Electrochemical Analysis of Heavy Metal Ions Using Conducting Polymer Interfaces
Abstract
1. Introduction
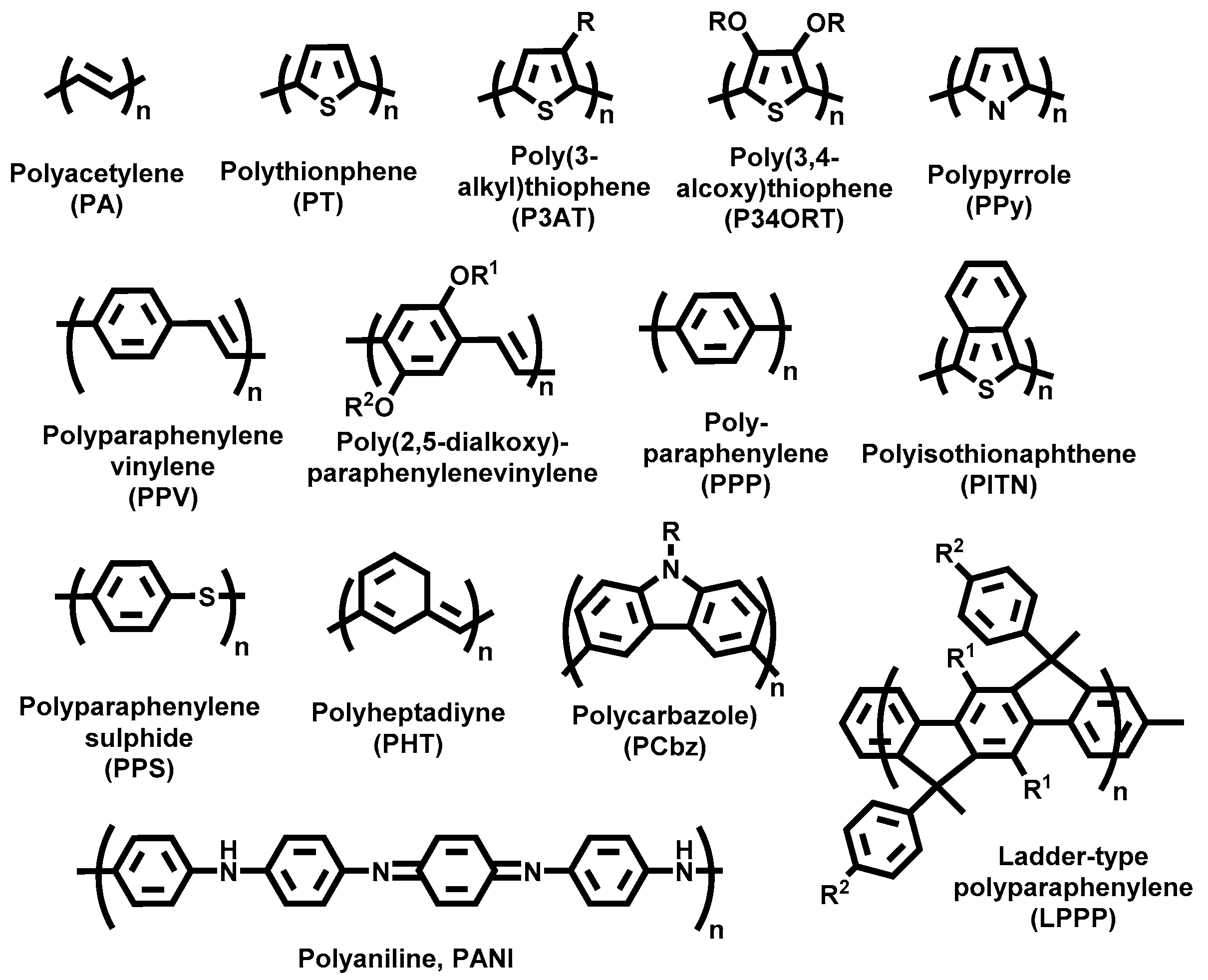
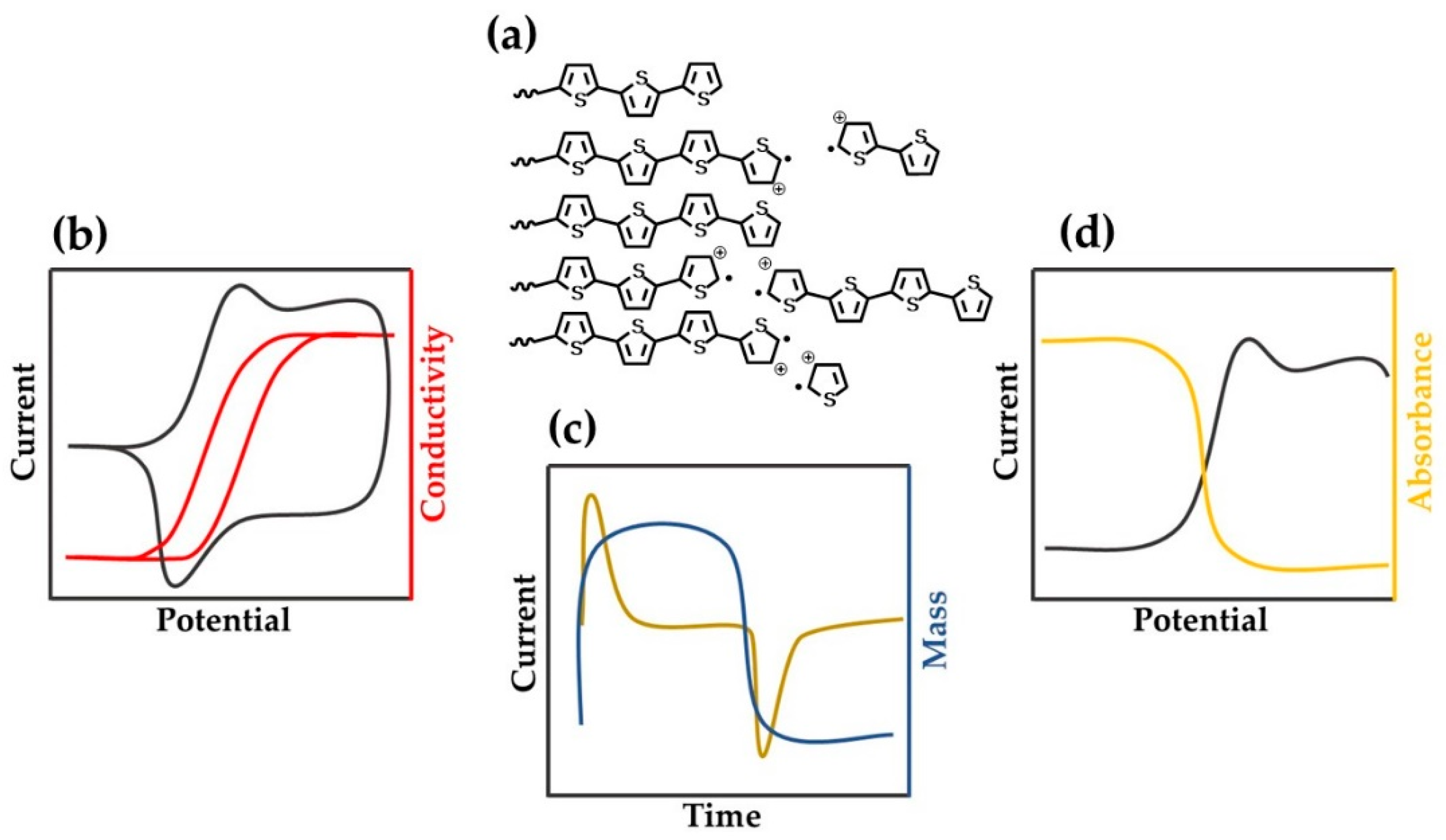
2. Electrochemistry of Conducting Polymers
3. Potentiometry
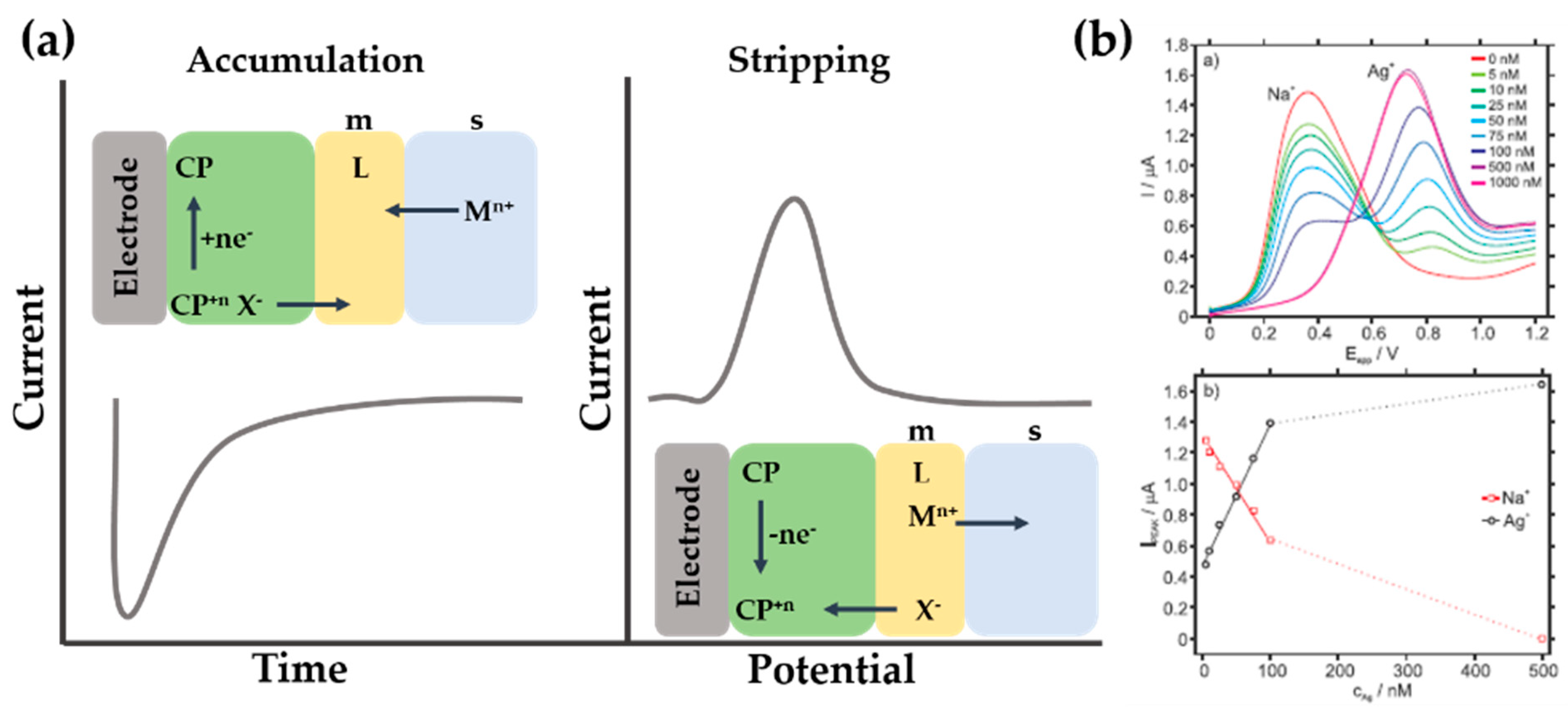
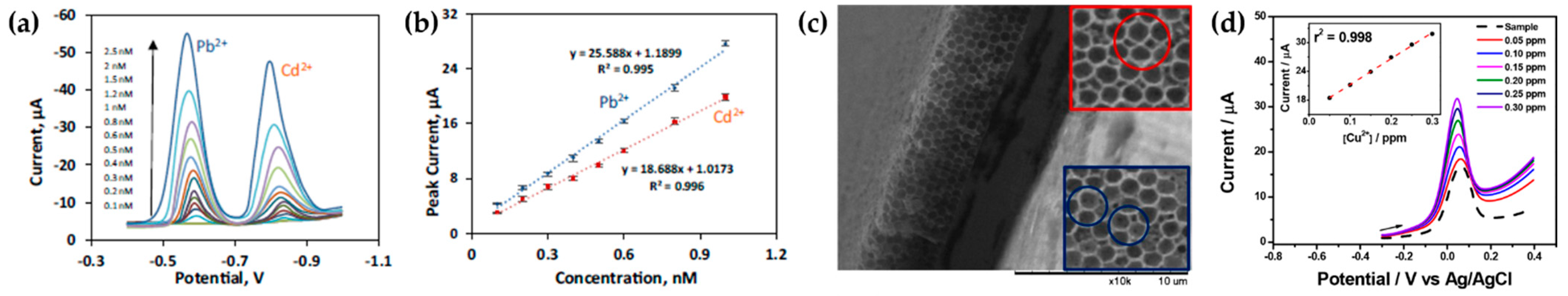
4. Pulse Anodic Stripping Voltammetry
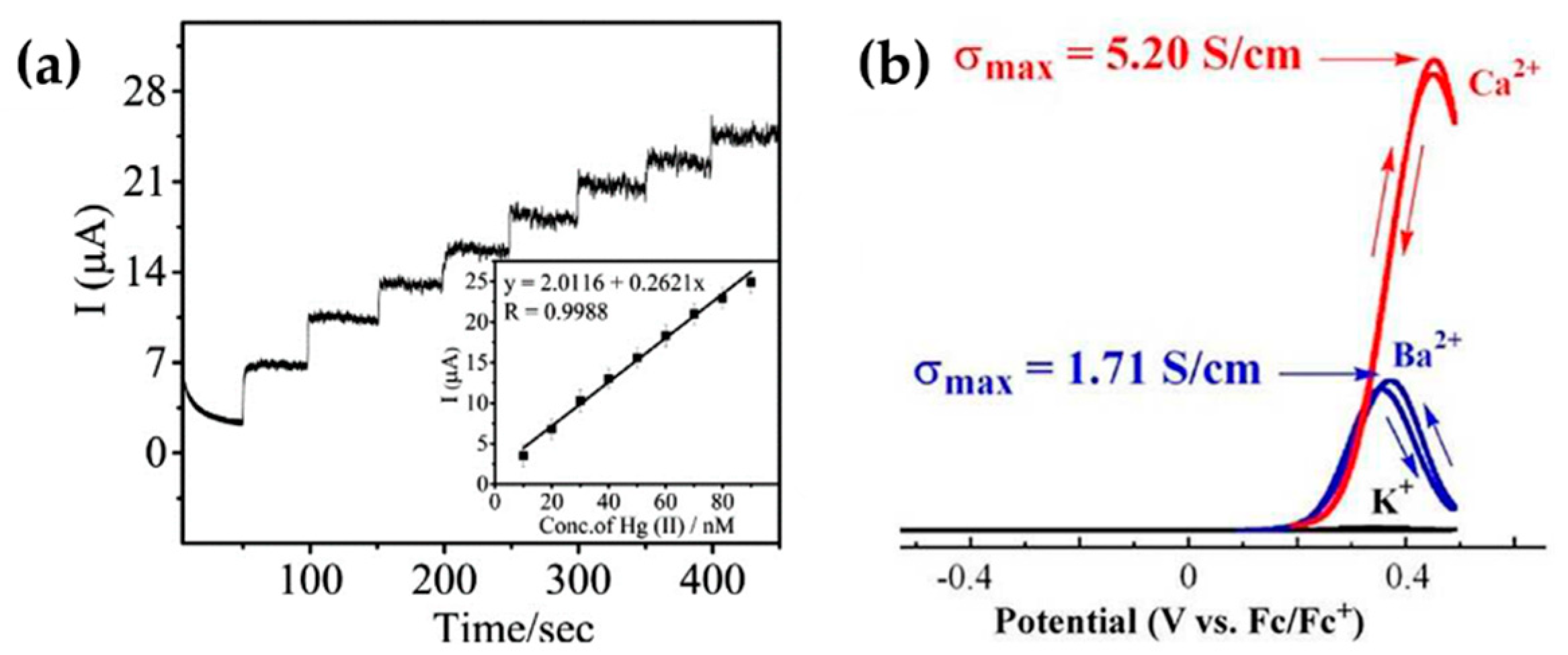
5. Alternative Non-Classical Electrochemical Methods
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Safiur Rahman, M.; Saha, N.; Hossain Molla, A.; Al-Reza, S.M. Assessment of Anthropogenic Influence on Heavy Metals Contamination in the Aquatic Ecosystem Components: Water, Sediment, and Fish. Soil Sediment. Contam. 2014, 23, 353–373. [Google Scholar] [CrossRef]
- Graudal, N.A.; Hubeck-Graudal, T.; Jurgens, G. Effects of low-sodium diet vs. high-sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Am. J. Hypertens. 2012, 25, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Johri, N.; Jacquillet, G.; Unwin, R. Heavy metal poisoning: The effects of cadmium on the kidney. Biometals 2010, 23, 783–792. [Google Scholar] [CrossRef]
- Teixeira, F.B.; de Oliveira, A.C.A.; Leão, L.K.R.; Fagundes, N.C.F.; Fernandes, R.M.; Fernandes, L.M.P.; da Silva, M.C.F.; Amado, L.L.; Sagica, F.E.S.; de Oliveira, E.H.C.; et al. Exposure to inorganic mercury causes oxidative stress, cell death, and functional deficits in the motor cortex. Front. Mol. Neurosci. 2018, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.L.; dos Santos Roldan, P.; Giné, M.F. Simultaneous preconcentration of copper, zinc, cadmium, and nickel in water samples by cloud point extraction using 4-(2-pyridylazo)-resorcinol and their determination by inductively coupled plasma optic emission spectrometry. J. Hazard. Mater. 2009, 171, 1133–1138. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, H.; Yu, A.; Jin, Q. Microwave plasma torch analytical atomic spectrometry. Microchem. J. 2000, 66, 147–170. [Google Scholar] [CrossRef]
- Lange, U.; Roznyatovskaya, N.V.; Mirsky, V.M. Conducting polymers in chemical sensors and arrays. Anal. Chim. Acta 2008, 614, 1–26. [Google Scholar] [CrossRef]
- Trojanowicz, M. Application of conducting polymers in chemical analysis. Microchim. Acta 2003, 143, 75–91. [Google Scholar] [CrossRef]
- Hosseini, S.; Ekramul Mahmud, N.H.M.; Binti Yahya, R.; Ibrahim, F.; Djordjevic, I. Polypyrrole conducting polymer and its application in removal of copper ions from aqueous solution. Mater. Lett. 2015, 149, 77–80. [Google Scholar] [CrossRef]
- Heitzmann, M.; Bucher, C.; Moutet, J.C.; Pereira, E.; Rivas, B.L.; Royal, G.; Saint-Aman, E. Characterization of metal cations-complexing polymer films interactions followed with anodic stripping voltammetry. J. Electroanal. Chem. 2007, 610, 147–153. [Google Scholar] [CrossRef]
- Tsakova, V. How to affect number, size, and location of metal particles deposited in conducting polymer layers. J. Solid State Electrochem. 2008, 12, 1421–1434. [Google Scholar] [CrossRef]
- Castillo-Lara, D.A.; Vasquez-Medrano, R.; Ibanez, J.G.; Frontana-Uribe, B.A.; Salinas, G. Electrochemical behavior of the Cu (II)/Cu (0) system on vitreous carbon electrodes modified with PEDOT electropolymerized in aqueous media. ECS Trans. 2018, 84, 9–14. [Google Scholar] [CrossRef][Green Version]
- Heinze, J.; Frontana-Uribe, B.A.; Ludwigs, S. Electrochemistry of Conducting Polymers Persistent Models and New Concepts. Chem. Rev. 2010, 110, 4724–4771. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, J.G.; Rincon, M.E.; Gutierrez-Granados, S.; Chahma, M.; Jaramillo-Quintero, O.A.; Frontana-Uribe, B.A. Conducting polymers in the fields of energy, environmental remediation, and chemical-chiral sensors. Chem. Rev. 2018, 118, 4731–4816. [Google Scholar] [CrossRef]
