Modelling the Current Response and Sensitivity of Oxidase Enzyme Electrodes, Monitored Amperometrically by the Consumption of Oxygen
Abstract
:1. Introduction
2. Mathematical Formulation and Analysis of the Problem
2.1. Mathematical Formulation
2.2. Schematic Representation
Boundary Conditions
2.3. Normalised Form
3. Analytical Expression of Concentrations of Mediator and Substrate under Steady-State Condition Using the AGM
4. Discussion
4.1. Previous Work
4.2. Numerical Simulation
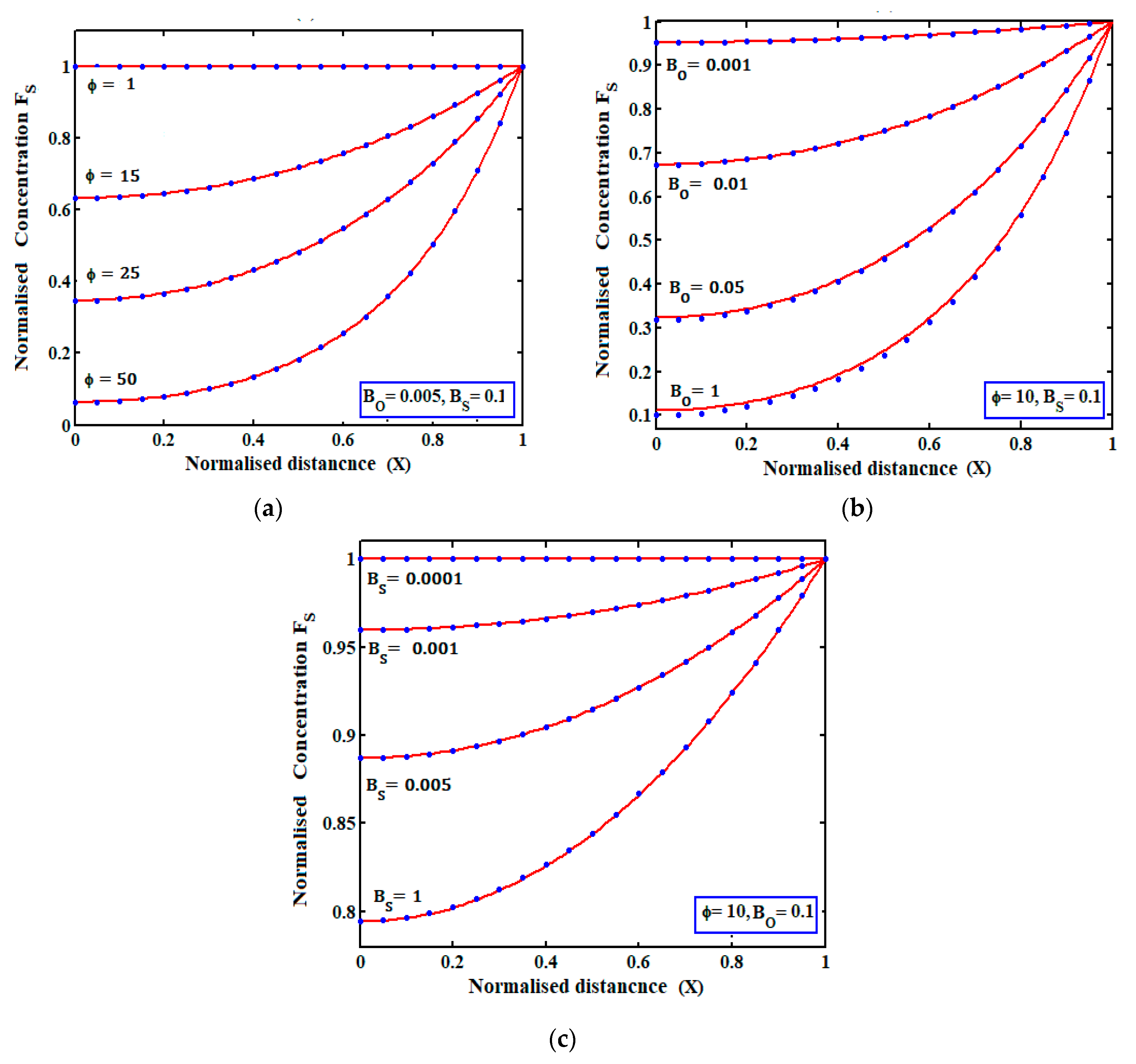
4.2.1. Concentrations of Mediator and Substrate
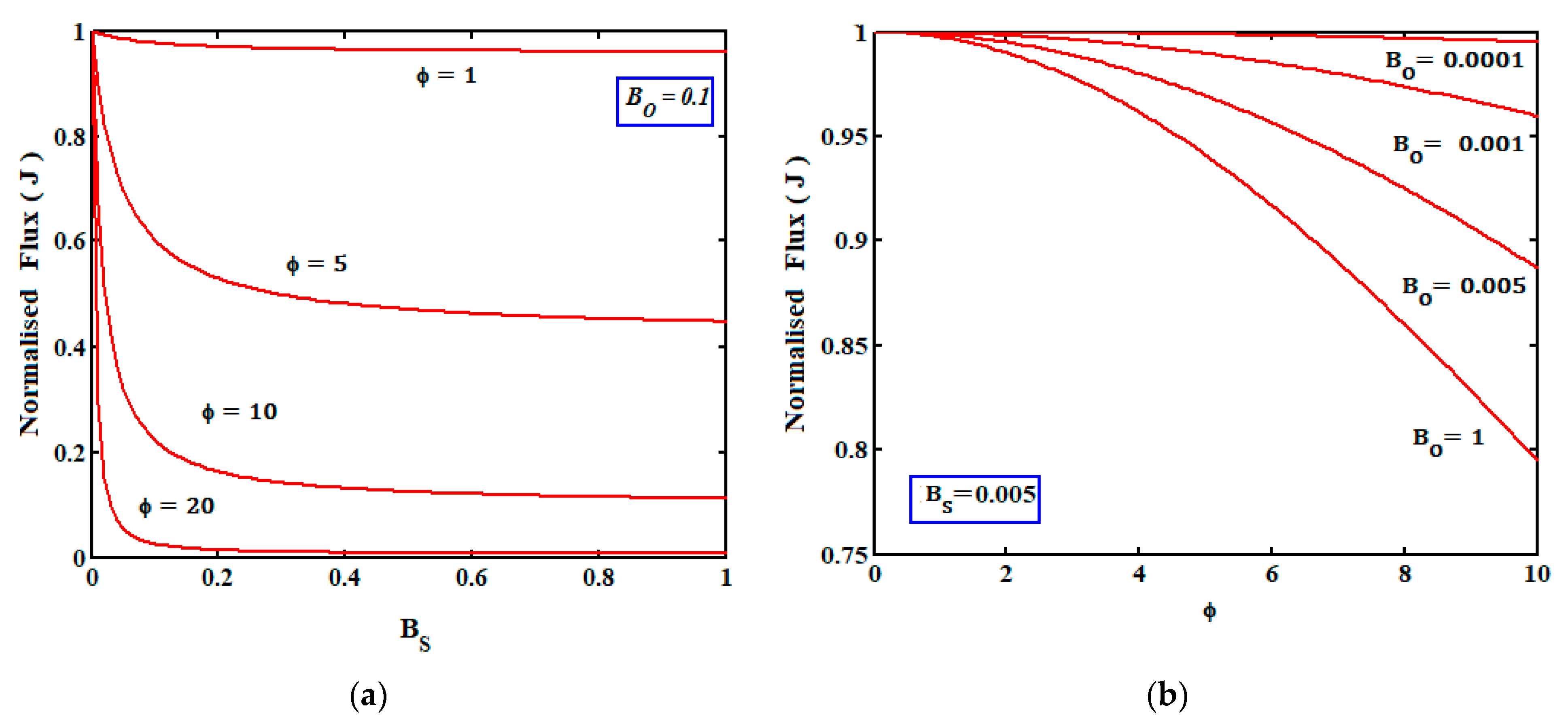
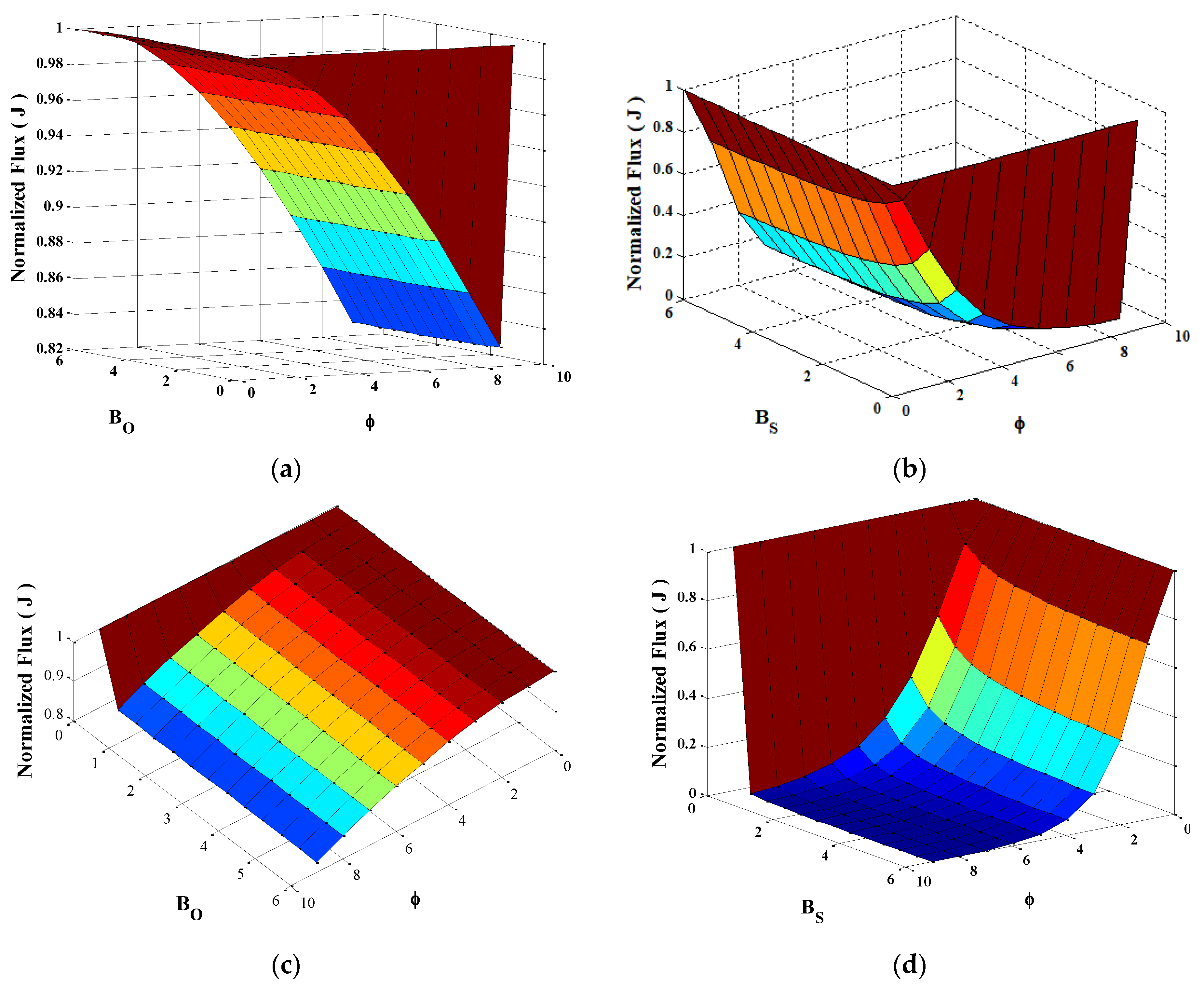
4.2.2. Effect of the Kinetic Parameters on the Current J
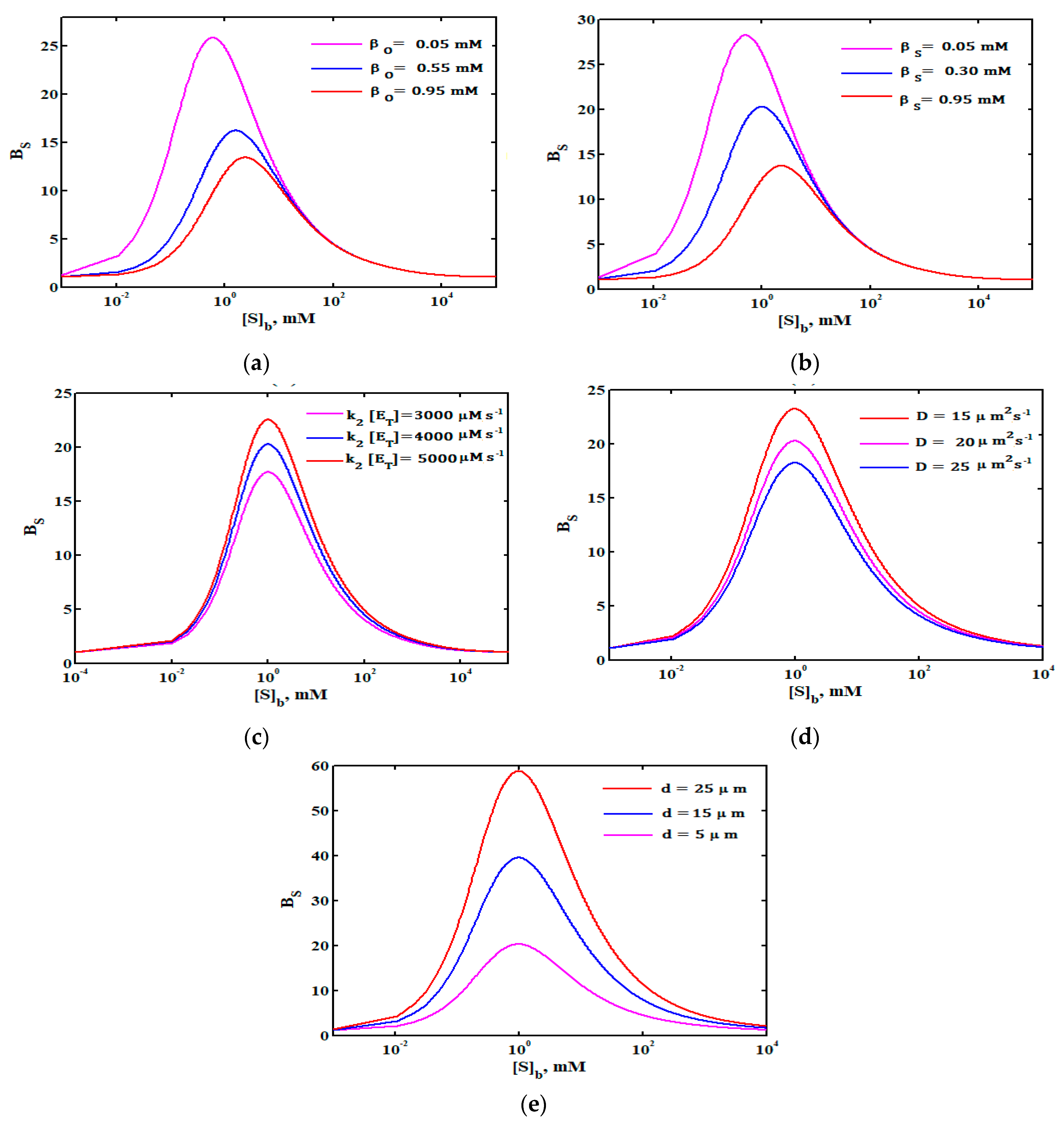
4.2.3. Sensitivity of Biosensor
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Notation
| Symbols | Definitions and Units | Experimental Values of Parameters [9] | Parameters Values Used in This Work |
| Total enzyme concentration in the matrix (μM) | 40 μM | ||
| Enzyme concentration of the oxygen (μM) | - | - | |
| Enzyme concentration of the substrate (μM) | - | - | |
| Reduced enzyme concentration (μM) | - | - | |
| Diffusion coefficient of oxygen (cm2s−1) | 1.90 × 10−7 cm2s−1 | ||
| Diffusion coefficient of substrate (cm2s−1) | 2.22 × 10−6 cm2s−1 | ||
| d | Thickness of the planar matrix (cm) | - | - |
| Concentration of oxygen at any position in the enzyme layer (mole cm−3) | - | - | |
| Oxygen concentration at the enzyme layer|electrode boundary (mM) | 0.25 mM | - | |
| Oxygen concentration in bulk solution (mole cm−3) | - | - | |
