Efficacy of the PlasmaShield®, a Non-Thermal, Plasma-Based Air Purification Device, in Removing Airborne Microorganisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. PlasmaShield Design
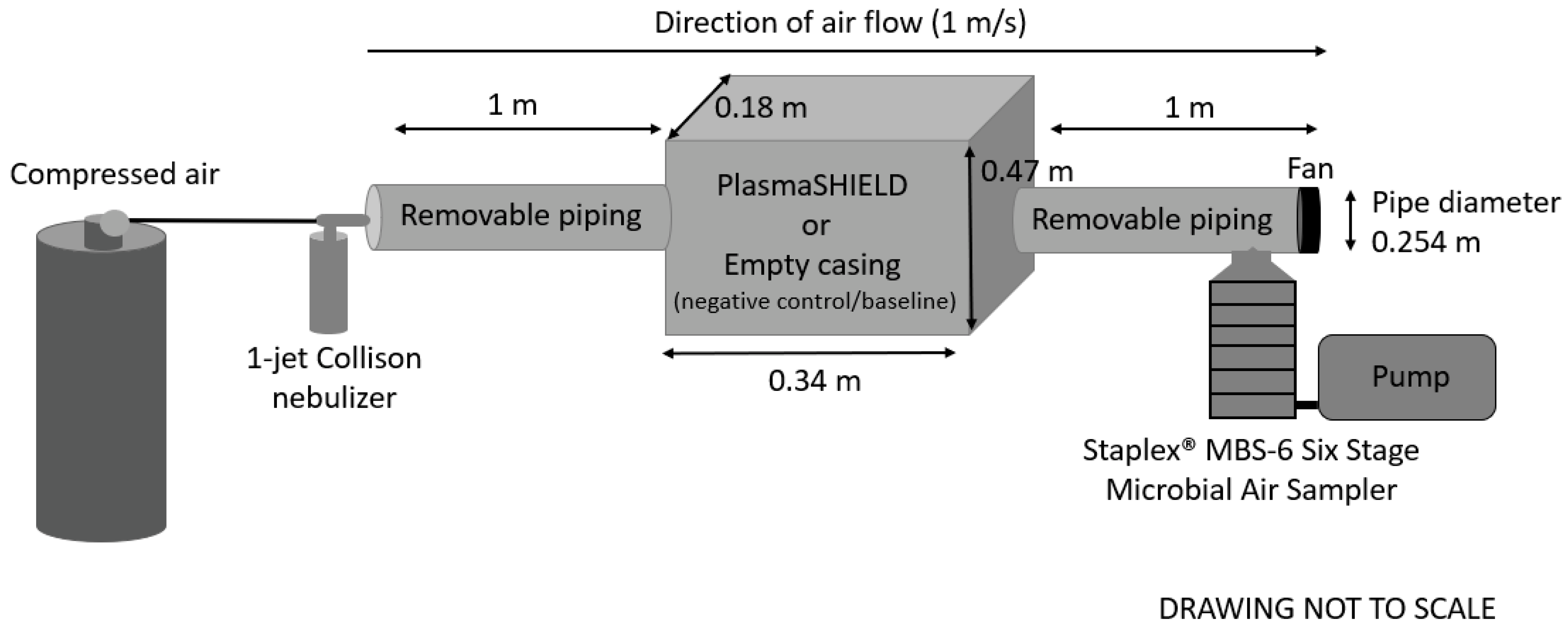
2.2. Experimental Setup
2.3. Escherichia coli
2.4. Staphylococcus epidermidis
2.5. Bacteriophage MS2
2.6. Cladosporium sp.
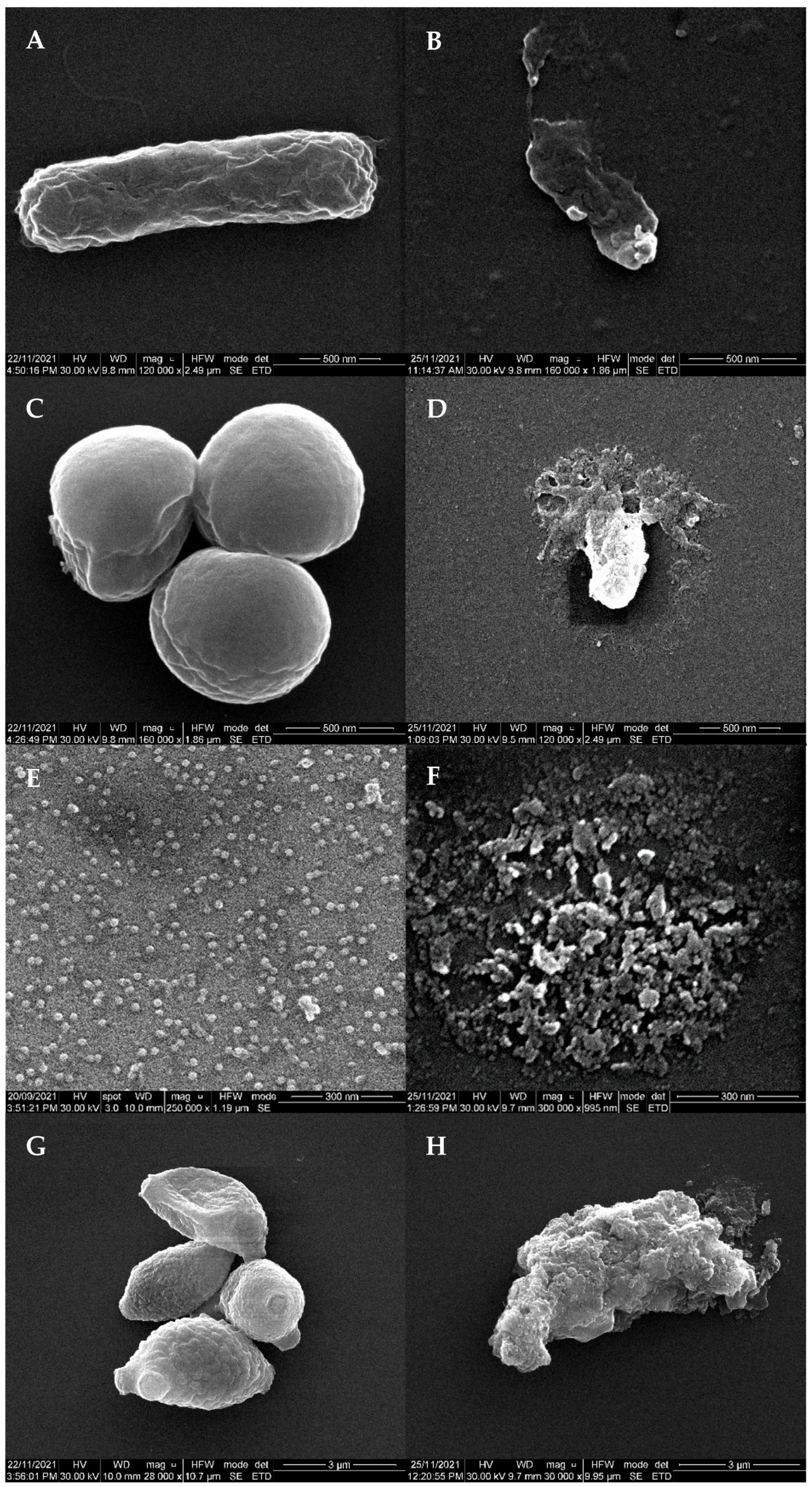
2.7. Scanning Electron Micrscopy (SEM)
3. Results
Efficacy of PlasmaShield® to Remove Airborne Escherichia coli, Staphylococcus epidermidis, Bacteriophage MS2 and Cladosporium sp.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tran, V.V.; Park, D.; Lee, Y.-C. Indoor air pollution, related human diseases, and recent trends in the control and improvement of indoor air quality. Int. J. Environ. Res. Public Health 2020, 17, 2927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouchtouri, V.A.; Rudge, J.W. Legionnaires’ disease in hotels and passenger ships: A systematic review of evidence, sources, and contributing factors. J. Travel Med. 2015, 22, 325–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomlinson, B.; Cockram, C. Sars: Experience at Prince of Wales hospital, Hong Kong. Lancet 2003, 361, 1486–1487. [Google Scholar] [CrossRef]
