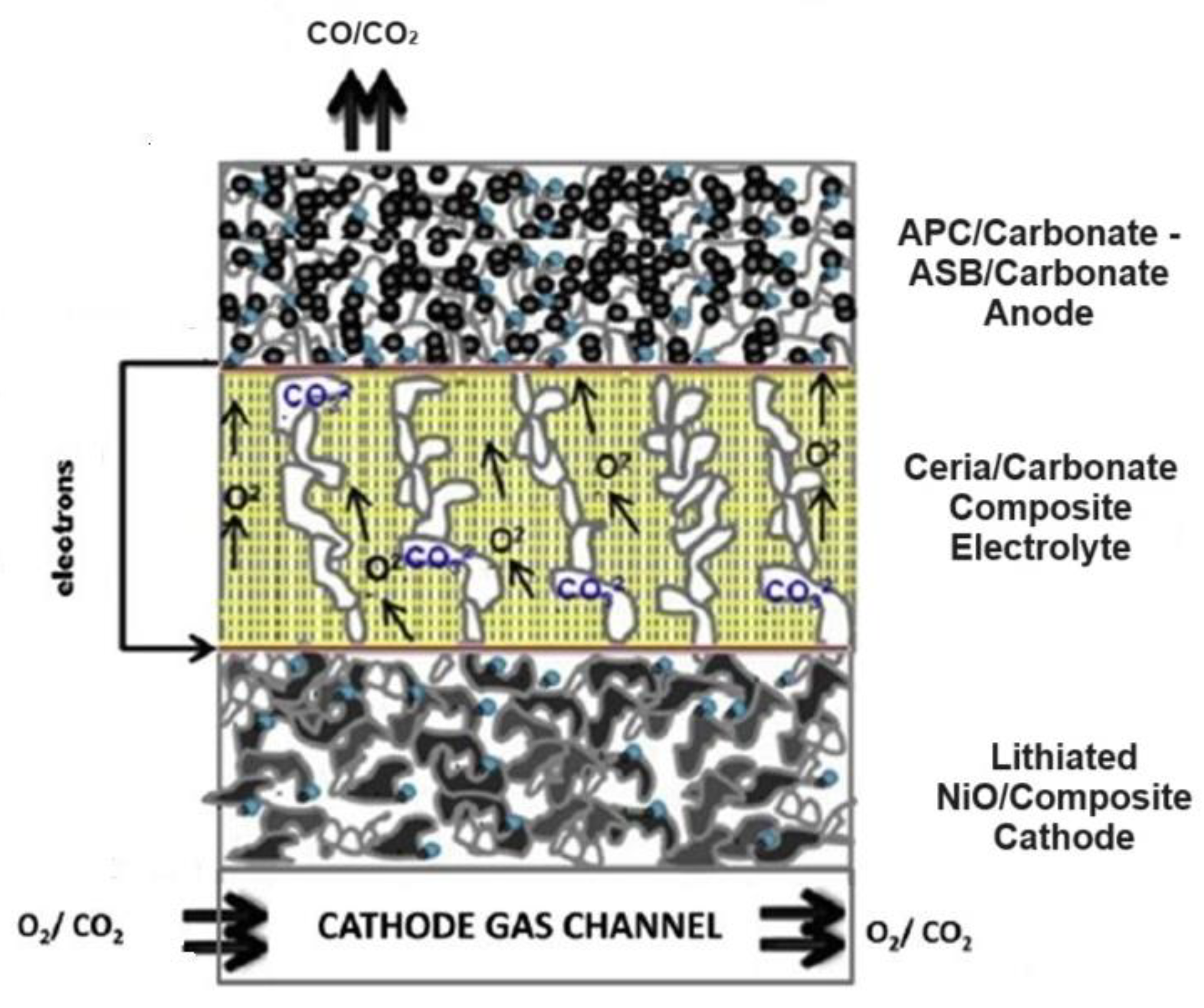
2.2. CO2 and CO/CO2 DCFC Models
CO
2 and CO/CO
2 producing DCFCs with molten carbonate electrolytes are advanced fuel cell technologies operating at high temperatures. They efficiently convert solid carbon-based fuels into electricity through oxidation at the anode, resulting in CO
2 production. Additionally, some designs can electrochemically reduce CO
2 at the anode, generating CO [
10,
28,
29,
30,
31,
32,
33]. In particular, when ASB is used as fuel in DCFCs, CO is the dominant gas at the anode [
5].
