Three-Dimensional Hybrid Nanostructures of Fe3O4 Nanoparticles/Vertically-Aligned Carbon Nanotubes for High-Performance Supercapacitors
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of VACNTs
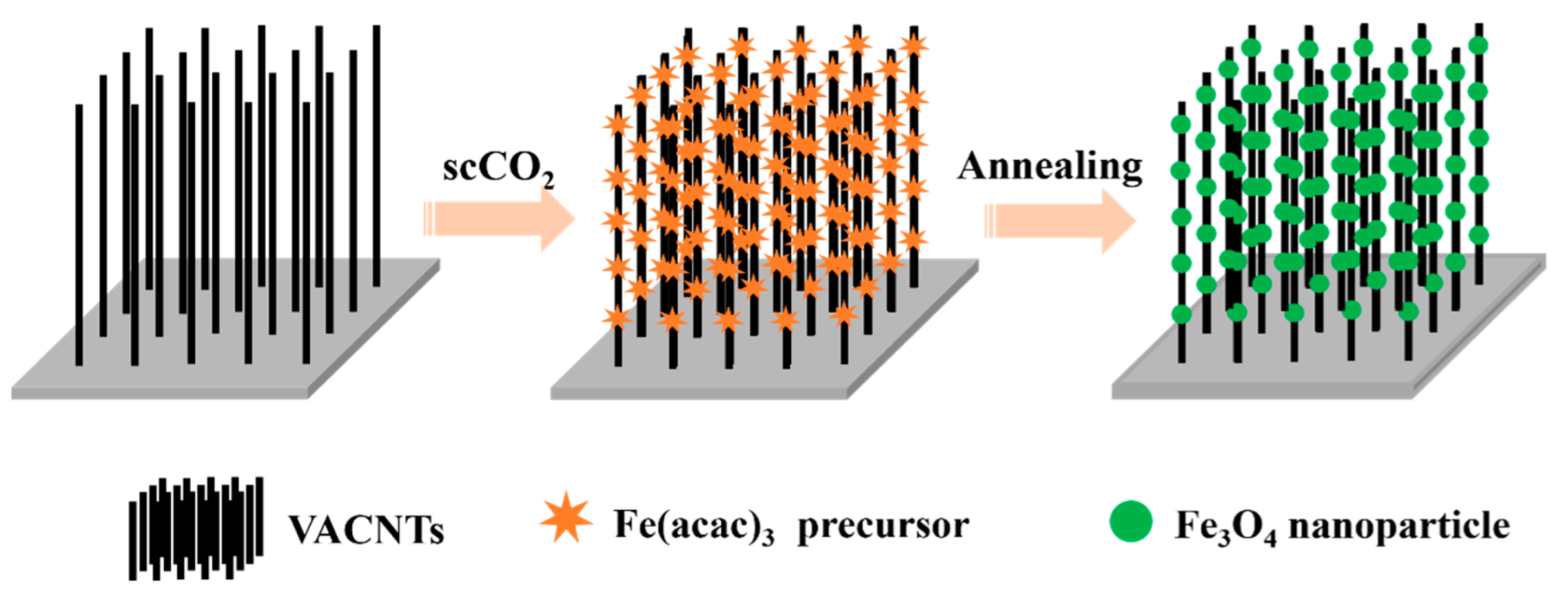
2.2. Fabrication of Fe3O4/VACNTs Composites
2.3. Characterization
2.4. Electrochemical Measurement
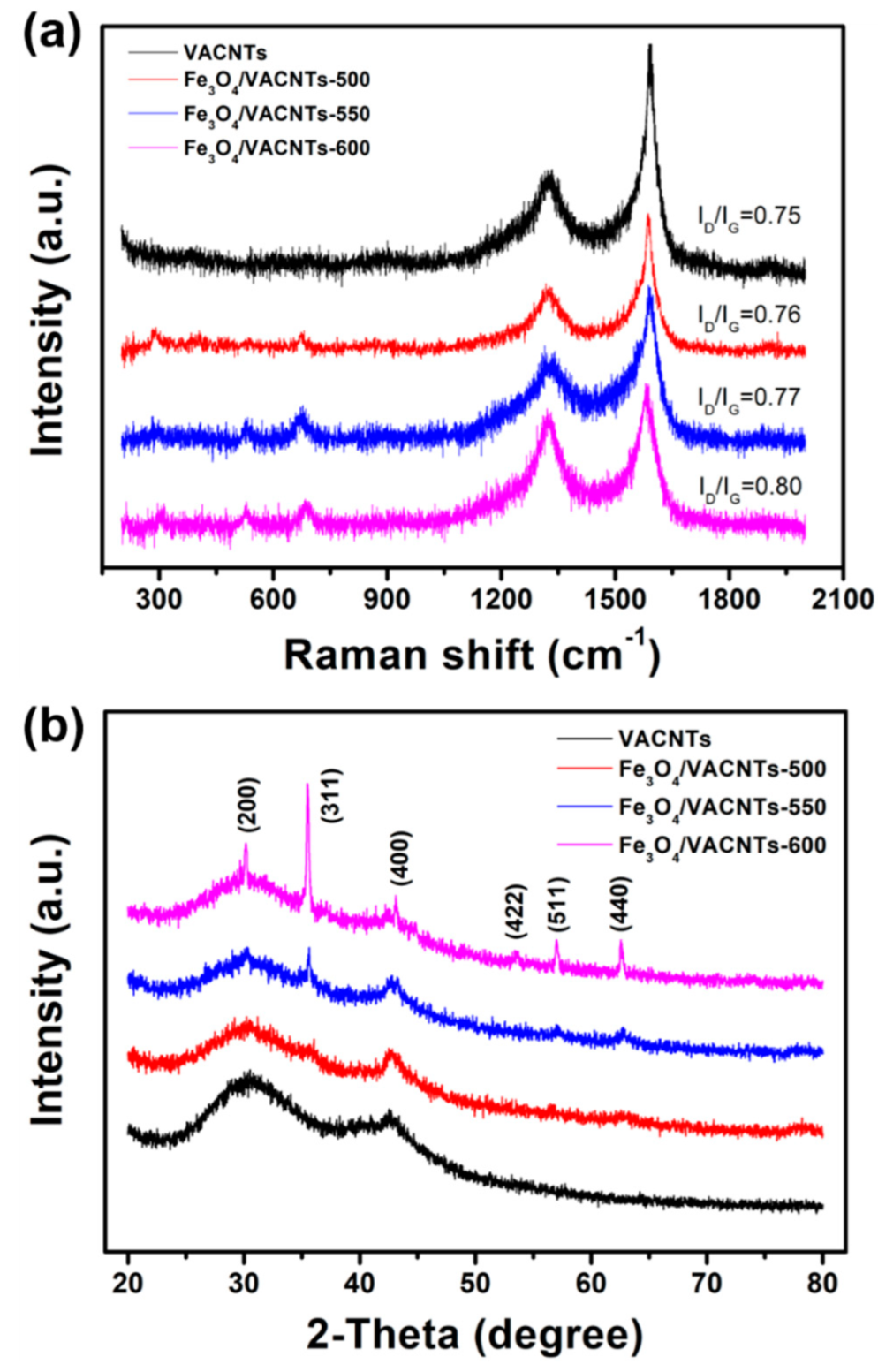
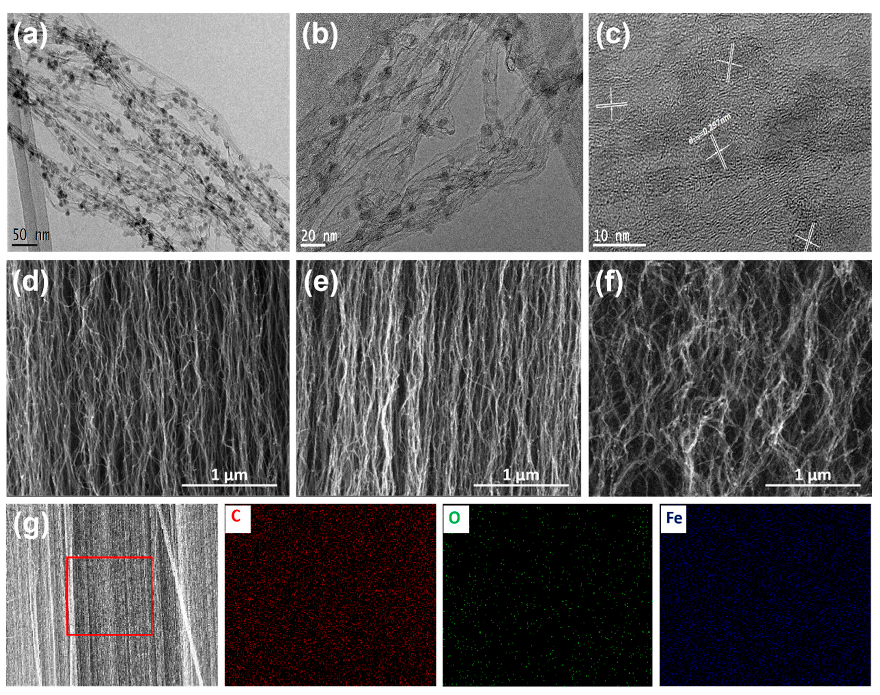
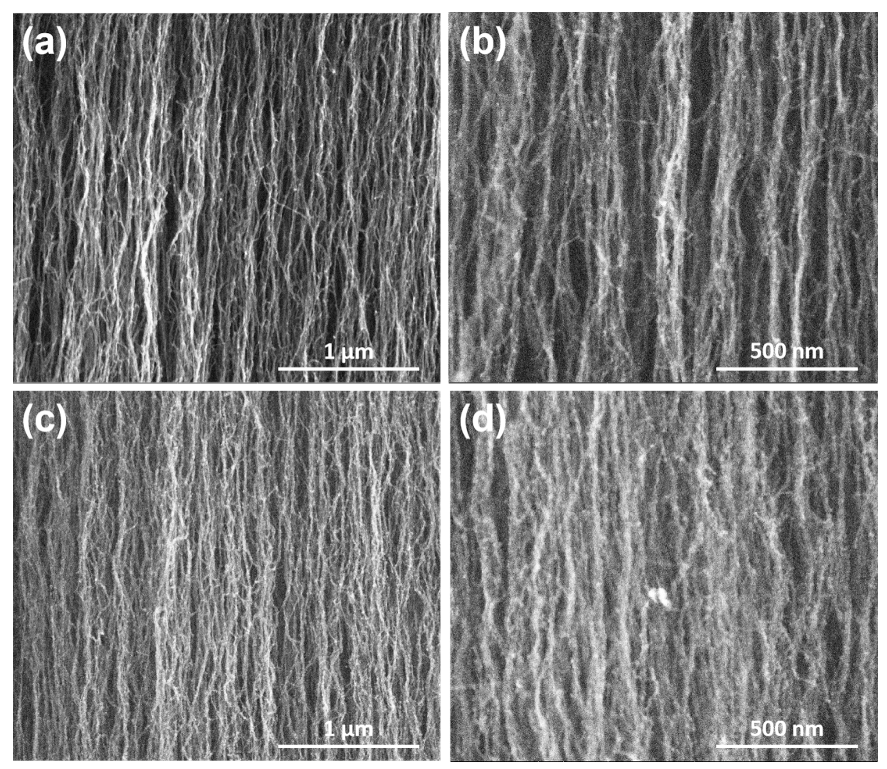
3. Results and Discussions
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, X.Y.; Hu, H.; Wang, Y.W.; Chen, H.Y.; Lou, X.W. Ultrathin MoS2 nanosheets supported on N-doped carbon nanoboxes with enhanced lithium storage and electrocatalytic properties. Angew. Chem. Int. Ed. 2015, 54, 7395–7398. [Google Scholar] [CrossRef]
- Peng, L.L.; Peng, X.; Liu, B.R.; Wu, C.Z.; Xie, Y.; Yu, G.H. Ultrathin two-dimensional MnO2/graphene hybrid nanostructures for high-performance, flexible planar supercapacitors. Nano Lett. 2013, 13, 2151–2157. [Google Scholar]
- Chen, Q.; Meng, Y.N.; Hu, C.G.; Zhao, Y.; Shao, H.B.; Chen, N.; Qu, L.T. MnO2-modified hierarchical graphene fiber electrochemical supercapacitor. J. Power Sources 2014, 247, 32–39. [Google Scholar] [CrossRef]
- Jia, X.L.; Chen, Z.; Cui, X.; Peng, Y.T.; Wang, X.L.; Wang, G.; Wei, F.; Lu, Y.F. Building robust architectures of carbon and metal oxide nanocrystals toward high performance anodes for lithium-ion batteries. ACS Nano 2012, 6, 9911–9919. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, J.; Yin, Z.; Cui, C.; Qian, W.; Wei, F. Carbon nanotube- and graphene-based nanomaterials and applications in high-voltage supercapacitor: A review. Carbon 2019, 141, 467–480. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M. Carbon-based supercapacitors produced by activation of graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Chen, Z.; Qin, Y.C.; Weng, D.; Xiao, Q.F.; Peng, Y.T.; Wang, X.L.; Li, H.X.; Wei, F.; Lu, Y.F. Design and synthesis of hierarchical nanowire composites for electrochemical energy storage. Adv. Funct. Mater. 2009, 19, 3420–3426. [Google Scholar] [CrossRef]
