Abstract
Renewable energy resources (wind, solar) are unpredictable, so it is wise to store the electricity they generate in an energy carrier X. Various PtX (power to useful energy-intensive raw material such as hydrogen, synthetic natural gas, fuel) applications have been proposed. At the heart of our work is widely used idea to convert residual CO2 from biogas plant into higher hydrocarbons using electricity from renewables (e.g., sun, wind, hydro). The specific goal is to produce ethylene-highly demanded hydrocarbon in plastics industry. The process itself is realised on electrocatalytic carbon/copper cathode which must be selective to reaction: 2CO2 + 12e− + 12H+→C2H4 + 4H2O. We propose a bottom-up approach to build catalyst from the smallest particles-graphene sheet stacks (GSS) coated with metallic copper nanocrystals. Composite GSS-Cu structure functions as a CO2 and proton absorber, facilitating hydrogenation and carbon–carbon coupling reactions on Cu-nanocluster/GSS for the formation of C2H4. In our design electrocatalytic electrode is made from nitrogen-doped graphene sheet stacks coated with copper nanostructures. The N-GSSitself can be drop-casted or electrophoretically incorporated onto the carbon paper and gas diffusion electrode. Electrochemical deposition method was recognized as successful and most promising to grow Cu nanocrystals on N-GSS incorporated in conducting carbon substrate. Gaseous products from CO2 electro-catalytic reformation on the cathode were investigated by mass-spectrometer but the electrode surface was analysed by SEM/EDS and XRD methods.
Keywords:
electrocatalysis; CO2; graphene sheet stacks; gas diffusion electrode; catalysts; ethylene 1. Introduction
The UN Climate Conference on COP26 is taking place in Glasgow, Scotland, from 31 October to 12 November 2021, at which countries must agree on action to curb global warming [1]. The leaders of almost 200 countries approved agreement, committing to fight climate change by cutting CO2 emissions to slow down the increase in its concentrations in the atmosphere, which has now reached 415 ppm [2].
Since CO2 is the final product of combustion of fossil fuels, its capture at the source (i.e., in exhaust sources), which is more implementable than directly capturing from the atmosphere and subsequent conversion using green electricity into useful products, have become an area of interest for researchers worldwide [3]. There are numerous catalyst-activated routes (thermal, photo, electrochemical and biologic) to convert anthropogenic CO2 into fuels and other chemicals employing renewable energy selected in number of projects [4,5].
Electrocatalytic CO2 reduction in electrochemical electrolysis cell is highly promising since the reduction process can be carried out at ambient conditions with green electricity and without the need of external hydrogen (since hydrogen is released at the cathode during the water electrolysis) [6]. Catalysts are needed to improve not only the selectivity but also the yield of all of these reactions, which involve transfer of two or more electrons and protons in multiple steps on three phase contact points—a catalyst surface, gas (CO2), and electrolyte. There are many catalyst materials screened up to now: Au, Ag, Zn primarily catalyse the formation of CO; Bi, Sn, In, Pb catalyse formic acid, and only Cu, copper oxides, and hydroxides drive the formation of C-C coupling and formation of multi-carbon molecules [6,7,8,9].
Copper (Cu) is identified as catalyst material that can produce different hydrocarbons and oxygenates carbon monoxide (CO), formic acid (HCOOH), methanol (CH3OH), methane (CH4), ethanol (C2H5OH), and ethylene (C2H4) [6,7,8,9]. Typical cathode reactions start with hydrogen evolution reaction (HER) and follows through CO2 reduction reaction (CO2RR) [7]:
2H+ + 2e− → H2(aq) E0 = 0.00 V vs. RHE*
CO2 + 2e− + 2H+ → CO(g) + H2O E0 = −0.11 V vs. RHE
CO2 + 2H+ + 2e− → HCOOH(aq) E0 = −0.12 V vs. RHE
CO2 + 6H+ + 6e− → CH3OH(aq) + H2O E0 = −0.03 V vs. RHE
CO2 + 8e− + 8H− → CH4(g) + 2H2O E0 = 0.17 V vs. RHE
2CO2 + 12e− + 12H+ → C2H5OH(aq) + 3H2O E0 = 0.09 V vs. RHE
2CO2 + 12e− + 12H+ → C2H4(g) + 4H2O E0 = 0.06 V vs. RHE
*RHE—reversible hydrogen electrode
These half-reaction standard potential values are very close, and thus slight changes in the environment (such as local pH, morphology, crystalline facets, doping and etc.) will determine the selectivity of reaction products. To ensure the desired product, the charge transfer must be led. However, this has proven to be a challenging problem.
