The Effect of Bulk Modification of the MF-4SK Membrane with Phosphorylated Hyper-Branched Dendrimer Bolthorn H20 on the Mechanisms of Electroconvection/Dissociation of Water and Specific Selectivity to Divalent Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. SPTFE Membrane
2.2. P-H20 Synthesis
2.3. Mechanical Properties
2.4. Ion-Exchange Capacity and Water Uptake
2.5. Conductivity and Diffusion Permeability Measurememnt
2.6. Current–Voltage Curves and Specific Permselectivity Measurement
Current–Voltage Curves of Bipolar Membranes
3. Results and Discussion
3.1. Dopant Distribution, Mechanical and Physicochemical Properties
3.2. Current–Voltage Curves and Mechanisms of Ion Transport
3.3. Prospects for Application
3.3.1. Monovalent/Divalent Selectivity
3.3.2. Bipolar Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of Ion Exchange Membranes: A Review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Helfferich, F.G. Ion Exchange; McGraw Hill Book Company, Inc.: New York, NY, USA, 1962; ISBN 0-486-68784-8. [Google Scholar]
- Zhang, Y.; Paepen, S.; Pinoy, L.; Meesschaert, B.; Van Der Bruggen, B. Selectrodialysis: Fractionation of Divalent Ions from Monovalent Ions in a Novel Electrodialysis Stack. Sep. Purif. Technol. 2012, 88, 191–201. [Google Scholar] [CrossRef]
- Galama, A.H.; Daubaras, G.; Burheim, O.S.; Rijnaarts, H.H.M.; Post, J.W. Fractioning Electrodialysis: A Current Induced Ion Exchange Process. Electrochim. Acta 2014, 136, 257–265. [Google Scholar] [CrossRef]
- Ge, L.; Wu, B.; Li, Q.; Wang, Y.; Yu, D.; Wu, L.; Pan, J.; Miao, J.; Xu, T. Electrodialysis with Nanofiltration Membrane (EDNF) for High-Efficiency Cations Fractionation. J. Membr. Sci. 2016, 498, 192–200. [Google Scholar] [CrossRef]
- Khoiruddin; Ariono, D.; Subagjo; Wenten, I.G.; Ariono, D.; Gede Wenten, I.; Khoiruddin; Ariono, D.; Subagjo; Wenten, I.G.; et al. Surface Modification of Ion-Exchange Membranes: Methods, Characteristics, and Performance. J. Appl. Polym. Sci. 2017, 134, 1–13. [Google Scholar] [CrossRef]
- Sata, T. Studies on Anion Exchange Membranes Having Permselectivity for Specific Anions in Electrodialysis—Effect of Hydrophilicity of Anion Exchange Membranes on Permselectivity of Anions. J. Membr. Sci. 2000, 167, 1–31. [Google Scholar] [CrossRef]
- Sata, T.T.; Sata, T.T.; Yang, W. Studies on Cation-Exchange Membranes Having Permselectivity between Cations in Electrodialysis. J. Membr. Sci. 2002, 206, 31–60. [Google Scholar] [CrossRef]