- Chmiola, J.; Yushin, G.; Gogotsi, Y.; Portet, C.; Simon, P.; Taberna, P.L. Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science 2006, 313, 1760–1763. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, Y.; Zeng, T.; Huang, D.; Wan, Q.; Yang, N. High-performance asymmetric supercapacitors using holey graphene electrodes and redox electrolytes. Carbon 2020, 157, 298–307. [Google Scholar] [CrossRef]
- Kumar, R.; Joanni, E.; Sahoo, S.; Shim, J.-J.; Tan, W.K.; Matsuda, M.; Singh, R.K. An overview of recent progress in nanostructured carbon-based supercapacitor electrodes: From zero to bi-dimensional materials. Carbon 2022, 193, 298–338. [Google Scholar] [CrossRef]
- Sun, Y.M.; Sills, R.B.; Hu, X.L.; Seh, Z.W.; Xiao, X.; Xui, H.H.; Luo, W.; Jin, H.Y.; Xin, Y.; Li, T.Q. A Bamboo-Inspired Nanostructure Design for Flexible, Foldable and Twistable Energy Storage Devices. Nano Lett. 2015, 15, 3899–3906. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Sun, G.; Yuan, R.; Chen, W.; Wang, Z.; Zhang, L.; Zhan, K.; Zhu, M.; Yang, J.H.; Zhao, B. Scalable fabrication of NiCo2O4/reduced graphene oxide composites by ultrasonic spray as binder-free electrodes for supercapacitors with ultralong lifetime. J. Mater. Sci. Technol. 2022, 99, 260–269. [Google Scholar] [CrossRef]
- Xiao, J.; Wan, L.; Yang, S.; Xiao, F.; Wang, S. Design hierarchical electrodes with highly conductive NiCo2S4 nanotube arrays grown on carbon fiber paper for high-performance pseudocapacitors. Nano Lett. 2014, 14, 831–838. [Google Scholar] [CrossRef]
- Sun, G.; Ren, H.; Shi, Z.; Zhang, L.; Wang, Z.; Zhan, K.; Yan, Y.; Yang, J.; Zhao, B. V2O5/vertically-aligned carbon nanotubes as negative electrode for asymmetric supercapacitor in neutral aqueous electrolyte. J. Colloid Interf. Sci. 2021, 588, 847–856. