Synthesis of Nickel Fumarate and Its Electrochemical Properties for Li-Ion Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Materials Characterisation
Electrochemical Characterisation
3. Results and Discussion
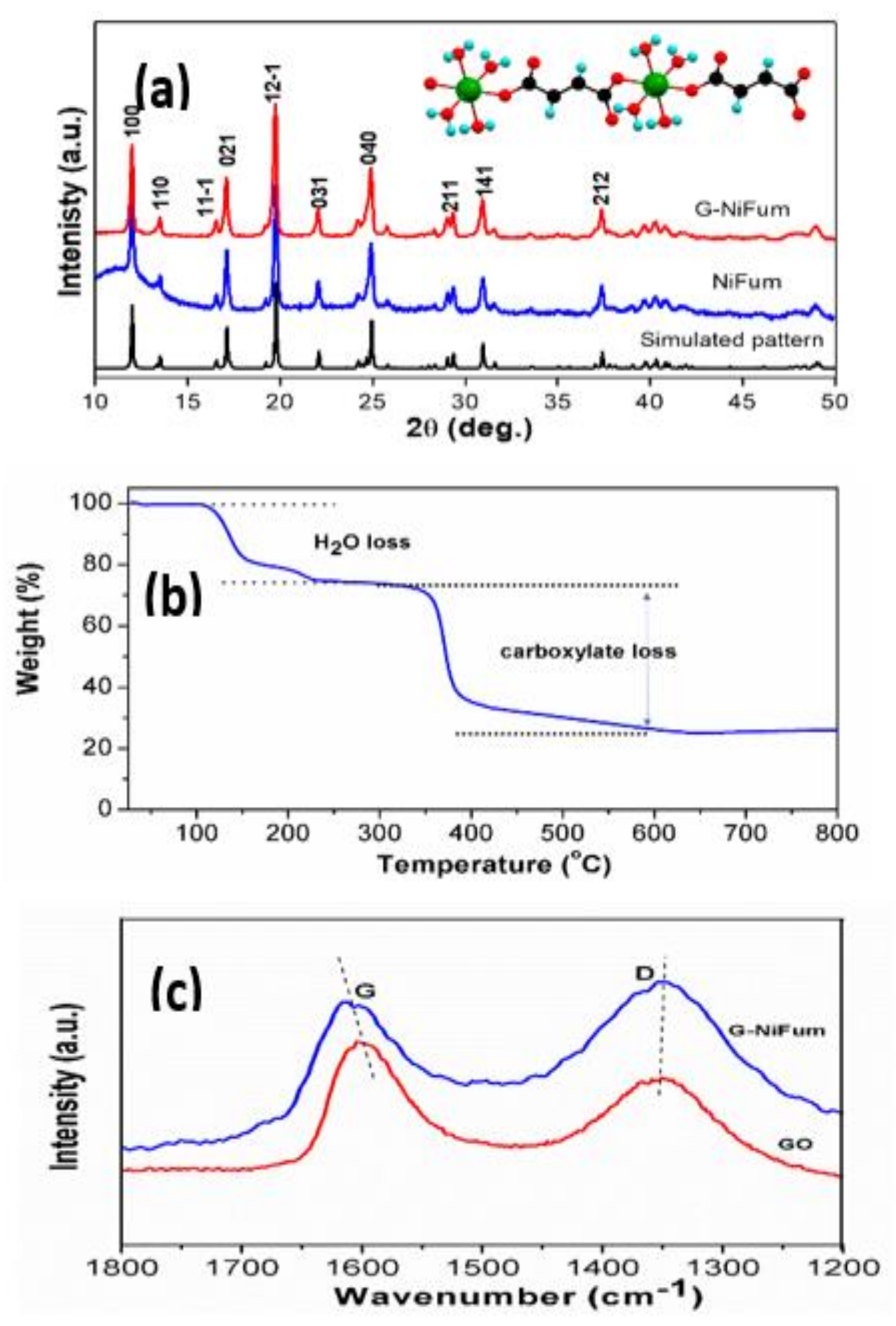
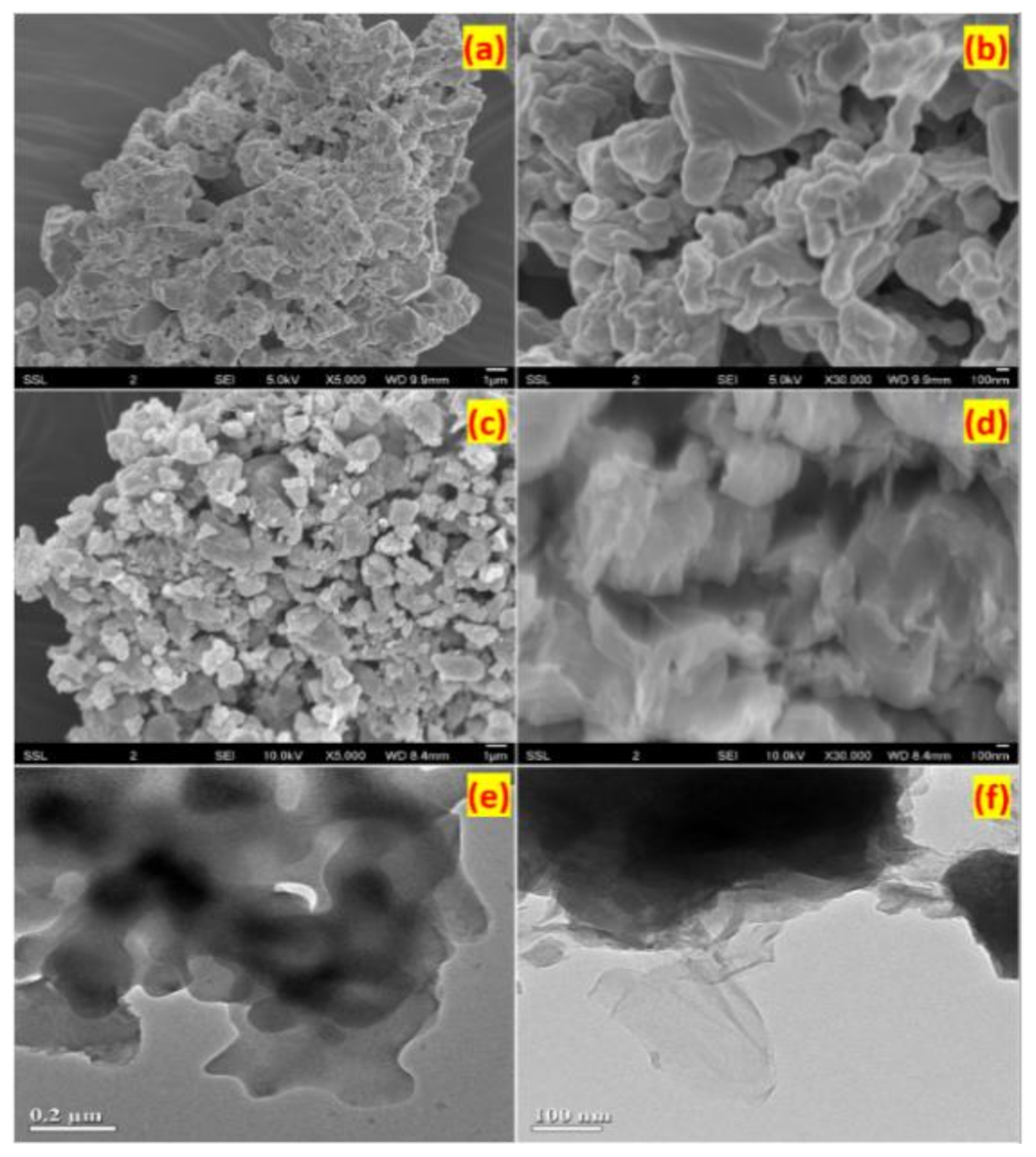
3.1. Structure and Morphology
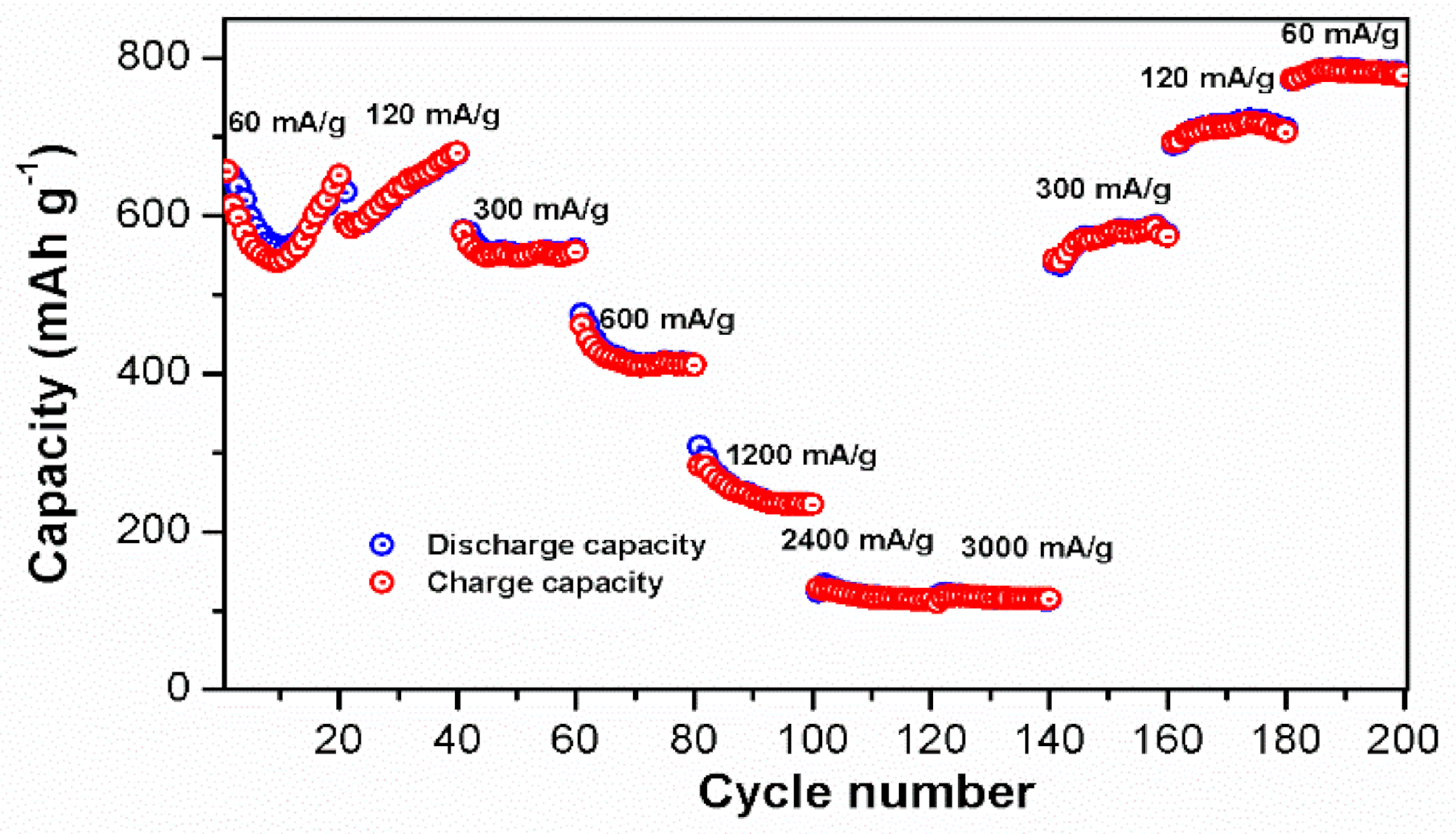
3.2. Electrochemical Studies
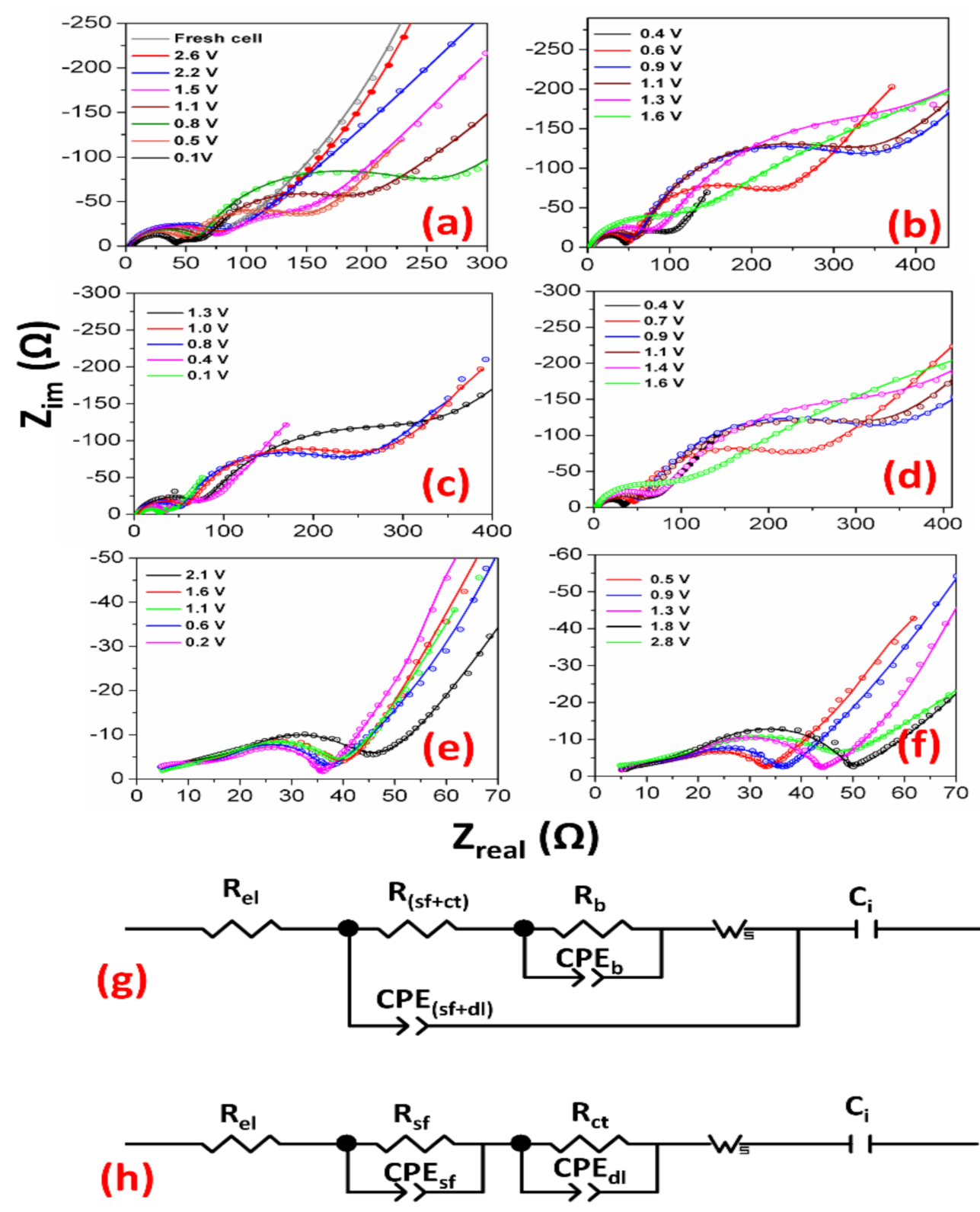
3.3. Electrochemical Impedance Spectroscopy (EIS)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, N.-S.; Chen, Z.; Freunberger, S.A.; Ji, X.; Sun, Y.-K.; Amine, K.; Yushin, G.; Nazar, L.F.; Cho, J.; Bruce, P.G. Challenges Facing Lithium Batteries and Electrical Double-Layer Capacitors. Angew. Chem. Int. Ed. 2012, 51, 9994–10024. [Google Scholar] [CrossRef] [PubMed]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Zaccaria, R.P.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.V.; Subba Rao, G.V.; Chowdari, B.V.R. Metal Oxides and Oxysalts as Anode Materials for Li Ion Batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [PubMed]
- Obrovac, M.N.; Chevrier, V.L. Alloy Negative Electrodes for Li-Ion Batteries. Chem. Rev. 2014, 114, 11444–11502. [Google Scholar] [CrossRef]
- Li, J.-Y.; Xu, Q.; Li, G.; Yin, Y.-X.; Wan, L.-J.; Guo, Y.-G. Research progress regarding Si-based anode materials towards practical application in high energy density Li-ion batteries. Mater. Chem. Front. 2017, 1, 1691–1708. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zeng, G.; Chen, J.; Lu, C.; Wen, Z. 3D graphene network encapsulating SnO2 hollow spheres as a high-performance anode material for lithium-ion batteries. J. Mater. Chem. A 2017, 5, 4535–4542. [Google Scholar] [CrossRef]
