Chitosan/Gold Nanoparticles Nanocomposite Film for Bisphenol A Electrochemical Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of AuNPs:PTS Nanoparticles
2.3. Layer-by-Layer (LbL) Films’ Fabrication and Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
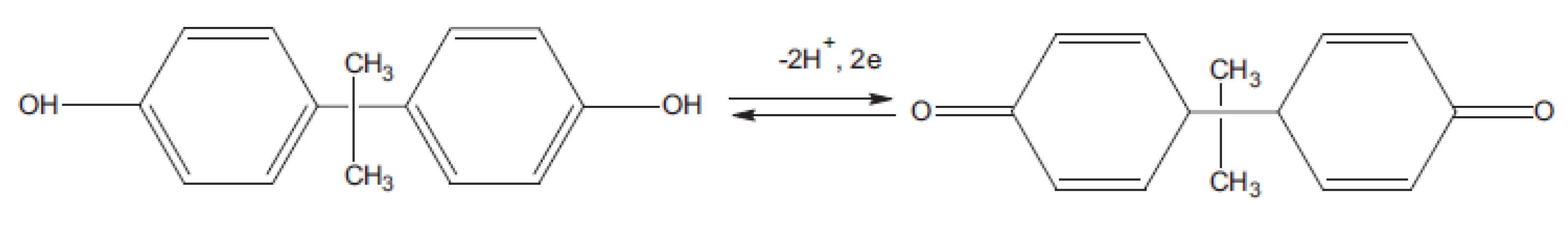
3.1. Choice of Materials
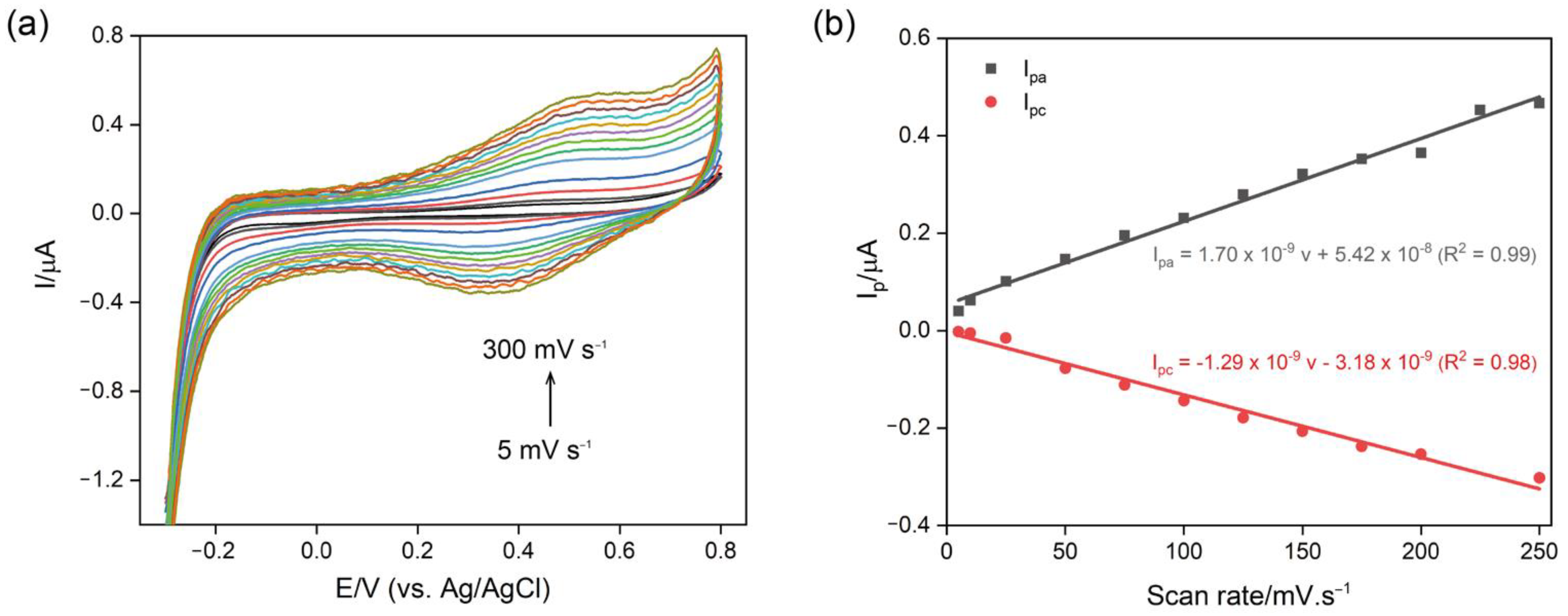
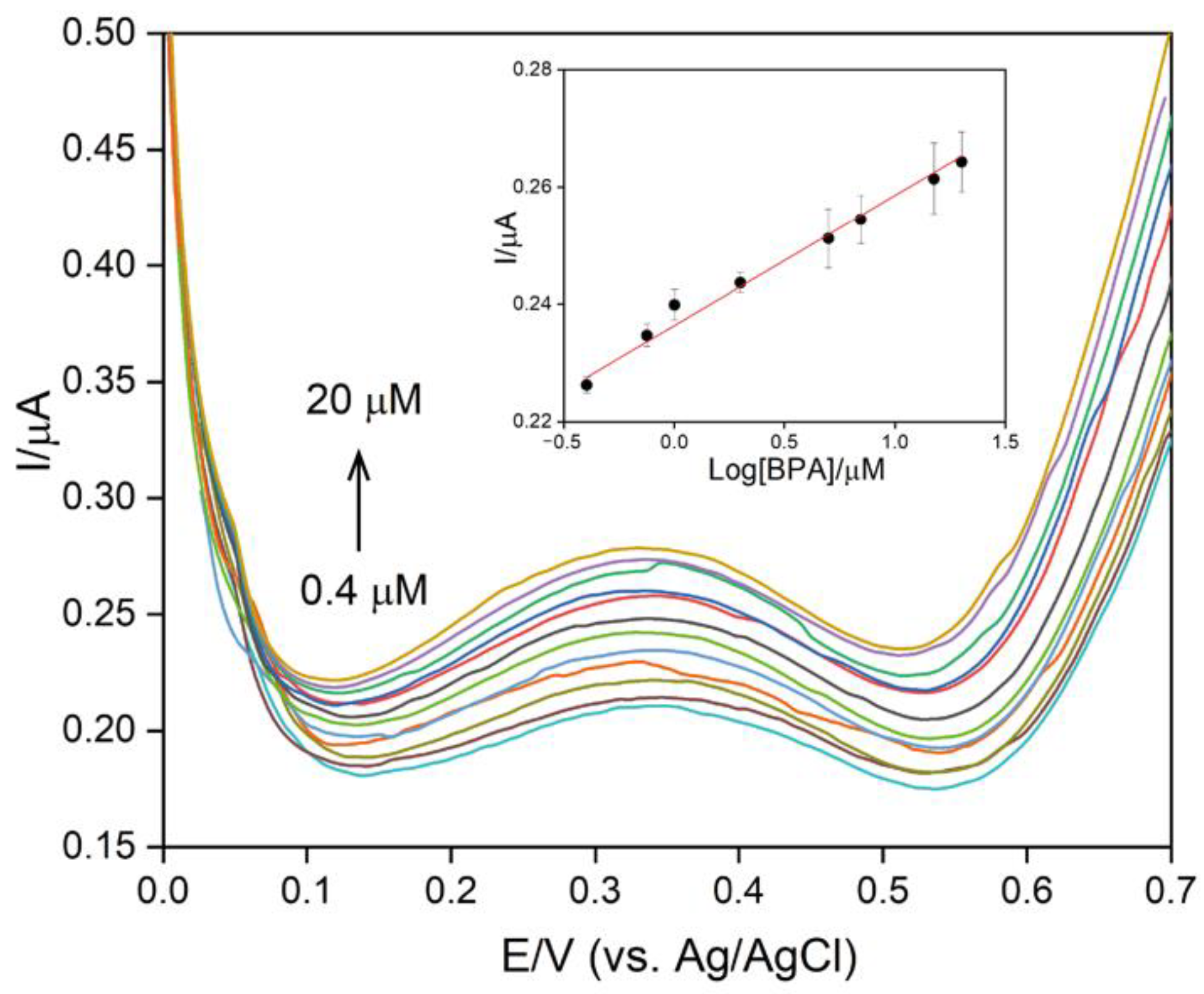
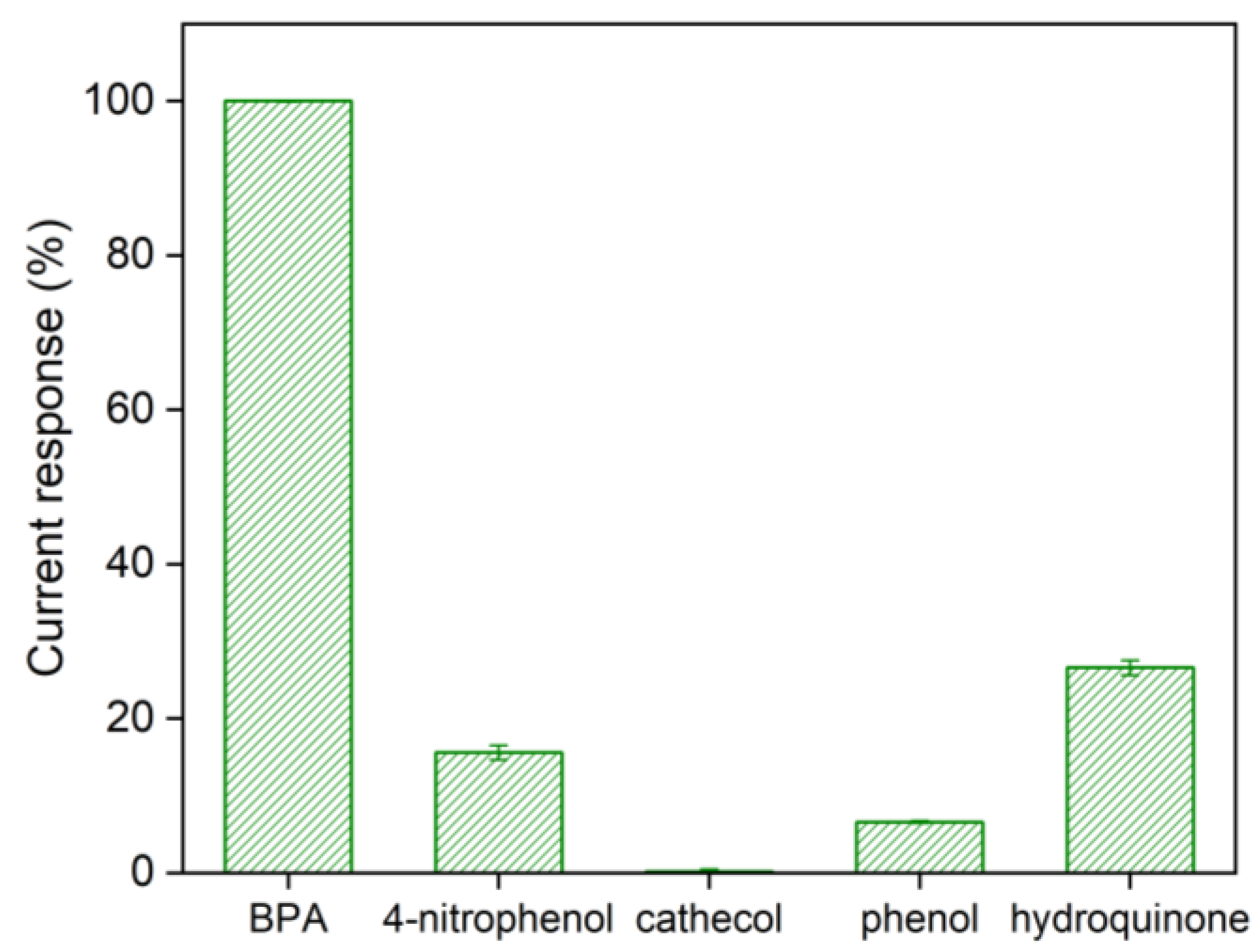
3.2. Characterization of Chi/AuNPs:PTS LbL Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xing, J.; Zhang, S.; Zhang, M.; Hou, J. A critical review of presence, removal and potential impacts of endocrine disruptors bisphenol A. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 254, 109275. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Chakraborty, P. A review on sources and health impacts of bisphenol A. Rev. Environ. Health 2020, 35, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Tarafdar, A.; Sirohi, R.; Balakumaran, P.A.; Reshmy, R.; Madhavan, A.; Sindhu, R.; Binod, P.; Kumar, Y.; Kumar, D.; Sim, S.J. The hazardous threat of Bisphenol A: Toxicity, detection and remediation. J. Hazard. Mater. 2022, 423, 127097. [Google Scholar] [CrossRef] [PubMed]
- Sivaranjanee, R.; Senthil Kumar, P.; Saravanan, R.; Govarthanan, M. Electrochemical sensing system for the analysis of emerging contaminants in aquatic environment: A review. Chemosphere 2022, 294, 133779. [Google Scholar] [CrossRef] [PubMed]
- Mirmira, P.; Evans-Molina, C. Bisphenol A, Obesity, and Type 2 Diabetes Mellitus: Genuine Concern or Unnecessary Preoccupation? Transl. Res. 2014, 164, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Sanko, V.; Şenocak, A.; Tümay, S.O.; Orooji, Y.; Demirbas, E.; Khataee, A. An Electrochemical Sensor for Detection of Trace-Level Endocrine Disruptor Bisphenol A Using Mo2Ti2AlC3 MAX Phase/MWCNT Composite Modified Electrode. Environ. Res. 2022, 212, 113071. [Google Scholar] [CrossRef]