- Alcaraz-Espinoza, J.J.; Ramos-Sanchez, G.; Sierra-Uribe, J.H.; Gonzalez, I. Supramolecular assembly of nanostructured conducting polymer hydrogels by hydrotropic agents for outstanding supercapacitive energy storage. ACS Appl. Energy Mater. 2021, 4, 9099–9110. [Google Scholar] [CrossRef]
- Paulsen, B.D.; Tybrandt, K.; Stavrinidou, E.; Rivnay, J. Organic mixed ionic-electronic conductors. Nat. Mater. 2020, 19, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, M.A.; Shirsat, M.D.; Ramanaviciene, A.; Ramanavicius, A. Composites based on conducting polymers and carbon nanomaterials for heavy metal ion sensing. Crit. Rev. Anal. Chem. 2018, 48, 293–304. [Google Scholar] [CrossRef]
- Bobaka, J. Conducting polymer-based solid-state ion-selective electrodes. Electroanalysis 2006, 18, 7–18. [Google Scholar] [CrossRef]
- Vazquez, M.; Bobaka, J.; Luostarinen, M.; Rissanen, K.; Lewenstam, A.; Ivaska, A. Potentiometric sensors based on poly(3,4-ethylenedioxythiophene) (PEDOT) doped with sulfonated calix [4]arene and calix[4]resorcarenes. J. Solid State Electrochem. 2005, 9, 312–319. [Google Scholar] [CrossRef]
- Prakash, R.; Srivastava, R.C.; Pandey, P.C. Copper(II) ion sensor based on electropolymerized undoped conducting polymers. J. Solid State Electrochem. 2006, 6, 203–208. [Google Scholar] [CrossRef]
- Zanganesh, A.R.; Amini, M.K. Polypyrrole-modified electrodes with induced recognition sites for potentiometric and voltammetric detection of copper(II) ion. Sens. Actuators B Chem. 2008, 135, 358–365. [Google Scholar] [CrossRef]
- Quintana, H.; Ramirez, J.L.; Rubio, E.F.; Marquez, E.; Gonzalez, G.; Gonzalez, G.; Uruchurtu, J. Electrochemical sensor based on polypyrrole for the detection of heavy metals in aqueous solutions. ECS Trans. 2013, 47, 265–273. [Google Scholar] [CrossRef]
- Yasri, N.G.; Halabi, A.J.; Istamboulie, G.; Noguer, T. Chronoamperometric determination of lead ions using PEDOT:PSS modified carbon electrodes. Talanta 2011, 85, 2528–2533. [Google Scholar] [CrossRef]
- Rahman, M.A.; Won, M.S.; Shim, Y.B. Characterization of an EDTA bonded conducting polymer modified electrode: Its application for the simultaneous determination of heavy metal ions. Anal. Chem. 2003, 75, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Heltzmann, M.; Basaez, L.; Brovelli, F.; Bucher, C.; Limosin, D.; Pereira, E.; Rivas, B.L.; Royal, G.; Saint-Aman, E.; Moutet, J.C. Voltammetric sensing of trace metals at a poly(pyrrole-malonic acid) film modified carbon electrode. Electroanalysis 2005, 17, 1970–1976. [Google Scholar] [CrossRef]
- Manisankar, P.; Vedhi, C.; Selvanathan, G.; Arumugam, P. Differential pulse stripping voltammetric determination of heavy metals simultaneously using new polymer modified glassy carbon electrodes. Microchim. Acta 2008, 163, 289–295. [Google Scholar] [CrossRef]
- Zejli, H.; Hidalgo-Hidalgo de Cisneros, J.L.; Naranjo-Rodriguez, I.; Temsamani, K.R. Stripping voltammetry of silver ions at polythiophene-modified platinum electrodes. Talanta 2007, 71, 1594–1598. [Google Scholar] [CrossRef]
- Yoo, K.S.; Woo, S.B.; Jyoung, J.Y. Trace mercury determination by differential pulse anodic stripping voltammetry using polythiophene-quinoline/glassy carbon modified electrode. Bull. Korean Chem. Soc. 2003, 24, 27–31. [Google Scholar] [CrossRef]