- Wang, M.; Gao, C. The State-of-The-Art of the Cation Exchange Membrane Having Monovalent Ion Selectivity—A Patent Review. Recent Pat. Chem. Eng. 2011, 4, 132–140. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion Exchange Membranes: New Developments and Applications. J. Membr. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Ge, L.; Wu, B.; Yu, D.; Mondal, A.N.; Hou, L.; Afsar, N.U.; Li, Q.; Xu, T.T.; Miao, J.; Xu, T.T. Monovalent Cation Perm-Selective Membranes (MCPMs): New Developments and Perspectives. Chin. J. Chem. Eng. 2017, 25, 1606–1615. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the Right Stuff: The Trade-off between Membrane Permeability and Selectivity. Science 2017, 356, 1138–1148. [Google Scholar] [CrossRef]
- Chen, Q.B.; Ren, H.; Tian, Z.; Sun, L.; Wang, J. Conversion and Pre-Concentration of SWRO Reject Brine into High Solubility Liquid Salts (HSLS) by Using Electrodialysis Metathesis. Sep. Purif. Technol. 2019, 213, 587–598. [Google Scholar] [CrossRef]
- Shaposhnik, V.A.; Kesore, K. An Early History of Electrodialysis with Permselective Membranes. J. Membr. Sci. 1997, 136, 35–39. [Google Scholar] [CrossRef]
- Culcasi, A.; Gurreri, L.; Zaffora, A.; Cosenza, A.; Tamburini, A.; Cipollina, A.; Micale, G. Ionic Shortcut Currents via Manifolds in Reverse Electrodialysis Stacks. Desalination 2020, 485, 114450. [Google Scholar] [CrossRef]
- Bazinet, L.; Ippersiel, D.; Montpetit, D.; Mahdavi, B.; Amiot, J.; Lamarche, F. Effect of Membrane Permselectivity on the Fouling of Cationic Membranes during Skim Milk Electroacidification. J. Membr. Sci. 2000, 174, 97–110. [Google Scholar] [CrossRef]
- White, N.; Misovich, M.; Alemayehu, E.; Yaroshchuk, A.; Bruening, M.L. Highly Selective Separations of Multivalent and Monovalent Cations in Electrodialysis through Nafion Membranes Coated with Polyelectrolyte Multilayers. Polymer 2015, 103, 478–485. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, J.; Ding, J.; Van der Bruggen, B.; Shen, J.; Gao, C. Electric-Pulse Layer-by-Layer Assembled of Anion Exchange Membrane with Enhanced Monovalent Selectivity. J. Membr. Sci. 2018, 548, 81–90. [Google Scholar] [CrossRef]
- Melnikov, S.; Bondarev, D.; Nosova, E.; Melnikova, E.; Zabolotskiy, V. Water Splitting and Transport of Ions in Electromembrane System with Bilayer Ion-Exchange Membrane. Membranes 2020, 10, 346. [Google Scholar] [CrossRef]
- Abdu, S.; Wessling, M.; Martí-Calatayud, M.-C.; Wong, J.E.; García-Gabaldón, M.; Wessling, M. Layer-by-Layer Modification of Cation Exchange Membranes Controls Ion Selectivity and Water Splitting. ACS Appl. Mater. Interfaces 2014, 6, 1843–1854. [Google Scholar] [CrossRef]
- White, N.; Misovich, M.; Yaroshchuk, A.; Bruening, M.L. Coating of Nafion Membranes with Polyelectrolyte Multilayers to Achieve High Monovalent/Divalent Cation Electrodialysis Selectivities. ACS Appl. Mater. Interfaces 2015, 7, 6620–6628. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.; Wijeratne, S.; Cheng, C.; Baker, G.L.; Bruening, M.L. Facilitated Ion Transport through Polyelectrolyte Multilayer Fi Lms Containing Metal-Binding Ligands. J. Membr. Sci. 2014, 459, 169–176. [Google Scholar] [CrossRef]