- Majedi, S.M.; Lai, E.P.C. Mass Spectrometric Analysis of Bisphenol A Desorption from Titania Nanoparticles: Ammonium Acetate, Fluoride, Formate, and Hydroxide as Chemical Desorption Agents. Methods Protoc. 2018, 1, 26. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, M.A.M.; André, L.C.; de Cardeal, Z.L. Hollow fiber liquid-phase microextraction-gas chromatography-mass spectrometry method to analyze bisphenol A and other plasticizer metabolites. J. Chromatogr. A 2017, 1481, 31–36. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Bai, J.; Peng, Y.; Ning, B.; Shi, H.; Kang, W.; Zhou, H.; Gao, Z. Determination of Bisphenol A by High-Performance Liquid Chromatography Based on Graphene Magnetic Dispersion Solid Phase Extraction. J. Chromatogr. Sci. 2020, 58, 280–286. [Google Scholar] [CrossRef]
- Mercante, L.A.; Iwaki, L.E.O.; Scagion, V.P.; Oliveira, O.N.; Mattoso, L.H.C.; Correa, D.S. Electrochemical Detection of Bisphenol A by Tyrosinase Immobilized on Electrospun Nanofibers Decorated with Gold Nanoparticles. Electrochem 2021, 2, 41–49. [Google Scholar] [CrossRef]
- Furquim, F.C.; Santos, E.N.; Mercante, L.A.; Amaral, M.M.; Pavinatto, A.; Rodrigues, B.V.M. Green and low-cost electrospun membranes from polycaprolactone/graphene oxide for Bisphenol A sensing. Mater. Lett. 2020, 274, 128014. [Google Scholar] [CrossRef]
- Gugoasa, L.A.D. Review—Electrochemical Sensors for Determination of the Endocrine Disruptor, Bisphenol A. J. Electrochem. Soc. 2020, 167, 37506. [Google Scholar] [CrossRef]