In case of ethylene, a multiple-step 12 electron and proton transfer reaction (7) on copper-based electrodes proceed at fairly high Faradaic efficiencies (FE) but with low currents. FE in the presence of two or more concurrent reactions indicates the percentage of electrons (charge) that contributes to the formation of a particular product.
More efforts have to be devoted to developing an electrode with a 3D structure and mixed hydrophobic/hydrophilic surfaces for powerful catalysts for efficient CO2 reduction to higher hydrocarbons. Single crystal metal surfaces or foil electrodes are incapable of this, due to the mass transport limitation of CO2, due to the poor solubility of CO2 in aqueous electrolytes (33 mM at 298 K and 1 atm.). The mass transport constraint can be managed by using gas diffusion electrodes (GDE). GDEs in electrocatalytic electrolysis cell provide environment in which a solid catalyst promotes the electrochemical reaction between the liquid and gaseous phases [7].
Another challenge is to keep the system stable over a long period of time—the falling-off and corrosion of the catalyst coating must be prevented. To this end, researchers propose different solutions, for example B-doped Cu and integrated sacrificial zinc anode [10]. Despite some more efforts on oxygen plasma-activated Cu [11], electro-redeposited Cu [12] and a copper catalyst modified with a polymer [13] the stabilisation issue of Cu under CO2RR condition remains challenging. In cases where the required CO2 reforming products are gaseous (CO, C2H4), the membrane-electrode system helps to hold the catalyst in place on the electrode surface—the catalyst (GSS/Cu) would be enclosed at the interface between the gas diffusion electrode and the proton conducting membrane [14]. No catholyte is needed; to power carbon dioxide electroreduction process, moist CO2 gas has to flow onto the cathode side in the proton exchange membrane cell. As concluded by the authors of [15], much effort has been devoted to achieving a stable CO2 electroreduction performance by catalyst electrode design. The biggest issue is low productivity and difficulty in product separation in low current regime and catalyst lose at higher currents. Through the introduction of zero-gap electrolyser, the amount of electrolyte reduces and accelerates the CO2 conversion reaction rate.
The graphene/nanoparticle hybrid structures exhibit additional advantageous and often synergistic properties that greatly augment their potential for catalysis applications. There are number of studies about modification of the graphene oxide surface by copper nanoparticles to develop efficient electrocatalysts for CO2 reduction to liquid multi-carbon products (see as example [16,17,18]). N-doped graphene supported Cu nanoparticle catalysts [16] prepared by simple wet chemical method shows high selectivity for ethanol with FE of 25.72% at −1.0 V (vs. RHE). The defective vertical graphene and stabilized Cu/CuxO enhances CO2 adsorption and promotes electron transfer to the adsorbed CO2 and intermediates on the catalyst surface, thus improving the overall CO2 reduction performance [17]. The Cu-terminated armchair graphene nanoribbons were more efficient catalysts for producing methanol from CO2, offering the advantages of a lower over-potential and higher selectivity than bulk Cu and other graphene-supported Cu structures [18]. Only a few studies can be found on the use of graphene/copper heterostructure for the synthesis of ethylene in the process of CO2 electro-reduction. For example, the authors of [19] claimed that the manifestation of several parameters including oxide contents in an electrocatalyst, Raman peaks ID/IG ratio due to GO support and mode of fabrication of working electrodes along with porous microstructures of the nanocomposite contributes crucially towards CO2 electro-reduction to ethylene.
Carbon-based materials are potentially interesting catalyst carriers for the CO2 reduction reaction due to their low cost and especially due to their ability to form a wide range of hybrid nanostructures [20,21]. They are chemically inactive at negative potential ranges and present high overpotentials for the hydrogen evolution reaction compared to metal surfaces [21]. Pristine graphene does not exhibit any activity. However, introducing dopants and defects during the synthesis tailors the electronic structure and catalytic properties of nanostructured carbon materials. In particular, N-doping has been shown to significantly enhance the CO2 reduction activity [21].