- Porozhnyy, M.V.; Shkirskaya, S.A.; Butylskii, D.Y.; Dotsenko, V.V.; Safronova, E.Y.; Yaroslavtsev, A.B.; Deabate, S.; Huguet, P.; Nikonenko, V.V. Physicochemical and Electrochemical Characterization of Nafion-Type Membranes with Embedded Silica Nanoparticles: Effect of Functionalization. Electrochim. Acta 2021, 370, 137689. [Google Scholar] [CrossRef]
- Patnaik, P.; Hossain, S.M.; Pal, S.; Sarkar, S.; Sharma, R.; Chatterjee, U. Controlling the Sub-Nano Ion Channels by Crosslinking in PVDF-Based Anion Exchange Membrane for Enhanced Mono/Bivalent Anion Permselectivity and Acid Reclamation. J. Membr. Sci. 2023, 688, 122105. [Google Scholar] [CrossRef]
- Pacini, A.; Nitti, A.; Vitale, M.; Pasini, D. Polylactic-Containing Hyperbranched Polymers through the CuAAC Polymerization of Aromatic AB2 Monomers. Int. J. Mol. Sci. 2023, 24, 7620. [Google Scholar] [CrossRef] [PubMed]
- Zabolotsky, V.; Utin, S.; Bespalov, A.; Strelkov, V. Modification of Asymmetric Bipolar Membranes by Functionalized Hyperbranched Polymers and Their Investigation during PH Correction of Diluted Electrolytes Solutions by Electrodialysis. J. Membr. Sci. 2015, 494, 188–195. [Google Scholar] [CrossRef]
- Hsu, W.Y.; Gierke, T.D. Ion Transport and Clustering in Nafion Perfluorinated Membranes. J. Membr. Sci. 1983, 13, 307–326. [Google Scholar] [CrossRef]
- Žagar, E.; Žigon, M. Aliphatic Hyperbranched Polyesters Based on 2,2-Bis(Methylol)Propionic Acid—Determination of Structure, Solution and Bulk Properties. Prog. Polym. Sci. 2011, 36, 53–88. [Google Scholar] [CrossRef]
- Li, Y.S.; Zhao, T.S.; Yang, W.W. Measurements of Water Uptake and Transport Properties in Anion-Exchange Membranes. Int. J. Hydrogen Energy 2010, 35, 5656–5665. [Google Scholar] [CrossRef]
- Karpenko, L.V.; Demina, O.A.; Dvorkina, G.A.; Parshikov, S.B.; Larchet, C.; Auclair, B.; Berezina, N.P. Comparative Study of Methods Used for the Determination of Electroconductivity of Ion-Exchange Membranes. Russ. J. Electrochem. 2001, 37, 287–293. [Google Scholar] [CrossRef]
- Berezina, N.P.; Kononenko, N.A.; Dyomina, O.A.; Gnusin, N.P. Characterization of Ion-Exchange Membrane Materials: Properties vs Structure. Adv. Colloid Interface Sci. 2008, 139, 3–28. [Google Scholar] [CrossRef]
- Falina, I.; Loza, N.; Loza, S.; Titskaya, E.; Romanyuk, N. Permselectivity of Cation Exchange Membranes Modified by Polyaniline. Membranes 2021, 11, 227. [Google Scholar] [CrossRef]