| Concentration of substrate at any position in the enzyme layer (mole cm−3) | - | - | |
| Substrate concentration at the enzyme layer|electrode boundary (mM) | - | - | |
| Substrate concentration in bulk solution (mole cm−3) | - | - | |
| Equilibrium partition coefficients for the oxygen (dimensionless) | 1 | - | |
| Equilibrium partition coefficients for the substrate | 1 | - | |
| Normalised surface concentration of mediator (dimensionless) | - | 0.0001–1 | |
| Normalised surface concentration of the substrate (dimensionless) | - | 0.0001–1 | |
| Normalised concentration of the mediator (dimensionless) | 0 to 1 | - | |
| Normalised concentration of the substrate (dimensionless) | 0 to 1 | - | |
| X = y/d | Normalised distance (dimensionless) | - | - |
| Dimensionless flux (dimensionless) | - | - | |
| , | Rate constants (M−1s−1) | 14,000 M−1s−1, 1.95 × 106 M−1s−1 | - |
| , | Rate constants (s−1) | 0 s−1, 1000 s−1 | - |
| Greek symbols | - | ||
| Thiele modulus for the mediator (dimensionless) | - | 1–2500 | |
| Thiele modulus for the substrate (dimensionless) | - | 1–2500 | |
| Subscripts | |||
| Oxidised | - | - | |
| T | Total | - | - |
| red | Reduced | - | - |
| o | Oxygen | - | - |
| s | Substrate | - | - |
| b | Boundary | - | - |
| Bulk | - | - |
Appendix A
References
- Tong, H.D.; Chen, S.; van der Wiel, W.G.; Carlen, E.T.; van den Berg, A. Novel top-down wafer-scale fabrication of single crystal silicon nanowires. Nanoletters 2009, 9, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhu, Z.; Lai, Z.; Wang, R.; Guo, X.; Wu, X.; Zhang, G.; Zhang, Z.; Wang, Y.; Chen, Z. Planar amperometric glucose sensor based on glucose oxidase immobilized by chitosan film on Prussian blue layer. Sensors 2002, 2, 127–136. [Google Scholar] [CrossRef]