The CO
2 model can be described using the following electrochemical electrode reactions:
The CO/CO
2 model can be described using the following electrochemical electrode reactions:
The Nernst equation determines the ideal potential of a CO
2 and a CO/CO
2 producing DCFC and depends on the partial pressure of the present gaseous species. For the CO
2 model, it is expressed as follows [
28,
34,
35]:
For the CO/CO
2 model, it is expressed as follows [
30,
36,
37,
38]:
At low current densities in a CO
2 producing DCFC, activation polarization plays a significant role. This polarization arises from the electrochemical reaction at the carbon–molten carbonate electrolyte interfaces, where the reactants need to overcome an energy barrier known as the activation energy in order to facilitate electron transfer. The activation polarization is accurately described by the Butler–Volmer equation:
Therefore, the activation losses are given by the following:
where
and
are, respectively, the charge transfer coefficients in the anode and cathode serve as dimensionless parameters that elucidate the symmetry of electrochemical reactions related to electron transfer. The determination of these coefficients often involves experimental methods such as electrochemical impedance spectroscopy for the anodic coefficient or cyclic voltammetry and chronoamperometry for the cathodic coefficient. Additionally, fitting experimental data to the Butler–Volmer equation is a common approach to ascertain these crucial parameters, giving arbitrary values between 0 and 1 (depending on the symmetry of the transition state in the electrochemical reactions). In this work, we propose the prediction of the optimal near-real values of these coefficients using new AI tools.
According to Arrhenius law, the exchange current density (i
0,a) at which the rate of the forward reaction (oxidation) equals the rate of the reverse reaction (reduction) at the anode under equilibrium conditions is as follows:
The pre-exponential factor of the backward reaction is K
B = 5.8 × 10
9 A·m
−2 and the activation energy of the backward reaction is E
B = 22.175 K
−1 [
36].
The anodic exchange current density is influenced by several operating factors such as the temperature, the anode material and the specific electrochemical reaction taking place on the anode side.
The cathodic exchange current density (i0,c) is the rate at which the backward reaction (reduction) equals the rate of the forward reaction (oxidation) at the cathode side under equilibrium conditions. As far as the anodic exchange current density, the cathodic exchange current density is also affected by the operating factors like the temperature, the cathode material and the specific electrochemical reaction occurring at the cathode. As multi-parameters and nonlinear electrochemical variables, i0,a and i0,c cannot be predicted accurately using classical numerical methods. An advanced identification AI algorithm and modern computational approaches may be used to anticipate the properties of the complex materials as well as the optimization of the DCFC process.
Ohmic polarization emerges as a result of resistance encountered by ions and electrons within the single domain cell, impeding their flow. This polarization follows Ohm’s law and is mathematically expressed by the Equation (14) [
32,
33,
34,
35,
36,
37]:
where δ
g = 10
−3 m and σ
g (S·m
−1) are, respectively, the thickness and the global conductivity of the cell.
The global electrical conductivity of a cell (σg) in the context of ohmic polarization refers to the overall ability of the cell to conduct electrical current and ions in electrodes and electrolytes, respectively. It depends on the materials of the electrodes and electrolytes and the operating temperature. Ohmic polarization occurs when the dominant factor limiting the flow of the current in an electrochemical cell is the resistance of the electrolyte.
Concentration polarization in a CO
2 producing DCFC can arise from the slow diffusion of gas species (i.e., CO
2, O
2) from the cathode inlet to the reaction zone, from the motion of un-reacted O
2 and CO
2 from the cathode and from the diffusion of reactants and products through the electrolyte to and from the electrochemical reaction sites. Although concentration polarization is typically formulated based on thermodynamic principles, Basio et al. [
38] demonstrated a more consistent kinetic expression. The concentration polarization in the CO
2 producing DCFC is defined using Fick’s law as follows:
Therefore, using (1) and (2):
The CO/CO
2 producing DCFC follows the same principle as the CO
2 system, with one key difference being that it involves an additional anodic reaction producing a mixture of CO and CO
2. Regarding modeling, the CO/CO
2 producing DCFC employs the same activation and ohmic losses formulation discussed in the previous section. The only difference is the number of electrons involved, which reduces from four to two due to the concurrent formation of CO and CO
2 on the anode side. Hence, the concentration polarization and ideal potential of the CO/CO
2 producing DCFC are determined while considering the simultaneous formation of both CO and CO
2 on the anode side. The ideal potential of the CO/CO
2 producing DCFC is given by Equation (8) and the concentration polarization is given by Equation (18) according to the electrochemical reaction Equations (4) and (5):
The limit current densities (, , ) of the gas spices in the cathode and anode refer to the maximum current densities that can be sustained at an electrode interface when mass transport limitations become the dominant factor. As the current density increases beyond the limit, the concentration of reactants or products near the electrode surface deviates significantly from the bulk concentration, leading to a drop in the current efficiency and other undesirable effects.