- Zabolotsky, V.I.; Nikonenko, V.V. Effect of Structural Membrane Inhomogeneity on Transport Properties. J. Membr. Sci. 1993, 79, 181–198. [Google Scholar] [CrossRef]
- Sharafan, M.V.; Zabolotsky, V.I. Study of Electric Mass Transfer Peculiarities in Electromembrane Systems by the Rotating Membrane Disk Method. Desalination 2014, 343, 194–197. [Google Scholar] [CrossRef]
- Zabolotskii, V.I.; Sharafan, M.V.; Sheldeshov, N.V.; Lovtsov, E.G. Electric Mass Transport of Sodium Chloride through Cation-Exchange Membrane MK-40 in Dilute Sodium Chloride Solutions: A Rotating Membrane Disk Study. Russ. J. Electrochem. 2008, 44, 141–146. [Google Scholar] [CrossRef]
- Zabolotskii, V.I.; Bugakov, V.V.; Sharafan, M.V.; Chermit, R.K. Transfer of Electrolyte Ions and Water Dissociation in Anion-Exchange Membranes under Intense Current Conditions. Russ. J. Electrochem. 2012, 48, 650–659. [Google Scholar] [CrossRef]
- Zabolotsky, V.I.; Achoh, A.R.; Lebedev, K.A.; Melnikov, S.S. Permselectivity of Bilayered Ion-Exchange Membranes in Ternary Electrolyte. J. Membr. Sci. 2020, 608, 118152. [Google Scholar] [CrossRef]
- Achoh, A.R.; Zabolotsky, V.I.; Lebedev, K.A.; Sharafan, M.V.; Yaroslavtsev, A.B. Electrochemical Properties and Selectivity of Bilayer Ion-Exchange Membranes in Ternary Solutions of Strong Electrolytes. Membr. Membr. Technol. 2021, 3, 52–71. [Google Scholar] [CrossRef]
- Kononenko, N.A.; Loza, N.V.; Shkirskaya, S.A.; Falina, I.V.; Khanukaeva, D.Y. Influence of Conditions of Polyaniline Synthesis in Perfluorinated Membrane on Electrotransport Properties and Surface Morphology of Composites. J. Solid State Electrochem. 2015, 19, 2623–2631. [Google Scholar] [CrossRef]
- Golubenko, D.V.; Yaroslavtsev, A.B. Effect of Current Density, Concentration of Ternary Electrolyte and Type of Cations on the Monovalent Ion Selectivity of Surface-Sulfonated Graft Anion-Exchange Membranes: Modelling and Experiment. J. Membr. Sci. 2021, 635, 119466. [Google Scholar] [CrossRef]
- Zhang, Z.; Qiao, C.; Zhang, J.; Zhang, W.; Yin, J.; Wu, Z. Synthesis of Unimolecular Micelles with Incorporated Hyperbranched Boltorn H30 Polyester Modified with Hyperbranched Helical Poly(Phenyl Isocyanide) Chains and Their Enantioselective Crystallization Performance. Macromol. Rapid Commun. 2017, 38, 1700315. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B.; Karavanova, Y.A.; Safronova, E.Y. Ionic Conductivity of Hybrid Membranes. Pet. Chem. 2011, 51, 473–479. [Google Scholar] [CrossRef]
- Chintapalli, M.; Timachova, K.; Olson, K.R.; Mecham, S.J.; Devaux, D.; Desimone, J.M.; Balsara, N.P. Relationship between Conductivity, Ion Diffusion, and Transference Number in Perfluoropolyether Electrolytes. Macromolecules 2016, 49, 3508–3515. [Google Scholar] [CrossRef]