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, B.; Yin, Y.; Yin, T.; Cheng, J.; Zhan, K.; Yan, Y.; Yang, J.; Li, J. Fe2O3-decorated millimeter-long vertically aligned carbon nanotube arrays as advanced anode materials for asymmetric supercapacitors with high energy and power densities. J. Mater. Chem. A 2016, 4, 19026–19036. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, Y.Q.; Chao, D.; Guan, C.; Zhang, Y.; Li, L.; Ge, X.; Bacho, I.M.; Tu, J.; Fan, H.J. Solution Synthesis of Metal Oxides for Electrochemical Energy Storage Applications. Nanoscale 2014, 6, 5008–5048. [Google Scholar] [CrossRef]
- Bhattarai, R.M.; Chhetri, K.; Saud, S.; Teke, S.; Kim, S.J.; Mok, Y.S. Eco-Friendly Synthesis of Cobalt Molybdenum Hydroxide 3d Nanostructures on Carbon Fabric Coupled with Cherry Flower Waste-Derived Activated Carbon for Quasi-Solid-State Flexible Asymmetric Supercapacitors. ACS Appl. Nano Mater. 2022, 5, 160–175. [Google Scholar] [CrossRef]
- Qu, G.; Wang, Z.; Zhang, X.; Zhao, S.; Wang, C.; Zhao, G.; Hou, P.; Xu, X. Designing flexible asymmetric supercapacitor with high energy density by electrode engineering and charge matching mechanism. Chem. Eng. J. 2022, 429, 132406. [Google Scholar] [CrossRef]
- Lu, X.H.; Wang, G.M.; Zhai, T.; Yu, M.H.; Xie, S.L.; Ling, Y.C.; Liang, C.L.; Tong, Y.X.; Li, Y. Stabilized TiN Nanowire Arrays for High-Performance and Flexible Supercapacitors. Nano Lett. 2012, 12, 5376–5381. [Google Scholar] [CrossRef]
- Yin, Y.; Xu, Y.; Zhou, Y.; Yan, Y.; Zhan, K.; Yang, J.; Li, J.; Zhao, B. Millimeter-Long Vertically Aligned Carbon-Nanotube-Supported Co3O4 Composite Electrode for High-Performance Asymmetric Supercapacitor. ChemElectroChem 2018, 5, 1394–1400. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, X.; Chen, P.; Zhu, K.; Cheng, K.; Ye, K.; Wang, G.; Cao, D.; Yan, J. Creating oxygen-vacancies in MoO3-x nanobelts toward high volumetric energy-density asymmetric supercapacitors with long lifespan. Nano Energy 2019, 58, 455–465. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, L.; Zhang, J.; Miao, T.; Yuan, R.; Chen, W.; Wang, Z.; Yang, J.; Zhao, B. Na+ pre-intercalated Na0.11MnO2 on three-dimensional graphene as cathode for aqueous zinc ion hybrid supercapacitor with high energy density. Carbon 2022, 198, 46–56. [Google Scholar] [CrossRef]
