Separator Materials for Lithium Sulfur Battery—A Review
Abstract
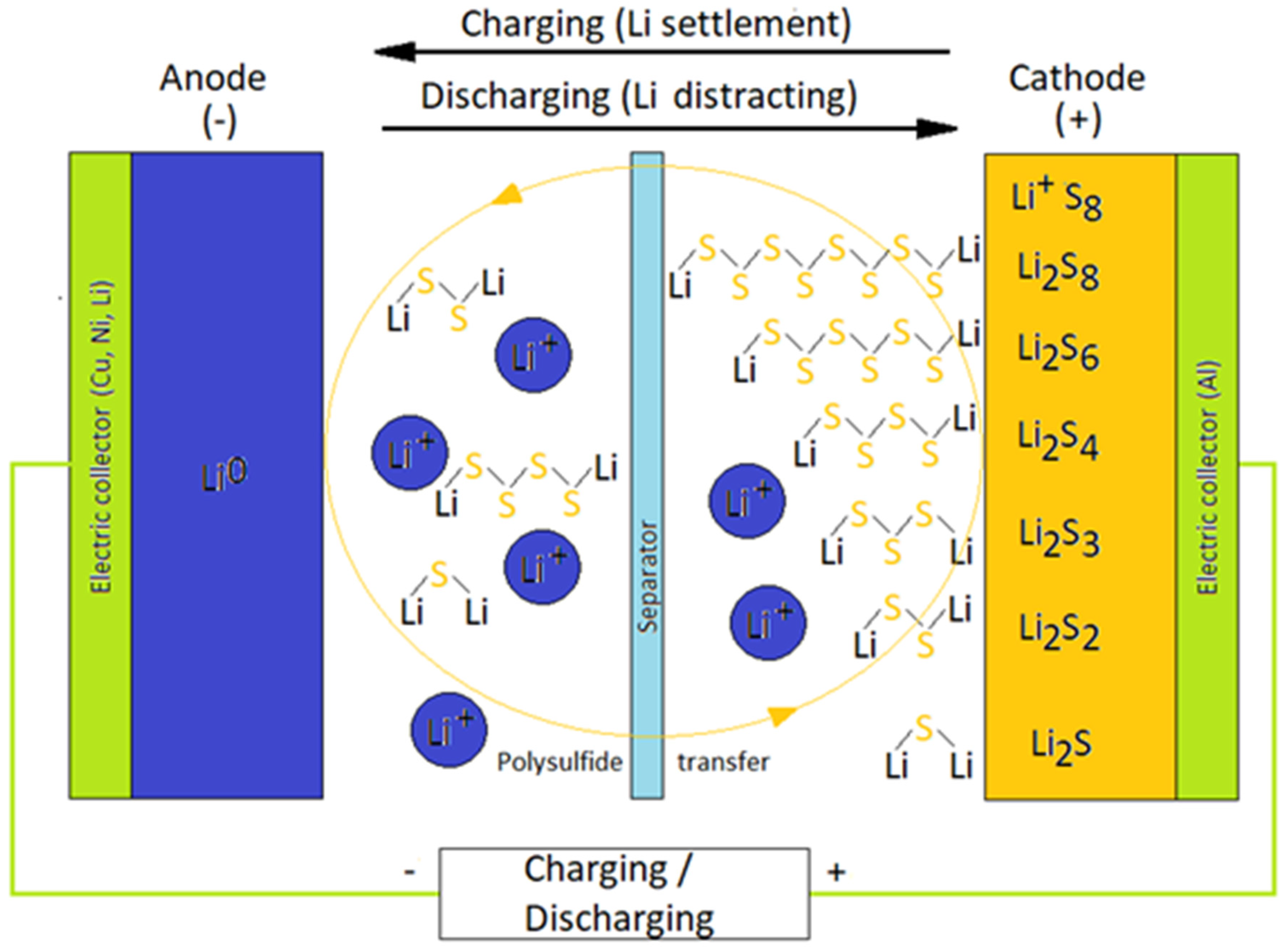
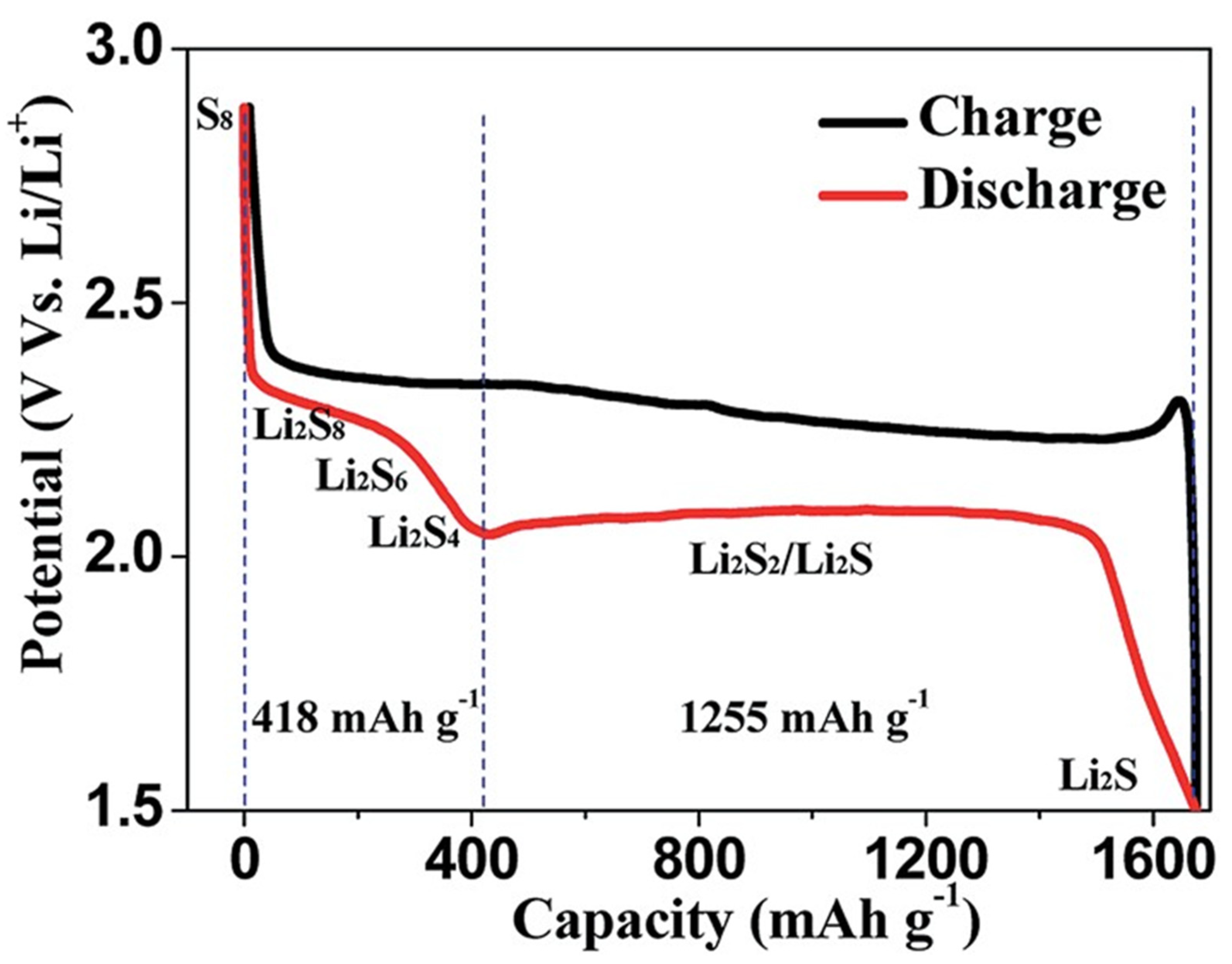
:1. Introduction
2. Separators Classified by Materials
2.1. Separator Material Facing Anode Side
2.1.1. Separator Material: Metal
2.1.2. Separator Material: Ceramic
2.1.3. Separator Material: Solid Electrolyte
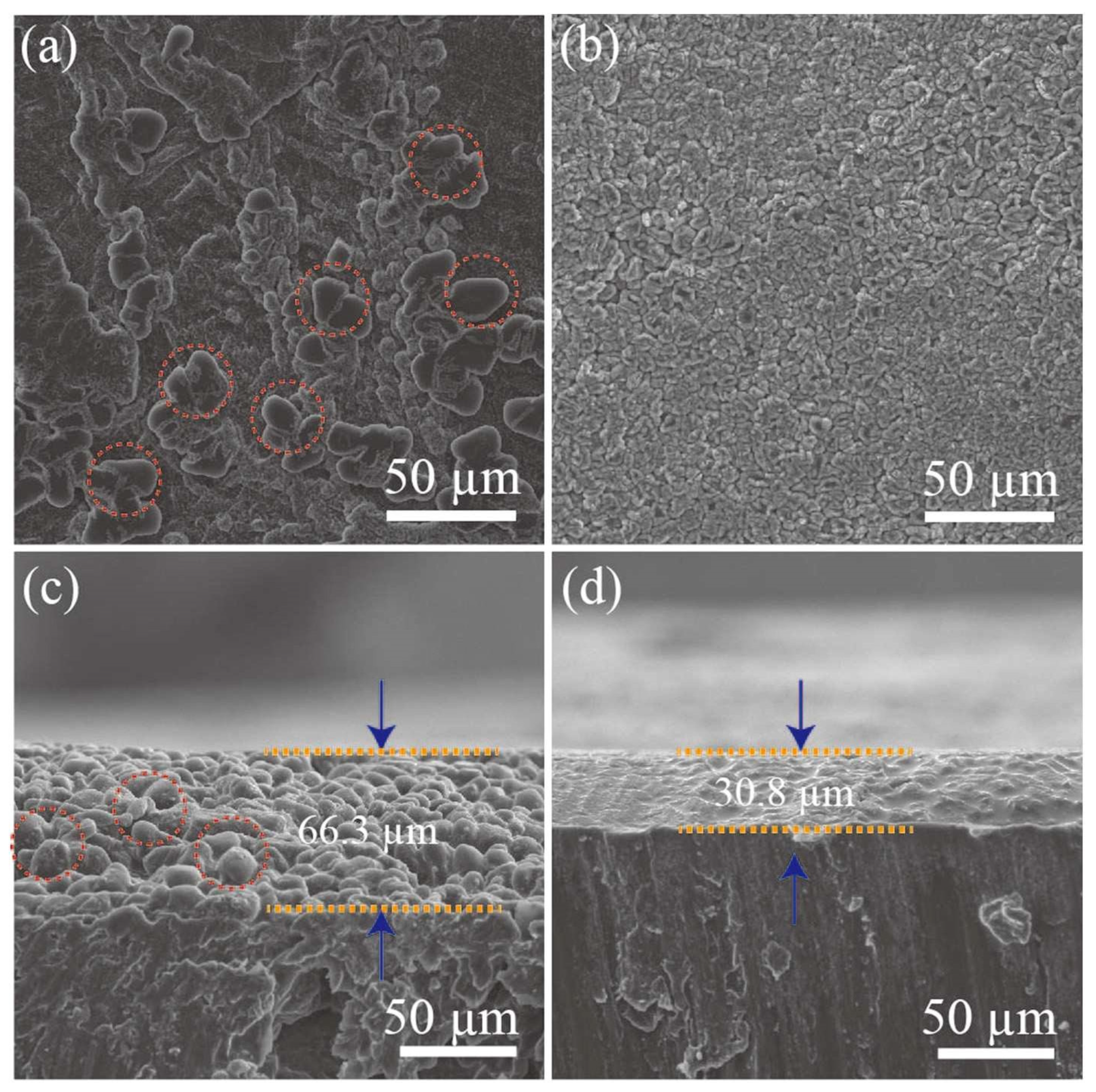
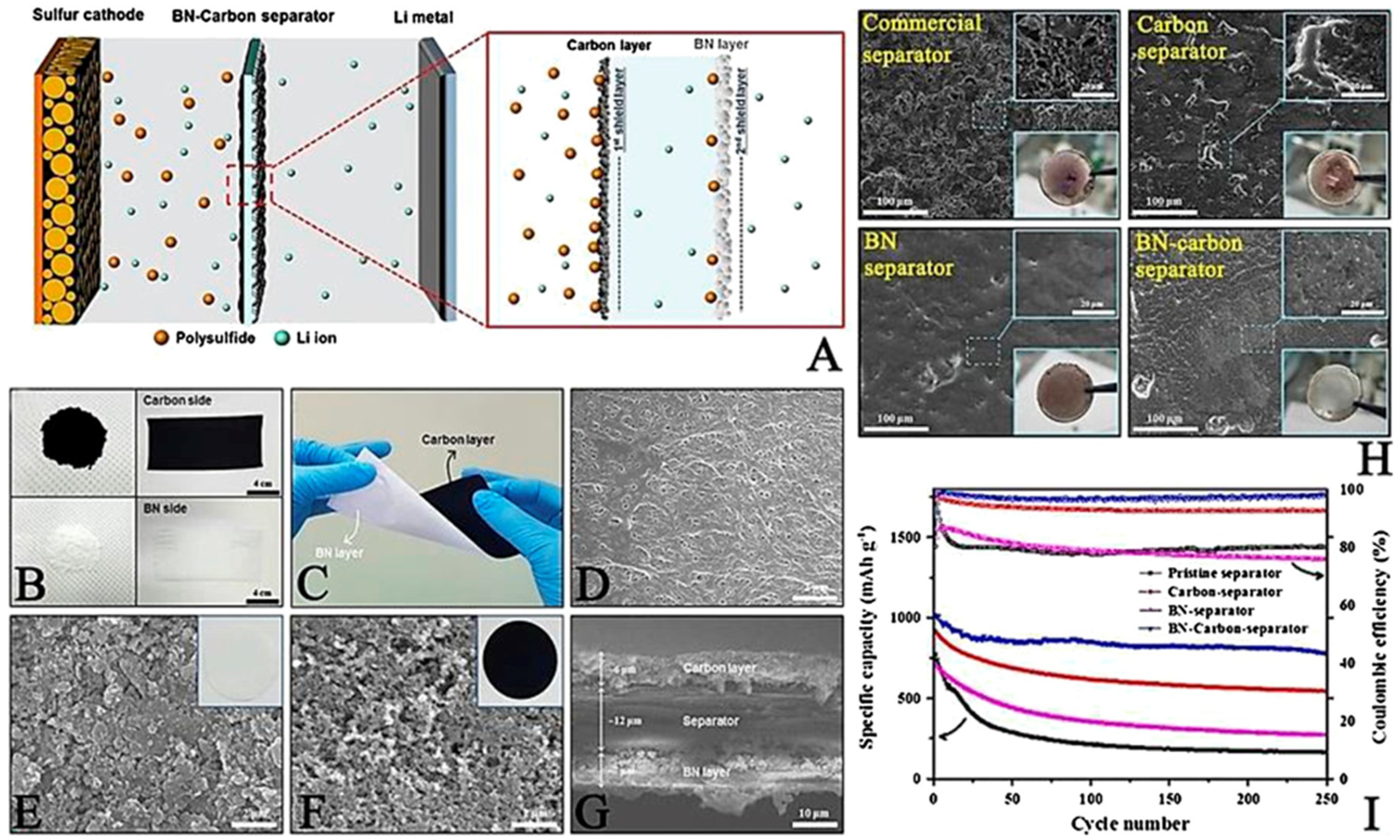
2.1.4. Other Functional Separator Materials
2.2. Separator Material Facing Cathode Side
2.2.1. Separator Material: Carbonaceous Materials
2.2.2. Separator Material: Metal Oxide
2.2.3. Separator Material: Metal Sulfide
2.2.4. Separator Material: Metal Carbide
2.2.5. Separator Material: Nitride
2.2.6. Separator Material: Phosphide
2.2.7. Separator Material: Metal Organic Framework-Based Materials
2.2.8. Separator Material: Quantum Dot
2.2.9. Separator Material: Mxenes
2.2.10. Other Functional Separator Materials
3. Necessary Properties for LSB Separators
3.1. Separator Porosity, Pore Size, and Thickness
3.2. Tortuosity and Permeability
3.3. Wettability
3.4. Mechanical Properties and Thermal Behavior
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, T.; Song, W.; Son, D.Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Xu, G.; Ding, B.; Pan, J.; Nie, P.; Shen, L.; Zhang, X. High performance lithium–sulfur batteries: Advances and challenges. J. Mater. Chem. A 2014, 2, 12662–12676. [Google Scholar] [CrossRef]
- Kang, W.; Deng, N.; Ju, J.; Li, Q.; Wu, D.; Ma, X.; Li, L.; Naebe, M.; Cheng, B. A review of recent developments in rechargeable lithium–sulfur batteries. Nanoscale 2016, 8, 16541–16588. [Google Scholar] [CrossRef]
- Lopez, C.V.; Maladeniya, C.P.; Smith, R.C. Lithium-sulfur batteries: Advances and trends. Electrochem 2020, 1, 226–259. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.H.; Zu, C.; Su, Y.S. Rechargeable lithium–sulfur batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef]
- Li, G.; Li, Z.; Zhang, B.; Lin, Z. Developments of electrolyte systems for lithium–sulfur batteries: A review. Front. Energy Res. 2015, 3, 1. [Google Scholar] [CrossRef]
- Guo, J.; Liu, J. A binder-free electrode architecture design for lithium–sulfur batteries: A review. Nanoscale Adv. 2019, 1, 2104–2122. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, G.; Cui, Y. A membrane-free lithium/polysulfide semi-liquid battery for large-scale energy storage. Energy Environ. Sci. 2013, 6, 1552. [Google Scholar] [CrossRef]
- Zhang, S.S.; Read, J.A. A new direction for the performance improvement of rechargeable lithium/sulfur batteries. J. Power Sources 2012, 200, 77–82. [Google Scholar] [CrossRef]
- Barchasz, C.; Molton, F.; Duboc, C.; Leprêtre, J.C.; Patoux, S.; Alloin, F. Lithium/sulfur cell discharge mechanism: An original approach for intermediate species identification. Anal. Chem. 2012, 84, 3973–3980, Corrigendum in 2020, 92, 13610. [Google Scholar] [CrossRef] [PubMed]
- Yamaki, J.I.; Tobishima, S.I.; Sakurai, Y.; Saito, K.I.; Hayashi, K. Safety evaluation of rechargeable cells with lithium metal anodes and amorphous V2O5 cathodes. J. Appl. Electrochem. 1998, 28, 135–140. [Google Scholar] [CrossRef]
- Cao, R.; Xu, W.; Lv, D.; Xiao, J.; Zhang, J.G. Anodes for rechargeable lithium–sulfur batteries. Adv. Energy Mater. 2015, 5, 1402273. [Google Scholar] [CrossRef]
- Brieske, D.M.; Warnecke, A.; Sauer, D.U. Modeling the volumetric expansion of the lithium-sulfur battery considering charge and discharge profiles. Energ. Storage Mater. 2023, 55, 289–300. [Google Scholar] [CrossRef]
- Kamisan, A.I.; Kudin, T.I.T.; Kamisan, A.S.; Omer, A.F.C.; Taib, M.F.M.; Hassan, O.H.; Ali, A.M.M.; Yahya, M.Z.A. Recent advances on graphene-based materials as cathode materials in lithium–sulfur batteries. Int. J. Hydrogen Energy 2022, 47, 8630–8657. [Google Scholar] [CrossRef]
- Choi, Y.J.; Chung, Y.D.; Baek, C.Y.; Kim, K.W.; Ahn, H.J.; Ahn, J.H. Effects of carbon coating on the electrochemical properties of sulfur cathode for lithium/sulfur cell. J. Power Sources 2008, 184, 548–552. [Google Scholar] [CrossRef]
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef]
- Liu, X.; Huang, J.Q.; Zhang, Q.; Mai, L. Nanostructured metal oxides and sulfides for lithium–sulfur batteries. Adv. Mater. 2017, 29, 1601759. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Zhang, Y.; Xiang, M.; Wu, H.; Liu, H.; Dou, S. Interwoven V2O5 nanowire/graphene nanoscroll hybrid assembled as efficient polysulfide-trapping-conversion interlayer for long-life lithium sulfur batteries. J. Mater. Chem. A 2018, 6, 19358–19370. [Google Scholar] [CrossRef]
- Hu, N.; Lv, X.; Dai, Y.; Fan, L.; Xiong, D.; Li, X. SnO2/reduced graphene oxide interlayer mitigating the shuttle effect of Li–S batteries. ACS Appl. Mater. Interfaces 2018, 10, 18665–18674. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Ryu, S.; Gong, Y.J.; Pyo, S.; Yun, H.; Kim, H.; Lee, J.; Yoo, J.; Kim, Y.S. Nitrogen-doped MoS2 as a catalytic sulfur host for lithium–sulfur batteries. Chem. Eng. J. 2022, 439, 135568. [Google Scholar] [CrossRef]
- Zhong, Y.; Xia, X.; Shi, F.; Zhan, J.; Tu, J.; Fan, H.J. Transition metal carbides and nitrides in energy storage and conversion. Adv. Sci. 2016, 3, 1500286. [Google Scholar] [CrossRef]