- Chan, C.K.; Zhang, X.F.; Cui, Y. High Capacity Li Ion Battery Anodes Using Ge Nanowires. Nano Lett. 2008, 8, 307–309. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects, and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Su, X.; Wu, Q.; Li, J.; Xiao, X.; Lott, A.; Lu, W.; Sheldon, B.W.; Wu, J. Silicon-Based Nanomaterials for Lithium-Ion Batteries: A Review. Adv. Energy Mater. 2014, 4, 1300882. [Google Scholar] [CrossRef]
- Reddy, M.V.; Yu, T.; Sow, C.-H.; Shen, Z.X.; Lim, C.T.; Subba Rao, G.V.; Chowdari, B.V.R. α-Fe2O3 nanoflakes as an anode material for Li-ion batteries. Adv. Funct. Mater. 2007, 17, 2792–2799. [Google Scholar] [CrossRef]
- Han, C.-G.; Zhu, C.; Sheng, N.; Aoki, Y.; Habazaki, H.; Akiyama, T. A facile one-pot synthesis of FeOx/carbon/graphene composites as superior anode materials for lithium-ion batteries. Electrochim. Acta 2017, 235, 88–97. [Google Scholar] [CrossRef]
- Jiao, F.; Bruce, P.G. Mesoporous crystalline β-MnO2—A reversible positive electrode for rechargeable lithium batteries. Adv. Mater. 2007, 19, 657–660. [Google Scholar] [CrossRef]
- Lu, B.; Liu, J.; Hu, R.; Wang, H.; Liu, J.; Zhu, M. Facile synthesis of self-supported Mn3O4@C nanotube arrays constituting an ultrastable and high-rate anode for flexible Li-ion batteries. J. Mater. Chem. A 2017, 5, 8555–8565. [Google Scholar] [CrossRef]
- Hameed, A.S.; Reddy, M.V.; Chowdari, B.V.R.; Vittal, J.J. Preparation of rGO-wrapped magnetite nanocomposites and their energy storage properties. RSC Adv. 2014, 4, 64142–64150. [Google Scholar] [CrossRef]
- Li, L.; Cheah, Y.; Ko, Y.; Teh, P.; Wee, G.; Wong, C.; Peng, S.; Srinivasan, M. The facile synthesis of hierarchical porous flower-like NiCo2O4 with superior lithium storage properties. J. Mater. Chem. A 2013, 1, 10935–10941. [Google Scholar] [CrossRef]