These preceding studies have led us to elaborate a theoretical model for stable C2H4-selective electrocatalyst based on copper nanocluster decorated graphene and understand how this selectivity came to fruition [22]. In this paper, we report the design of electrocatalytic electrode from graphene sheet stacks (GSS) coated with copper nanocrystals to follow our theoretical predictions [22,23]. The novelty of this study is in obtaining stacks of graphene sheets (GSS) using high-frequency modulated DC pulses for electrochemical exfoliation. The exfoliation process is also useful to dope the resulting material with selected atoms, such as nitrogen, though, a suitable electrolyte must be found. Industrial graphite waste is taken as the starting material. Two methods have been tested for the application of the copper catalyst-chemical and electrochemical deposition. It has been found that the size of the copper crystals and the uniformity of the coating can be controlled by the length and amplitude of the current pulses, which prefers the electrochemical deposition method to obtain good catalyst electrodes. Carbon paper and a gas diffusion layer (GDL) were used as substrates to design the electrocatalyst cathode. A gas diffusion layer electrode is shown as best to perform production of ethylene with satisfying FE at high currents.
2. Materials and Methods
2.1. Reagents, Raw Materials and Substrates
The following chemicals were used as received without further purification: deionized water (Crystal 7, Adrona Ltd., Riga, Latvia), ethanol, Polyvinylidene Fluoride (PVDF), Dimethylformamide (DMF), N-Methyl-2-pyrrolidone (NMP), hydrazine (N2H4), CuSO4, copper sulphate pentahydrate CuSO4·5H2O, copper foil (all from Sigma-Aldrich, St. Louis, MO, USA, chemical grade). Waste graphite crucibles were obtained from Termopulss Ltd. (Adazi, Latvia); carbon paper from AvCarb MGL190 from Ballard Power (Canada) and GDL from Fuel Cell Store (USA).
2.2. Synthesis of Graphene Sheet Stacks
Stacks of graphene sheets (GSS) were obtained using high-frequency modulated DC pulses for electrochemical exfoliation. The peak duration is 5 s, amplitude 10 V, modulated with 100 kHz small amplitude (100 mV) signal. Electrodes were made from industrial graphite waste, and obtained micro-structured GSS were separated and annealed at 500 °C in Ar/H2 (95/5 vol%) atmosphere. The exfoliation process is also grateful to dope the resulting material with selected atoms, such as nitrogen—a suitable electrolyte must be found. The presence of N-C bonds in the GSS material thus obtained is shown by our previous work [24].
2.3. Copper Chemical Deposition
Chemical deposition of Cu nanocrystals onto GSS was performed reducing Cu2+ simultaneously with non-reduced GSS in aqueous hydrazine (N2H4) solution (modified setup from [25]). For hydrazine reduced Cu on GSS we used CuSO4 solution as a Cu source. The GSS on Carbon paper was submerged in CuSO4 and then droplets of hydrazine were added as drop casting method. The hydrazine reacted with CuSO4 and Cu nanocrystals and microcrystals could be formed. The procedure was performed until the surface of Carbon paper was completely coated with Cu.
2.4. Electrode Formation
In our research on the development of a selective electrode for the electrocatalytic conversion of CO2 to ethylene, carbon paper and gas diffusion layer (GDL) have been used as substrates. They are first electrophoretically coated with GSS and then copper microcrystals by chemical and/or electrochemical deposition.
2.5. Copper Electrochemical Deposition
Copper coatings with different structures are electrochemically deposited (0.05 M CuSO4 aqueous solution) on the carbon paper with GSS particles. Electrochemical deposition of copper on Freudenberg-GDL substrate (catalyst area 3.2 cm2) was carried out. It was proved that more stable and selective to ethylene formation catalyst layer are obtained in pulse deposition process with 0.3 mA/cm2 current pulse length 7 ms. As electrolyte the 1.25 M CuSO4 solution was used: deposition time 2 h, total charge–30 C, thickness of deposited Cu layer around 3 µm, coverage or uniformity of coating 77%.
2.6. Analyses of Morphology and Structure
SEM analysis of GSS was performed with Lyra3 TESCAN. XRD analyses were used to characterize composition and structure of deposited copper layers using XRD X’Pert PRO (PANalytical) equipment.