- Pérez-Fernández, B.; Muñiz, A. de la E. Electrochemical biosensors based on nanomaterials for aflatoxins detection: A review (2015–2021). Anal. Chim. Acta 2022, 6, 339658. [Google Scholar] [CrossRef]
- Baig, N.; Sajid, M.; Saleh, T.A. Recent trends in nanomaterial-modified electrodes for electroanalytical applications. TrAC—Trends Anal. Chem. 2019, 111, 47–61. [Google Scholar] [CrossRef]
- Ahmad, R.; Wolfbeis, O.S.; Hahn, Y.B.; Alshareef, H.N.; Torsi, L.; Salama, K.N. Deposition of nanomaterials: A crucial step in biosensor fabrication. Mater. Today Commun. 2018, 17, 289–321. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Gu, M.; Park, M.; Kim, T.; Kim, B.S. Layer-by-layer assembly for photoelectrochemical nanoarchitectonics. Mol. Syst. Des. Eng. 2019, 4, 65–77. [Google Scholar] [CrossRef]
- Sanfelice, R.C.; Pavinatto, A.; Gonçalves, V.C.; Correa, D.S.; Mattoso, L.H.C.; Balogh, D.T. Synthesis of a nanocomposite containing a water-soluble polythiophene derivative and gold nanoparticles. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1245–1254. [Google Scholar] [CrossRef]
- Pavinatto, A.; Mercante, L.A.; Leandro, C.S.; Mattoso, L.H.C.; Correa, D.S. Layer-by-Layer assembled films of chitosan and multi-walled carbon nanotubes for the electrochemical detection of 17α-ethinylestradiol. J. Electroanal. Chem. 2015, 755, 215–220. [Google Scholar] [CrossRef]
- Mercante, L.A.; Scagion, V.P.; Pavinatto, A.; Sanfelice, R.C.; Mattoso, L.H.C.; Correa, D.S. Electronic Tongue Based on Nanostructured Hybrid Films of Gold Nanoparticles and Phthalocyanines for Milk Analysis. J. Nanomater. 2015, 2015, 402. [Google Scholar] [CrossRef]