- Clark, L.C.; Lyons, L. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Mell, C.D.; Maloy, J.T. A model for the amperometric enzyme electrode obtained through digital simulation and applied to the immobilized glucose oxidase system. Anal. Chem. 1975, 47, 294–299. [Google Scholar] [CrossRef]
- Mell, C.D.; Maloy, J.T. Amperometric response enhancement of the immobilized glucose oxidase enzyme electrode. Anal. Chem. 1976, 48, 1597–1601. [Google Scholar] [CrossRef] [PubMed]
- Schuulmeister, T. Mathematical modelling of the dynamic behaviour of amperometric enzyme electrodes. Sel. Electrode Rev. 1990, 12, 203–260. [Google Scholar]
- Schulmeister, T. Mathematical treatment of concentration profiles and anodic current of amperometric multilayer enzyme electrodes. Anal. Chim. Acta 1987, 198, 223–229. [Google Scholar] [CrossRef]
- Leypoldt, J.K.; Gough, D.A. Model of a two-substrate enzyme electrode for glucose. Anal. Chem. 1984, 56, 2896–2904. [Google Scholar] [CrossRef]
- Gooding, J.J.; Hall, E.A.H. Parameters in the design of oxygen detecting oxidase enzyme electrodes. Electroanalysis 1996, 8, 407–413. [Google Scholar] [CrossRef]
- Gros, P.; Bergel, A. Improved model of a polypyrrole glucose oxidase modified electrode. J. Electroanal. Chem. 1995, 386, 65–73. [Google Scholar] [CrossRef]