2.7. Electrochemical Tests
Electrochemical tests and gaseous product analysis were carried out in three electrode self-made cell (in case of carbon paper-based cathode) and in FlexCell® PP cell (Gaskatel -electrochemical test cell made of polypropylene in case of GDL based cathode) with Nafion 117 proton exchange membrane. 0.5 M KHCO3 solution saturated with CO2 was used as electrolyte, RHE as hydrogen reference electrode and Pt wire with Pt-black coating was used as counter electrode. Different measurements–linear sweep voltammetry, chrono-potentiometry were carried out with VoltaLab PGZ-301 potentiostat. Selected CO2RR experiments were performed also with potentiostat PGSTAT302N (Metrohm Autolab).
2.8. Gas Analyses
The evolved gases from the cathode were analysed in a mass spectrometer RGA-100 (Pfeifer). In case of solid cathode (carbon paper) the headspace gases from catholyte compartment were collected and analysed from a gas syringe. Gas flow by the GDL electrode were collected from the back-side of electrode into gasbag for further analysis. Results were normalized vs. total consumed CO2 and total amount of detected products represented in results table. More information in Supplementary Materials Section S1.
2.9. Faraday Efficiency (FE) Calculations
It is known that CO2RR to produce ethylene requires at least 12 electron and proton transfer. Even though, the specific pathway is not definitively known, one can assume 12 single electron transfer steps through adsorbed carbene (CH2) mechanism [23]: . Thus, knowing supplied current, charge and produced amount of gasses, Faradaic efficiency for ethylene can be calculated [7] from Equation (8)
where e is the number of electrons participating in the reaction (12 for ethylene), n the number of products in moles, F the Faraday constant, Q the supplied charge, I the current, and t is time. More information can be in the Supplementary Materials Section S2.
3. Results and Discussion
As it is shown from theoretical model developed by one from authors of this article Sergei Piskunov [22,23], the Cu7 cluster is quite strongly physically adsorbed to the graphene layer with binding energy of −1.54 eV/Cu atom. The negative binding energy means energy is released after the substrate-adsorbate coupling. Single Cu atoms tend to adsorb at the hollow sites of graphene with the binding energy of −2.65 eV/Cu atom. Thus, Cu atoms deposited at graphene could reproduce the facets of the most stable Cu (111) surface [22]. Nevertheless, Cu deposited at graphene forms quite weak Cu-C graphene bonds, with a bond population of 80 millielectrons. The presence of the nitrogen atoms at graphene support allows for strong chemisorption of Cu atom with the bond population of Cu-N = 303 millielectrons and N-C graphene = 344 millielectrons [23].
The SEM micrographs of exfoliated GSS powder is shown below in Figure 1. Clear GSS edges are visible. As it is seen in SEM image (Figure 2), due to chemical deposition process the Cu coating can be rare, with chaotically scattered copper nanocrystals of various sizes (range from 20 to 200 nm).

Figure 1.
SEM inspection of graphene sheet stacks.

Figure 2.
Copper (white structures in pictures) grown on rGSS deposited on carbon paper. SEM images of (a) distinct rGSS particle with copper on it and (b) small coper crystals attached to the edges (yellow arrow) of rGSS flakes.
The concentration and temperature of the reaction solutions should be optimized. Perhaps exfoliated graphene is too reduced and has insufficient amount of defect sites with oxygen/nitrogen atoms attached to its surface which acts as seed forming centres. The following experiments are performed to optimize the material, and mainly Electrochemical deposition pulse method can be noted as a solution to the concerns raised above.
Integration of rGSS with carbon paper/gas diffusion electrode is performed by electrophoretic deposition of rGSS–SEM micrographs clearly shows GSS flakes on the substrate (Figure 2a).
Electrochemical copper catalyst deposition on carbon paper and GDL electrodes with GSS flakes gives coatings with different structures (needles, wires, flowers, cubes). From SEM and XRD results it has been concluded that copper creates dendrite and needle such as Cu/Cu2O structures when high current density (up to 100 mA/cm2) is applied, on the other hand micron dimension grains with developed crystalline Cu/Cu2O structures (Figure 3 and Figure 4) appear when low current densities (0.1–0.01 mA/cm2) with shorter current pulses and longer growth times are applied. Upon long exposition, the crystals grow in size until dense coverage is reached (as shown in Figure 3). It is important to note that in this process the copper coating is formed as the monovalent copper oxide Cu2O, which at negative potential is reduced to copper without changing morphology (SEM (Figure 3) and XRD (Figure 4) images before and after 60 min of electrolysis).