- Gao, J.; Lowe, M.A.; Kiya, Y.; Abruna, H.D. Effects of liquid electrolytes on the charge-discharge performance of rechargeable lithium/sulfur batteries: Electrochemical and in-situ X-ray absorption spectroscopic studies. J. Phys. Chem. C 2011, 115, 25132–25137. [Google Scholar] [CrossRef]
- Zhang, S.S. Liquid electrolyte lithium/sulfur battery: Fundamental chemistry, problems, and solutions. J. Power Sources 2013, 231, 153–162. [Google Scholar] [CrossRef]
- Aurbach, D.; Pollak, E.; Elazari, R.; Salitra, G.; Kelley, C.S.; Affinito, J. On the surface chemical aspects of very high energy density, rechargeable Li-sulfur batteries. J. Electrochem. Soc. 2009, 156, A694. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, L.; Liu, T. Multifunctional second barrier layers for lithium–sulfur batteries. Mater. Chem. Front. 2018, 2, 235–252. [Google Scholar] [CrossRef]
- Chang, C.H.; Chung, S.H.; Manthiram, A. Ultra-lightweight PANiNF/MWCNT-functionalized separators with synergistic suppression of polysulfide migration for Li–S batteries with pure sulfur cathodes. J. Mater. Chem. A 2015, 3, 18829–18834. [Google Scholar] [CrossRef]
- Kong, W.; Yan, L.; Luo, Y.; Wang, D.; Jiang, K.; Li, Q.; Fan, S.; Wang, J. Ultrathin MnO2/graphene oxide/carbon nanotube interlayer as efficient polysulfide trapping shield for high-performance Li–S batteries. Adv. Funct. Mater. 2017, 27, 1606663. [Google Scholar] [CrossRef]
- Huang, J.Q.; Zhang, Q.; Wei, F. Multi-functional separator/interlayer system for high-stable lithium–sulfur batteries: Progress and prospects. Energy Storage Mater. 2015, 1, 127–145. [Google Scholar] [CrossRef]
- Fiedler, M.; Cangaz, S.; Hippauf, F.; Dörfler, S.; Abendroth, T.; Althues, H.; Kastel, S. Mechanistic Insights into the Cycling Behavior of Sulfur Dry-Film Cathodes. Adv. Sustain. Syst. 2023, 7, 2200439. [Google Scholar] [CrossRef]
- Xu, J.; Shui, J.; Wang, J.; Wang, M.; Liu, H.-K.; Dou, S.X.; Jeon, I.-Y.; Seo, J.-M.; Baek, J.-B.; Dai, L. Sulfur–Graphene Nanostructured Cathodes via Ball-Milling for High-Performance Lithium–Sulfur Batteries. ACS Nano 2014, 8, 10920–10930. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, M.; Du, W.; Ni, B.; Wu, Y.; Liu, Y.; Shang, C.; Guo, Z.; Pan, H. A mechanochemical synthesis of submicron-sized Li2S and a mesoporous Li2S/C hybrid for high performance lithium/sulfur battery cathodes. J. Mater. Chem. A 2017, 5, 6471–6482. [Google Scholar] [CrossRef]
- Younes, H.; Hong, H.; Peterson, G.P. A Novel Approach to Fabricate Carbon Nanomaterials–Nanoparticle Solids through Aqueous Solutions and Their Applications. Nanomanuf. Metrol. 2021, 4, 226–236. [Google Scholar] [CrossRef]
- Li, J.; Xiao, Z.; Chen, A.; Zhang, W.; Zhu, D.; Jin, Y.; Mao, Q.; Wang, G.; He, J.; Xia, Y. Functionally modified polyolefin-based separators for lithium–sulfur batteries: Progress and prospects. Front. Energy Res. 2020, 8, 593640. [Google Scholar] [CrossRef]
- Yan, J.; Liu, X.; Li, B. Capacity fade analysis of sulfur cathodes in lithium–sulfur batteries. Adv. Sci. 2016, 3, 1600101. [Google Scholar] [CrossRef]
- Peng, H.J.; Huang, J.Q.; Cheng, X.B.; Zhang, Q. Review on high loading and high-energy lithium–sulfur batteries. Adv. Energy Mater. 2017, 7, 1700260. [Google Scholar] [CrossRef]
- Deng, N.; Liu, Y.; Li, Q.; Yan, J.; Lei, W.; Wang, G.; Wang, L.; Liang, Y.; Kang, W.; Cheng, B. Functional mechanism analysis and customized structure design of interlayers for high performance Li–S battery. Energy Storage Mater. 2019, 23, 314–349. [Google Scholar] [CrossRef]
- Liu, Y.; Xiong, S.; Wang, J.; Jiao, X.; Li, S.; Zhang, C.; Song, Z.; Song, J. Dendrite-free lithium metal anode enabled by separator engineering via uniform loading of lithiophilic nucleation sites. Energy Storage Mater. 2019, 19, 24–30. [Google Scholar] [CrossRef]
- Zuo, Z.; Zhuang, L.; Xu, J.; Shi, Y.; Su, C.; Lian, P.; Tian, B. Lithiophilic silver coating on lithium metal surface for inhibiting lithium dendrites. Front. Chem. 2020, 8, 109. [Google Scholar] [CrossRef]
- Yang, C.P.; Yin, Y.X.; Zhang, S.F.; Li, N.W.; Guo, Y.G. Accommodating lithium into 3D current collectors with a submicron skeleton towards long-life lithium metal anodes. Nat. Comm. 2015, 6, 8058. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhu, S.; Lu, Y. 3D Porous Cu Current Collector/Li-Metal Composite Anode for Stable Lithium-Metal Batteries. Adv. Funct. Mater. 2017, 27, 1606422. [Google Scholar] [CrossRef]
- Kim, P.J.H.; Seo, J.; Fu, K.; Choi, J.; Liu, Z.; Kwon, J.; Hu, L.; Paik, U. Synergistic protective effect of a BN-carbon separator for highly stable lithium sulfur batteries. NPG Asia Mater. 2017, 9, e375. [Google Scholar] [CrossRef]
- Favors, Z.; Wang, W.; Bay, H.H.; George, A.; Ozkan, M.; Ozkan, C. Stable cycling of SiO2 nanotubes as high-performance anodes for lithium-ion batteries. Sci. Rep. 2014, 4, 4605. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Wang, F.; Zhong, H.; Li, Y.; Wang, Y.; Hu, L.; Chen, Q. Hollow porous SiO2 nanocubes towards high-performance anodes for lithium-ion batteries. Sci. Rep. 2013, 3, 1568. [Google Scholar] [CrossRef] [PubMed]
- Suriyakumar, S.; Stephan, A.M.; Angulakshmi, N.; Hassan, M.H.; Alkordi, M.H. Metal–organic framework@SiO2 as permselective separator for lithium–sulfur batteries. J. Mater. Chem. A 2018, 6, 14623–14632. [Google Scholar] [CrossRef]
- Fu, D.; Luan, B.; Argue, S.; Bereau, M.N.; Davidson, I.J. Nano SiO2 particle formation and deposition on polypropylene separators for lithium-ion batteries. J. Power Sources 2012, 206, 325–333. [Google Scholar] [CrossRef]