- Hall, E.A.H.; Gooding, J.J.; Hall, C.E. Redox enzyme linked electrochemical sensors: Theory meets practice. Mikrochim. Acta 1995, 121, 119–145. [Google Scholar] [CrossRef]
- Martens, N.; Hall, E.A.H. Model for an immobilized oxidase enzyme electrode in the presence of two oxidants. Anal. Chem. 1994, 66, 2763–2770. [Google Scholar] [CrossRef]
- Bartlett, P.N.; Pratt, K.F.E. Modeling of processes in enzyme electrodes. Biosens. Bioelectron. 1993, 8, 451–462. [Google Scholar] [CrossRef]
- Cambiaso, A.; Delfino, L.; Grattarola, M.; Verreschi, G.; Ashworth, D.; Maines, A.; Vadgama, P. Modelling and simulation of a diffusion limited glucose biosensor. Sens. Actuators B Chem. 1996, 33, 203–207. [Google Scholar] [CrossRef]
- Mackey, D.; Killard, A.J.; Ambrosi, A.; Smyth, M.R. Optimizing the ratio of horseradish peroxidase and glucose oxidase on a bienzyme electrode: Comparison of a theoretical and experimental approach. Sens. Actuators B 2007, 122, 605–612. [Google Scholar] [CrossRef]
- Jobst, G.; Moser, I.; Urban, G. Numerical simulation of multi-layered enzymatic sensors. Biosens. Bioelectron. 1996, 11, 1079–1111. [Google Scholar] [CrossRef]
- Iliev, I.; Atanasov, P.; Gamburzev, S.; Kaisheva, A. Transient response of electrochemical biosensors with asymmetrical sandwich membranes. Sens. Actuators B 1992, 8, 6–8. [Google Scholar] [CrossRef]
- Jochum, P.; Kowalski, B.R. A coupled two-compartment model for immobilized enzyme electrodes. Anal. Chim. Acta 1982, 144, 25–38. [Google Scholar] [CrossRef]
- Lemke, K. Mathematical simulation of an enzyme-based glucose sensor with pO2-basic sensor. Biomed. Biochim. Acta 1989, 48, 867–877. [Google Scholar]