- Hameed, A.S.; Bahiraei, H.; Reddy, M.V.; Shoushtari, M.Z.; Vittal, J.J.; Ong, C.K.; Chowdari, B.V.R. Lithium Storage Properties of Pristine and (Mg, Cu) Codoped ZnFe2O4 Nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 10744–10753. [Google Scholar] [CrossRef] [PubMed]
- Cabana, J.; Monconduit, L.; Larcher, D.; Palacin, M.R. Beyond Intercalation-Based Li-Ion Batteries: The State of the Art and Challenges of Electrode Materials Reacting through Conversion Reactions. Adv. Mater. 2010, 22, E170–E192. [Google Scholar] [CrossRef] [PubMed]
- Kucinskis, G.; Bajars, G.; Kleperis, J. Graphene in lithium ion battery cathode materials: A review. J. Power Sources 2013, 240, 66–79. [Google Scholar] [CrossRef]
- Xin, S.; Guo, Y.-G.; Wan, L.-J. Nanocarbon Networks for Advanced Rechargeable Lithium Batteries. Acc. Chem. Res. 2012, 45, 1759–1769. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Zhou, G.; Yin, L.-C.; Ren, W.; Li, F.; Cheng, H.-M. Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 2012, 1, 107–131. [Google Scholar] [CrossRef]
- Inagaki, M. Carbon coating for enhancing the functionalities of materials. Carbon 2012, 50, 3247–3266. [Google Scholar] [CrossRef]
- Fedele, L.; Sauvage, F.; Gottis, S.; Davoisne, C.; Salager, E.; Chotard, J.-N.; Becuwe, M. 2D-Layered Lithium Carboxylate Based on Biphenyl Core as Negative Electrode for Organic Lithium-Ion Batteries. Chem. Mater. 2017, 29, 546–554. [Google Scholar] [CrossRef]
- Liang, Y.; Tao, Z.; Chen, J. Organic electrode materials for rechargeable lithium batteries. Adv. Energy Mater. 2012, 2, 742–769. [Google Scholar] [CrossRef]