- Song, R.; Fang, R.; Wen, L.; Shi, Y.; Wang, S.; Li, F. A trilayer separator with dual function for high performance lithium–sulfur batteries. J. Power Sources 2016, 301, 179–186. [Google Scholar] [CrossRef]
- Hou, T.Z.; Chen, X.; Peng, H.G.; Huang, J.Q.; Li, B.Q.; Zhang, Q.; Li, B. Design principles for heteroatom-doped nanocarbon to achieve strong anchoring of polysulfides for lithium–sulfur batteries. Small 2016, 12, 3283–3291. [Google Scholar] [CrossRef]
- He, Y.; Wu, S.; Li, Q.; Zhou, H. Designing a multifunctional separator for high-performance Li–S batteries at elevated temperature. Small 2019, 15, 1904332. [Google Scholar] [CrossRef]
- Huo, H.; Li, X.; Chen, Y.; Liang, J.; Deng, S.; Gao, X.; Doyle-Davis, K.; Li, R.; Guo, X.; Shen, Y.; et al. Bifunctional composite separator with a solid-state-battery strategy for dendrite-free lithium metal batteries. Energy Storage Mater. 2020, 29, 361–366. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Liu, X.; Mao, E.; Wang, M.; Li, G.; Fu, L.; Li, Z.; Eng, A.Y.S.; Seh, Z.W.; et al. Engineering stable electrode-separator interfaces with ultrathin conductive polymer layer for high-energy-density Li–S batteries. Energy Storage Mater. 2019, 23, 261–268. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Liu, X.; Zeng, R.; Li, M.; Huang, Y.; Hu, X. Constructing hierarchical tectorum-like α-Fe2O3/PPy nanoarrays on carbon cloth for solid-state asymmetric supercapacitors. Angew. Chem. Int. Ed. 2017, 129, 1125–1130. [Google Scholar] [CrossRef]
- Feng, J.X.; Xu, H.; Ye, S.H.; Ouyang, G.; Tong, Y.X.; Li, G.R. Silica.polypyrrole hybrids as high-performance metal-free electrocatalysts for the hydrogen evolution reaction in neutral media. Angew. Chem. Int. Ed. 2017, 56, 8120–8124. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Q.; Zheng, G.; Seh, Z.W.; Yao, H.; Cui, Y. Understanding the role of different conductive polymers in improving the nanostructured sulfur cathode performance. Nano Lett. 2013, 13, 5534–5540. [Google Scholar] [CrossRef]
- Kong, L.; Li, B.; Peng, H.; Zhang, R.; Xie, J.; Huang, J.; Zhang, Q. Porphyrin-derived graphene-based nanosheets enabling strong polysulfide chemisorption and rapid kinetics in lithium–sulfur batteries. Adv. Energy Mater. 2018, 8, 1800849. [Google Scholar] [CrossRef]
- Li, C.; Zhang, P.; Dai, J.; Shen, X.; Peng, Y.; Zhang, Y.; Zhao, J. Rational method for improving the performance of lithium–sulfur batteries: Coating the separator with lithium fluoride. ChemElectroChem 2017, 4, 1535–1543. [Google Scholar] [CrossRef]
- Wang, Y.; Ai, R.; Wang, F.; Hu, X.; Zeng, Y.; Hou, J.; Zhao, J.; Zhang, Y.; Zhang, Y.; Li, X. Research Progress on Multifunctional Modified Separator for Lithium–Sulfur Batteries. Polymers 2023, 15, 993. [Google Scholar] [CrossRef]
- Zhou, G.; Li, L.; Wang, D.W.; Shan, X.Y.; Pei, S.; Li, F.; Cheng, H.M. A flexible sulfur-graphene-polypropylene separator integrated electrode for advanced Li–S batteries. Adv. Mater. 2015, 27, 641–647. [Google Scholar] [CrossRef]
- Zhao, D.; Qian, X.; Jin, L.; Yang, X.X.; Wang, S.; Shen, X.; Yao, S.; Rao, D.; Zhou, Y.; Xi, X. Separator modified by Ketjen black for enhanced electrochemical performance of lithium–sulfur batteries. RSC Adv. 2016, 17, 13680–13685. [Google Scholar] [CrossRef]
- Chung, S.H.; Manthiram, A. A polyethylene glycol-supported microporous carbon coating as a polysulfide trap for utilizing pure sulfur cathodes in lithium–sulfur batteries. Adv. Mater. 2014, 26, 7352–7357. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Yen, Y.J.; Tseng, Y.H.; Chung, S.H. Module-designed carbon-coated separators for high-loading, high-sulfur-utilization cathodes in lithium–sulfur batteries. Molecules 2022, 77, 228. [Google Scholar] [CrossRef] [PubMed]
- Ponraj, R.; Kannan, A.G.; Ahn, J.H.; Lee, J.H.; Kang, J.; Han, B.; Kim, D.W. Effective trapping of lithium polysulfides using a functionalized carbon nanotube-coated separator for lithium–sulfur cells with enhanced cycling stability. ACS Appl. Mater. Interfaces 2017, 9, 38445–38454. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dong, Z.H.; Wang, D.; Zhang, F.X.; Jin, J. Covalent bond glued sulfur nanosheet-based cathode integration for long-cycle-life Li–S batteries. Nano Lett. 2013, 13, 6244–6250. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, D.; Zhang, F.; Jin, J. Interface chemistry guided long-cycle life Li–S battery. Nano Lett. 2013, 13, 4206–4211. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhao, T.; Lu, J.; Wu, F.; Li, L.; Chen, J.; Tan, G.; Ye, Y.; Amine, K. Graphene-based three-dimensional hierarchical sandwich-type architecture for high-performance Li/S batteries. Nano Lett. 2013, 13, 4642–4649. [Google Scholar] [CrossRef]
- Yu, X.; Joseph, J.; Manthiram, A. Suppression of the polysulfide-shuttle behavior in Li–S batteries through the development of a facile functional group on the polypropylene separator. Mater. Horiz. 2016, 3, 314–319. [Google Scholar] [CrossRef]
- Vizintin, A.; Lozinšek, M.; Chellappan, R.K.; Foix, D.; Krajnc, A.; Mali, G.; Drazic, G.; Genorio, B.; Dedryvère, R.; Dominko, R. Fluorinated reduced graphene oxide as an interlayer in Li–S batteries. Chem. Mater. 2015, 27, 7070–7081. [Google Scholar] [CrossRef]