- Xia, H.; Hong, C.Y.; Li, B.; Zhao, B.; Lin, Z.X.; Zheng, M.B.; Savilov, S.V.; Aldoshin, S.M. Facile Synthesis of Hematite Quantum-Dot/Functionalized Graphene-Sheet Composites as Advanced Anode Materials for Asymmetric Supercapacitors. Adv. Funct. Mater. 2015, 25, 627–635. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, S.; Xie, H.; Dai, Y.; Meng, X. Hierarchically porous MnO2 microspheres doped with homogeneously distributed Fe3O4 nanoparticles for supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 17637–17646. [Google Scholar] [CrossRef]
- Lei, C.; Han, F.; Sun, Q.; Li, W.-C.; Lu, A.-H. Confined nanospace pyrolysis for the fabrication of coaxial Fe3O4@C hollow particles with a penetrated mesochannel as a superior anode for Li-Ion batteries. Chem. Eur. J. 2014, 20, 139–145. [Google Scholar] [CrossRef]
- Chen, Y.; Song, B.; Li, M.; Lu, L.; Xue, J. Fe3O4 nanoparticles embedded in uniform mesoporous carbon spheres for superior high-rate battery applications. Adv. Funct. Mater. 2014, 24, 319–326. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Deb, P. Hybrid nanostructured C-dot decorated Fe3O4 electrode materials for superior electrochemical energy storage performance. Dalton Trans. 2015, 44, 9221–9229. [Google Scholar] [CrossRef]
- Yu, X.; Wang, M.; Gagnoud, A.; Fautrelle, Y.; Moreau, R.; Li, X. Fabrication and electrochemical properties of a graphene-enhanced hierarchical porous network of Fe3O4/carbon nanobelts. Electrochim. Acta 2017, 248, 150–159. [Google Scholar] [CrossRef]