- Ahonen, H.J.; Ukkari, J.L.; Kankare, J. n-and p-doped poly (3, 4-ethylenedioxythiophene): Two electronically conducting states of the polymer. Macromolecules 2000, 33, 6787–6793. [Google Scholar] [CrossRef]
- Grande, H.; Otero, T.F. Conformational movements explain logarithmic relaxation in conducting polymers. Electrochim. Acta 1999, 44, 1893–1900. [Google Scholar] [CrossRef]
- Otero, T.F.; Alfaro, M.; Martinez, V.; Perez, M.A.; Martinez, J.G. Biomimetic structural electrochemistry from conducting polymers: Processes, charges, and energies. coulovoltammetric results from films on metals revisited. Adv. Funct. Mater. 2013, 23, 3929–3940. [Google Scholar] [CrossRef]
- Villarroel Marquez, A.; Salinas, G.; Abarkan, M.; Idir, M.; Brochon, C.; Hadziioannou, G.; Raoux, M.; Kuhn, A.; Lang, J.; Cloutet, E. Design of potassium-selective mixed ion/electron conducting polymers. Macromol. Rapid Commun. 2020, 41, 2000134. [Google Scholar] [CrossRef] [PubMed]
- Salinas, G.; Ibanez, J.G.; Vásquez-Medrano, R.; Frontana-Uribe, B.A. Electrochemical behavior of poly-bithiophene, poly-3,4-ethylendioxythiophene and poly-3,4-orthoxylendioxythiophene in EtOH/H2O (1:1) mixture. Synth. Met. 2018, 237, 65–72. [Google Scholar] [CrossRef]
- Salinas, G.; Del-Oso, J.A.; Espinoza-Montero, P.J.; Heinze, J.; Frontana-Uribe, B.A. Electrochemical polymerization, characterization and in-situ conductivity studies of poly-3,4-ortho-xylendioxythiophene (PXDOT). Synth. Met. 2018, 245, 135–143. [Google Scholar] [CrossRef]
- Casalbore-Miceli, G.; Camaioni, N.; Geri, A.; Ridolfi, G.; Zanelli, A.; Gallazzi, M.C.; Maggini, M.; Benincori, T. Solid state charge trapping: Examples of polymer systems showing memory effect. J. Electroanal. Chem. 2007, 603, 227–234. [Google Scholar] [CrossRef]
- Hillman, A.R.; Daisley, S.J.; Bruckenstein, S. Ion and solvent transfers and trapping phenomena during n-doping of PEDOT films. Electrochim. Acta 2008, 53, 3763–3771. [Google Scholar] [CrossRef]
- Salinas, G.; Frontana-Uribe, B.A. Analysis of conjugated polymers conductivity by in situ electrochemical-conductance method. ChemElectroChem 2019, 6, 4105–4117. [Google Scholar] [CrossRef]
- Inzelt, G. Conducting Polymers: A New Era in Electrochemistry, 2nd ed.; Springer: Berlin, Germany, 2012; pp. 1–293. [Google Scholar]
- Cosnier, S.; Karyakin, A. Electropolymerization: Concepts, Materials and Applications; Wiley-VCH: Weinheim, Germany, 2010; pp. 1–296. [Google Scholar]
- Nicolini, T.; Villarroel Marquez, A.; Goudeau, B.; Kuhn, A.; Salinas, G. In situ spectroelectrochemical-conductance measurements as an efficient tool for the evaluation of charge trapping in conducting polymers. J. Phys. Chem. Lett. 2021, 12, 10422–10428. [Google Scholar] [CrossRef]
- Visy, C.; Tóth, P.S. Complementary nature of voltabsorptiometric, nanogravimetric and in situ conductance results for the interpretation of conducting polymers redox transformation. Synth. Met. 2018, 246, 260–266. [Google Scholar] [CrossRef]
- Isildak, O.; Ozbek, O. Application of potentiometric sensors in real samples. Crit. Rev. Anal. Chem. 2021, 51, 2018–2231. [Google Scholar] [CrossRef]
- Zdrachek, E.; Bakker, E. Potentiometric sensing. Anal. Chem. 2021, 93, 72–102. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Ying, Y.; Ping, J. Recent advances in solid-contact ion-selective electrodes: Functional materials, transduction mechanisms, and development trends. Chem. Soc. Rev. 2020, 49, 4405–4465. [Google Scholar] [CrossRef] [PubMed]
- Ayranci, R.; Ak, M. An electrochemical sensor platform for sensitive detection of Iron (III) ions based on pyrene-substituted poly(2,5-dithienylpyrrole). J. Electrochem. Soc. 2019, 166, B291–B296. [Google Scholar] [CrossRef]