- Walker, W.; Grugeon, S.; Mentre, O.; Laruelle, S.; Tarascon, J.-M.; Wudl, F. Ethoxycarbonyl-Based Organic Electrode for Li-Batteries. J. Am. Chem. Soc. 2010, 132, 6517–6523. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, L.; Zhang, K.; Zhu, Z.; Tao, Z.; Chen, J. Organic Li4C8H2O6 Nanosheets for Lithium-Ion Batteries. Nano Lett. 2013, 13, 4404–4409. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, Z.; Ma, L.; Wang, J.; Yang, S. Graphene oxide-templated growth of MOFs with enhanced lithium-storage properties. New J. Chem. 2017, 41, 14209–14216. [Google Scholar] [CrossRef]
- He, S.; Zhou, X.; Li, Z.; Wang, J.; Ma, L.; Yang, S. Fluorine Doping Strengthens the Lithium-Storage Properties of the Mn-Based Metal-Organic Framework. ACS Appl. Mater. Interfaces 2017, 9, 26907–26914. [Google Scholar] [CrossRef]
- Li, X.; Cheng, F.; Zhang, S.; Chen, J. Shape-controlled synthesis and lithium-storage study of metal-organic frameworks Zn4O(1,3,5-benzenetribenzoate)2. J. Power Sources 2006, 160, 542–547. [Google Scholar] [CrossRef]
- Thakur, A.K.; Majumder, M.; Patole, S.P.; Zaghib, K.; Reddy, M.V. Metal–organic framework-based materials: Advances, exploits, and challenges in promoting post Li-ion battery technologies. Mater. Adv. 2021, 2, 2457–2482. [Google Scholar] [CrossRef]
- Wang, L.; Han, Y.; Feng, X.; Zhou, J.; Qi, P.; Wang, B. Metal-organic frameworks for energy storage: Batteries and supercapacitors. Coord. Chem. Rev. 2016, 307, 361–381. [Google Scholar] [CrossRef]
- Long, J.R.; Yaghi, O.M. The pervasive chemistry of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1213–1214. [Google Scholar] [CrossRef] [PubMed]
- Férey, G.; Millange, F.; Morcrette, M.; Serre, C.; Doublet, M.-L.; Grenèche, J.-M.; Tarascon, J.-M. Mixed-Valence Li/Fe-Based Metal–Organic Frameworks with Both Reversible Redox and Sorption Properties. Angew. Chem. Int. Ed. 2007, 46, 3259–3263. [Google Scholar] [CrossRef]
- Maiti, S.; Pramanik, A.; Manju, U.; Mahanty, S. Reversible Lithium Storage in Manganese 1,3,5-Benzenetricarboxylate Metal–Organic Framework with High Capacity and Rate Performance. ACS Appl. Mater. Interfaces 2015, 7, 16357–16363. [Google Scholar] [CrossRef]