- Hoge, C.W.; Reichler, M.R.; Dominguez, E.A.; Bremer, J.C.; Mastro, T.D.; Hendricks, K.A.; Musher, D.M.; Elliott, J.A.; Facklam, R.R.; Breiman, R.F. An epidemic of pneumococcal disease in an overcrowded, inadequately ventilated jail. N. Engl. J. Med. 1994, 331, 643–648. [Google Scholar] [CrossRef]
- Liu, Y.; Ning, Z.; Chen, Y.; Guo, M.; Liu, Y.; Gali, N.K.; Sun, L.; Duan, Y.; Cai, J.; Westerdahl, D. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 2020, 582, 557–560. [Google Scholar] [CrossRef]
- Li, Y.; Qian, H.; Hang, J.; Chen, X.; Cheng, P.; Ling, H.; Wang, S.; Liang, P.; Li, J.; Xiao, S. Probable airborne transmission of SARS-CoV-2 in a poorly ventilated restaurant. Build. Environ. 2021, 196, 107788. [Google Scholar] [CrossRef]
- Noorimotlagh, Z.; Jaafarzadeh, N.; Martínez, S.S.; Mirzaee, S.A. A systematic review of possible airborne transmission of the COVID-19 virus (SARS-CoV-2) in the indoor air environment. Environ. Res. 2021, 193, 110612. [Google Scholar] [CrossRef]
- Griffiths, W.D.; Bennett, A.; Speight, S.; Parks, S. Determining the performance of a commercial air purification system for reducing airborne contamination using model micro-organisms: A new test methodology. J. Hosp. Infect. 2005, 61, 242–247. [Google Scholar] [CrossRef]
- enHealth Council. Guidance on Use of Rainwater Tanks; National Public Health Partnership; Australian Government Department of Health and Ageing: Canberra, Australia, 2004; Volume 3432-JN8304.