- Lin, J.; Liang, H.; Jia, H.; Chen, S.; Guo, J.; Qi, J.; Qu, C.; Cao, J.; Fei, W.; Feng, J. In situ encapsulated Fe3O4 nanosheet arrays with graphene layers as an anode for high-performance asymmetric supercapacitors. J. Mater. Chem. A 2017, 5, 24594–24601. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Vaz, A.R.; Savu, R.; Moshkalev, S.A. Self-Assembled and One-Step Synthesis of Interconnected 3D Network of Fe3O4/Reduced Graphene Oxide Nanosheets Hybrid for High-Performance Supercapacitor Electrode. ACS Appl. Mater. Interfaces 2017, 9, 8880–8890. [Google Scholar] [CrossRef]
- Jiang, H.; Lee, P.S.; Li, C.Z. 3D carbon based nanostructures for advanced supercapacitors. Energy Environ. Sci. 2013, 6, 41–53. [Google Scholar] [CrossRef]
- Futaba, D.N.; Hata, K.; Yamada, T.; Hiraoka, T.; Hayamizu, Y.; Kakudate, Y.; Tanaike, O.; Hatori, H.; Yumura, M.; Iijima, S. Shape-engineerable and highly densely packed single-walled carbon nanotubes and their application as super-capacitor electrodes. Nat. Mater. 2006, 5, 987–994. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, G.P.; Yang, Y.S. Carbon nanotube arrays and their composites for electrochemical capacitors and lithium-ion batteries. Energy Environ. Sci. 2009, 2, 932–943. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, X.M.; Jin, Z.P.; Liu, Z.; Nie, H.G.; Chen, X.A.; Huang, S.M. A Facile and General Approach for the Direct Fabrication of 3D, Vertically Aligned Carbon Nanotube Array/Transition Metal Oxide Composites as Non-Pt Catalysts for Oxygen Reduction Reactions. Adv. Mater. 2014, 26, 3156–3161. [Google Scholar] [CrossRef]
- Gong, K.P.; Du, F.; Xia, Z.H.; Durstock, M.; Dai, L.M. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef]
- Casella, I.G. Electrodeposition of cobalt oxide films from carbonate solutions containing Co (II)–tartrate complexes. J. Electroanal. Chem. 2002, 520, 119–125. [Google Scholar] [CrossRef]
- Cui, X.W.; Hu, F.P.; Wei, W.F.; Chen, W.X. Dense and long carbon nanotube arrays decorated with Mn3O4 nanoparticles for electrodes of electrochemical supercapacitors. Carbon 2011, 49, 1225–1234. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Zhao, B.; Zhang, W.K.; Shi, F.; Zheng, G.P.; Zhang, D.Q.; Yang, J.H. High-Performance Supercapacitor Applications of NiO-Nanoparticle-Decorated Millimeter-Long Vertically Aligned Carbon Nanotube Arrays via an Effective Supercritical CO2-Assisted Method. Adv. Funct. Mater. 