- Maiti, S.; Pramanik, A.; Unnikrishnan, M.; Mahanty, S. Cu3(1,3,5-benzenetricarboxylate)2 Metal-Organic Framework: A Promising Anode Material for Lithium-ion Battery. J. Microporous Mesoporous Mater. 2016, 226, 353–359. [Google Scholar] [CrossRef]
- Hu, H.; Lou, X.; Li, C.; Hu, X.; Li, T.; Chen, Q.; Shen, M.; Hu, B. A thermally activated manganese 1,4-benzenedicarboxylate metal organic framework with high anodic capability for Li-ion batteries. New J. Chem. 2016, 40, 9746–9752. [Google Scholar] [CrossRef]
- Saravanan, K.; Nagarathinam, M.; Balaya, P.; Vittal, J.J. Lithium storage in a metal organic framework with diamondoid topology—A case study on metal formats. J. Mater. Chem. 2010, 20, 8329–8335. [Google Scholar] [CrossRef]
- Gou, L.; Liu, P.-G.; Liu, D.; Wang, C.-Y.; Lei, H.-Y.; Li, Z.-Y.; Fan, X.-Y.; Li, D.-L. Rational synthesis of Ni3(HCOO)6/CNT ellipsoids with enhanced lithium storage performance: Inspired by the time evolution of the growth process of a nickel formate framework. Dalton Trans. 2017, 46, 6473–6482. [Google Scholar] [CrossRef]
- Oh, H.-J.; Jo, C.-H.; Yoon, C.S.; Yashiro, H.; Kim, S.-J.; Passerini, S.; Sun, Y.-K.; Myung, S.-T. Nickel oxalate dihydrate nanorods attached to reduced graphene oxide sheets as a high-capacity anode for rechargeable lithium batteries. NPG Asia Mater. 2016, 8, e270. [Google Scholar] [CrossRef] [Green Version]
- López, M.C.; Tirado, J.L.; Pérez Vicente, C. Structural and comparative electrochemical study of M(II) oxalates, M = Mn, Fe, Co, Ni, Cu, Zn. J. Power Sources 2013, 227, 65–71. [Google Scholar] [CrossRef]