- Sorochinskii, V.V.; Kurganov, B.I. Steady-state kinetics of cyclic conversions of substrate in amperometric bienzyme sensors. Biosens. Bioelectron. 1996, 11, 225–238. [Google Scholar] [CrossRef]
- Kulys, J.J.; Sorochinskii, V.V.; Vidziunaite, R.A. Transient response of bienzyme electrodes. Biosensors 1986, 2, 135–146. [Google Scholar] [CrossRef]
- Manimozhi, P.; Subbiah, A.; Rajendran, L. Solution of steady-state substrate concentration in the action of biosensor response at mixed enzyme kinetics. Sens. Actuators B 1992, 8, 6–11. [Google Scholar] [CrossRef]
- Flexer, V.; Pratt, K.F.E.; Garay, F.; Bartlett, P.N.; Calvo, E.J. Relaxation and simplex mathematical algorithms applied to the study of steady-state electrochemical response of immobilized enzyme biosensors: Comparison with experiments. J. Electroanal. Chem. 2008, 616, 87–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolev, S.D. Mathematical modelling of single-line flow-injection analysis systems with single-layer enzyme electrode detection. Anal. Chim. Acta 1990, 241, 43–53. [Google Scholar] [CrossRef]
- Baronas, R.; Ivanauskas, F.; Kaunietis, I.; Laurinavicius, V. Mathematical modeling of plate-gap biosensors with an outer porous membrane. Sensors 2006, 6, 727–745. [Google Scholar] [CrossRef] [Green Version]
- Parker, J.W.; Schwartz, C.S. Modeling the kinetics of immobilized glucose oxidase. Biotech. Bioeng. 1987, 30, 724–735. [Google Scholar] [CrossRef]
- Athinson, B.; Lester, D.E. An enzyme rate equation for the overall rate of reaction of gel-immobilized glucose oxidase particles under buffered conditions. Biotech. Bioeng. 1974, 16, 1299–1320. [Google Scholar] [CrossRef]