- Kovalenko, A.V.; Nikonenko, V.V.; Chubyr, N.O.; Urtenov, M.K. Mathematical Modeling of Electrodialysis of a Dilute Solution with Accounting for Water Dissociation-Recombination Reactions. Desalination 2023, 550, 116398. [Google Scholar] [CrossRef]
- Mikhaylin, S.; Nikonenko, V.; Pismenskaya, N.; Pourcelly, G.; Choi, S.; Kwon, H.J.; Han, J.; Bazinet, L. How Physico-Chemical and Surface Properties of Cation-Exchange Membrane Affect Membrane Scaling and Electroconvective Vortices: Influence on Performance of Electrodialysis with Pulsed Electric Field. Desalination 2016, 393, 102–114. [Google Scholar] [CrossRef]
- Bondarev, D.; Melnikov, S.; Zabolotskiy, V. New Homogeneous and Bilayer Anion-Exchange Membranes Based on N,N-Diallyl-N,N-Dimethylammonium Chloride and Ethyl Methacrylate Copolymer. J. Membr. Sci. 2023, 675, 121510. [Google Scholar] [CrossRef]
- Dukhin, S.S. Electrokinetic Phenomena of the Second Kind and Their Applications. Adv. Colloid Interface Sci. 1991, 35, 173–196. [Google Scholar] [CrossRef]
- Nikonenko, V.V.; Mareev, S.A.; Pis’menskaya, N.D.; Uzdenova, A.M.; Kovalenko, A.V.; Urtenov, M.K.; Pourcelly, G. Effect of Electroconvection and Its Use in Intensifying the Mass Transfer in Electrodialysis (Review). Russ. J. Electrochem. 2017, 53, 1122–1144. [Google Scholar] [CrossRef]
- Akberova, E.M.; Vasil’eva, V.I.; Zabolotsky, V.I.; Novak, L. Effect of the Sulfocation-Exchanger Dispersity on the Surface Morphology, Microrelief of Heterogeneous Membranes and Development of Electroconvection in Intense Current Modes. J. Membr. Sci. 2018, 566, 317–328. [Google Scholar] [CrossRef]
- Rubinstein, I. Electroconvection at an Electrically Inhomogeneous Permselective Interface. Phys. Fluids A 1991, 3, 2301–2309. [Google Scholar] [CrossRef]
- Simons, R. Strong Electric Field Effects on Proton Transfer between Membrane Bound Amines and Water. Nature 1979, 280, 824–826. [Google Scholar] [CrossRef]
- Zabolotskii, V.I.; Shel, N.V.; Gnusin, N.P. Dissociation of Water Molecules in Systems with Ion-Exchange Membranes. Russ. Chem. Rev. 1988, 57, 801–808. [Google Scholar] [CrossRef]
- Mishchuk, N.A.; Takhistov, P.V. Electroosmosis of the Second Kind. Colloids Surf. A Physicochem. Eng. Asp. 1995, 95, 119–131. [Google Scholar] [CrossRef]
- Balster, J.; Yildirim, M.H.; Stamatialis, D.F.; Ibanez, R.; Lammertink, R.G.H.; Jordan, V.; Wessling, M. Morphology and Microtopology of Cation-Exchange Polymers and the Origin of the Overlimiting Current. J. Phys. Chem. B 2007, 111, 2152–2165. [Google Scholar] [CrossRef] [PubMed]
- Nikonenko, V.V.; Kovalenko, A.V.; Urtenov, M.K.; Pismenskaya, N.D.; Han, J.; Sistat, P.; Pourcelly, G. Desalination at Overlimiting Currents: State-of-the-Art and Perspectives. Desalination 2014, 342, 85–106. [Google Scholar] [CrossRef]