- Khan, A.; Parwaz Khan, A.A.; Asiri, A.M.; Kosa, S.A. Preparation and properties of novel quaternized metal-polymer matrix nanocomposites. Polym. Plast. Technol. Eng. 2015, 54, 1615–1624. [Google Scholar] [CrossRef]
- Ansari, R.; Delavar, A.F.; Aliakbar, A.; Mohammad-jhah, A. Solid-state Cu (II) ion-selective electrode based on polyaniline-conducting polymer film doped with copper carmoisine dye complex. J. Solid. State Electrochem. 2012, 16, 869–875. [Google Scholar] [CrossRef]
- Migdalski, J.; Blaz, T.; Lewenstam, A. Conducting polymers—Mechanisms of cationic sensitivity and the methods of inducing thereof. Electrochim. Acta 2014, 133, 316–324. [Google Scholar] [CrossRef]
- Ansari, R.; Delavar, A.F.; Mohammad-jhah, A. Solid-state ion selective electrode based on polypyrrole conducting polymer nanofilm as a new potentiometric sensor for Zn2+ ion. J. Solid State Electrochem. 2012, 16, 3315–3322. [Google Scholar] [CrossRef]
- Ansari, R.; Mosayebzadeh, Z.; Arvand, M.; Mohammad-jhah, A. A potentiometric solid state copper electrode based on nanostructure polypyrrole conducting polymer film doped with 5-sulfosalicylic acid. J. Nanostructure Chem. 2013, 3, 33. [Google Scholar] [CrossRef]
- Huang, M.R.; Ding, Y.B.; Li, X.G. Lead-ion potentiometric sensor based on electrically conducting microparticles of sulfonic phenylenediamine copolymer. Analyst 2013, 138, 3820–3829. [Google Scholar] [CrossRef]
- Singh, V.K.; Kushwaha, C.S.; Shukla, S.K. Potentiometric detection of copper ion using chitin grafted polyaniline electrode. Int. J. Biol. Macromol. 2020, 147, 250–257. [Google Scholar] [CrossRef]
- Crespo, G.A.; Cuartero, M.; Bakker, E. Thin layer ionophore-based membrane for multianalyte ion activity detection. Anal. Chem. 2015, 87, 7729–7737. [Google Scholar] [CrossRef] [PubMed]
- Michalska, A. Optimizing the analytical performance and construction of ion-selective electrodes with conducting polymer-based ion-to-electron transducers. Anal. Bioanal. Chem. 2006, 384, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Vanamo, U.; Bobacka, J. Electrochemical control of the standard potential of solid-contact ion-selective electrodes having a conducting polymer as ion-to-electron transducer. Electrochim. Acta 2014, 122, 316–321. [Google Scholar] [CrossRef]
- Han, T.; Mattinen, U.; Bobacka, J. Improving the sensitivity of solid-contact ion-selective electrodes by using coulometric signal transduction. ACS Sens. 2019, 4, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Mousavi, Z.; Mattinen, U.; Bobacka, J. Coulometric response characteristics of solid contact ion-selective electrodes for divalent cations. J. Solid State Electrochem. 2020, 24, 2975–2983. [Google Scholar] [CrossRef]
- Kim, Y.; Amemiya, S. Stripping analysis of nanomolar perchlorate in drinking water with a voltammetric ion-selective electrode based on thin-layer liquid membrane. Anal. Chem. 2008, 80, 6056–6065. [Google Scholar] [CrossRef]
- Kabagambe, B.; Garada, M.B.; Ishimatsu, R.; Amemiya, S. Electrochemical mechanism of ion-ionophore recognition at plasticized polymer membrane/water interfaces. Anal. Chem. 2014, 86, 7939–7946. [Google Scholar] [CrossRef]
- Xu, K.; Cuartero, M.; Crespo, G.A. Lowering the limit of detection of ion-selective membranes backside contacted with a film of poly(3-octylthiophene). Sens. Actuators B Chem. 2019, 297, 126781. [Google Scholar] [CrossRef]
- Xu, K.; Crespo, G.A.; Cuartero, M. Subnanomolar detection of ions using thin voltammetric membranes with reduced Exchange capacity. Sens. Actuators B Chem. 2020, 321, 128453. [Google Scholar] [CrossRef]