- Mercante, L.A.; Pavinatto, A.; Iwaki, L.E.O.; Scagion, V.P.; Zucolotto, V.; Oliveira, O.N.; Mattoso, L.H.C.; Correa, D.S. Electrospun polyamide 6/poly(allylamine hydrochloride) nanofibers functionalized with carbon nanotubes for electrochemical detection of dopamine. ACS Appl. Mater. Interfaces 2015, 7, 4784–4790. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Akbari, A. A novel and high sensitive MWCNTs-nickel carbide/hollow fiber-pencil graphite modified electrode for in situ ultra-trace analysis of bisphenol A. J. Electroanal. Chem. 2018, 817, 9–17. [Google Scholar] [CrossRef]
- Arabali, V.; Ebrahimi, M.; Gheibi, S.; Khaleghi, F.; Bijad, M.; Rudbaraki, A.; Abbasghorbani, M.; Ganjali, M.R. Bisphenol A Analysis in Food Samples Using Modified Nanostructure Carbon Paste Electrode as a Sensor. Food Anal. Methods 2016, 9, 1763–1769. [Google Scholar] [CrossRef]
- Chan, Y.Y.; Yue, Y.; Li, Y.; Webster, R.D. Electrochemical/chemical oxidation of bisphenol A in a four-electron/two- proton process in aprotic organic solvents. Electrochim. Acta 2013, 112, 287–294. [Google Scholar] [CrossRef]
- Smyth, W.F. Analytical Electrochemistry; Wiley: New York, NY, USA, 1995; Volume 311, ISBN 0471282723. [Google Scholar]
- Li, H.; Wang, W.; Lv, Q.; Xi, G.; Bai, H.; Zhang, Q. Disposable paper-based electrochemical sensor based on stacked gold nanoparticles supported carbon nanotubes for the determination of bisphenol A. Electrochem. Commun. 2016, 68, 104–107. [Google Scholar] [CrossRef]
- Su, B.; Shao, H.; Li, N.; Chen, X.; Cai, Z.; Chen, X. A sensitive bisphenol A voltammetric sensor relying on AuPd nanoparticles/graphene composites modified glassy carbon electrode. Talanta 2017, 166, 126–132. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, J.; Li, D.; Li, Y. An electrochemical aptasensor based on gold nanoparticles dotted graphene modified glassy carbon electrode for label-free detection of bisphenol A in milk samples. Food Chem. 2014, 162, 34–40. [Google Scholar] [CrossRef]