- Lei, T.; Lei, T.; Chen, W.; Lv, W.; Huang, J.; Zhu, J.; Chu, J.; Yan, C.; Wu, C.; Yan, Y.; et al. Inhibiting polysulfide shuttling with a graphene composite separator for highly robust lithium–sulfur batteries. Joule 2018, 2, 2091–2104. [Google Scholar] [CrossRef]
- Zhao, Y.; Gu, Z.; Weng, W.; Zhou, D.; Liu, Z.; Fan, W.; Deng, S.; He, H.; Yao, X. Nitrogen doped hollow carbon nanospheres as efficient polysulfide restricted layer on commercial separators for high-performance lithium-sulfur batteries. Chinese Chem. Lett. 2023, 34, 107232. [Google Scholar] [CrossRef]
- Wu, P.; Hu, H.-Y.; Xie, N.; Wang, C.; Wu, F.; Pan, M.; Li, H.-F.; Wang, X.-D.; Zeng, Z.; Deng, S.; et al. A N-doped graphene-cobalt nickel sulfide aerogel as a sulfur host for lithium–sulfur batteries. RSC Adv. 2019, 9, 32247–32257. [Google Scholar] [CrossRef]
- Li, Y.; Fan, J.; Zhang, J.; Yang, J.; Yuan, R.; Chang, J.; Zheng, M.; Dong, Q. A honeycomb-like Co@N–C composite for ultrahigh sulfur loading Li–S batteries. ACS Nano 2017, 11, 11417–11424. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, Y.; Yuan, L.; Yi, Z.; Wu, C.; Liu, Y.; Strasser, P.; Huang, Y. A highly ordered meso@microporous carbon-supported sulfur@smaller sulfur core–shell structured cathode for Li–S batteries. ACS Nano 2014, 8, 9295–9303. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Tan, L.; Wang, X.D.; Liao, P.; Liu, Z.; Hou, P.P.; Zhou, Q.Y.; Jin, X.J.; Chao, M.; Shao, X.R.; et al. Porous 3D nitrogen-doped rGO/Co-Ni-S composite modified separator for high-capacity and stable lithium–sulfur batteries. Mater. Res. Bull. 2022, 145, 111550. [Google Scholar] [CrossRef]
- Fang, R.; Zhao, S.; Pei, S.; Qian, X.; Hou, P.-X.; Cheng, H.-M.; Liu, C.; Li, F. Toward more reliable lithium–sulfur batteries: An all-graphene cathode structure. ACS Nano 2016, 10, 8676–8682. [Google Scholar] [CrossRef] [PubMed]
- Díez, N.; Sevilla, M.; Fuertes, A.B. N/S-Co-doped porous carbon nanoparticles serving the dual function of sulfur host and separator coating in lithium–sulfur batteries. ACS Appl. Energy Mater. 2020, 3, 3397–3407. [Google Scholar] [CrossRef]
- Song, X.; Wang, S.; Chen, G.; Gao, T.; Bao, Y.; Ding, L.X.; Wang, H. Fe-N-doped carbon nanofiber and graphene modified separator for lithium–sulfur batteries. Chem. Eng. J. 2018, 333, 564–571. [Google Scholar] [CrossRef]
- Zhang, Z.; Lai, Y.; Zhang, Z.; Zhang, K.; Li, J. Al2O3-coated porous separator for enhanced electrochemical performance of lithium sulfur batteries. Electrochim. Acta 2014, 129, 55–61. [Google Scholar] [CrossRef]
- Han, H.; Niu, S.; Zhao, Y.; Tan, T.; Zhang, Y. TiO2/porous carbon composite-decorated separators for lithium/sulfur battery. Nanoscale Res. Lett. 2019, 14, 176. [Google Scholar] [CrossRef]
- Gao, Z.; Xue, Z.; Miao, Y.; Chen, B.; Xu, J.; Shi, H.; Tang, T.; Zhao, X. TiO2@Porous carbon nanotubes modified separator as polysulfide barrier for lithium–sulfur batteries. J. Alloy. Compd. 2022, 906, 164249. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Zhang, S.; Jia, W.; Wang, X.; Guo, Y.; Jia, D.; Wang, L. Decoration of silica nanoparticles on polypropylene separator for lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2017, 9, 7499–7504. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, C.; Zhou, G.; Lv, W.; Ling, G.; Zhi, L.; Yang, Q.H. Catalytic effects in lithium–sulfur batteries: Promoted sulfur transformation and reduced shuttle effect. Adv. Sci. 2018, 5, 1700270. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cen, Y.; Xiang, Q.; Aslam, M.K.; Hu, B.; Li, W.; Tang, Y.; Yu, Q.; Liu, Y.; Chen, C. Vanadium dioxide-reduced graphene oxide binary host as an efficient polysulfide plague for high-performance lithium–sulfur batteries. J. Mater. Chem. 2019, 7, 1658–1668. [Google Scholar] [CrossRef]
- Yuan, H.; Peng, H.J.; Li, B.Q.; Xie, J.; Kong, L.; Zhao, M.; Chen, X.; Huang, J.Q.; Zhang, Q. Conductive and catalytic triple-phase interfaces enabling uniform nucleation in high-rate lithium–sulfur batteries. Adv. Energy Mater. 2019, 9, 1802768. [Google Scholar] [CrossRef]
- Qian, X.; Zhao, D.; Jin, L.; Shen, X.; Yao, S.; Rao, D.; Zhou, Y.; Xi, X.M. Hollow spherical Lanthanum oxide coated separator for high electrochemical performance lithium–sulfur batteries. Mater. Res. Bull. 2017, 94, 104–112. [Google Scholar] [CrossRef]
- Tan, L.; Li, X.; Wang, Z.; Guo, H.; Wang, J. Lightweight reduced graphene Oxide@MoS2 interlayer as polysulfide barrier for high-performance lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2018, 10, 3707–3713. [Google Scholar] [CrossRef]
- Fan, H.J.; Knez, M.; Scholz, R.; Nielsch, K.; Pippel, E.; Hesse, D.; Zacharias, M.; Gösele, U. Monocrystalline spinel nano-tube fabrication based on the kirkendall efect. Nat. Mater. 2006, 5, 627–631. [Google Scholar] [CrossRef]
- Zheng, N.; Jiang, G.; Chen, X.; Mao, J.; Jiang, N.; Li, Y. Battery separators functionalized with edge-rich MoS2/C hollow microspheres for the uniform deposition of Li2S in high-performance lithium–sulfur batteries. Nano-Micro Lett. 2019, 11, 43. [Google Scholar] [CrossRef]
- Mao, L.; Mao, J. Ultralow-decay lithium–sulfur batteries: Modified separator with graphene/ZnS(en)0.5 exfoliation nanosheets. J. Solid State Chem. 2020, 290, 121555. [Google Scholar] [CrossRef]
- Yao, W.; Zheng, W.; Xu, J.; Tian, C.; Han, K.; Sun, W.; Xiao, S. ZnS-SnS@NC Heterostructure as robust lithiophilicity and sulfiphilicity mediator toward high-rate and long-life lithium–sulfur batteries. ACS Nano 2021, 15, 7114–7130. [Google Scholar] [CrossRef]