- Zhu, W.W.; Li, N.B.; Luo, H.Q. Anodic stripping voltammetry determination of Pb (II) and Cd (II) at a bismuth/poly (aniline) film electrode. Anal. Lett. 2006, 39, 2273–2284. [Google Scholar] [CrossRef]
- Compton, G.R.; Banks, C.E. Understanding Voltammetry, 3rd ed.; Word Scientific: London, UK, 2018; pp. 355–376. [Google Scholar]
- Somerset, V.; Leaner, J.; Mason, R.; Iwuoha, E.; Morrin, A. Development and application of a poly (2, 2′-dithiodianiline)(PDTDA)-coated screen-printed carbon electrode in inorganic mercury determination. Electrochim. Acta 2010, 55, 4240–4246. [Google Scholar] [CrossRef]
- Dong, Y.P.; Ding, Y.; Zhou, Y.; Chen, J.; Wang, C.M. Differential pulse anodic stripping voltammetric determination of Pb ion at a montmorillonites/polyaniline nanocomposite modified glassy carbon electrode. J. Electroanal. Chem. 2014, 717, 206–212. [Google Scholar] [CrossRef]
- Joseph, A.; Subramanian, S.; Ramamurthy, P.C.; Sampath, S.; Kumar, R.V.; Schwandt, C. Iminodiacetic acid functionalized polypyrrole modified electrode as Pb(II) sensor: Synthesis and DPASV studies. Electrochim. Acta 2014, 137, 557–563. [Google Scholar] [CrossRef]
- Kumar, P.; Joseph, A.; Ramamurthy, P.C.; Subramanian, S. Lead ion sensor with electrodes modified by imidazole-functionalized polyaniline. Microchim. Acta 2012, 177, 317–323. [Google Scholar] [CrossRef]
- Guo, Z.; Li, S.; Liu, X.M.; Gao, Y.P.; Zhang, W.W.; Ding, X.P. Mesoporous carbon-polyaniline electrode: Characterization and application to determination of copper and lead by anodic stripping voltammetry. Mater. Chem. Phys. 2011, 128, 238–242. [Google Scholar] [CrossRef]
- Su, Z.; Liu, Y.; Xie, Q.; Chen, L.; Huang, Y.; Fu, Y.; Meng, Y.; Li, X.; Ma, M.; Yao, S. Thiol-ene chemistry guided preparation of thiolated polymeric nanocomposite for anodic stripping voltammetric analysis of Cd2+ and Pb2+. Analyst 2013, 138, 1180–1186. [Google Scholar] [CrossRef]
- Somerset, V.S.; Hernandez, L.H.; Iwuoha, E.I. Stripping voltammetric measurement of trace metal ions using screen-printed carbon and modified carbon paste electrodes on river water from the Eerste-Kuils River System. J. Environ. Sci. Health A 2011, 46, 17–32. [Google Scholar] [CrossRef]
- Tang, L.; Chen, J.; Zeng, G.; Zhu, Y.; Zhang, Y.; Zhou, Y.; Xie, X.; Yang, G.; Zhang, S. Ordered mesoporous carbon and thiolated polyaniline modified electrode for simultaneous determination of Cadmium(II) and Lead(II) by anodic stripping voltammetry. Electroanalysis 2014, 26, 2283–2291. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Gicevicius, M.; Ramanaviciene, A.; Mahendra, D.S.; Viter, R.; Ramanavicius, A. Hybrid electrochemical/electrochromic Cu(II) ion sensor prototype based on PANI/ITO-electrode. Sens. Actuators B Chem. 2017, 248, 527–535. [Google Scholar] [CrossRef]
- Dong, Y.P.; Zhou, Y.; Ding, Y.; Chu, X.F.; Wang, C.M. Sensitive detection of Pb(II) at gold nanoparticle/polyaniline/graphene modified electrode using differential pulse anodic stripping voltammetry. Anal. Methods 2014, 6, 9367–9374. [Google Scholar] [CrossRef]
- Joseph, A.; Subramanian, S.; Ramamurthy, P.C.; Sampath, S.; Kumar, R.V.; Schwandt, C. Amine functionalized polyaniline grafted to exfoliated graphite oxide: Synthesis, characterization and multi-element sensor studies. J. Electroanal. Chem. 2015, 757, 137–143. [Google Scholar] [CrossRef]