- United States Environmental Protection Agency. Recreational Water Quality Criteria; Office of Water, Ed.; United Stated Environmental Protection Agency: Washington, DC, USA, 2012.
- Food Standards Australia New Zealand. Australian and New Zealand Food Standards Code; Australian Department of Health: Canberra, Australia, 2015.
- Blount, Z.D. The natural history of model organisms: The unexhausted potential of E. coli. Elife 2015, 4, e05826. [Google Scholar] [CrossRef]
- Gomes, F.; Teixeira, P.; Oliveira, R. Mini-review: Staphylococcus epidermidis as the most frequent cause of nosocomial infections: Old and new fighting strategies. Biofouling 2014, 30, 131–141. [Google Scholar] [CrossRef]
- Weßels, C.; Strommenger, B.; Klare, I.; Bender, J.; Messler, S.; Mattner, F.; Krakau, M.; Werner, G.; Layer, F. Emergence and control of linezolid-resistant Staphylococcus epidermidis in an ICU of a German hospital. J. Antimicrob. Chemother. 2018, 73, 1185–1193. [Google Scholar] [CrossRef]
- Büttner, H.; Mack, D.; Rohde, H. Structural basis of Staphylococcus epidermidis biofilm formation: Mechanisms and molecular interactions. Front. Cell. Infect. Microbiol. 2015, 5, 14. [Google Scholar]
- Wojtyczka, R.D.; Orlewska, K.; Kępa, M.; Idzik, D.; Dziedzic, A.; Mularz, T.; Krawczyk, M.; Miklasińska, M.; Wąsik, T.J. Biofilm formation and antimicrobial susceptibility of Staphylococcus epidermidis strains from a hospital environment. Int. J. Environ. Res. Public Health 2014, 11, 4619–4633. [Google Scholar] [CrossRef] [Green Version]
- McMinn, B.R.; Ashbolt, N.J.; Korajkic, A. Bacteriophages as indicators of fecal pollution and enteric virus removal. Lett. Appl. Microbiol. 2017, 65, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Kohn, T.; Mattle, M.J.; Minella, M.; Vione, D. A modeling approach to estimate the solar disinfection of viral indicator organisms in waste stabilization ponds and surface waters. Water Res. 2016, 88, 912–922. [Google Scholar] [CrossRef]
- Silverman, A.I.; Nguyen, M.T.; Schilling, I.E.; Wenk, J.; Nelson, K.L. Sunlight inactivation of viruses in open-water unit process treatment wetlands: Modeling endogenous and exogenous inactivation rates. Environ. Sci. Technol. 2015, 49, 2757–2766. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, S.R.S.; Dovom, M.R.E.; Yavarmanesh, M.; Abbaszadegan, M. Thermal inactivation of ms2 bacteriophage as a surrogate of enteric viruses in cow milk. J. Consum. Prot. Food Saf. 2017, 12, 341–347. [Google Scholar] [CrossRef]
- Turgeon, N.; Toulouse, M.-J.; Martel, B.; Moineau, S.; Duchaine, C. Comparison of five bacteriophages as models for viral aerosol studies. Appl. Environ. Microbiol. 2014, 80, 4242–4250. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Z.; Kuehn, T.H.; Bekele, A.Z.; Mor, S.K.; Verma, H.; Goyal, S.M.; Raynor, P.C.; Pui, D.Y. Survival of airborne ms2 bacteriophage generated from human saliva, artificial saliva, and cell culture medium. Appl. Environ. Microbiol. 2014, 80, 2796–2803. [Google Scholar] [CrossRef] [Green Version]