- Aritomi, T.; van den Boomgaard, T.; Strathmann, H. Current-Voltage Curve of a Bipolar Membrane at High Current Density. Desalination 1996, 104, 13–18. [Google Scholar] [CrossRef]
- Balster, J.; Stamatialis, D.F.; Wessling, M. Electro-Catalytic Membrane Reactors and the Development of Bipolar Membrane Technology. Chem. Eng. Process. Process Intensif. 2004, 43, 1115–1127. [Google Scholar] [CrossRef]
- Zabolotskii, V.I.; Nikonenko, V.V.; Korzhenko, N.M.; Seidov, R.R.; Urtenov, M.K. Mass Transfer of Salt Ions in an Electromembrane System with Violated Electroneutrality in the Diffusion Layer: The Effect of a Heterolytic Dissociation of Water. Russ. J. Electrochem. 2002, 38, 810–818. [Google Scholar] [CrossRef]
- Melnikov, S.S.; Nosova, E.N.; Melnikova, E.D.; Zabolotsky, V.I. Reactive Separation of Inorganic and Organic Ions in Electrodialysis with Bilayer Membranes. Sep. Purif. Technol. 2021, 268, 118561. [Google Scholar] [CrossRef]
- Sharafan, M.V.; Zabolotskii, V.I.; Bugakov, V.V. Electric Mass Transport through Homogeneous and Surface-Modified Heterogeneous Ion-Exchange Membranes at a Rotating Membrane Disk. Russ. J. Electrochem. 2009, 45, 1162–1169. [Google Scholar] [CrossRef]
- Belashova, E.D.; Melnik, N.; Pismenskaya, N.D.; Shevtsova, K.; Nebavsky, A.; Lebedev, K.A.; Nikonenko, V.V. Overlimiting Mass Transfer through Cation-Exchange Membranes Modified by Nafion Film and Carbon Nanotubes. Electrochim. Acta 2012, 59, 412–423. [Google Scholar] [CrossRef]
- Gil, V.; Porozhnyy, M.; Rybalkina, O.; Butylskii, D.; Pismenskaya, N. The Development of Electroconvection at the Surface of a Heterogeneous Cation-Exchange Membrane Modified with Perfluorosulfonic Acid Polymer Film Containing Titanium Oxide. Membranes 2020, 10, 125. [Google Scholar] [CrossRef]
- Zyryanova, S.; Mareev, S.; Gil, V.; Korzhova, E.; Pismenskaya, N.; Sarapulova, V.; Rybalkina, O.; Boyko, E.; Larchet, C.; Dammak, L.; et al. How Electrical Heterogeneity Parameters of Ion-Exchange Membrane Surface Affect the Mass Transfer and Water Splitting Rate in Electrodialysis. Int. J. Mol. Sci. 2020, 21, 973. [Google Scholar] [CrossRef] [PubMed]
- Titorova, V.D.; Moroz, I.A.; Mareev, S.A.; Pismenskaya, N.D.; Sabbatovskii, K.G.; Wang, Y.; Xu, T.; Nikonenko, V.V. How Bulk and Surface Properties of Sulfonated Cation-Exchange Membranes Response to Their Exposure to Electric Current during Electrodialysis of a Ca2+ Containing Solution. J. Membr. Sci. 2022, 644, 120149. [Google Scholar] [CrossRef]