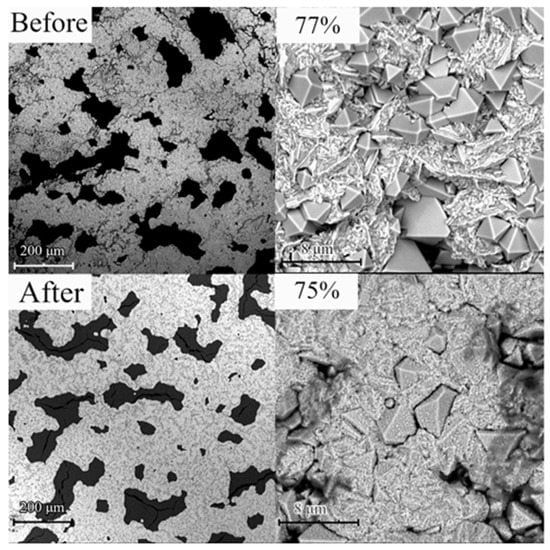
Figure 3.
SEM pictures of electrodeposited copper catalyst layer with 0.3 mA/cm2 short pulse (7 ms) current before and after 60 min electrolysis (in the pictures at the top and bottom, left and right is the same layer in two different magnifications, respectively scales 200 and 8 µm).
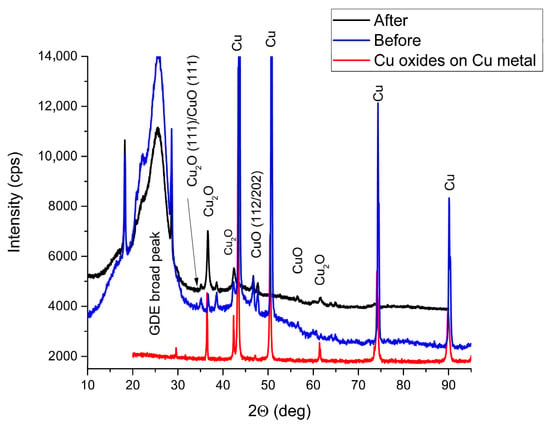
Figure 4.
XRD curves of oxidised copper foil (red) and electrochemically deposited Cu coating on GDL/N-GSS before (blue) and after (black) 1 h electrolysis.
The best electrodes with higher stability in 1 h tests and higher FE for ethylene production in our experiments were Freudenberg GDL/N-GSS with Cu/Cu2O coating (2–3 microns thick) obtained in pulse electrodeposition process (0.3 mA/cm2 current pulses for 7 ms in length and separated by 400 ms). As it is seen in Figure 4, after prolonged electrolysis at higher currents XRD diffractograms of electrochemically deposited Cu coatings on GDL/GSS is with smaller coper oxide peaks. Some etching of copper crystals can be seen after prolonged CO2 reduction reaction, SEM pictures before and after process are shown in Figure 3 (up) and (below) accordingly.
Electrocatalytic tests were carried out for three different electrodes:
(a) with 2D electrodes (carbon paper/GSS/Cu). Before starting experiment an electrolyte 0.5 M KHCO3 was saturated with CO2 by purging it for 20 min. The time of electrolysis experiments (2–3 V, 6–60 mA/cm2) was 1 h, and gas samples were collected in syringe (volume 12 cm3) from catholyte chamber. Low concentrations of ethylene were detected (<0.5 vol%). As it was found in the mass spectra, about 60% was carbon dioxide, about 15% -air. The rest 25% of the gases selected for analysis were H2, CO, C2H4, HCOOH. (b) with 3D electrodes (GDL/N-GSS/Cu). Experiment was performed at fixed current of 150 mA/cm2 in 1.0 M KHCO3 electrolyte and CO2 was introduced to cathode through the non-electrolyte side of cathode at flow rate of 20 cm3/min. Gas collection was carried out using Tedlar gas bag. MS analyse showed the presence of ethylene in concentration 2.8 vol% (FE~27% for both C2H4 and H2—see Figure S4).
(c) with 2D electrodes (N2H4 assisted chemical Cu deposition on carbon paper/GSS). Before starting experiments an electrolyte 0.5 M KHCO3 was saturated with CO2 by purging it for 20 min. The time of electrolysis experiments (2–3 V, 6–60 mA/cm2) was 1 h, and gas samples were collected in syringe (volume 12 cm3) from catholyte chamber.