- Wang, G.; Jiao, Q.; Zhang, Z.; Zhao, Y.; Lin, C.; Zhang, X.; Ma, H.; Dai, S.; Xu, T. Improved electrochemical behavior of Li–S battery with functional WS2@PB–PPy–modified separator. Chem. Eng. J. Adv. 2021, 8, 100145. [Google Scholar] [CrossRef]
- Xiao, Y.; Hwang, J.Y.; Sun, Y.K. Transition metal carbide-based materials: Synthesis and applications in electrochemical energy storage. J. Mater. Chem. A 2016, 4, 10379–10393. [Google Scholar] [CrossRef]
- Cui, Z.; Yao, J.; Mei, T.; Zhou, S.; Hou, B.; Li, J.; Li, J.; Wang, J.; Qian, J.; Wang, X. Strong lithium polysulfides chemical trapping of TiC-TiO2/S composite for long-cycle lithium–sulfur batteries. Electrochim. Acta 2019, 298, 43–51. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, P.; Li, B.; Zhang, S.; Liu, K.; Hou, R.; Zhang, X.; Silva, S.R.; Shao, G. Vertically aligned graphene nanosheets on multi-yolk/shell structured TiC@C nanofibers for stable Li–S batteries. Energy Storage Mater. 2020, 27, 159–168. [Google Scholar] [CrossRef]
- Liu, S.; Luo, J.; Xiong, Y.; Chen, Z.; Zhang, K.; Rui, G.; Wang, L.; Hu, G.; Jiang, J.; Mei, T. Taming polysulfides in an Li–S battery with low-temperature one-step chemical synthesis of titanium carbide nanoparticles from waste PTFE. Front. Chem. 2021, 9, 638557. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, Z. Flexible TiC modified separators trapping polysulfide for high performance lithium–sulfur batteries. J. Alloys Compd. 2021, 856, 156609. [Google Scholar] [CrossRef]
- Moon, S.-H.; Kim, J.-H.; Shin, J.-H.; Jang, J.-S.; Kim, S.-B.; Lee, S.-N.; Kwon, S.-H.; Park, K.-W. High absorption and fast polysulfides conversion of duel functional separator based on mesoporous-WC/rGO composite for lithium–sulfur batteries. J. Alloy. Compd. 2022, 904, 164120. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, Z.; He, H.; Yu, Y.; Li, X.; Xie, W.; Yang, Z.; Cai, J. Cobalt-tungsten bimetallic carbide nanoparticles as efficient catalytic material for high-performance lithium–sulfur batteries. ChemSusChem 2019, 12, 4866–4873. [Google Scholar] [CrossRef]
- Cai, W.; Li, G.; Zhang, K.; Xiao, G.; Wang, C.; Ye, K.; Chen, Z.; Zhu, Y.; Qian, Y. Conductive nanocrystalline niobium carbide as high efficiency polysulfides tamer for lithium–sulfur batteries. Adv. Funct. Mater. 2017, 28, 1704865. [Google Scholar] [CrossRef]
- Abghoui, Y.; Skúlasson, E. Transition Metal Nitride Catalysts for Electrochemical Reduction of Nitrogen to Ammonia at Ambient Conditions. Procedia Computer Sci. 2015, 51, 1897–1906. [Google Scholar] [CrossRef]
- Lan, L.; Chen, D.; Yao, Y.; Peng, X.; Wu, J.; Li, Y.; Ping, J.; Ying, Y. Phase-dependent fluorescence quenching efficiency of MoS2 nanosheets and their applications in multiplex target biosensing. ACS Appl. Mater. Interfaces 2018, 10, 42009–42017. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Song, X.; Wang, S.; Chen, X.; Wang, H. Two dimensional molybdenum nitride nanosheets modified Celgard separator with multifunction for Li–S batteries. J. Power Sources 2018, 408, 58–64. [Google Scholar] [CrossRef]
- Kim, H.S.; Kang, H.J.; Lim, H.; Hwang, H.J.; Park, J.W.; Lee, T.G.; Cho, S.Y.; Jang, S.G.; Jun, Y.S. Boron nitride nanotube-based separator for high-performance lithium–sulfur batteries. Nanomaterials 2022, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kang, Q.; Jiang, P.; Huang, X. Rapid, high-efficient and scalable exfoliation of high-quality boron nitride nanosheets and their application in lithium–sulfur batteries. Nano Res. 2021, 14, 2424–2431. [Google Scholar] [CrossRef]
- Luo, M.; Bai, Y.; Sun, R.; Wang, Z.; Sun, W.; Lin, P.; Dai, X.; Sun, K. Enhanced performance of lithium–sulfur batteries with Co-doped g-C3N4 nanosheet-based separator. Ind. Eng. Chem. Res. 2021, 60, 1231–1240. [Google Scholar] [CrossRef]
- Huang, S.; Huixiang, E.; Yang, Y.; Zhang, Y.; Ye, M.; Li, C.C. Transition metal phosphides: New generation cathode host/separator modifier for Li–S batteries. J. Mater. Chem. A 2021, 9, 7458–7480. [Google Scholar] [CrossRef]
- Chen, X.; Ding, X.; Wang, C.; Feng, Z.; Xu, L.; Gao, X.; Zhai, Y.; Wang, D. A multi-shelled CoP nanosphere modified separator for highly efficient Li–S batteries. Nanoscale 2018, 10, 13694–13701. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, K.; Zhu, Z.; Zhang, R.; Li, N.; Zhao, C. CoP/C nanocubes-modified separator suppressing polysulfide dissolution for high-rate and stable lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2019, 12, 2497–2504. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, H.; Cheng, X.; Ren, R.; Meng, Z.; Wang, X. Multifunctional FeP/spongy carbon modified separator with enhanced polysulfide immobilization and conversion for flame-retardant lithium–sulfur batteries. Chem. Select 2021, 6, 7098–7102. [Google Scholar] [CrossRef]
- Mao, J.; Niu, D.; Huang, G.; Jin, X.; Wei, C.; Cai, J.; Li, Y.; Shi, J. A Ni/Ni2P heterostructure in modified porous carbon separator for boosting polysulfide catalytic conversion. Sci. China Mater. 2022, 65, 2453–2462. [Google Scholar] [CrossRef]
- Zhou, G.; Tian, H.; Jin, Y.; Tao, X.; Liu, B.; Zhang, R.; Seh, Z.W.; Zhuo, D.; Liu, Y.; Sun, J.; et al. Catalytic oxidation of Li2S on the surface of metal sulfides for Li−S batteries. PNAS 2017, 114, 840–845. [Google Scholar] [CrossRef]