- Niu, X.; Yang, W.; Wang, G.; Ren, J.; Guo, H.; Gao, J. A novel electrochemical sensor of bisphenol A based on stacked graphene nanofibers/gold nanoparticles composite modified glassy carbon electrode. Electrochim. Acta 2013, 98, 167–175. [Google Scholar] [CrossRef]
- Santana, E.R.; de Lima, C.A.; Piovesan, J.V.; Spinelli, A. An original ferroferric oxide and gold nanoparticles-modified glassy carbon electrode for the determination of bisphenol A. Sens. Actuators B Chem. 2017, 240, 487–496. [Google Scholar] [CrossRef]
- Ben Messaoud, N.; Ghica, M.E.; Dridi, C.; Ben Ali, M.; Brett, C.M.A. Electrochemical sensor based on multiwalled carbon nanotube and gold nanoparticle modified electrode for the sensitive detection of bisphenol A. Sens. Actuators B Chem. 2017, 253, 513–522. [Google Scholar] [CrossRef]
- Safavi Gerdin, H.; Sarhadi, H.; Tajik, S. Determination of bisphenol A in real samples using modified graphite screen-printed electrode. Int. J. Environ. Anal. Chem. 2020, 1–10. [Google Scholar] [CrossRef]
- Sá, A.C.; Barbosa, S.C.; Raymundo-Pereira, P.A.; Wilson, D.; Shimizu, F.M.; Raposo, M.; Oliveira, O.N., Jr. Flexible carbon electrodes for electrochemical detection of bisphenol-A, hydroquinone and catechol in water samples. Chemosensors 2020, 8, 103. [Google Scholar] [CrossRef]


| Electrode | Electroanalytical Method | Detection Limit (µmol L−1) | Linear Range (µmol L−1) | Ref. |
|---|---|---|---|---|
| MWCNTs a/AuNP/Paper | LSV | 0.131 | 0.876–87.6 | [25] |