- Flannigan, B.; Samson, R.A.; Miller, J.D. Microorganisms in Home and Indoor Work Environments: Diversity, Health Impacts, Investigation and Control; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Verde, S.C.; Almeida, S.M.; Matos, J.; Guerreiro, D.; Meneses, M.; Faria, T.; Botelho, D.; Santos, M.; Viegas, C. Microbiological assessment of indoor air quality at different hospital sites. Res. Microbiol. 2015, 166, 557–563. [Google Scholar] [CrossRef]
- Yilmaz, O.; Asan, A.; Aydogdu, H.; Sen, B. Airborne fungal diversity inside a nursing home in Edirne, Turkey. Fresenius Environ. Bull. 2017, 26, 7025–7033. [Google Scholar]
- Reddy, M.K.; Srinivas, T. Mold allergens in indoor play school environment. Energy Procedia 2017, 109, 27–33. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Z.; Zhu, Y.; Xu, W.; Li, H. Investigation of dust loading and culturable microorganisms of HVAC systems in 24 office buildings in Beijing. Energy Build. 2015, 103, 166–174. [Google Scholar] [CrossRef]
- Khazaei, S.V. Gas Purifying Apparatus. U.S. Patent 10,744,515, 2020. Washington, DC: U.S. Patent and Trademark Office. Available online: https://patents.google.com/patent/US10744515B2/en (accessed on 21 April 2022).
- Zhao, W.; Alwahabi, Z.T. Diagnostics of air purification plasma device by spatially resolved emission spectroscopy. Plasma 2022, 5, 206–220. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, S.; Langenbacher, J. Ozone Test Report (Report Number: 210304019gzu-001); Intertek: Guangzhou, China, 2021. [Google Scholar]
- The California Air Resources Board. List of Carb-Certified Air Cleaning Devices. Available online: https://ww2.arb.ca.gov/list-carb-certified-air-cleaning-devices (accessed on 21 April 2022).
- Macher, J.M. Positive-hole correction of multiple-jet impactors for collecting viable microorganisms. Am. Ind. Hyg. Assoc. J. 1989, 50, 561–568. [Google Scholar] [CrossRef]
- Schroder, T.; Gaskin, S.; Ross, K.; Whiley, H. Antifungal activity of essential oils against fungi isolated from air. Int. J. Occup. Environ. Health 2018, 23, 181–186. [Google Scholar] [CrossRef]
- Zhang, Y.; Mo, J.; Li, Y.; Sundell, J.; Wargocki, P.; Zhang, J.; Little, J.C.; Corsi, R.; Deng, Q.; Leung, M.H. Can commonly-used fan-driven air cleaning technologies improve indoor air quality? A literature review. Atmos. Environ. 2011, 45, 4329–4343. [Google Scholar] [CrossRef]
- Earnest, G.S.; Gressel, M.G.; Mickelsen, R.L.; Moyer, E.S.; Reed, L.D.; Karwacki, C.J.; Morrison, R.W.; Tevault, D.E. Guidance for Filtration and Air-Cleaning Systems to Protect Building Environments from Airborne Chemical, Biological, or Radiological Attacks; Department of Health and Human Services, National Institute for Occupational Safety and Health, Eds.; Department of Health and Human Services: Cincinatti, OH, USA, 2003; p. 78.