- Mahadik, M.; Patil, H.; Bodkhe, G.; Ingle, N.; Sayyad, P.; Al-Gahaouri, T.; Shirsat, S.M.; Shirsat, M. EDTA modified PANI/GO composite based detection of Hg (II) ions. Front. Mater. 2020, 7, 81. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Patil, H.K.; Bodkhe, G.A.; Yasuzawa, M.; Koinkar, P.; Ramanaviciene, A.; Shirsat, M.D.; Ramanavicius, A. EDTA-modified PANI/SWNTs nanocomposite for differential pulse voltammetry based determination of Cu(II) ions. Sens. Actuators B Chem. 2018, 260, 331–338. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Celiesiute, R.; Ramanaviciene, A.; Shirsat, M.D.; Ramanavicius, A. EDTA_PANI/SWCNTs nanocomposite modified electrode for electrochemical determination of copper (II), lead (II) and mercury (II) ions. Electrochim. Acta 2018, 259, 930–938. [Google Scholar] [CrossRef]
- Desmukh, M.A.; Patil, H.K.; Bodkhe, G.A.; Yasuzawa, M.; Koinkar, P.; Ramanavicius, A.; Pandey, S.; Shirsat, D. EDA modified PANI/SWNTs nanocomposite for determination of Ni(II) metal ions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 303–309. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Bodkhe, G.A.; Shirsat, S.; Ramanavicius, A.; Shirsat, M.D. Nanocomposite platform based on EDTA modified Ppy/SWNTs for the sensing of Pb(II) ions by electrochemical method. Front. Chem. 2018, 6, 451. [Google Scholar] [CrossRef]
- Kumar, P.; Saravana, S.; Ranjith, K.; Ramamurthy, P.C. D–A–D-structured conducting polymer-modified electrodes for detection of lead(II) ions in water. J. Appl. Electrochem. 2014, 44, 133–139. [Google Scholar] [CrossRef]
- Yu, L.; Cui, X.; Li, H.; Lu, J.; Kang, Q.; Shen, D. A ratiometric electrochemical sensor for multiplex detection of cancer biomarkers using bismuth as an internal reference and metal sulfide nanoparticles as signal tags. Analyst 2019, 144, 4073–4080. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Huang, W.; Zhang, T.; Hu, X.; Perman, J.A.; Ma, S. A metal–organic framework and conducting polymer based electrochemical sensor for high performance cadmium ion detection. J. Mater. Chem. A 2017, 5, 8385–8393. [Google Scholar] [CrossRef]
- Poudel, A.; Sunder, G.S.S.; Rohanifar, A.; Adhikari, S.; Kirchhoff, J.R. Electrochemical determination of Pb2+ and Cd2+ with a poly(pyrrole-1-carboxylic acid) modified electrode. J. Electroanal. Chem. 2022, 911, 116221. [Google Scholar] [CrossRef]
- Shojaei, J.; Zanganeh, A.R. Electrochemically imprinted self-doped polyaniline as highly sensitive voltammetric sensor for determination of bismuth in water, wastewater, and pharmaceutical samples. J. Mater. Sci. Mater. Electron. 2020, 31, 7182–7192. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, E.; Gu, D.; Wang, Y. Glassy carbon electrode coated with polyaniline-functionalized carbon nanotubes for detection of trace lead in acetate solution. Thin Solid Films 2011, 519, 5280–5284. [Google Scholar] [CrossRef]
- Chen, L.; Su, Z.; He, X.; Liu, Y.; Qin, C.; Zhou, Y.; Li, Z.; Wang, L.; Xie, Q.; Yao, S. Square wave anodic stripping voltammetric determination of Cd and Pb ions at a Bi/Nafion/thiolated polyaniline/glassy carbon electrode. Electrochem. Commun. 2012, 15, 34–37. [Google Scholar] [CrossRef]
- Wang, Z.; Li, L.; Liu, E. Graphene ultrathin film electrodes modified with bismuth nanoparticles and polyaniline porous layers for detection of lead and cadmium ions in acetate buffer solutions. Thin Solid Films 2013, 544, 362–367. [Google Scholar] [CrossRef]
- de Barros, A.; Ferreira, M.; Constantino, C.J.L.; Ferreira, M. Nanocomposites based on LbL films of polyaniline and sodium montmorillonite clay. Synth. Met. 2014, 197, 119–125. [Google Scholar] [CrossRef]
- Dutta, K.; Panda, S. Identification of the Levels of Interference of Ions toward heavy metal detection in electrochemical sensors using the barrier width technique. J. Electrochem. Soc. 2018, 165, B378–B389. [Google Scholar] [CrossRef]
