH = 5.0 in buffer) | [206] |
| 5,10,15,20-tetraphenyl(porphyrin)zinc(II) | SO2− | −59.5 | 3.7 µM | - | 95–104 | - | Wine (pH = 1.6 in buffer) | [208] |
| Fe3O4 nanoparticles | SO42− | −29.7 | 3.1 µM | - | - | Drinking water | [209] |
| Material | Ion | Sensitivity (mV Log[ClO4−]) | LOD | pH Range | Recovery (%) | Selectivity | Sample | Ref. |
|---|---|---|---|---|---|---|---|---|
| Neocuproine | ClO4− | −53.0 | 0.1 µM | 3.0–11.0 | - | Drinking water | [229] | |
| bis(dibenzoylmethanato) nickel(II) | ClO4− | −58.5 | 0.7 µM | 3.0–9.0 | 92–128 | Drinking water | [230] | |
| Bisnaphthalimidopropyl 4,4′-diaminodiphenyl methane | ClO4− | - | 0.3 nM | - | 95–110 | Potato, lettuce, tomato, red pepper, beans | [231] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Gonzalez, A. Ion-Selective Electrodes in the Food Industry: Development Trends in the Potentiometric Determination of Ionic Pollutants. Electrochem 2024, 5, 178-212. https://doi.org/10.3390/electrochem5020012
Ruiz-Gonzalez A. Ion-Selective Electrodes in the Food Industry: Development Trends in the Potentiometric Determination of Ionic Pollutants. Electrochem. 2024; 5(2):178-212. https://doi.org/10.3390/electrochem5020012
Chicago/Turabian StyleRuiz-Gonzalez, Antonio. 2024. "Ion-Selective Electrodes in the Food Industry: Development Trends in the Potentiometric Determination of Ionic Pollutants" Electrochem 5, no. 2: 178-212. https://doi.org/10.3390/electrochem5020012
APA StyleRuiz-Gonzalez, A. (2024). Ion-Selective Electrodes in the Food Industry: Development Trends in the Potentiometric Determination of Ionic Pollutants. Electrochem, 5(2), 178-212. https://doi.org/10.3390/electrochem5020012