2015, 25, 7381–7391. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, B.; Wang, X.Y.; Liang, Y.X.; Qiu, H.X.; Zheng, G.P.; Yang, J.H. Gas Transport in Vertically-aligned Carbon Nanotube/parylene Composite Membranes. Carbon 2014, 66, 11. [Google Scholar] [CrossRef]
- Bhattarai, R.M.; Chhetri, K.; Natarajan, S.; Saud, S.; Kim, S.J.; Mok, Y.S. Activated carbon derived from cherry flower biowaste with a self-doped heteroatom and large specific surface area for supercapacitor and sodium-ion battery applications. Chemsphere 2022, 303, 135290. [Google Scholar] [CrossRef]
- Majumder, S.; Sardar, M.; Satpati, B.; Kumar, S.; Banerjee, S. Magnetization Enhancement of Fe3O4 by Attaching onto Graphene Oxide: An Interfacial Effect. J. Phys. Chem. C 2018, 122, 21356–21365. [Google Scholar] [CrossRef]
- Wu, N.; Wang, S.; Han, C.; Wu, D.; Shiue, L. Electrochemical capacitor of magnetite in aqueous electrolytes. J. Power Sources 2003, 113, 173–178. [Google Scholar] [CrossRef]
- Yang, J.E.; Jang, I.; Kim, M.; Baeck, S.H.; Hwang, S.; Shim, S.E. Electrochemically polymerized vine-like nanostructured polyaniline on activated carbon nanofibers for supercapacitor. Electrochim. Acta 2013, 111, 136–143. [Google Scholar] [CrossRef]
- Lei, Z.; Shi, F.; Lu, L. Incorporation of MnO2-coated carbon nanotubes between graphene sheets as supercapacitor electrode. ACS Appl. Mater. Interfaces 2012, 4, 1058–1064. [Google Scholar] [CrossRef]
- Zhou, Y.; Wen, L.; Zhan, K.; Yan, Y.; Zhao, B. Three-dimensional porous graphene/nickel cobalt mixed oxide composites for high-performance hybrid supercapacitor. Ceram. Int. 2018, 44, 21848–21854. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, J.; Wang, B.; Feng, H.; Ito, K.; Sakai, E. Fe3O4/biocarbon composites with superior performance in supercapacitors. J. Electroanal. Chem. 2017, 804, 232–239. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, D.; Jing, C.; Liu, X.; Li, K.; Yu, M.; Qi, S.; Zhang, Y. Biotemplate Synthesis of Fe3O4/Polyaniline for Supercapacitor. J. Energy Storage 2020, 30, 101554. [Google Scholar] [CrossRef]