- Alshawi, J.M.S.; Mohammed, M.Q.; Alesary, H.F.; Ismail, H.K.; Barton, S. Voltammetric Determination of Hg2+, Zn2+, and Pb2+ Ions Using a PEDOT/NTA-Modified Electrode. ACS Omega 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Park, M.O.; Noh, H.B.; Park, D.S.; Yoon, J.H.; Shim, Y.B. Long-life heavy metal ions sensor based on graphene oxide-anchored conducting polymer. Electroanalysis 2017, 29, 514–520. [Google Scholar] [CrossRef]
- Akhtar, M.; Tahir, A.; Zulfiqar, S.; Hanif, F.; Farooq Warsi, M.; Agboola, P.O.; Shakir, I. Ternary hybrid of polyaniline-alanine-reduced graphene oxide for electrochemical sensing of heavy metal ions. Synth. Met. 2020, 265, 116410. [Google Scholar] [CrossRef]
- Lu, Z.; Dai, W.; Liu, B.; Mo, G.; Zhang, J.; Ye, J.; Ye, J. One pot synthesis of dandelion-like polyaniline coated gold nanoparticles composites for electrochemical sensing applications. J. Colloid Interface Sci. 2018, 525, 86–96. [Google Scholar] [CrossRef]
- Salinas, G.; Frontana-Uribe, B.A.; Reculusa, S.; Garrigue, P.; Kuhn, A. Highly ordered macroporous poly-3,4-ortho-xylendioxythiophene electrodes as a sensitive analytical tool for heavy metal quantification. Anal. Chem. 2018, 90, 11770–11774. [Google Scholar] [CrossRef] [PubMed]
- Demeter, D.; Blanchard, P.; Allain, M.; Grosu, I.; Roncali, J. Synthesis and metal cation complexing properties of crown-annelated terthiophenes containing 3, 4-ethylenedioxythiophene. J. Org. Chem. 2007, 72, 5285–5290. [Google Scholar] [CrossRef] [PubMed]
- Sobkowiak, M.; Gabrielsson, R.; Inganäs, O.; Milczarek, G. Amperometric detection of iron (III) on electroconductive hydrogelbased on polypyrrole and alkoxysulfonatedpoly(3,4-ethylenedioxythiophene) (PEDOT-S). Synth. Met. 2014, 194, 170–175. [Google Scholar] [CrossRef]
- Palanna, M.; Aralekallu, S.; Prabhu, C.P.K.; Sajjan, V.A.; Mounesh; Sannegowda, L.K. Nanomolar detection of mercury(II) using electropolymerized phthalocyanine film. Electrochim. Acta 2021, 367, 137519. [Google Scholar] [CrossRef]
- Marsella, M.J.; Newland, R.J.; Carroll, P.J.; Swager, T.M. Ionoresistivity as a highly sensitive sensory probe: Investigations of polythiophenes functionalized with Calix [4] arene-based ion receptors. J. Am. Chem. Soc. 1995, 117, 9842–9848. [Google Scholar] [CrossRef]
- Salinas, G.; Villarroel Marquez, A.; Idir, M.; Shinde, S.; Frontana-Uribe, B.A.; Raoux, M.; Lang, J.; Cloutet, E.; Kuhn, A. Sodium-ion selectivity study of a crown-ether-functionalized PEDOT analog. ChemElectroChem 2020, 7, 2826–2830. [Google Scholar] [CrossRef]
- Yu, H.-H.; Pullen, A.E.; Buschel, M.G.; Swager, T.M. Charge-specific interactions in segmented conducting polymers: An approach to selective ionoresistive responses. Angew. Chem. Int. Ed. 2004, 43, 3700–3703. [Google Scholar] [CrossRef]
- Zhu, S.S.; Swager, T.M. Conducting polymetallorotaxanes: Metal ion mediated enhancements in conductivity and charge localization. J. Am. Chem. Soc. 1997, 119, 12568–12577. [Google Scholar] [CrossRef]
- Buey, J.; Swager, T.M. Three-strand conducting ladder polymers: Two-step electropolymerization of metallorotaxanes. Angew. Chem. Int. Ed. 2000, 112, 622–626. [Google Scholar] [CrossRef]

| Electrochemical Method | Conducting Polymer | Cation | LOD | Ref |
|---|---|---|---|---|
| Potentiometry | PANI | Hg2+ | 0.2 ppb | [46] |
| Cu2+ | 0.128 ppm | [47] | ||
| Cu2+ | 13.77 ppm | [52] | ||
| Cu2+ | 0.64 ppb | [21] | ||
| Ppy | Zn2+ | 0.52 ppm | [49] | |
| Cu2+ | 0.34 ppm | [50] | ||
| Poly-Dithienyl pyrrole | Fe3+ | 9.7 ppb | [45] | |
| PEDOT | Ag+ | ND | [19] | |