- Peng, H.J.; Zhang, G.; Chen, X.; Zhang, Z.W.; Xu, W.T.; Huang, J.Q.; Zhang, Q. Enhanced electrochemical kinetics on conductive polar mediators for lithium–sulfur batteries. Angew. Chem. Int. Ed. 2016, 55, 12990–12995. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, C.; Valdez, J.; Motta Meira, D.; He, W.; Wang, Y.; Harnagea, C.; Lu, Q.; Guner, T.; Wang, H.; et al. Phase-enabled metal-organic framework homojunction for highly selective CO2. Photoreduction. Nat. Comm. 2021, 12, 1231. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, Y.; Ban, Y.; Jin, H.; Jiao, W.; Liu, X.; Yang, W. Metal-organic framework nanosheets as building blocks for molecular sieving membranes. Science 2014, 346, 1356–1359. [Google Scholar] [CrossRef]
- Wang, B.; Cote, A.P.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. Colossal cages in zeolitic imidazolate frameworks as selective carbon dioxide reservoirs. Nature 2008, 453, 207–211. [Google Scholar] [CrossRef]
- Bai, S.; Sheng, T.; Tan, C.; Zhu, Q.; Huang, Y.; Jiang, H.; Hu, S.; Fu, R.; Wu, X. Distinct anion sensing by a 2D self-assembled Cu(I)-based metal–organic polymer with versatile visual colorimetric responses and efficient selective separations via anion exchange. J. Mater. Chem. A 2013, 1, 2970. [Google Scholar] [CrossRef]
- Mori, R. Cathode materials for lithium–sulfur battery: A review. J. Solid State Electrochem. 2023, 27, 813–839. [Google Scholar] [CrossRef]
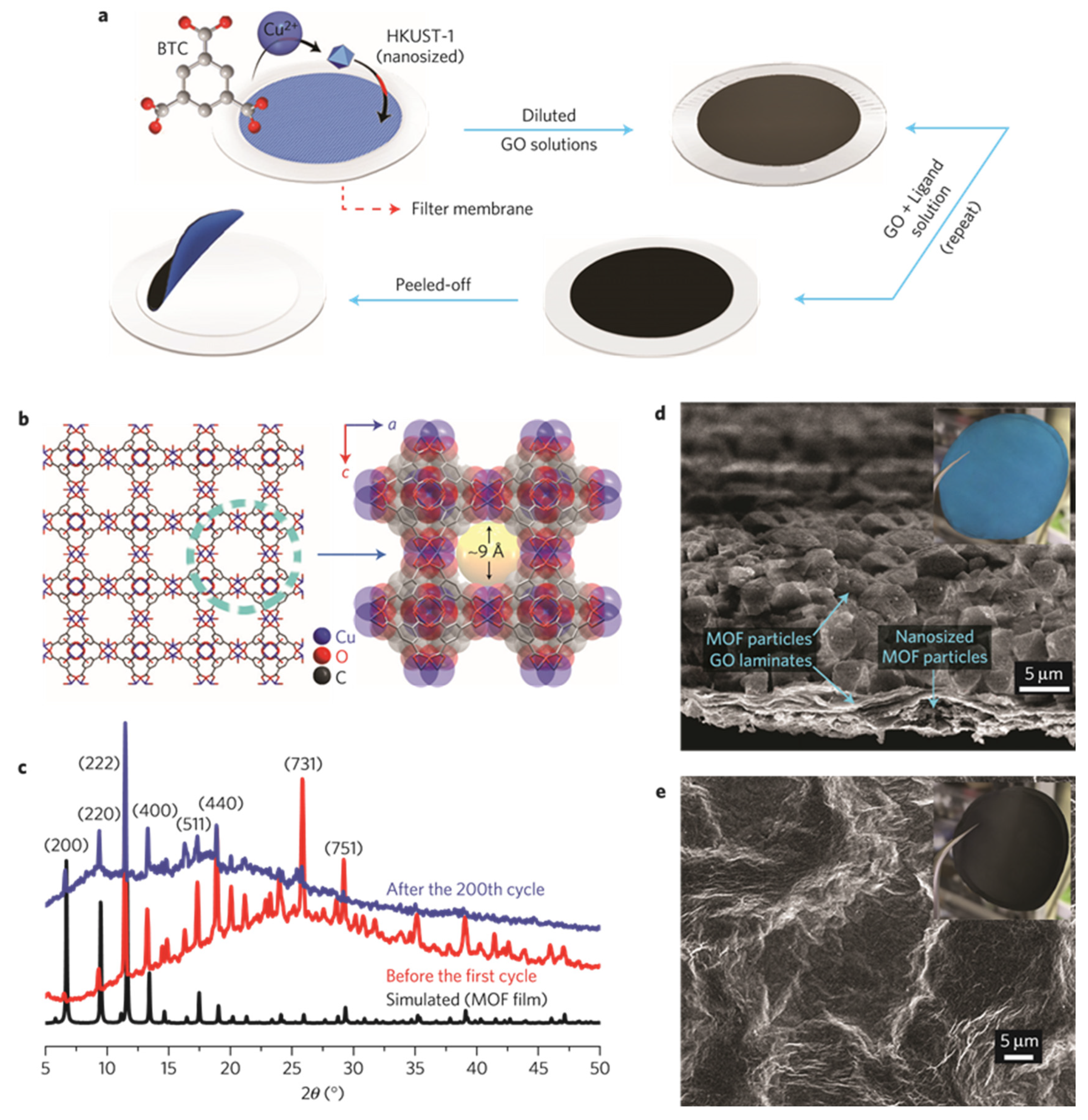
- Bai, S.; Liu, X.; Zhu, K.; Wu, S.; Zhou, H. Metal-organic framework-based separator for lithium–sulfur batteries. Nat. Energy 2016, 1, 16094. [Google Scholar] [CrossRef]
- Dang, B.; Li, Q.; Luo, Y.; Zhao, R.; Li, J.; Wu, F. Metal-organic framework-based glass fiber separator as an efficacious polysulfide barrier and dendrite suppressor for lithium–sulfur batteries. J. Alloys Compd. 2022, 915, 165375. [Google Scholar] [CrossRef]
- Su, Y.; Wang, W.; Wang, W.; Wang, A.; Huang, Y.; Guan, Y. Cerium-based MOF as a separator coating for high-performance lithium–sulfur batteries. J. Electrochem. Soc. 2022, 169, 030528. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, X.; Deng, X.; Huang, S.; Xiao, M.; Wang, S.; Han, D.; Meng, Y. Construction of KB@ZIF-8/PP composite separator for lithium–sulfur batteries with enhanced electrochemical performance. Polymers 2021, 13, 4210. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Fowley, C.; Nomikou, N.; McHale, A.P.; McCaughan, B.; Callan, J.F. Extending the tissue penetration capability of conventional photosensitisers: A carbon quantum dot–protoporphyrin IX conjugate for use in two-photon excited photodynamic therapy. Chem Commun (Camb) 2013, 49, 8934–8936. [Google Scholar] [CrossRef]
- Xu, Q.; Niu, Y.; Li, J.; Yang, Z.; Gao, J.; Ding, L.; Ni, H.; Zhu, P.; Liu, Y.; Tang, Y.; et al. Recent progress of quantum dots for energy storage applications. Carbon. Neutrality 2022, 1, 13. [Google Scholar] [CrossRef]
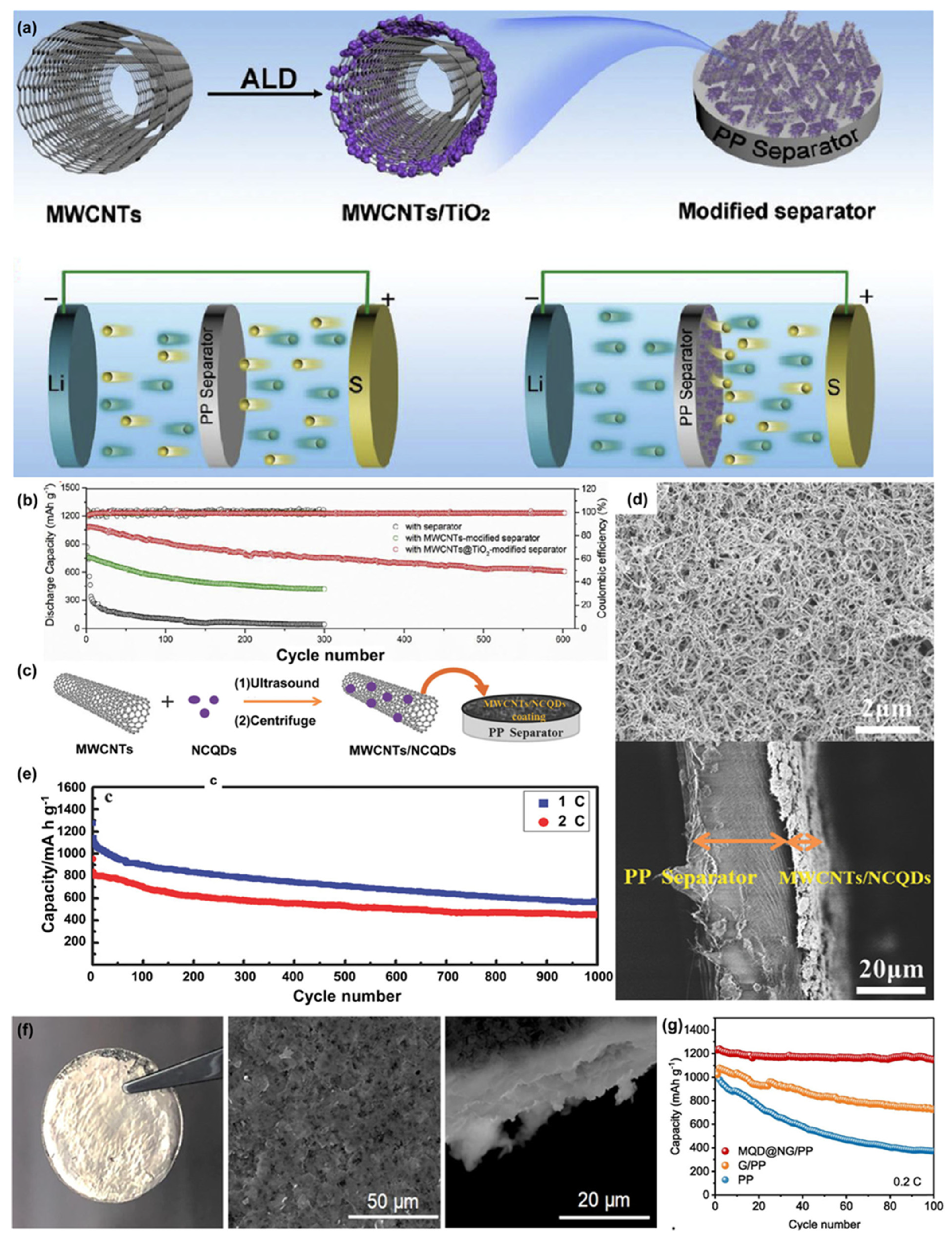
- Ding, H.; Zhang, Q.; Liu, Z.; Wang, J.; Ma, R.; Fan, L.; Wang, T.; Zhao, J.; Ge, J.; Lu, X.; et al. TiO2 quantum dots decorated multi-walled carbon nanotubes as the multifunctional separator for highly stable lithium sulfur batteries. Electrochim. Acta 2018, 284, 314–320. [Google Scholar] [CrossRef]
- Pang, Y.; Wei, J.; Wang, Y.; Xia, Y. Synergetic protective effect of the ultralight MWCNTs/NCQDs modified separator for highly stable lithium–sulfur batteries. Adv. Energy Mater. 2018, 8, 1702288. [Google Scholar] [CrossRef]
- Chung, S.H.; Manthiram, A. High-performance Li–S batteries with an ultra-lightweight MWCNT-coated separator. J. Phys. Chem. Lett. 2014, 5, 197. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Chen, D.; Wang, Z.; Qi, F.; Zhang, X.; Wang, X.; Hu, Y.; Wang, B.; Zhang, W.; Chen, Y.; et al. Mo2C quantum dots@graphene functionalized separator toward high-current-density lithium metal anodes for ultrastable Li–S batteries. Chem. Eng. J. 2020, 399, 125837. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, Z.; Jiang, X.; Zhang, Y.; Wang, X.; Zhang, S. Multi-functional ZnS quantum Dots/Graphene aerogel modified separator for high performance lithium–sulfur batteries. Electrochim. Acta 2022, 422, 140496. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, Y.; Chen, H.; Wang, Y.; Chen, Q.; Hou, G.; Wen, M.; Tang, Y. MoP quantum dot-modified N,P-carbon nanotubes as a multifunctional separator coating for high-performance lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2022, 14, 16289–16299. [Google Scholar] [CrossRef]
- Liu, Q.; Han, X.; Park, H.; Kim, J.; Xiong, P.; Yuan, H.; Yeon, J.S.; Kang, Y.; Park, J.M.; Dou, Q.; et al. Layered double hydroxide quantum dots for use in a bifunctional separator of lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2021, 13, 179780. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.R.; Gao, G.; Li, F.T.; Ma, T.Y.; Du, A.; Qiao, S.Z. Ti3C2 MXene co-catalyst on metal sulfide photo-absorbers for enhanced visible-light photocatalytic hydrogen production. Nat. Commun. 2017, 8, 13907. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.S.; Meng, X.; Zhu, K.; Du, F.; Chen, G.; Wei, Y.J.; Gogotsi, Y.; Gao, Y. Li-ion uptake and increase in interlayer spacing of Nb4C3 MXene. Energy Storage Mater. 2017, 8, 42–48. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef]