- Nikonenko, V.V.; Zabolotskii, V.I.; Lebedev, K.A. Model for the Competitive Transport of Ions through Ion Exchange Membranes with a Modified Surface. Russ. J. Electrochem. 1996, 32, 234–236. [Google Scholar]
- Kovalev, N.V.; Karpenko, T.V.; Sheldeshov, N.V.; Zabolotskii, V.I. Ion Transport through a Modified Heterogeneous Bipolar Membrane and Electromembrane Recovery of Sulfuric Acid and Sodium Hydroxide from a Sodium Sulfate Solution. Membr. Membr. Technol. 2020, 2, 391–398. [Google Scholar] [CrossRef]
- Mareev, S.A.; Evdochenko, E.; Wessling, M.; Kozaderova, O.A.; Niftaliev, S.I.; Pismenskaya, N.D.; Nikonenko, V.V. A Comprehensive Mathematical Model of Water Splitting in Bipolar Membranes: Impact of the Spatial Distribution of Fixed Charges and Catalyst at Bipolar Junction. J. Membr. Sci. 2020, 603, 118010. [Google Scholar] [CrossRef]
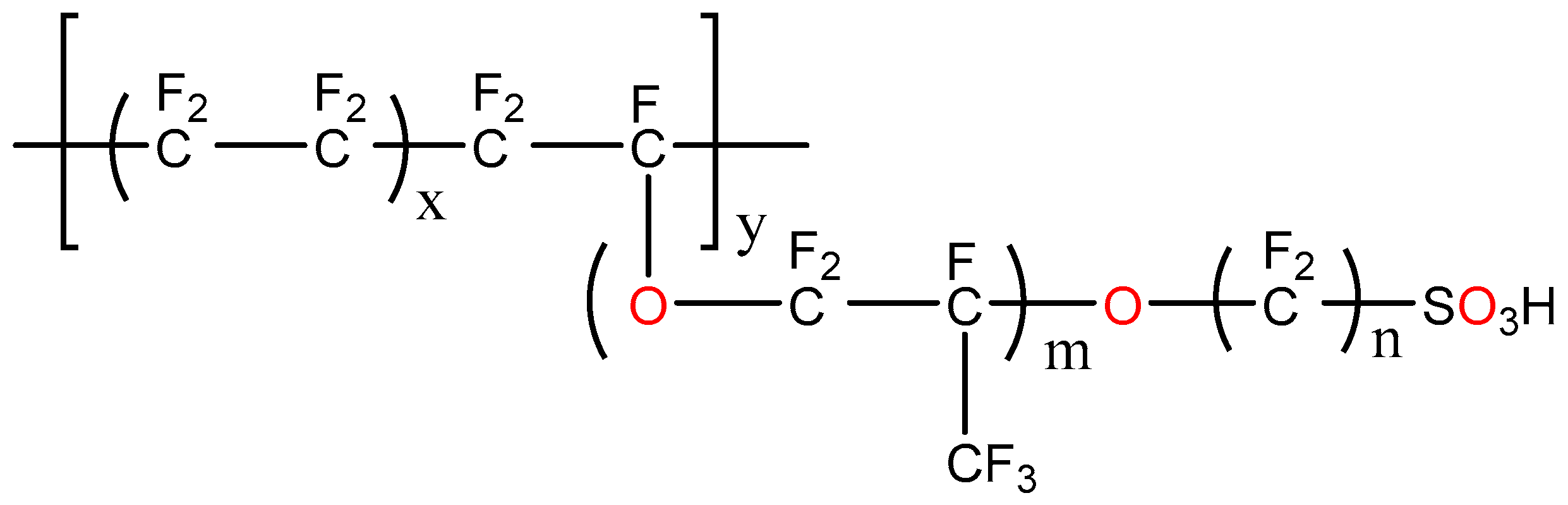


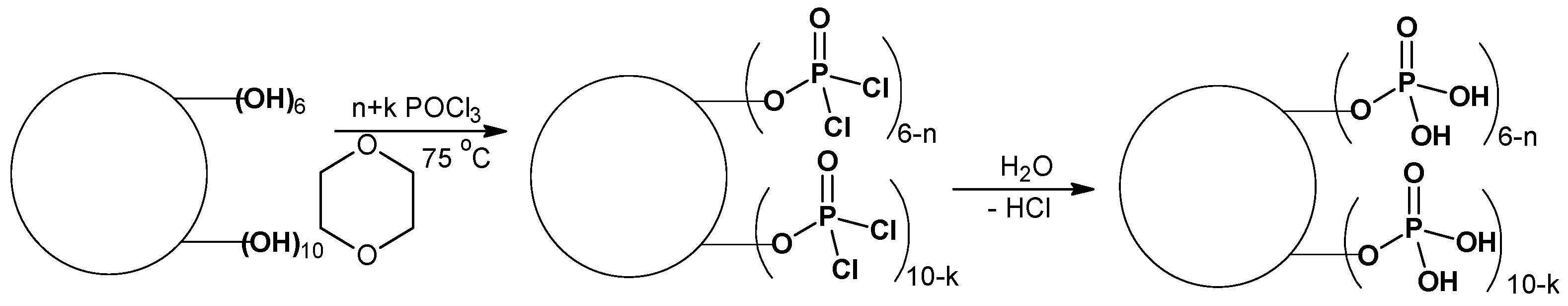
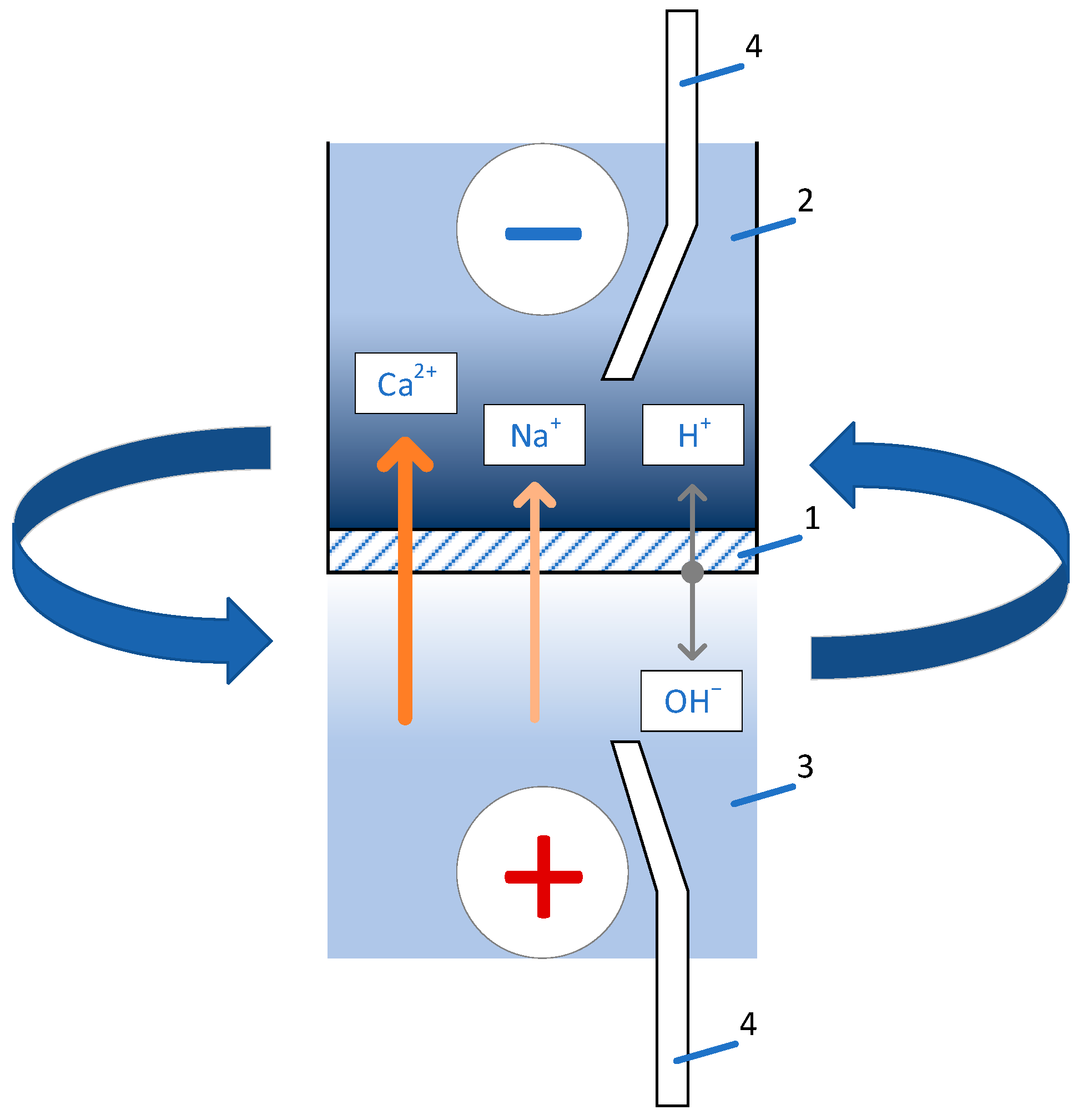

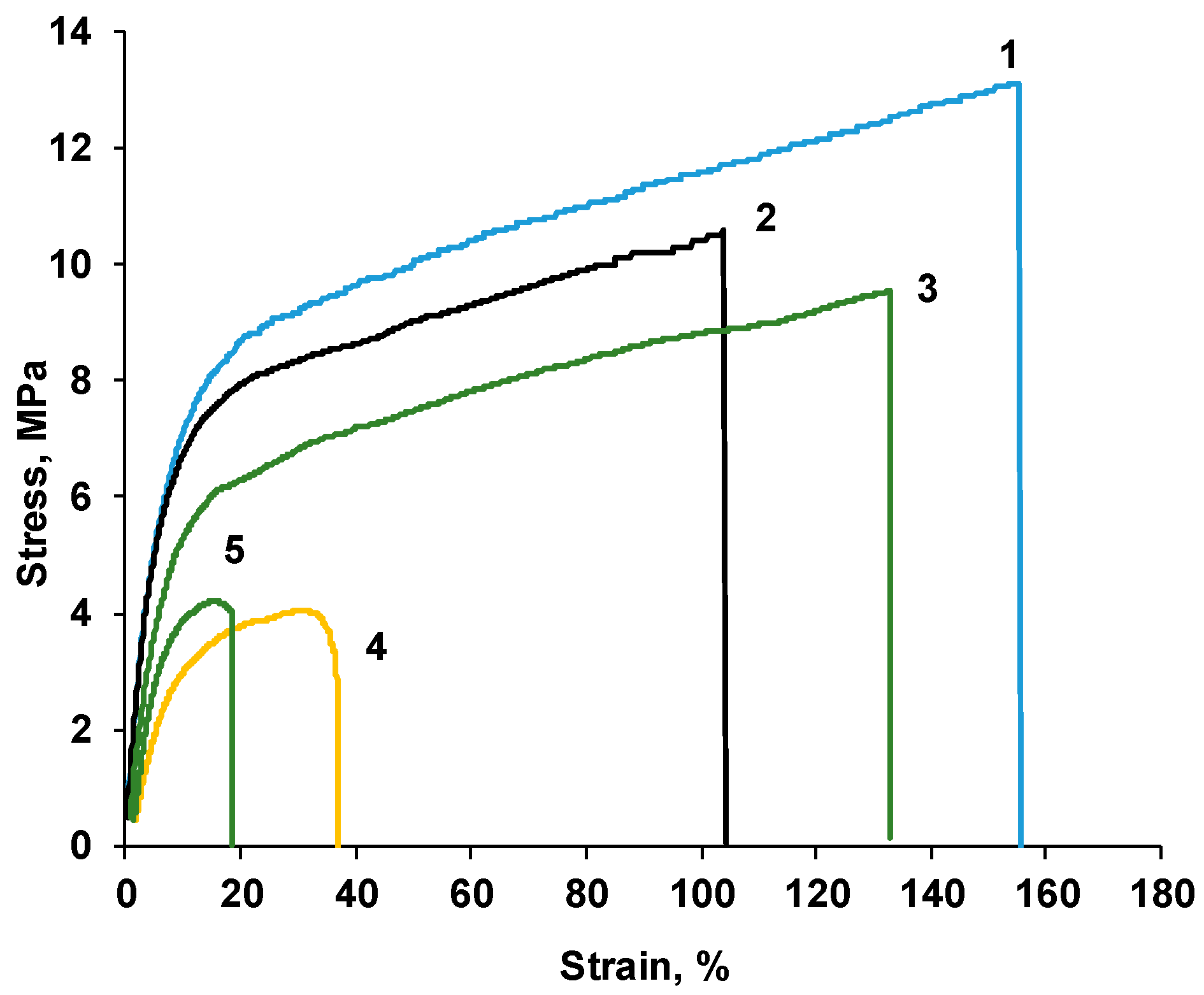


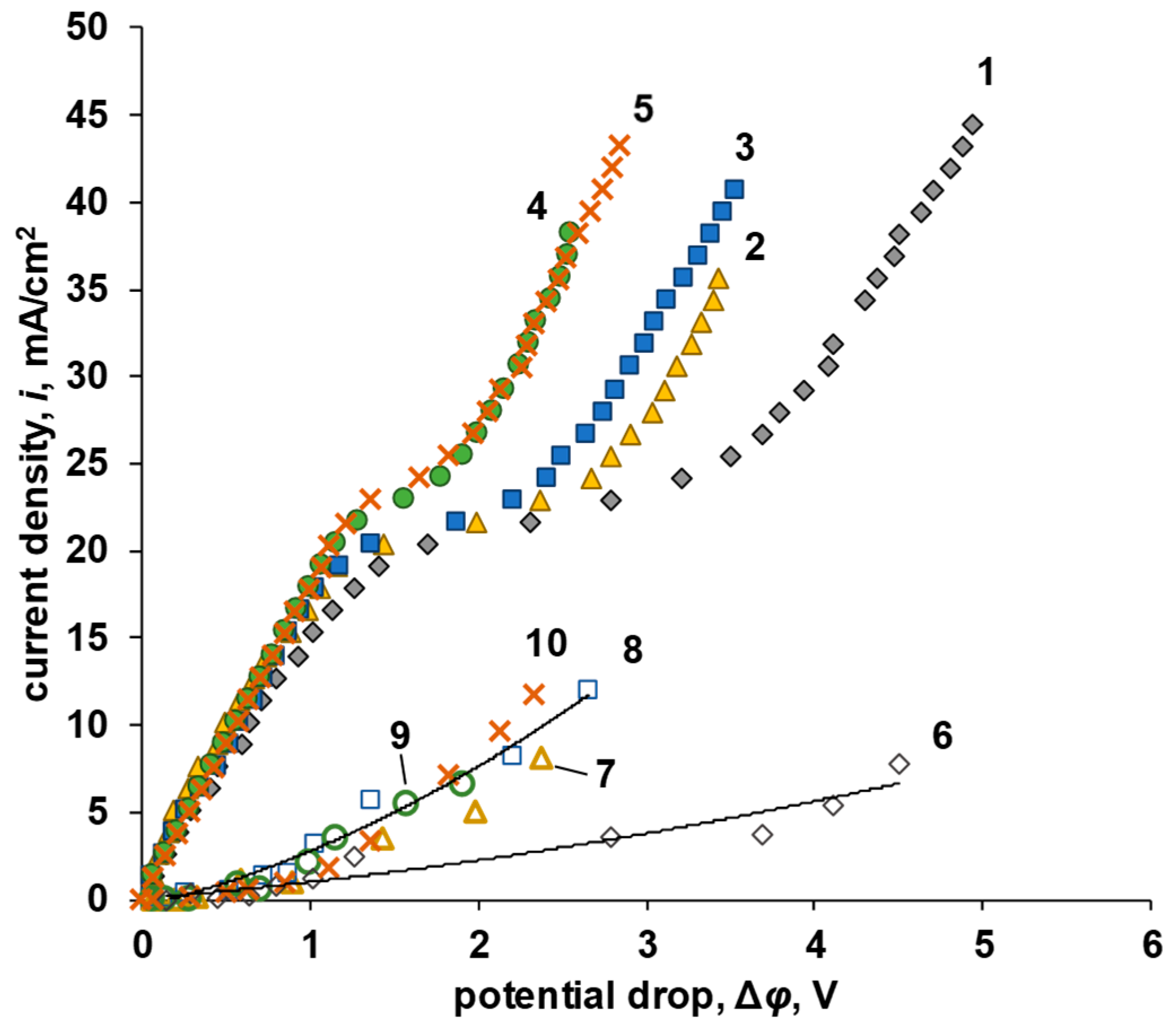
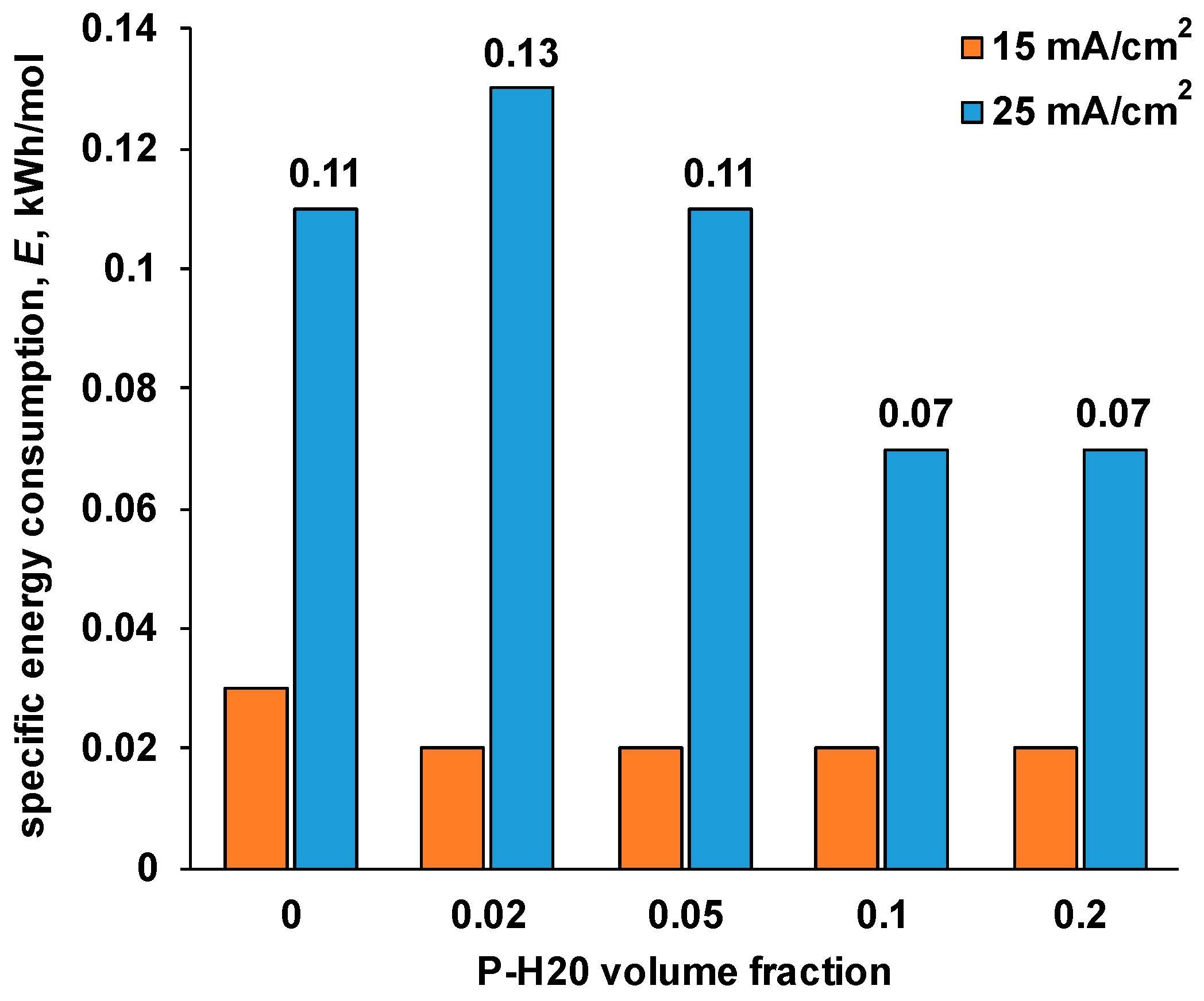
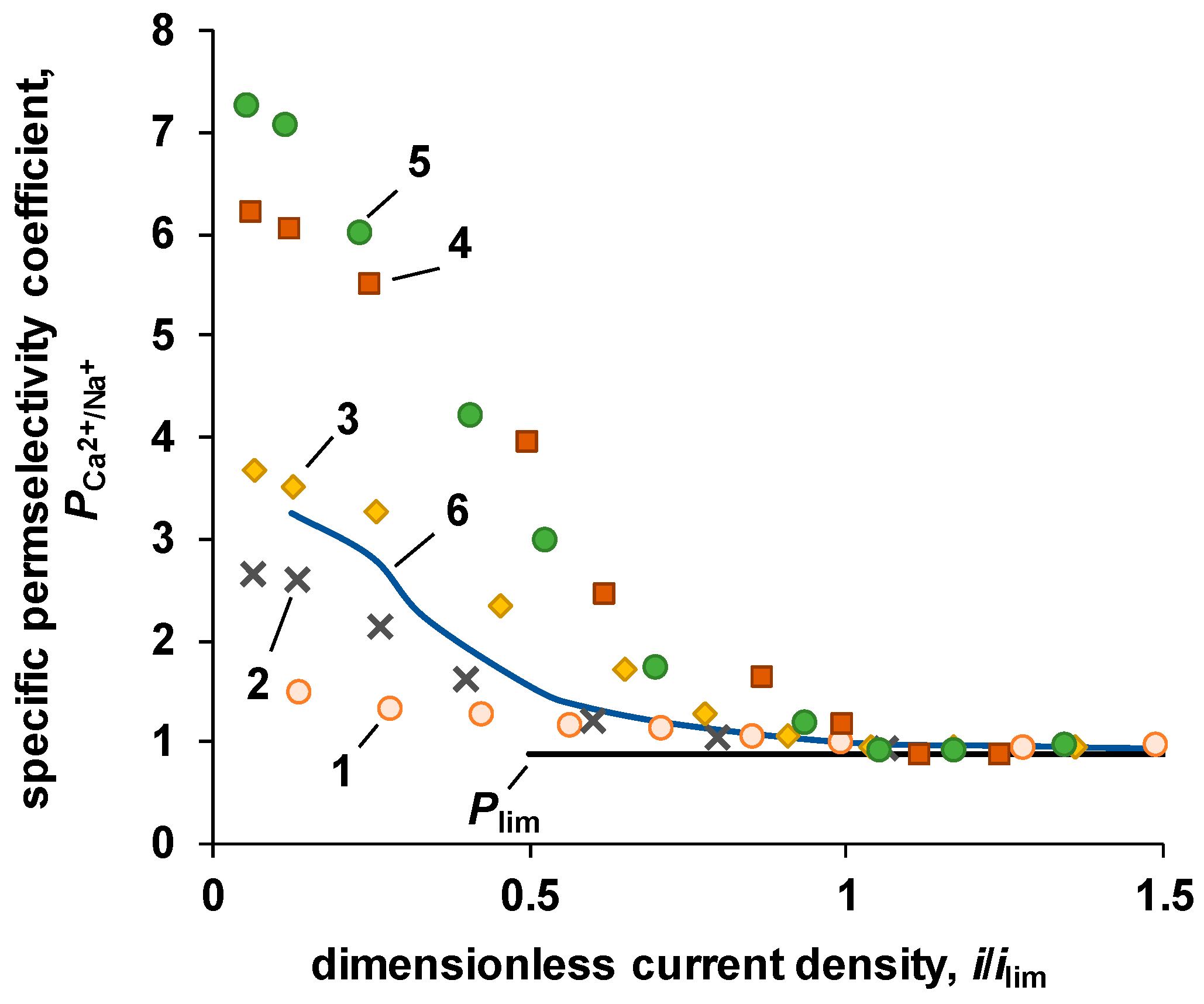
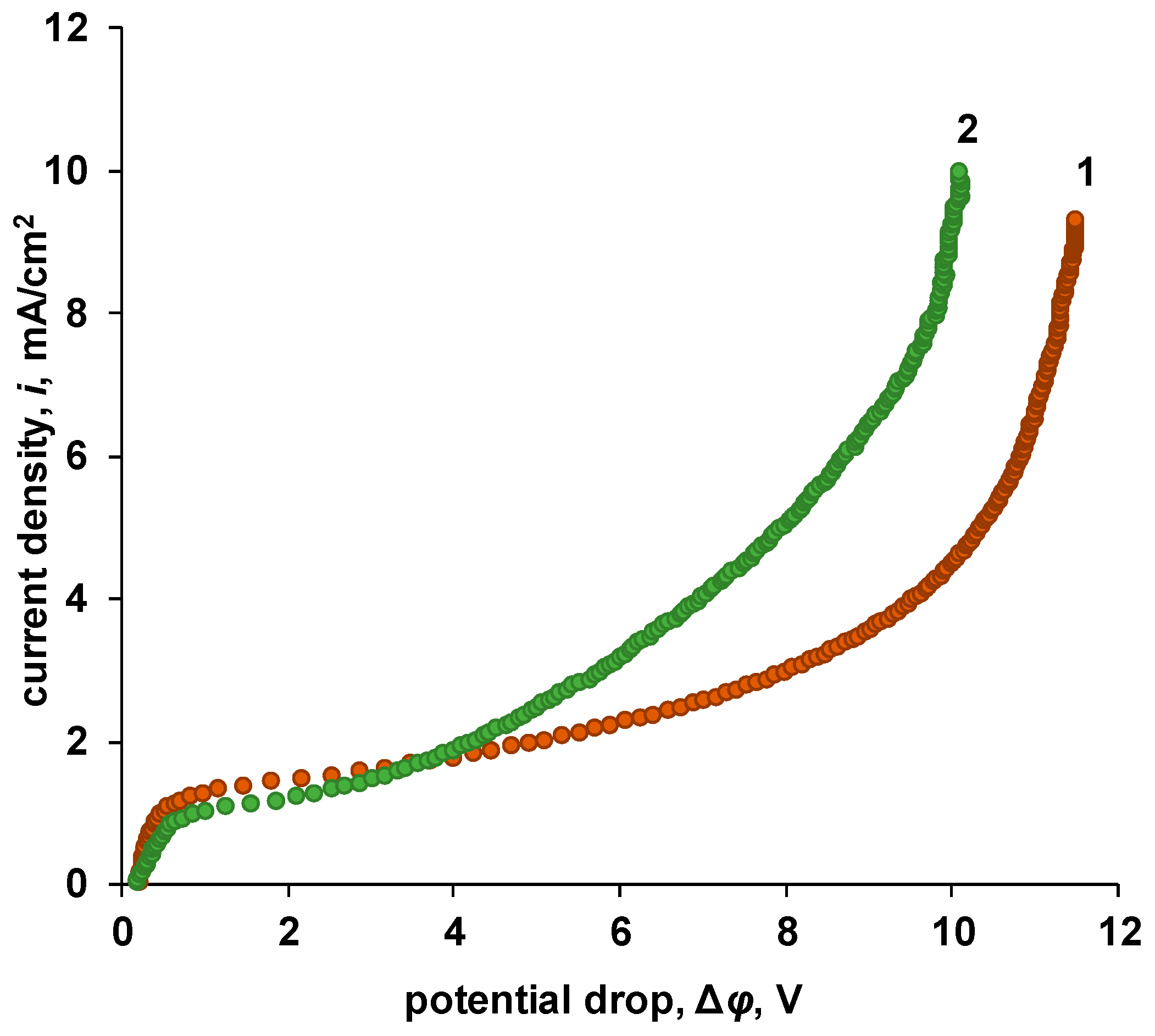
| P-H20 Content | Stress, MPa | Strain at Rupture, % | Young’s Modulus, MPa | Yield Strength, MPa |
|---|---|---|---|---|
| 0% | 12.0 ± 1.1 | 118 ± 44 | 159 ± 12 | 8.0 ± 0.3 |
| 2% | 10.4 ± 0.7 | 112 ± 7 | 142 ± 8 | 7.2 ± 0.3 |
| 5% | 11.0 ± 1.3 | 138 ± 10 | 116 ± 8 | 6.5 ± 0.6 |
| 10% | 3.4 ± 1.4 | 22 ± 9 | 52 ± 22 | 2.8 ± 1.1 |
| 20% | 5.3 ± 1.0 | 23 ± 12 | 110 ± 31 | 3.7 ± 0.7 |
| Volume Fraction of P-H20, % | 0 | 2 | 5 | 10 | 20 |
|---|---|---|---|---|---|
| Ion-exchange capacity, mmol/gwet | 0.83 ± 0.05 | 0.81 ± 0.05 | 0.79 ± 0.05 | 0.72 ± 0.05 | 0.66 ± 0.05 |
| Water uptake, % | 15.6 ± 0.3 | 15.4 ± 0.3 | 14.7 ± 0.3 | 12.5 ± 0.3 | 10.3 ± 0.3 |
| Gel conductivity, kiso, mS/cm | 9.7 | 5.3 | 4.2 | 3.7 | 2.8 |
| Intergel fraction, f2 | 0.02 | 0.06 | 0.07 | 0.07 | 0.07 |
| Integral diffusion permeability in 1 M NaCl, Pm, 106·cm2/s | 0.1 ± 0.05 | 0.3 ± 0.1 | 0.5 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.1 |
| Potential Drop | Membrane | SPTFE | SPTFE+10% P-H20 |
|---|---|---|---|
| Volume fraction of P-H20, % | 0 | 10 | |
| Limiting current density, mA/cm2 | 18 | 17 | |
| Δφ = 1 V | Total current, mA/cm2 | 14.8 | 16.4 |
| Salt ion current, mA/cm2 | 13.6 | 14.6 | |
| Water-splitting product current, mA/cm2 | 1.3 | 1.8 | |
| Non-equilibrium electroconvection current, mA/cm2 | - | - | |
| Exaltation current, mA/cm2 | 0.2 | 0.3 | |
| Δφ = 2 V | Total current, mA/cm2 | 21.3 | 21.7 |
| Salt ion current, mA/cm2 | 19.1 | 16.9 | |
| Water-splitting product current, mA/cm2 | 2.2 | 4.9 | |
| Non-equilibrium electroconvection current, mA/cm2 | 1.8 | - | |
| Exaltation current, mA/cm2 | 0.3 | 0.7 | |