Faraday efficiency describes the efficiency with which charge (electrons) is transferred in a system facilitating electrochemical reactions on both-cathode and anode. In case of CO2 reduction in electrocatalysis on cathode, several gaseous and liquid products may be formed (gaseous: H2, CO, CH4, C2H4, C2H6; liquid: formic acid, methyl and ethyl alcohols and more). Dissolved products, especially lower hydrocarbons, are usually volatile and may be present in the collected gas sample. It is more difficult to estimate their amount, but if their presence in the gas is not noticeable, then it is assumed that they do not occur. The analysis of the mass spectrometer shows that the gas samples collected at our cathode for our synthesized electrodes contain 30–60% of overflowed CO2, 30–50% of H2 generated by electrolysis and the rest of the volume is occupied by CO, HCOOH and C2H4. By determining the partial volumes of the sample for the gases produced by the electrocatalysis process and assuming it as 100%, the Faraday efficiencies (FE) for the particular reactions can be calculated (see Table 1).

Table 1.
FE of hydrogen and ethylene in gas products from catholyte chamber for selected 3 samples (a), (b) and (c).
It can be seen from Table 1 that the highest FE of C2H4 in the tested samples is for the GDL/N-GSS/Cu sample, where on the 3D gas diffusion layer/nitrogen-doped GSS substrate a fine-grained copper electrode was obtained in the electrochemical deposition process. Compared to the two other samples tested which are on carbon paper, it can be concluded that both the higher currents and the higher FE are given to the substrate of the gas diffusion layer for ethylene and the lowest FE for hydrogen. It also plays a role of N-doped GSS, since as our calculations show, the composite graphene/Cu structure functions as a CO2 and proton absorber, facilitating hydrogenation and carbon–carbon coupling reactions through carbene mechanism (CH2 + CH2 [23]) on graphene/Cu-nanocluster for the formation of C2H4. The authors are convinced that carbon, which is in close contact with copper catalyst, plays an important role in the electrocatalysis of CO2 for higher hydrocarbons, serving as proton absorber.
Research work on GDL/N-GSS/Cu electrode (b) will be continued. In our experiments of the CO2 electrocatalysis process, N-GSS flakes are the first to delaminate from the electrode surface. One should think of strengthening N-GSS flakes in the carbon fabric with additional binder. Therefore, further research should focus on the development of an ink-based N-GSS slurry on an electrically conductive carbon fabric to form 3D electrode substrate for electrochemical Cu deposition to be applied for electrocatalytic CO2 reforming.
4. Conclusions
In our design electrocatalytic electrode was made from nitrogen-doped graphene sheet stacks coated with copper nano- micro structures. The novelty of this study is to obtain nitrogen-doped stacks of graphene sheets (N-GSS) using high-frequency modulated DC pulses for electrochemical exfoliation. The exfoliation process is grateful to dope the resulting material with selected atoms, such as nitrogen—a suitable electrolyte must be used. Industrial graphite waste is taken as the starting material.
Two methods have been tested for the application of the copper catalyst—chemical and electrochemical deposition. Although chemical deposition makes it possible to obtain a suspension of particulate GSS/Cu material, which can be used to form an electrode, the deposition of copper on the surface of GSS sheets is very fragmentary, covering the edges of the sheets more. Further research and improvement of this method is needed.
It has been found that the size of the copper crystals and the uniformity of the coating can be controlled by the length and amplitude of the current pulses, which prefers the electrochemical deposition method to obtain good catalyst electrodes.
Theoretical calculations show that the composite N-doped graphene/Cu structure functions as a CO2 and proton absorber, facilitating hydrogenation and carbon–carbon coupling reactions on Cu-nanocluster/graphene for the formation of C2H4.
In this research, electrochemical deposition method was recognized as successful and most promising to grow Cu crystals on conducting carbon substrates. Further research can focus on the two tasks need to be solved—how to strengthen the bonding of graphene sheet stacks with the carbon substrate and how to increase adhesion of copper nanocrystals to graphene plates.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/electrochem3020015/s1, Section S1: Gas analyses with mass spectrometer; Section S2: Faraday efficiency calculations from mass spectra analysis.