- Park, J.-S.; Jo, J.-H.; Yashiro, H.; Kim, S.-S.; Kim, S.-J.; Sun, Y.-K.; Myung, S.-T. Synthesis and Electrochemical Reaction of Tin Oxalate-Reduced Graphene Oxide Composite Anode for Rechargeable Lithium Batteries. ACS Appl. Mater. Interfaces 2017, 9, 25941–25951. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qu, Q.; Liu, G.; Battaglia, V.S.; Zheng, H. Aluminum fumarate-based metal organic frameworks with tremella-like structure as ultrafast and stable anode for lithium-ion batteries. Nano Energy 2017, 39, 200–210. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Yaghtin, A.; Masoudpanah, S.M.; Hasheminiasari, M.; Salehi, A.; Safanama, D.; Ong, C.K.; Adams, S.; Reddy, M.V. Effect of Reducing Agent on Solution Synthesis of Li3V2(PO4)3 Cathode Material for Lithium Ion Batteries. Molecules 2020, 25, 3746. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.Z.; Zheng, Y.Q.; Wu, Q.S. Crystal structure of tetraaquafumaratonickel(II), Ni(H2O)4(C4H2O4). Z. Krist. New Cryst. Struct. 2003, 218, 111–112. [Google Scholar]
- Petnikota, S.; Marka, S.K.; Banerjee, A.; Reddy, M.V.; Srikanth, V.V.S.S.; Chowdari, B.V.R. Graphenothermal reduction synthesis of ‘exfoliated graphene oxide/iron (II) oxide’ composite for anode application in lithium ion batteries. J. Power Sources 2015, 293, 253–263. [Google Scholar] [CrossRef]
- Hameed, A.S.; Reddy, M.V.; Nagarathinam, M.; Runčevski, T.; Dinnebier, R.E.; Adams, S.; Chowdari, B.; Vittal, J.J. Room temperature large-scale synthesis of layered frameworks as low-cost 4 V cathode materials for lithium ion batteries. Sci. Rep. 2015, 5, 16270. [Google Scholar] [CrossRef] [Green Version]
- Petnikota, S.; Maseed, H.; Srikanth, V.V.S.S.; Reddy, M.V.; Adams, S.; Srinivasan, M.; Chowdari, B.V.R. Experimental Elucidation of a Graphenothermal Reduction Mechanism of Fe2O3: An Enhanced Anodic Behavior of an Exfoliated Reduced Graphene Oxide/Fe3O4 Composite in Li-Ion Batteries. J. Phys. Chem. C 2017, 121, 3778–3789. [Google Scholar] [CrossRef]