Typically, at low current density, the main source of voltage loss in a fuel cell is activation polarization. However, as the current density rises, ohmic polarization takes over as the primary cause of voltage drop. Eventually, at high current densities, concentration polarization becomes the dominant factor contributing to the voltage loss. It is worth noting that the theoretical output voltage of the fuel cell is as follows:
It can be seen that the nonlinear Equation (20) contains several unknown parameters affecting the electrochemical conversion mechanism of the DCFC. Hence, appropriate AI optimization strategies should be used for the effective and accurate prediction of these parameters. Accordingly, the differential evolution (DE) algorithm is proposed here to determine the following seven unknown parameters: the anode charge transfer coefficient (), the cathode charge transfer coefficient (), the global electrical conductivity of the single-domain DCFC (), the cathode exchange current density () and the limit current densities of oxygen (), the carbon dioxide on the cathode side () and the carbon dioxide on the anode side Taking into account that these seven parameters will considerably affect the DCFC electrochemical behavior, they must thus be accurately estimated to fulfill the actual I–V and I-P characteristics of the DCFC.
2.3. Differential Evolution (DE) Algorithm
DE, belonging to the evolutionary algorithm family, generates new solutions by recombining existing ones, making it robust and governed by few algorithm-specific parameters. DE outperforms other optimization methods in tackling challenging problems with nonlinear, multimodal, and non-separable features. It was consistently ranked as one of the top performers in optimization competitions, demonstrating its potential for real-world applications with competitive accuracy and convergence speed. Notably, DE’s lower space complexity grants it superior scalability, particularly for handling large-scale and computationally intensive optimization problems. These advantages make DE a favored choice among researchers and practitioners for solving diverse sets of real-world optimization challenges effectively and efficiently [
21,
22,
23,
24,
25,
26,
27].
Figure 2 illustrates the scheme of DE calculation used in this paper. The DE algorithm consists of four phases which are as follows: initialization; mutation; crossover and evaluation; and population update. In the version used in this paper, we added a boundary refinement phase.
Population initialization: This is the first process step of DE-generating random individuals within the specified boundaries, which are vectors in a D-dimensional space. Each ith individual solution of DE can be represented as a D-dimensional vector as follows [
23]:
The specified boundaries are given by the following Equations [
25]:
For each ith DE solution, the jth dimensional component can be initialized by randomly generating as follows [
23]:
Mutation: For each individual in the population, the algorithm selects three distinct individuals (candidates), Xi,1t,Xi,2t and Xi,3t, randomly from the population (excluding the current individual) to calculate the mutant vector:
where SF is the scaling factor and X
it ≠ X
i,1t ≠ X
i,2t ≠ X
i,3t.
The crossover operation is performed with a probability of a crossover rate [
23]:
Evaluation and population update: The fitness of the trial vector is evaluated using the objective function. If the fitness of the trial vector is better (lower) than the fitness of the current individual, the current individual is replaced by the trial vector [
23,
27]:
In this study, the population size was set to 100, CR = 0.8, SF = 0.2 and the maximum number of iterations was set to 100.
In our DCFC case, the process consisted of finding the values of the vector of the seven predefined unknown parameters
that minimize the root mean square error (RMSE) between the model’s predictions and the experimental data in order to fulfill the actual I-V and I-P characteristics:
Indeed, the DE algorithm is an evolutionary optimization algorithm that iteratively explores the parameter space by generating new candidate solutions and evaluating their fitness based on RMSEs by minimizing the errors between the experimental and the estimated voltage values. The algorithm gradually refines the parameter values until a satisfactory convergence is achieved, yielding the best-fitted model to the experimental data. This optimization approach allows for robust and efficient parameter estimation, leading to a more accurate representation of the underlying relationship between the model and the experimental data.