| Ca2+ | ND | [57] | ||
| Pb2+ | ND | |||
| Polycarbzole (PCbz) | Cu2+ | 0.65 ppm | [20] | |
| Co-Polysulfonic phenylenediamine | Pb2+ | 0.026 ppm | [51] | |
| POT | Ca2+ | 0.84 ppm | [53] | |
| Ca2+ | 0.012 ppb | [60,61] | ||
| Differential Pulse Anodic Stripping Voltammetry | PANI | Hg2+ | 56.37 ppb | [64] |
| Pb2+ | 0.21 ppb | [65] | ||
| Pb2+ | 20 ppb | [67] | ||
| Pb2+ | 0.83 ppb | [68] | ||
| Cu2+ | 0.38 ppb | |||
| Pb2+ Cu2+ | 0.01 ppb | [69] | ||
| 0.04 ppb | ||||
| Hg2+ | 7.6–0.03 ppm | [70] | ||
| Cd2+ | 0.16–0.03 ppm | |||
| Pb2+ | 13–0.04 ppm | |||
| Ni2+ | 0.06–0.02 ppm | |||
| Pb2+ | 0.033 ppb | [71] | ||
| Cd2+ | 0.029 ppb | |||
| Cu2+ | 1.27 ppm | [72] | ||
| Pb2+ | 0.1 ppb | [73] | ||
| Hg2+ | 0.44 ppm | [74] | ||
| Cd2+ | 0.15 ppm | |||
| Pb2+ | 2.03 ppm | |||
| Hg2+ | 0.612 ppb | [75] | ||
| Cu2+ | 0.088 ppm | [76] | ||
| Pb2+ | 0.34 ppm | [77] | ||
| Hg2+ | 0.13 ppm | |||
| Ni2+ | 0.058 ppm | [78] | ||
| Cd2+ | 0.3 ppb | [82] | ||
| Bi3+ | 0.48 nM | [84] | ||
| Ppy | Pb2+ | 1.98 ppb | [66] | |
| Pb2+ | 0.07 µM | [79] | ||
| Pb2+ | 0.014 ppm | [83] | ||
| Cd2+ | 0.023 nM | |||
| PTh | Ag+ | 60 ppb | [27] | |
| Cu2+ | ND | [28] | ||
| Hg2+ | ND | |||
| Pb2+ | 20.7 ppb | [80] | ||
| PEDOT | Zn2+ | 2 ppm | [26] | |
| Cd2+ | 0.6 ppm | |||
| Pb2+ | 0.5 ppm | |||
| Cu2+ | 0.6 ppm | |||
| As3+ | 0.5 ppm | |||
| Poly(2-amino terephthalic acid) | Hg2+ | 0.49 ppb | [81] | |
| Cd2+ | 0.13 ppb | |||
| Pb2+ | 0.16 ppb | |||
| Zn2+ | 0.089 ppb | |||
| Square Wave Anodic Stripping Voltammetry | PANI | Pb2+ | ND | [85] |
| Pb2+ | 0.05 ppb | [86] | ||
| Cd2+ | 0.04 ppb | |||
| Pb2+ | 0.069 ppb | [87] | ||
| Pb2+ | 0.267 ppb | [88] | ||
| Cu2+ | 0.283 ppb | |||
| Cd2+ | 0.097 ppb | |||
| Cd2+ | 50 ppb | [89] | ||
| Cu2+ | 0.063 nM | [92] | ||
| Pb2+ | 0.045 nM | |||
| Cd2+ | 0.03 nM | |||
| Pb2+ | 0.62 ppb | [93] | ||
| Cu2+ | 0.51 ppb | |||
| Ppy | Cu2+ | 0.32 ppb | [25] | |
| Pb2+ | 0.1 ppb | |||
| Cd2+ | 5.6 ppb | |||
| Hg2+ | 40 ppb | |||
| PTh | Pb2+ | 0.12 ppb | [24] | |
| Cu2+ | 0.013 ppb | |||
| Hg2+ | 0.1 ppb | |||
| PEDOT | Pb2+ | 2.33 μg L−1 | [90] | |
| Hg2+ | 1.73 μg L−1 | |||
| Zn2+ | 1.99 μg L−1 | |||
| Poly-terthiophene | Zn2+ | 0.05 ppb | [91] | |
| Cd2+ | 0.08 ppb | |||
| Pb2+ | 0.2 ppb | |||
| Cu2+ | 0.09 ppb | |||
| Hg2+ | 0.1 ppb | |||
| PXDOT | Cu2+ | ND | [94] | |
| Cyclic Voltammetry | Ppy | Cu2+ | ND | [22] |
| Pb2+ | ND | |||
| Cd2+ | ND | |||
| Zn2+ | ND | |||
| PTh-PEDOT | Pb2+ | ND | [95] | |
| Chronoamperometry | PEDOT | Pb2+ | 0.04 ppb | [23] |
| Ppy, PEDOT | Fe2+ | 0.8 µM | [96] | |
| Poly-phthalocyanine | Hg2+ | 3.8 nM | [97] | |
| In situ Electrochemical-Conductance | PBTh | Ba2+ | ND | [100] |
| Ca2+ | ND | |||
| PTh-PEDOT | Cu2+ | ND | [101,102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salinas, G.; Frontana-Uribe, B.A. Electrochemical Analysis of Heavy Metal Ions Using Conducting Polymer Interfaces. Electrochem 2022, 3, 492-506. https://doi.org/10.3390/electrochem3030034
Salinas G, Frontana-Uribe BA. Electrochemical Analysis of Heavy Metal Ions Using Conducting Polymer Interfaces. Electrochem. 2022; 3(3):492-506. https://doi.org/10.3390/electrochem3030034
Chicago/Turabian StyleSalinas, Gerardo, and Bernardo A. Frontana-Uribe. 2022. "Electrochemical Analysis of Heavy Metal Ions Using Conducting Polymer Interfaces" Electrochem 3, no. 3: 492-506. https://doi.org/10.3390/electrochem3030034
APA StyleSalinas, G., & Frontana-Uribe, B. A. (2022). Electrochemical Analysis of Heavy Metal Ions Using Conducting Polymer Interfaces. Electrochem, 3(3), 492-506. https://doi.org/10.3390/electrochem3030034