- Akbari, M.R.; Ganji, D.D.; Nimafar, M.; Ahmadi, A.R. Significant progress in solution of nonlinear equations at displacement of structure and heat transfer extended surface by new AGM approach. Front. Mech. Eng. 2014, 9, 390–401. [Google Scholar] [CrossRef]
- Dharmalingam, K.M.; Veeramuni, M. Akbari-Ganji’s Method (AGM) for solving non-linear reaction-Diffusion equation in the electroactive polymer film. J. Electroanal. Chem. 2019, 844, 1–5. [Google Scholar] [CrossRef]
- He, J.H. Some asymptotic methods for strongly nonlinear equations. Int. J. Mod. Phys. B 2006, 20, 1141–1199. [Google Scholar] [CrossRef] [Green Version]
- Loghambal, S.; Rajendran, L. Mathematical modeling in amperometric oxidase enzyme membrane electrodes. J. Mem. Sci. 2011, 373, 20–28. [Google Scholar] [CrossRef]
- Loghambal, S.; Rajendran, L. Mathematical modeling of diffusion and kinetics of amperometric immobilized enzyme electrodes. Electrochim. Acta 2010, 55, 5230–5238. [Google Scholar] [CrossRef]
- Meena, A.; Rajendran, L. Mathematical modeling of amperometric and potentiometric biosensors and system of non-linear equations—Homotopy perturbation approach. J. Electroanal. Chem. 2010, 644, 50–59. [Google Scholar] [CrossRef]
- Eswari, A.; Rajendran, L. Analytical solution of steady state current an enzyme modified microcylinder electrodes. J. Electroanal. Chem. 2010, 648, 36–46. [Google Scholar] [CrossRef]