| AuPdNPs/GNs b | DPV | 0.008 | 0.05–10 | [26] |
| AuNps-GR c/GCE | DPV | 0.005 | 0.01–10 | [27] |
| AuNps/SGNF d/GCE | LSV | 0.035 | 0.08 to 250 | [28] |
| Fe3O4 NPs-Si4Pic+Cl− e/AuNps-Si4Pic+Cl−/GCE | DPV | 0.007 | 0.02 to 1.4 | [29] |
| AuNP/MWCNT/GCE | DPV | 0.004 | 0.01 to 0.7 | [30] |
| La3+/ZnO NFs/GSPE | DPV | 0.25 | 0.8 to 300.0 | [31] |
| Flexible SPE f electrochemically treated with H2SO4 | DPV | 0.95 | 2-50 | [32] |
| (Chi/AuNPs:PTS)3 | DPV | 0.32 | 0.4 to 20 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, L.A.; Rodrigues, B.V.M.; Balogh, D.T.; Sanfelice, R.C.; Mercante, L.A.; Frade-Barros, A.F.; Pavinatto, A. Chitosan/Gold Nanoparticles Nanocomposite Film for Bisphenol A Electrochemical Sensing. Electrochem 2022, 3, 239-247. https://doi.org/10.3390/electrochem3020016
Almeida LA, Rodrigues BVM, Balogh DT, Sanfelice RC, Mercante LA, Frade-Barros AF, Pavinatto A. Chitosan/Gold Nanoparticles Nanocomposite Film for Bisphenol A Electrochemical Sensing. Electrochem. 2022; 3(2):239-247. https://doi.org/10.3390/electrochem3020016
Chicago/Turabian StyleAlmeida, Leandro A., Bruno V. M. Rodrigues, Debora T. Balogh, Rafaela C. Sanfelice, Luiza A. Mercante, Amanda F. Frade-Barros, and Adriana Pavinatto. 2022. "Chitosan/Gold Nanoparticles Nanocomposite Film for Bisphenol A Electrochemical Sensing" Electrochem 3, no. 2: 239-247. https://doi.org/10.3390/electrochem3020016
APA StyleAlmeida, L. A., Rodrigues, B. V. M., Balogh, D. T., Sanfelice, R. C., Mercante, L. A., Frade-Barros, A. F., & Pavinatto, A. (2022). Chitosan/Gold Nanoparticles Nanocomposite Film for Bisphenol A Electrochemical Sensing. Electrochem, 3(2), 239-247. https://doi.org/10.3390/electrochem3020016