- Er, D.; Li, J.; Naguib, M.; Gogotsi, Y.; Shenoy, V.B. Ti3C2 MXene as a high capacity electrode material for metal (Li, Na, K, Ca) ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 11173–11179. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef]
- Saeed, M.A.; Shahzad, A.; Rasool, K.; Mateen, F.; Oh, J.; Shim, J.W. 2D MXene: A potential candidate for photovoltaic cells? a critical review. Adv. Sci. 2020, 9, 2104743. [Google Scholar] [CrossRef]
- Chen, X.; Shi, Z.; Tian, Y.; Lin, P.; Wu, D.; Li, X.; Dong, B.; Xu, W.; Fang, X. Two-dimensional Ti3C2 MXene-based nanostructures for emerging optoelectronic applications. Mater. Horiz. 2021, 8, 2929–2963. [Google Scholar] [CrossRef]
- Wojciechowski, T.; Rozmysłowska-Wojciechowska, A.; Matyszczak, G.; Wrzecionek, M.; Olszyna, A.; Peter, A.; Mihaly-Cozmuta, A.; Nicula, C.; Mihaly-Cozmuta, L.; Podsiadło, S.; et al. Ti2C MXene modified with ceramic oxide and noble metal nanoparticles: Synthesis, morphostructural properties, and high photocatalytic activity. Inorg. Chem. 2019, 58, 7602–7614. [Google Scholar] [CrossRef]
- Gao, L.; Chen, H.; Kuklin, A.V.; Wageh, S.; Al-Ghamdi, A.A.; Ågren, H.; Zhang, H. Optical properties of few-layer Ti3CN MXene: From experimental observations to theoretical calculations. ACS Nano 2022, 16, 3059–3069. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Mashtali, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
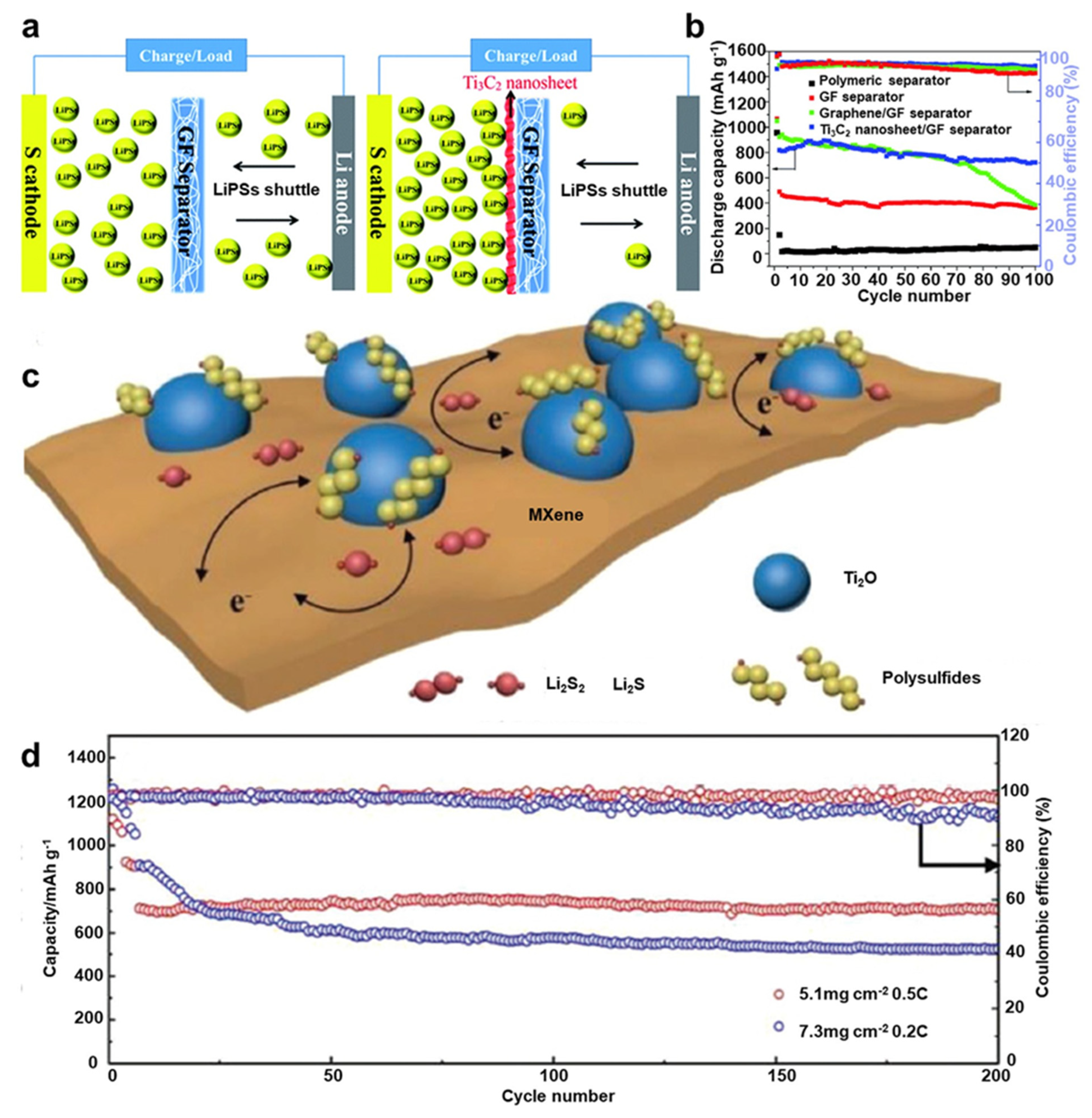
- Lin, C.; Zhang, W.; Wang, L.; Wang, Z.; Zhao, W.; Duan, W.; Zhao, Z.; Liu, B.; Jin, J. A few-layered Ti3C2 nanosheet/glass fiber composite separator as a lithium polysulphide reservoir for high-performance lithium–sulfur batteries. J. Mater. Chem. 2016, 4, 5993–5998. [Google Scholar] [CrossRef]
- Jiao, L.; Zhang, C.; Geng, C.; Wu, S.; Li, H.; Lv, W.; Tao, Y.; Chen, Z.; Zhou, G.; Li, J.; et al. Capture and catalytic conversion of polysulfides by in situ built TiO2-MXene heterostructures for lithium–sulfur batteries. Adv. Energy Mater. 2019, 9, 190021. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, S.; Jia, X.; Yang, J.; Li, Y.; Liao, J.; Song, H. Freestanding sandwich-like hierarchically TiS2–TiO2/Mxene bi-functional interlayer for stable Li–S batteries. Carbon 2022, 188, 533–542. [Google Scholar] [CrossRef]
- Li, P.; Lv, H.; Li, Z.; Meng, X.; Lin, Z.; Wang, R.; Li, X. The Electrostatic Attraction and Catalytic Effect Enabled by Ionic–Covalent Organic Nanosheets on MXene for Separator Modification of Lithium–Sulfur Batteries. Adv. Mater. 2021, 33, 2007803. [Google Scholar] [CrossRef]
- Wang, J.; Zhai, P.; Zhao, T.; Li, M.; Yang, Z.; Zhang, H.; Huang, J. Laminar MXene-Nafion-modified separator with highly inhibited shuttle effect for long-life lithium–sulfur batteries. Electrochimica Acta 2019, 320, 134558. [Google Scholar] [CrossRef]
- He, Y.; Qiao, Y.; Chang, Z.; Cao, X.; Jia, M.; He, P.; Zhou, H. Developing A “polysulfide-phobic” strategy to restrain shuttle effect in lithium–sulfur batteries. Angew. Chem. Int. Ed. 2019, 58, 11774–11778. [Google Scholar] [CrossRef]
- Huang, J.Q.; Zhang, Q.; Peng, H.J.; Liu, X.Y.; Qian, W.Z.; Wei, F. Ionic shield for polysulfides towards highly-stable lithium–sulfur batteries. Energy Environ. Sci. 2013, 7, 347–353. [Google Scholar] [CrossRef]
- Zhang, Z.; Yi, S.; Wei, Y.; Bian, H.; Wang, R.; Min, Y. Lignin nanoparticle-coated celgard separator for high-performance lithium–sulfur batteries. Polymers 2019, 11, 1946. [Google Scholar] [CrossRef]
- Henriksson, M.; Berglund, L.A.; Isaksson, P.; Lindstrom, T.; Nishino, T. Cellulose nanopaper structures of high toughness. Biomacromolecules 2008, 9, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Pavlin, N.; Hribernik, S.; Kapun, G.; Talian, S.D.; Njel, C.; Dedryvère, R.; Dominko, R. The role of cellulose based separator in lithium sulfur batteries. J. Electrochem. Soc. 2019, 166, A5237–A5243. [Google Scholar] [CrossRef]
- Tian, Y.W.; Zhang, Y.J.; Wu, L.; Dong, W.D.; Huang, R.; Dong, P.Y.; Yan, M.; Liu, J.; Mohamed, H.S.H.; Chen, L.H.; et al. Bifunctional Separator with Ultra-Lightweight MnO2 Coating for Highly Stable Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2023, 15, 6877–6887. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, Y.; Zhan, H. Advanced separators for lithium–ion batteries. IOP Conf. Ser. Earth Environ. Sci. 2022, 1011, 012009. [Google Scholar] [CrossRef]
- Huang, X. Separator technologies for lithium-ion batteries. J. Solid. State Electrochem. 2011, 15, 649–662. [Google Scholar] [CrossRef]
- Deimede, V.; Elmasides, C. Separators for lithium-ion batteries: A review on the production processes and recent developments. Energy Technol. 2015, 3, 453–468. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Yang, M.; Chen, W. High-safety separators for lithium-ion batteries and sodium-ion batteries: Advances and perspective, energy storage materials. ScienceDirect 2021, 41, 522–545. [Google Scholar]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Francis, C.F.J.; Kyratzis, I.L.; Best, A.S. Lithium-ion battery separators for ionic-liquid. electrolytes: A review. Adv. Mater. 2020, 32, 1904205. [Google Scholar] [CrossRef]
- Xie, Y.; Zou, H.; Xiang, H.; Xia, R.; Liang, D.; Shi, P.; Dai, S.; Wang, H. Enhancement on the wettability of lithium battery separator toward nonaqueous electrolytes. J. Membr. Sci. 2016, 503, 25–30. [Google Scholar] [CrossRef]