- Fisk, W.J.; Faulkner, D.; Palonen, J.; Seppanen, O. Performance and costs of particle air filtration technologies. Indoor Air 2002, 12, 223–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly-Wintenberg, K.; Sherman, D.M.; Tsai, P.-Y.; Gadri, R.B.; Karakaya, F.; Chen, Z.; Roth, J.R.; Montie, T.C. Air filter sterilization using a one atmosphere uniform glow discharge plasma (the volfilter). IEEE Trans. Plasma Sci. 2000, 28, 64–71. [Google Scholar] [CrossRef]
- Tanaka, Y.; Fujino, K.; Larkins, G.A.; Osawa, A.; Hayashi, Y.; Taharaguchi, S. Preventing the spread of norovirus-like infections by the airborne route using plasma assisted catalytic technology (pact). J. Vet. Med. Sci. 2018, 80, 1459–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly-Wintenberg, K.; Hodge, A.; Montie, T.; Deleanu, L.; Sherman, D.; Reece Roth, J.; Tsai, P.; Wadsworth, L. Use of a one atmosphere uniform glow discharge plasma to kill a broad spectrum of microorganisms. J. Vac. Sci. Technol. A Vac. Surf. Film. 1999, 17, 1539–1544. [Google Scholar] [CrossRef]
- Yu, Q.; Huang, C.; Hsieh, F.H.; Huff, H.; Duan, Y. Bacterial inactivation using a low-temperature atmospheric plasma brush sustained with argon gas. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 80, 211–219. [Google Scholar] [CrossRef]
- Murray, B.K.; Ohmine, S.; Tomer, D.P.; Jensen, K.J.; Johnson, F.B.; Kirsi, J.J.; Robison, R.A.; O’Neill, K.L. Virion disruption by ozone-mediated reactive oxygen species. J. Virol. Methods 2008, 153, 74–77. [Google Scholar] [CrossRef]
- Yusupov, M.; Bogaerts, A.; Huygh, S.; Snoeckx, R.; Van Duin, A.C.; Neyts, E.C. Plasma-induced destruction of bacterial cell wall components: A reactive molecular dynamics simulation. J. Phys. Chem. 2013, 117, 5993–5998. [Google Scholar] [CrossRef]
| Microorganism | Test Concentration (CFU/mL or PFU/mL) | Average Positive Hole Corrected CFU or PFU (±2SD) | Log10 Removal | Percentage Removal (%) + | |
|---|---|---|---|---|---|
| Negative Control | PlasmaShield® | ||||
| Escherichia coli | 107 | 2875 (±22) | 37 (±22) | 2 | 98 |
| 108 | 28,750 * | 16 (±4) | 3 | 99.9 | |
| 109 | 287,500 * | 15 (±2) | 4 | 99.99 | |
| Staphylococcus epidermidis | 106 | 2026 (±92) | 44 (±25) | 2 | 98 |
| 107 | 20,260 * | 34 (±11) | 3 | 99.8 | |
| 108 | 202,600 * | 165 (±20) | 3 | 99.9 | |
| 109 | 2,026,000 * | 625 (±153) | 4 | 99.97 | |
| Bacteriophage MS2 | 107 | 117 (±30) | 24 (±9) | 1 | 80 |
| 1010 | 117,000 * | 34 (±14) | 4 | 99.97 | |
| 1012 | 11,700,000 * | 91 (±30) | 5 | 99.999 | |
| 1015 | 11,700,000,000 * | 2757 (±886) | 7 | 99.99998 | |
| Cladosporium sp. | 107 | 4957 (±1809) | 10 (±1) | 3 | 99.8 |
| 109 | 495,700 * | 21 (±5) | 4 | 99.99 | |
| 1010 | 4,957,000 * | 54 (±27) | 5 | 99.999 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whiley, H.; Keerthirathne, T.P.; Kuhn, E.J.; Nisar, M.A.; Sibley, A.; Speck, P.; Ross, K.E. Efficacy of the PlasmaShield®, a Non-Thermal, Plasma-Based Air Purification Device, in Removing Airborne Microorganisms. Electrochem 2022, 3, 276-284. https://doi.org/10.3390/electrochem3020019
Whiley H, Keerthirathne TP, Kuhn EJ, Nisar MA, Sibley A, Speck P, Ross KE. Efficacy of the PlasmaShield®, a Non-Thermal, Plasma-Based Air Purification Device, in Removing Airborne Microorganisms. Electrochem. 2022; 3(2):276-284. https://doi.org/10.3390/electrochem3020019
Chicago/Turabian StyleWhiley, Harriet, Thilini P. Keerthirathne, Emma J. Kuhn, Muhammad Atif Nisar, Alex Sibley, Peter Speck, and Kirstin E. Ross. 2022. "Efficacy of the PlasmaShield®, a Non-Thermal, Plasma-Based Air Purification Device, in Removing Airborne Microorganisms" Electrochem 3, no. 2: 276-284. https://doi.org/10.3390/electrochem3020019
APA StyleWhiley, H., Keerthirathne, T. P., Kuhn, E. J., Nisar, M. A., Sibley, A., Speck, P., & Ross, K. E. (2022). Efficacy of the PlasmaShield®, a Non-Thermal, Plasma-Based Air Purification Device, in Removing Airborne Microorganisms. Electrochem, 3(2), 276-284. https://doi.org/10.3390/electrochem3020019