Author Contributions
Conceptualization, S.P. and J.K.; data curation, P.L., A.K., and L.J.; formal analysis, A.K.; funding acquisition, J.K.; investigation, P.L., A.K., and L.J.; methodology, P.L., A.K., and L.J.; project administration, J.K.; software, S.P.; writing—original draft, P.L.; writing—review and editing, J.K. All authors will be informed about each step of manuscript processing including submission, revision, revision reminder, etc. via emails from our system or assigned Assistant Editor. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by European Union’s Horizon 2020 research and innovation programme under grant agreement No 768789.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Acknowledgments
The authors would like to express their gratitude for funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 768789 (CO2EXIDE project). Calculations and research were performed in Center of Excellence at Institute of Solid State Physics, the University of Latvia, which is supported by European Union Horizon2020 Framework Programme H2020-WIDESPREAD-01-2016-2017-TeamingPhase2 under Grant Agreement No. 739508, project CAMART2. P.L. and J.K. thank ISSP UL research assistant Ingars Lukosevics for the experimental work and the results obtained.
Conflicts of Interest
The authors declare no conflict of interest.
References
- What Exactly Did the COP26 Climate Conference Do? Available online: https://www.cnbc.com/2021/11/16/un-cop26-climate-summit-what-was-accomplished.html (accessed on 13 December 2021).
- Daily CO2. Available online: https://www.co2.earth/daily-co2 (accessed on 13 December 2021).
- Perez, A.C.; Diaz-Perez, M.A.; Serrano-Ruiz, J.C. Electrochemical Reduction of CO2: Overcoming Chemical Inertness at Ambient Conditions. Electrochem 2020, 1, 56–59. [Google Scholar] [CrossRef] [Green Version]
- Ghiat, I.; Al-Ansari, T. A review of carbon capture and utilisation as a CO2 abatement opportunity within the EWF nexus. J. CO2 Util. 2021, 45, 101432. [Google Scholar] [CrossRef]
- Knoks, A.; Lesnicenoks, P.; Kleperis, J.; Grinberga, L.; Hodakovska, J.; Klavins, J.; Cikvaidze, G.; Lukosevics, I. Electro-catalytic and photo-catalytic reformation of CO2–reactions and efficiencies processes (Review). IOP Conf. Ser. Mater. Sci. Eng. 2019, 503, 12009. [Google Scholar] [CrossRef]
- Perry, S.C.; Leung, P.; Wang, L.; Ponce de León, C.A. Developments on carbon dioxide reduction: Their promise, achievements, and challenges. Curr. Opin. Electrochem. 2020, 20, 88–98. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, S.-X.; Gandionco, K.A.; Bond, A.M.; Zhang, J. Electrocatalytic carbon dioxide reduction: From fundamental principles to catalyst design. Mater. Today Adv. 2020, 7, 100074. [Google Scholar] [CrossRef]
- Li, H.; Gao, J.; Du, Q.; Shan, J.; Zhang, Y.; Wu, S.; Wang, Z. Direct CO2 electroreduction from NH4HCO3 electrolyte to syngas on bromine-modified Ag catalyst. Energy 2021, 216, 119250. [Google Scholar] [CrossRef]
- Burdyny, T.; Smith, W.A. CO2 reduction on gas-diffusion electrodes and why catalytic performance must be assessed at commercially-relevant conditions. Energy Environ. Sci. 2019, 12, 1442–1453. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Junqueira, J.R.; Sikdar, N.; Öhl, D.; Dieckhöfer, S.; Quast, T.; Seisel, S.; Masa, J.; Andronescu, C.; Schuhmann, W. B-Cu-Zn Gas Diffusion Electrodes for CO2 Electroreduction to C2+ Products at High Current Densities. Angew. Chem. Int. 2021, 60, 9135–9141. [Google Scholar] [CrossRef]