- Mori, R.; Yokokawa, K. Silicon Coupling Agent Treatment of Cathode Material for Lithium Sulfur Battery. JP Patent No. 2022-155874, 14 October 2022. [Google Scholar]

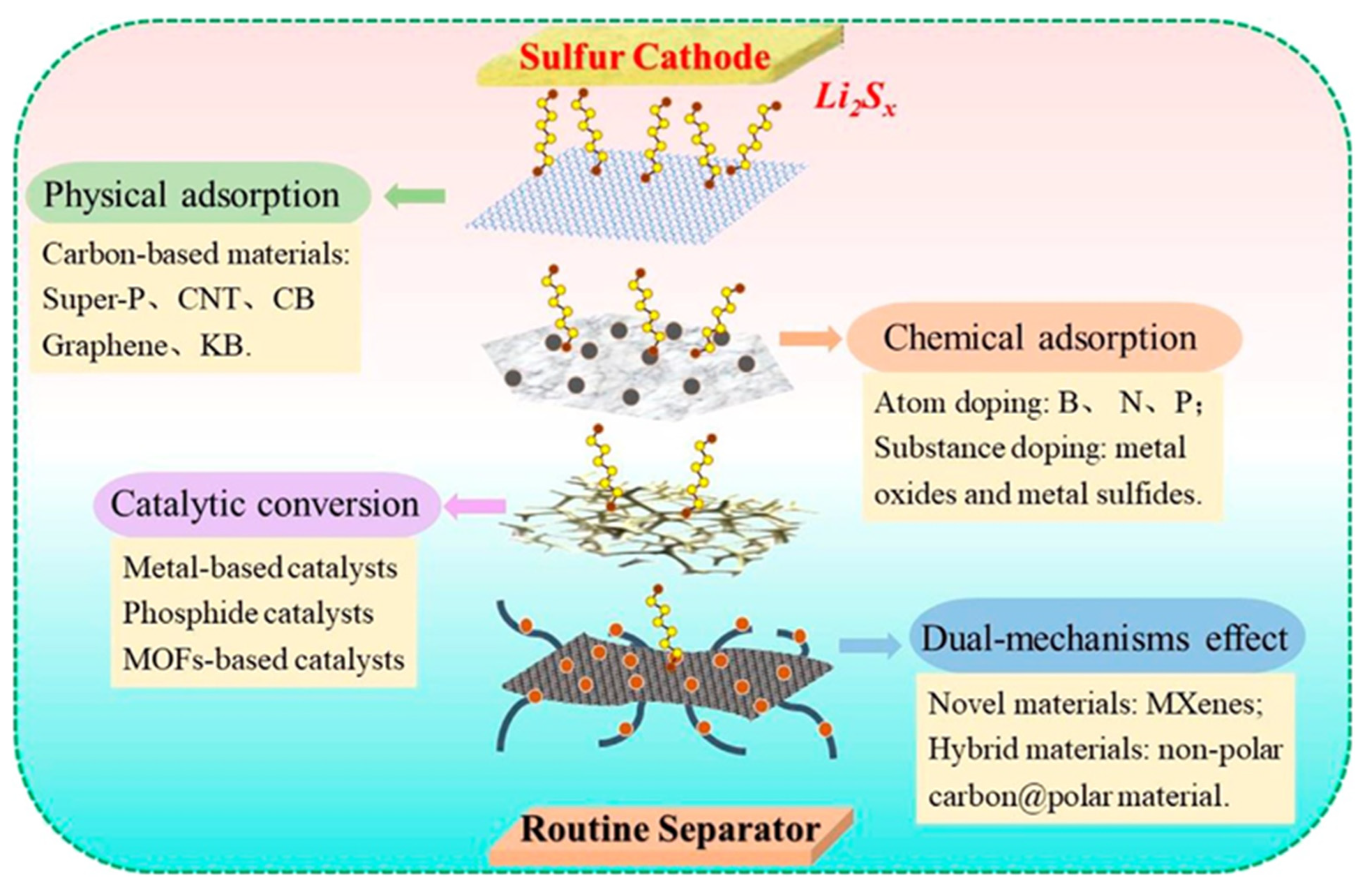

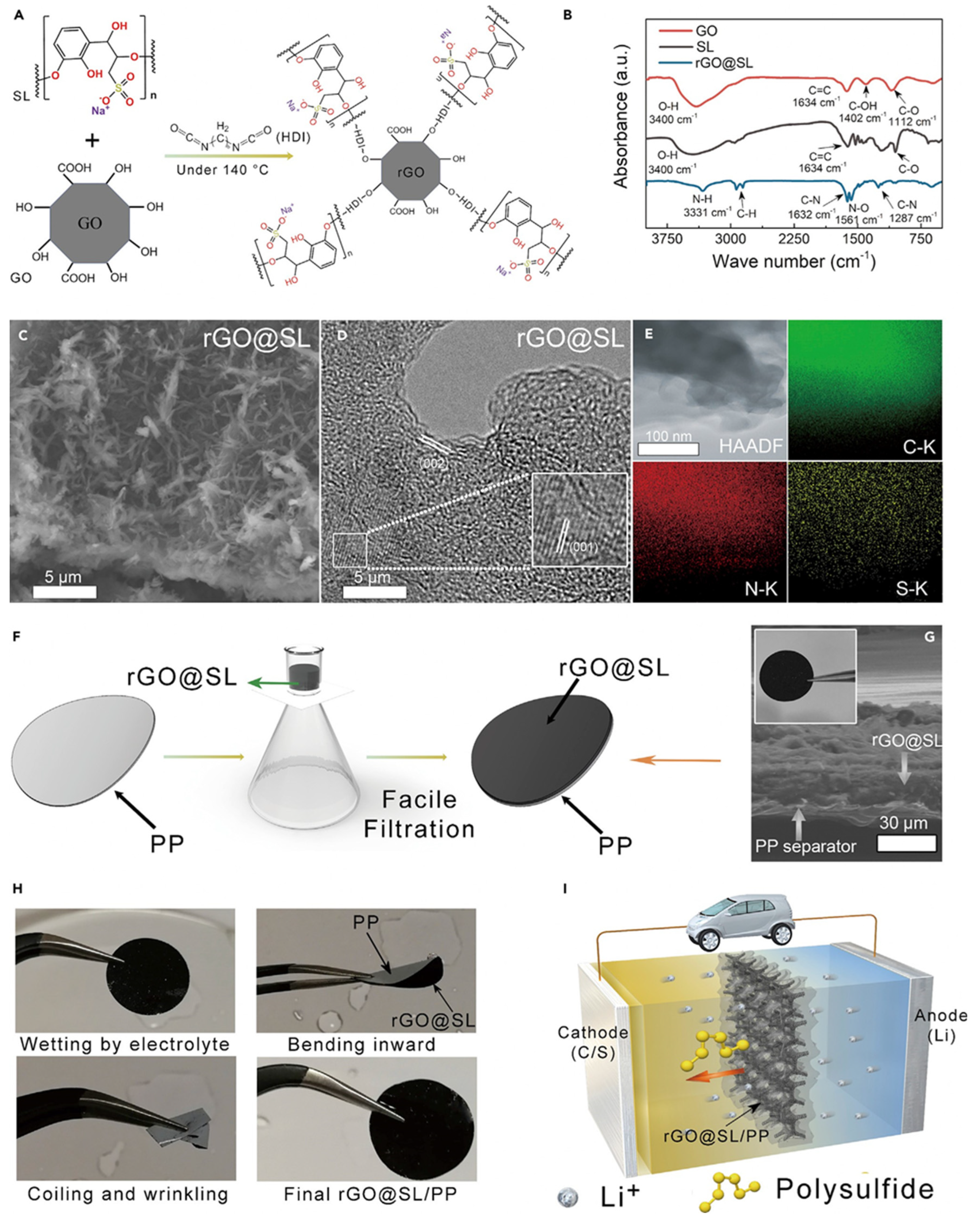
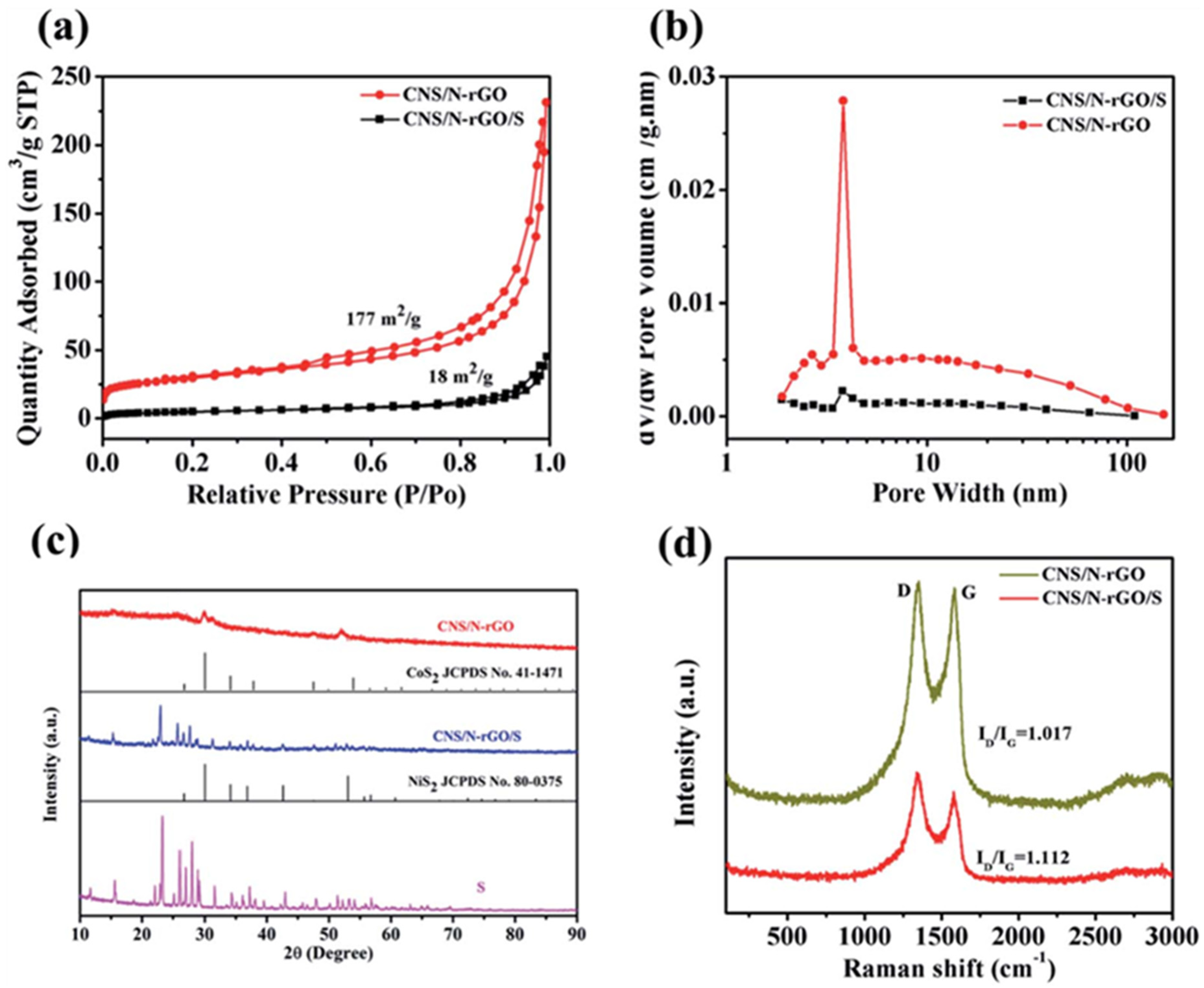


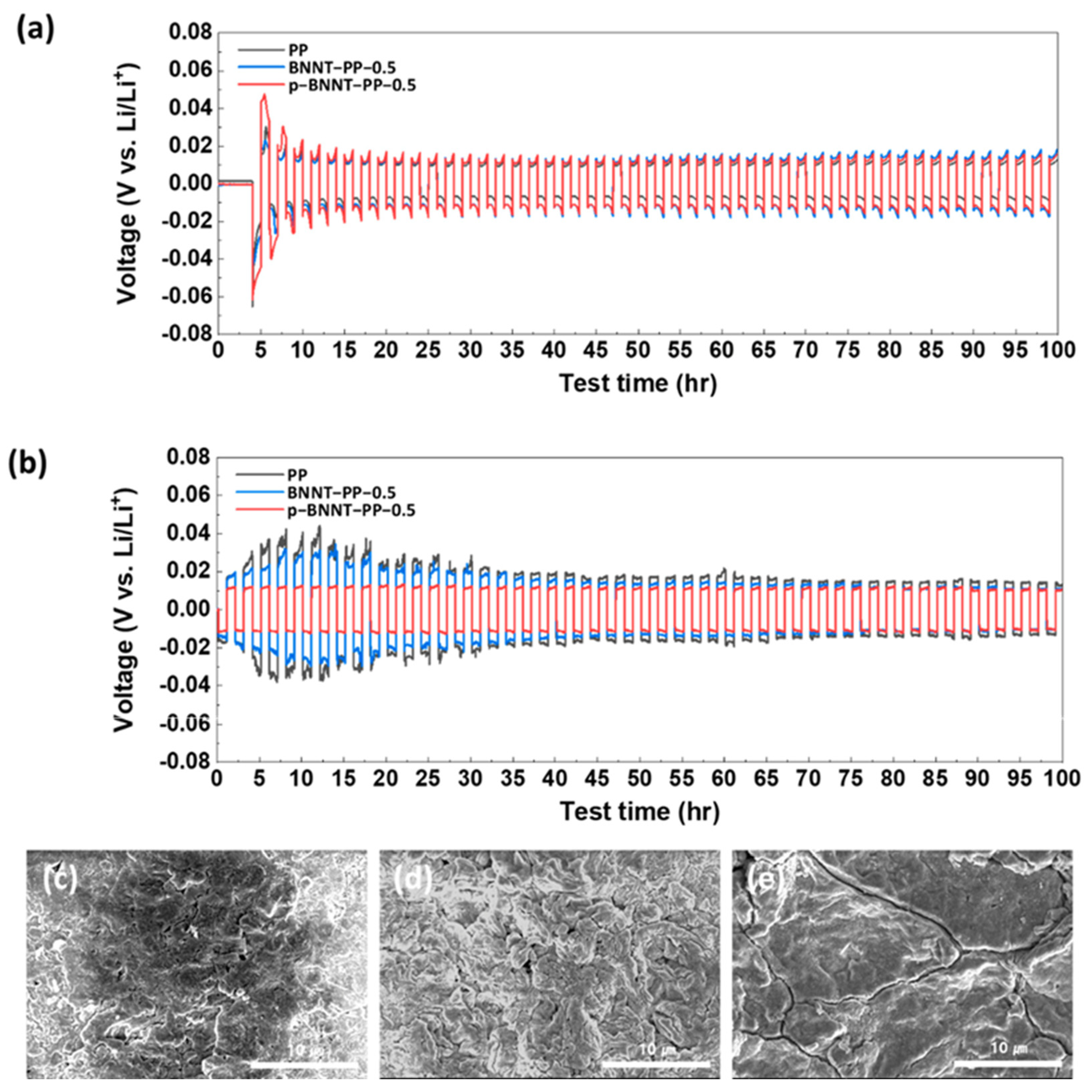

| Materials on Separator | Current Density (C, 1675 mAh g−1) | Cycle Number | Initial Discharge Capacity (mAh g−1) | Capacity Retention (%) (Fadeing Rate) | Reference |
|---|---|---|---|---|---|
| Metal | |||||
| Mg nanoparticles | 400 | 135–140 | 80 | [39] | |
| Silver | 0.5 | 300 | 131 | 92 | [40] |
| Ceramic | |||||
| Boron Nitride | 4 | 250 | 1018 | 69 | [43] |
| SiO2 Nanotubes | 0.5 | 100 | 1266 | [44] | |
| Hollow Porous SiO2 Nanocubes | 100 mAh g−1 | 30 | 919 (30th) | [45] | |
| SiO2 | 0.2 | 200 | 956.3 | 36 | [47] |
| Al2O3 | 0.2 | 100 | 1067.7 | 75 | [48] |
| Other Functional Materials | |||||
| polypyrrole | 0.5 | 250 | (0.083% per cycle) | [52] | |
| porphyrin-derived graphene | 0.5 | 300 | (0.099% per cycle) | [56] | |
| lithium fluoride | 0.2 | 200 | 69.30% | [57] | |
| Materials on Separator | Current Density (C, 1675 mAh g−1) | Cycle Number | Initial Discharge Capacity (mAh g−1) | Capacity Retention (%) (Fadeing Rate) | Reference |
|---|---|---|---|---|---|
| Carbonaceous Materials | |||||
| Ketjen black | 0.1 | 100 | 1318 | [60] | |
| carbon | 0.1 | 200 | 1112 | 63.8 | [62] |
| carbon nanotube | 0.5 | 400 | 1056 | (0.11% per cycle) | [63] |
| Graphene Composite | 2 | 1000 | 707 | 74.0% | [69] |
| nitrogen-doped reduced GO/Co-Ni-S composite | 0.1 | 350 | 1524 | [74] | |
| nitrogen and sulfur doped carbon | 2 | 500 | 841 (0.1C) | (0.089% per cycle) | [76] |
| Fe, nitrogen-doped carbon nanofibers and 2D graphene | 0.5 | 500 | 847.9 | (0.053% per cycle) | [77] |
| Metal Oxide | |||||
| Al2O3 | 0.2 | 50 | 967 | 70.0% | [78] |
| TiO2/carbon composite | 0.1 | 150 | 926 | 75.0% | [79] |
| TiO2 modified carbon nanotubes | 0.5 | 900 | 1103.9 | (0.066% per cycle) | [80] |
| SiO2 | 0.2 | 200 | 956.3 | 64.0% | [81] |
| CoSe2/grapheme oxide | 6 | 500 | 916 | 50.1% | [84] |
| Lanthanum oxide | 1 | 200 | 966 | 74.5% | [85] |
| Metal Sulfide | |||||
| reduced Graphene Oxide@MoS2 | 0.5 | 500 | 1122 | 0.116% per cycle | [86] |
| MoS2/C hollow microsphere | 1 | 1000 | 935 | (0.053% per cycle) | [88] |
| ZnS nanosheet/graphene | 0.1 | 100 | 1165.9 | 58.8% | [89] |
| WS2 Prussian Blue-Polypyrrole | 300 | 1050 | 62.0% | [91] | |
| Metal Carbide | |||||
| TiC-TiO2 | 1 | 500 | 1218 | 58.6% | [93] |
| WC/reduced Graphene Oxide composite | 1 | 300 | 83.0% | [97] | |
| Co3W3Carbide@C | 1 A g−1 | 500 | (0.06% per cycle) | [98] | |
| NbC | 5 | 1500 | (0.037% per cycle) | [99] | |
| Metal Nitride | |||||
| MoNx | 0.1 | 500 | 1298 | (0.063% per cycle) | [102] |
| BN Nanotube | 0.3 | 200 | 1429 | [103] | |
| Co-doped g-C3N4 | 0.2 | 100 | 1121 | 95.0% | [105] |
| Phosphide | |||||
| CoP nanosphere | 1 | 500 | (0.078% per cycle) | [107] | |
| CoP/C | 1 | 500 | 938 | (0.08% per cycle) | [108] |
| FeP/C | 1 | 400 | 526 | [109] | |
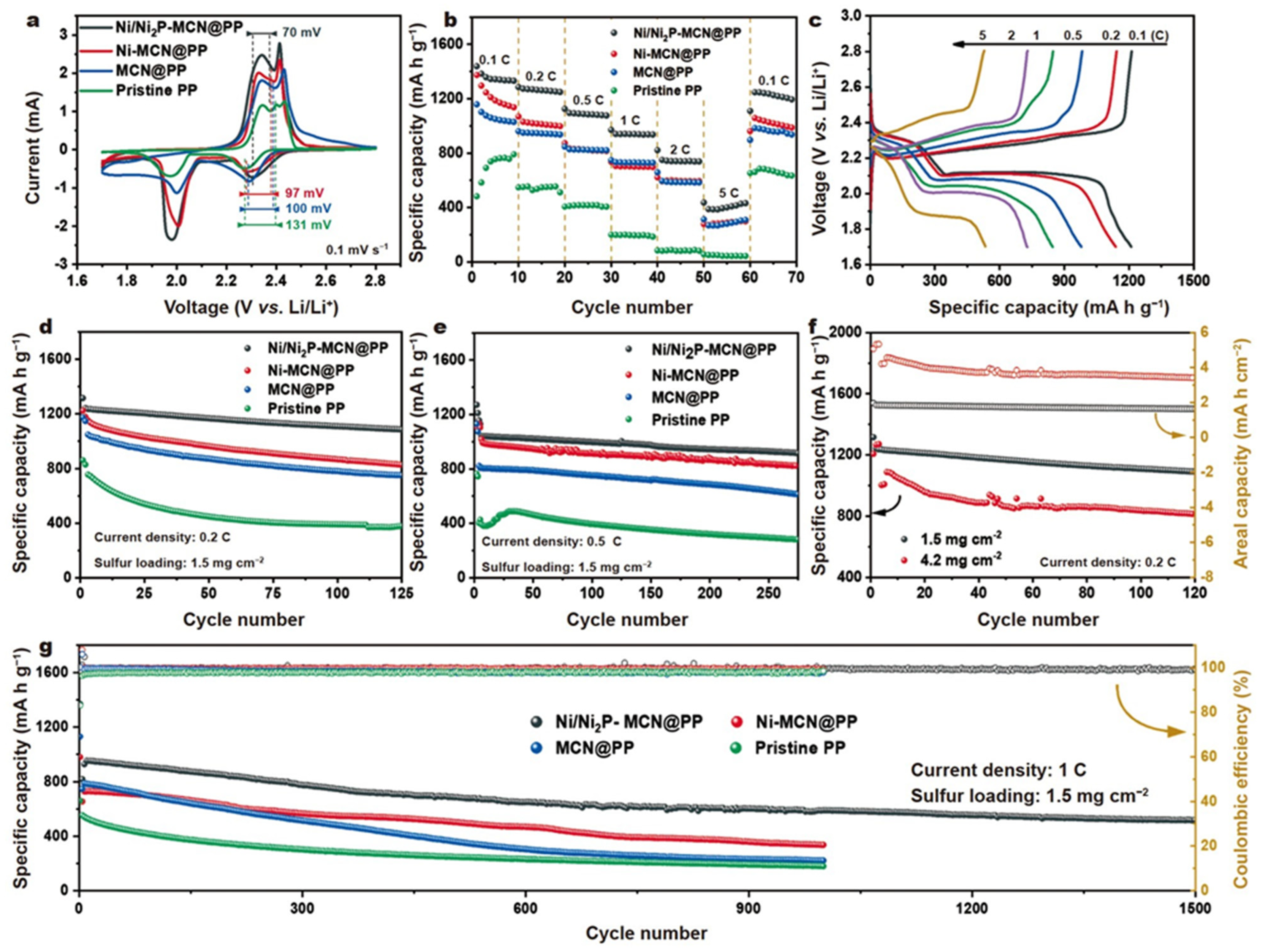
| Ni/Ni2P-Carbon | 5 | 1500 | 431 | (0.031% per cycle) | [110] |
| Metal Organic Framework | |||||
| Ce UiO-67 | 1 | 500 | 919 | 0.04%/cycle | [119] |
| Ce-UiO-66-NH2 | 0.2 | 300 | 1366.3 | 0.09%/cycle | [120] |
| ZIF-8 | 0.1 | 199 | 1235.6 | [121] | |
| Quantum Dot | |||||
| TiO2 Quantum Dot | 600 | 1083 | 0.072%/cycle | [125] | |
| MWCNT/Nitrogen Doped Carbon Quantum Dot | 0.5 | 1000 | 1330.8 | 0.05%/cycle | [126] |
| Mo2C quantum dot | 0.2 | 100 | 1230 | [128] | |
| ZnS Quantum Dot | 0.1 | 500 | 1211 | [129] | |
| MoP Quantum Dot | 1 | 600 | 500 | 0.052%/cycle | [130] |
| Mxene | |||||
| Ti3C2 | 100 | 820 | 0.879%/cycle | [143] | |
| TiO2-Ti3C2Tx | 0.5 | 200 | 662 | 0.035 %/cycle | [144] |
| TiS2/TiO2-Ti3C2Tx | 1 | 500 | 0.048 %/cycle | [145] | |
| Other Functional Materials | |||||
| Lignin | 1 | 500 | 487 | [150] | |
| Nano-fibrillated cellulose | 1 | 580 | [152] | ||
| Materials on Separator | Current Density (C, 1675 mAh g−1) | Cycle Number | Initial Discharge Capacity (mAh g−1) | Capacity Retention (%) (Fadeing Rate) | Reference |
|---|---|---|---|---|---|
| Lanthanum oxide | 1 | 200 | 966 | 74.5% | [85] |
| Co3W3Carbide@C | 1 | 500 | (0.06% per cycle) | [92] | |
| Co-doped g-C3N4 | 0.2 | 100 | 1121 | 95.0% | [99] |
| FeP/C | 1 | 400 | 526 | [109] | |
| Ni/Ni2P-Carbon | 5 | 1500 | 431 | (0.031% per cycle) | [110] |
| MOF and MOF Composite | [113] | ||||
| ZnS Quantum Dot | 0.1 | 500 | 1211 | [126] | |
| MoP Quantum Dot | 1 | 600 | 500 | 0.052%/cycle | [130] |
| TiO2-Mxene | [144] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mori, R. Separator Materials for Lithium Sulfur Battery—A Review. Electrochem 2023, 4, 485-522. https://doi.org/10.3390/electrochem4040032
Mori R. Separator Materials for Lithium Sulfur Battery—A Review. Electrochem. 2023; 4(4):485-522. https://doi.org/10.3390/electrochem4040032
Chicago/Turabian StyleMori, Ryohei. 2023. "Separator Materials for Lithium Sulfur Battery—A Review" Electrochem 4, no. 4: 485-522. https://doi.org/10.3390/electrochem4040032
APA StyleMori, R. (2023). Separator Materials for Lithium Sulfur Battery—A Review. Electrochem, 4(4), 485-522. https://doi.org/10.3390/electrochem4040032