| Δφ = 4 V | Total current, mA/cm2 | 30.4 | 44.5 |
| Salt ion current, mA/cm2 | 25.3 | 24.8 | |
| Water-splitting product current, mA/cm2 | 5.1 | 19.8 | |
| Non-equilibrium electroconvection current, mA/cm2 | 7.6 | 4.9 | |
| Exaltation current, mA/cm2 | 0.7 | 2.8 |
| Membrane | SPTFE | SPTFE+P-H20 | |||
|---|---|---|---|---|---|
| Volume fraction of P-H20, % | 0 | 2 | 5 | 10 | 20 |
| Limiting current density, mA/cm2 | 18 | 18.5 | 19 | 21 | 22 |
| Total current, mA/cm2 | 21.3 | 16.4 | 21.8 | 21.9 | 26.7 |
| Salt ion current, mA/cm2 | 19.1 | 14.6 | 16.1 | 14.3 | 19.2 |
| Water-splitting product current, mA/cm2 | 2.2 | 1.8 | 5.7 | 7.6 | 7.5 |
| Non-eqilibrium electroconvection current, mA/cm2 | 1.8 | - | - | - | - |
| Exaltation current, mA/cm2 | 0.3 | 0.3 | 1.5 | 2.0 | 2.0 |
| Membrane | SPTFE | SPTFE+P-H20 | |||
|---|---|---|---|---|---|
| Volume fraction of P-H20, % | 0 | 2 | 5 | 10 | 20 |
| Potential drop, V | 3.4 | 2.8 | 2.4 | 1.9 | 1.8 |
| Salt ion current, mA/cm2 | 21.5 | 14.8 | 14.3 | 18.7 | 18.6 |
| Water-splitting product current, mA/cm2 | 3.4 | 10.6 | 10.1 | 6.8 | 6.6 |
| Non-eqilibrium electroconvection current, mA/cm2 | 2.7 | - | - | - | - |
| Exaltation current, mA/cm2 | 0.9 | 2.8 | 2.7 | 1.8 | 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achoh, A.; Bondarev, D.; Nosova, E.; Melnikov, S. The Effect of Bulk Modification of the MF-4SK Membrane with Phosphorylated Hyper-Branched Dendrimer Bolthorn H20 on the Mechanisms of Electroconvection/Dissociation of Water and Specific Selectivity to Divalent Ions. Electrochem 2024, 5, 84-106. https://doi.org/10.3390/electrochem5010006
Achoh A, Bondarev D, Nosova E, Melnikov S. The Effect of Bulk Modification of the MF-4SK Membrane with Phosphorylated Hyper-Branched Dendrimer Bolthorn H20 on the Mechanisms of Electroconvection/Dissociation of Water and Specific Selectivity to Divalent Ions. Electrochem. 2024; 5(1):84-106. https://doi.org/10.3390/electrochem5010006
Chicago/Turabian StyleAchoh, Aslan, Denis Bondarev, Elena Nosova, and Stanislav Melnikov. 2024. "The Effect of Bulk Modification of the MF-4SK Membrane with Phosphorylated Hyper-Branched Dendrimer Bolthorn H20 on the Mechanisms of Electroconvection/Dissociation of Water and Specific Selectivity to Divalent Ions" Electrochem 5, no. 1: 84-106. https://doi.org/10.3390/electrochem5010006
APA StyleAchoh, A., Bondarev, D., Nosova, E., & Melnikov, S. (2024). The Effect of Bulk Modification of the MF-4SK Membrane with Phosphorylated Hyper-Branched Dendrimer Bolthorn H20 on the Mechanisms of Electroconvection/Dissociation of Water and Specific Selectivity to Divalent Ions. Electrochem, 5(1), 84-106. https://doi.org/10.3390/electrochem5010006