- Mistry, H.; Varela, A.S.; Bonifacio, C.S.; Zegkinoglou, I.; Sinev, I.; Choi, Y.W.; Kisslinger, K.; Stach, E.A.; Yang, J.C.; Strasser, P.; et al. Highly selective plasma-activated copper catalysts for carbon dioxide reduction to ethylene. Nat. Commun. 2016, 7, 12123. [Google Scholar] [CrossRef]
- Bisztyga-Szklarz, M.; Mech, K.; Marzec, M.; Kalendarev, R.; Szaciłowski, K. In Situ Regeneration of Copper-Coated Gas Diffusion Electrodes for Electroreduction of CO2 to Ethylene. Materials 2021, 14, 3171. [Google Scholar] [CrossRef]
- Perry, S.C.; Gateman, S.M.; Malpass-Evans, R.; McKeown, N.; Wegener, M.; Nazarovs, P.; Mauzeroll, J.; Wang, L.; de León, C.P. Polymers with Intrinsic Microporosity (PIMs) for targeted CO2 reduction to ethylene. Chemosphere 2020, 248, 125993. [Google Scholar] [CrossRef] [PubMed]
- Endrodi, B.; Kecsenovity, E.; Samu, A.; Darvas, F.; Jones, R.V.; Török, V.; Danyi, A.; Janáky, C. Multilayer Electrolyzer Stack Converts Carbon Dioxide to Gas Products at High Pressure with High Efficiency. ACS Energy Lett. 2019, 4, 1770–1777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Wijaya, D.T.; Na, J.; Lee, C.W. Towards the Large-Scale Electrochemical Reduction of Carbon Dioxide. Catalysts 2021, 11, 253. [Google Scholar] [CrossRef]
- Dongare, S.; Singh, N.; Bhunia, H. Nitrogen-doped graphene supported copper nanoparticles for electrochemical reduction of CO2. J. CO2 Util. 2021, 44, 101382. [Google Scholar] [CrossRef]
- Ma, Z.; Tsounis, C.; Kumar, P.V.; Han, Z.; Wong, R.J.; Toe, C.Y.; Zhou, S.; Bedford, N.M.; Thomsen, L.; Ng, Y.H.; et al. Enhanced electrochemical CO2 reduction of Cu@CuxO nanoparticles decorated on 3D vertical graphene with intrinsic sp3-type defect. Adv. Funct. Mater. 2020, 30, 910118. [Google Scholar] [CrossRef]
- Zhu, G.; Li, Y.; Zhu, H.; Su, H.; Chan, S.H.; Sun, Q. Enhanced CO2 electroreduction on armchair graphene nanoribbons edge-decorated with copper. Nano Res. 2021, 10, 1641–1650. [Google Scholar] [CrossRef]
- Rashid, N.; Bhat, M.A.; Goutamc, U.K.; Ingole, P.P. Electrochemical reduction of CO2 to ethylene on Cu/CuxO-GO composites in aqueous solution. RSC Adv. 2020, 10, 17572. [Google Scholar] [CrossRef]
- Siahrostami, S.; Jiang, K.; Karamad, M.; Chan, K.; Wang, H.; Nørskov, J. Theoretical Investigations into Defected Graphene for electrochemical Reduction of CO2. ACS Sustain. Chem. Eng. 2017, 5, 11080–11085. [Google Scholar] [CrossRef]
- Varela, A.S.; Ranjbar Sahraie, N.; Steinberg, J.; Ju, W.; Oh, H.S.; Strasser, P. Metal-Doped Nitrogenated Carbon as an Efficient Catalyst for Direct CO2 Electroreduction to CO and Hydrocarbons. Angew. Chem. 2015, 127, 10908–10912. [Google Scholar] [CrossRef]
- Piskunov, S.; Zhukovskii, Y.F.; Sokolov, M.N.; Kleperis, J. Ab Initio Calculations of CuN@Graphene (0001) Nanostructures for Electrocatalytic Applications. Latv. J. Phys. Tech. Sci. 2018, 6, 30–34. [Google Scholar] [CrossRef] [Green Version]
- Lisovski, O.; Piskunov, S.; Bocharov, D.; Zhukovskii, Y.F.; Kleperis, J.; Knoks, A.; Lesnicenoks, P. CO2 and CH2 Adsorption on Copper-Decorated Graphene: Predictions from First Principle Calculation. Crystals 2022, 12, 194. [Google Scholar] [CrossRef]
- Olins, R.; Lesnicenoks, P.; Kleperis, J.; Knoks, A.; Lukosevics, I. Electrochemical exfoliation-streamline method for synthesis of nitrogen doped graphene. Chemija 2021, 32, 9–16. [Google Scholar] [CrossRef]
- Ortega-Amaya, R.; Matsumoto, Y.; Espinoza-Rivas, A.M.; Pérez-Guzmán, M.A.; Ortega-López, M. Development of highly faceted reduced graphene oxide-coated copper oxide and copper nanoparticles on a copper foil surface. Beilstein J. Nanotechnol. 2016, 7, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).