- Aragón, M.J.; León, B.; Pérez Vicente, C.; Tirado, J.L. Synthesis and Electrochemical Reaction with Lithium of Mesoporous Iron Oxalate Nanoribbons. Inorg. Chem. 2008, 47, 10366–10371. [Google Scholar] [CrossRef]
- Ang, W.A.; Gupta, N.; Prasanth, R.; Madhavi, S. High-Performing Mesoporous Iron Oxalate Anodes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2012, 4, 7011–7019. [Google Scholar] [CrossRef] [PubMed]

| Cycle No | NiFum | G-NiFum | ||||
|---|---|---|---|---|---|---|
| Charge Capacity (mAh g−1) | Discharge Capacity (mAh g−1) | Coulombic Efficiency (%) | Charge Capacity (mAh g−1) | Discharge Capacity (mAh g−1) | Coulombic Efficiency (%) | |
| 1st cycle | 620 | 1066 | 58 | 658 | 1320 | 50 |
| 2nd cycle | 587 | 627 | 94 | 611 | 657 | 93 |
| 50th cycle | 485 | 491 | 99 | 797 | 805 | 99 |
| Voltage | R(sf+ct) (Ω) | CPE(sf+dl) (μF) | α | Rb (Ω) | CPEb (mF) | α’ | Ci (F) |
|---|---|---|---|---|---|---|---|
| First discharge cycle | |||||||
| Fresh cell | 66 | 37 | 0.67 | - | - | - | 0.01 |
| 2.6 V | 72 | 33 | 0.68 | - | - | - | 0.01 |
| 2.2 V | 84 | 46 | 0.64 | - | - | - | 0.06 |
| 1.5 V | 70 | 51 | 0.64 | - | - | - | 0.12 |
| 1.1 V | 63 | 40 | 0.70 | 97 | 1.9 | 0.89 | 0.09 |
| 0.8 V | 57 | 38 | 0.73 | 174 | 1.9 | 0.83 | 0.08 |
| 0.5 V | 48 | 36 | 0.76 | 83 | 3.5 | 0.83 | 0.11 |
| 0.1 V | 38 | 63 | 0.72 | 15 | 14 | 0.77 | 0.14 |
| First charge cycle | |||||||
| 0.4 V | 43 | 43 | 0.75 | 44 | 5.6 | 0.82 | 0.23 |
| 0.6 V | 47 | 36 | 0.77 | 143 | 2.6 | 0.87 | 0.19 |
| 0.9 V | 53 | 36 | 0.75 | 224 | 1.8 | 0.87 | 0.36 |
| 1.1 V | 59 | 53 | 0.70 | 228 | 1.6 | 0.88 | 0.28 |
| 1.3 V | 73 | 48 | 0.71 | 240 | 1.9 | 0.62 | 0.29 |
| 1.6 V | 127 | 71 | 0.63 | 360 | 1.9 | 0.55 | 0.78 |