- Margret, V.; Rajendran, L. Analytical expression of non-steady-state concentration profiles at planar electrode for the CE mechanism. Nat. Sci. 2010, 2, 13181325. [Google Scholar]
- Baronas, R.; Kulys, J.; Ivanauskas, F. Mathematical Modeling of Biosensors. In An Introduction for Chemists and Mathematicians; Springer: Dordrecht, The Netherlands; Berlin/Heidelberg, Germany; London, UK; New York, NY, USA, 2010. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirabaharan, P.; Devi, M.C.; Swaminathan, R.; Rajendran, L.; Lyons, M.E.G. Modelling the Current Response and Sensitivity of Oxidase Enzyme Electrodes, Monitored Amperometrically by the Consumption of Oxygen. Electrochem 2022, 3, 309-321. https://doi.org/10.3390/electrochem3020021
Pirabaharan P, Devi MC, Swaminathan R, Rajendran L, Lyons MEG. Modelling the Current Response and Sensitivity of Oxidase Enzyme Electrodes, Monitored Amperometrically by the Consumption of Oxygen. Electrochem. 2022; 3(2):309-321. https://doi.org/10.3390/electrochem3020021
Chicago/Turabian StylePirabaharan, Pandy, M. Chitra Devi, Rajagopal Swaminathan, Lakshmanan Rajendran, and Michael E. G. Lyons. 2022. "Modelling the Current Response and Sensitivity of Oxidase Enzyme Electrodes, Monitored Amperometrically by the Consumption of Oxygen" Electrochem 3, no. 2: 309-321. https://doi.org/10.3390/electrochem3020021
APA StylePirabaharan, P., Devi, M. C., Swaminathan, R., Rajendran, L., & Lyons, M. E. G. (2022). Modelling the Current Response and Sensitivity of Oxidase Enzyme Electrodes, Monitored Amperometrically by the Consumption of Oxygen. Electrochem, 3(2), 309-321. https://doi.org/10.3390/electrochem3020021