- Liu, D.; Wang, X.; Wang, X.; Tian, W.; Liu, J.; Zhi, C.; He, D.; Bando, Y.; Golberg, D. Ultrathin nanoporous Fe3O4-carbon nanosheets with enhanced supercapacitor performance. J. Mater. Chem. A 2013, 1, 1952–1955. [Google Scholar] [CrossRef]
- Liao, J.; Li, Y.; Wang, Z.; Lv, L.; Chang, L. In-situ preparation of Fe3O4/graphene nanocomposites and their electrochemical performances for supercapacitor. Mater. Chem. Phys. 2021, 258, 123995. [Google Scholar] [CrossRef]



| Composites | Special Capacitance (F/g) | Current Density (A/g) | Reference |
|---|---|---|---|
| RGO-Fe3O4 | 236 | 1 | [31] |
| Fe3O4/activated biocarbon | 342 | 1 | [47] |
| DE/Fe3O4/PANI | 242.9 | 0.5 | [48] |
| Nanoporous Fe3O4-carbon nanosheets | 163.4 | 1 | [49] |
| Fe3O4/graphene | 300 | 0.4 | [50] |
| Fe3O4/VACNTs | 364.2 | 0.5 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B. Three-Dimensional Hybrid Nanostructures of Fe3O4 Nanoparticles/Vertically-Aligned Carbon Nanotubes for High-Performance Supercapacitors. Electrochem 2022, 3, 507-519. https://doi.org/10.3390/electrochem3030035
Zhao B. Three-Dimensional Hybrid Nanostructures of Fe3O4 Nanoparticles/Vertically-Aligned Carbon Nanotubes for High-Performance Supercapacitors. Electrochem. 2022; 3(3):507-519. https://doi.org/10.3390/electrochem3030035
Chicago/Turabian StyleZhao, Bin. 2022. "Three-Dimensional Hybrid Nanostructures of Fe3O4 Nanoparticles/Vertically-Aligned Carbon Nanotubes for High-Performance Supercapacitors" Electrochem 3, no. 3: 507-519. https://doi.org/10.3390/electrochem3030035
APA StyleZhao, B. (2022). Three-Dimensional Hybrid Nanostructures of Fe3O4 Nanoparticles/Vertically-Aligned Carbon Nanotubes for High-Performance Supercapacitors. Electrochem, 3(3), 507-519. https://doi.org/10.3390/electrochem3030035