| Voltage | Rsf (Ω) | CPEsf (μF) | α | Rct (Ω) | CPEdl (μF) | α’ | Ci (F) |
|---|---|---|---|---|---|---|---|
| 201st discharge cycle | |||||||
| 2.1 V | 42 | 191 | 0.28 | 12 | 152 | 0.91 | 0.85 |
| 1.6 V | 31 | 160 | 0.30 | 12 | 190 | 0.87 | 0.47 |
| 1.1 V | 21 | 512 | 0.40 | 14 | 180 | 0.87 | 0.52 |
| 0.6 V | 21 | 343 | 0.42 | 14 | 162 | 0.86 | 0.45 |
| 0.2 V | 20 | 122 | 0.36 | 12 | 200 | 0.87 | 0.20 |
| 201st charge cycle | |||||||
| 0.5 V | 21 | 410 | 0.43 | 24 | 69 | 0.91 | 1.51 |
| 0.9 V | 19 | 423 | 0.46 | 20 | 94 | 0.88 | 0.75 |
| 1.3 V | 19 | 409 | 0.43 | 14 | 147 | 0.86 | 0.68 |
| 1.8 V | 16 | 381 | 0.47 | 13 | 180 | 0.84 | 0.46 |
| 2.8 V | 27 | 812 | 0.36 | 19 | 182 | 0.81 | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hameed, S.A.; Petnikota, S.; Hassan, N.S.; Al-Qaradawi, S.Y.; Karim, Z.; Reddy, M.V. Synthesis of Nickel Fumarate and Its Electrochemical Properties for Li-Ion Batteries. Electrochem 2021, 2, 439-451. https://doi.org/10.3390/electrochem2030029
Hameed SA, Petnikota S, Hassan NS, Al-Qaradawi SY, Karim Z, Reddy MV. Synthesis of Nickel Fumarate and Its Electrochemical Properties for Li-Ion Batteries. Electrochem. 2021; 2(3):439-451. https://doi.org/10.3390/electrochem2030029
Chicago/Turabian StyleHameed, Shahul A., Shaikshavali Petnikota, Nusyba S. Hassan, Siham Y. Al-Qaradawi, Zaghib Karim, and M. V. Reddy. 2021. "Synthesis of Nickel Fumarate and Its Electrochemical Properties for Li-Ion Batteries" Electrochem 2, no. 3: 439-451. https://doi.org/10.3390/electrochem2030029
APA StyleHameed, S. A., Petnikota, S., Hassan, N. S., Al-Qaradawi, S. Y., Karim, Z., & Reddy, M. V. (2021). Synthesis of Nickel Fumarate and Its Electrochemical Properties for Li-Ion Batteries. Electrochem, 2(3), 439-451. https://doi.org/10.3390/electrochem2030029









