Developments of the Electroactive Materials for Non-Enzymatic Glucose Sensing and Their Mechanisms
Abstract
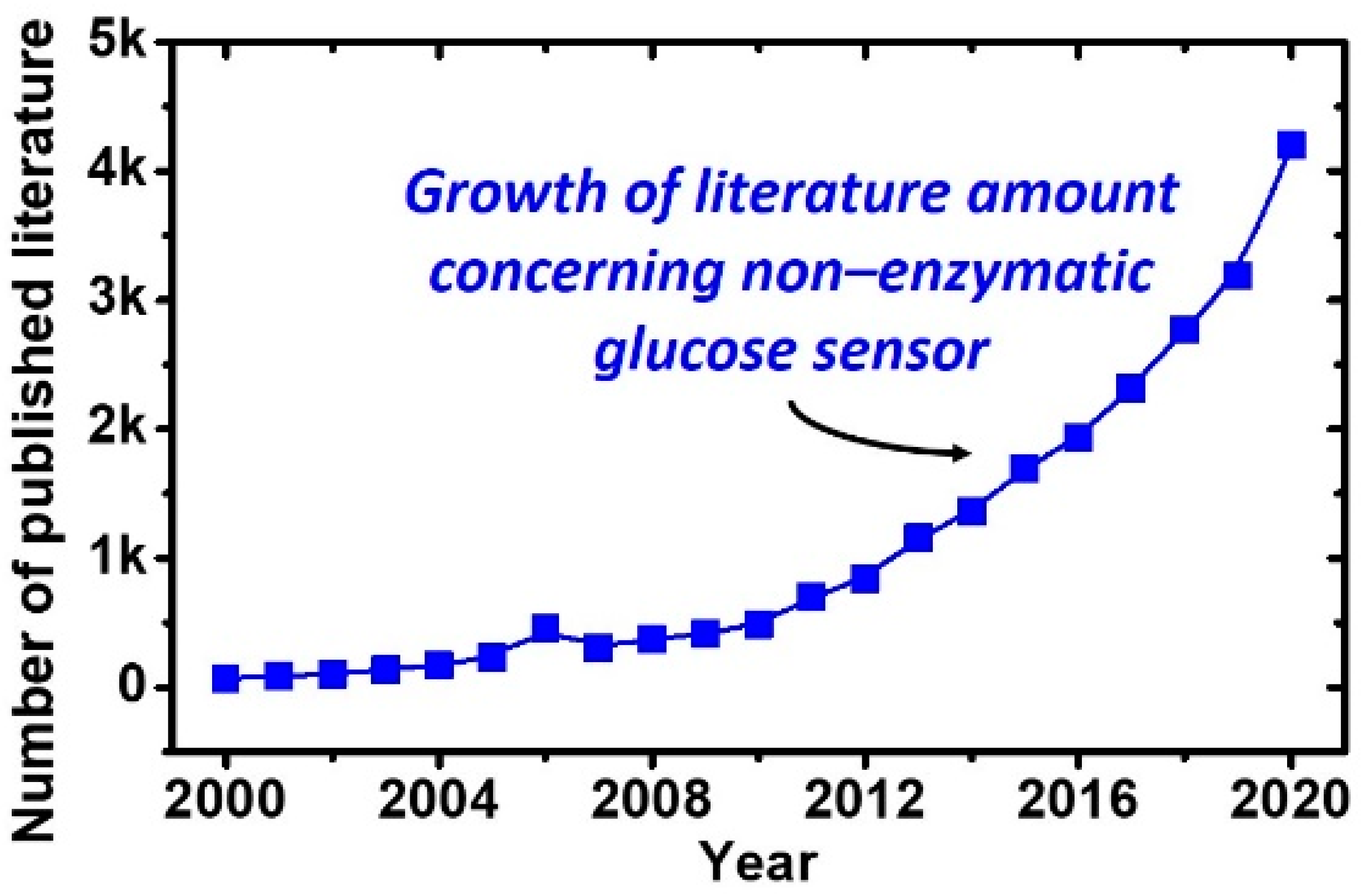
:1. Introduction
2. Types of Glucose-Sensing Devices
2.1. Invasive Glucose Sensor Devices
2.2. Non-Invasive Glucose Sensor Devices
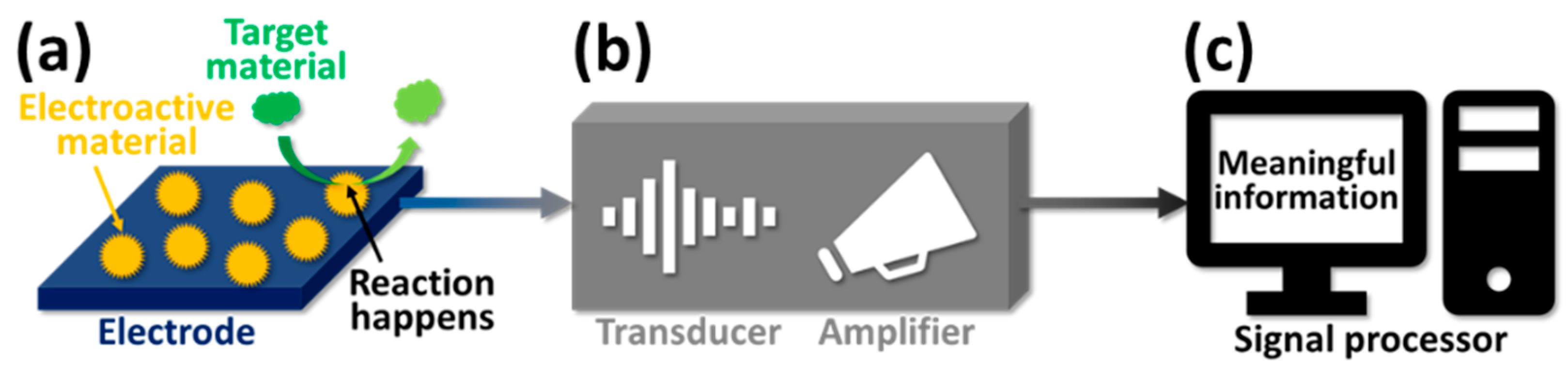
3. Electrochemical Technique for Glucose Sensing
3.1. Three-Electrode System of the Electrochemical Technique
3.2. Various Measurements via the Electrochemical System for Glucose-Sensing
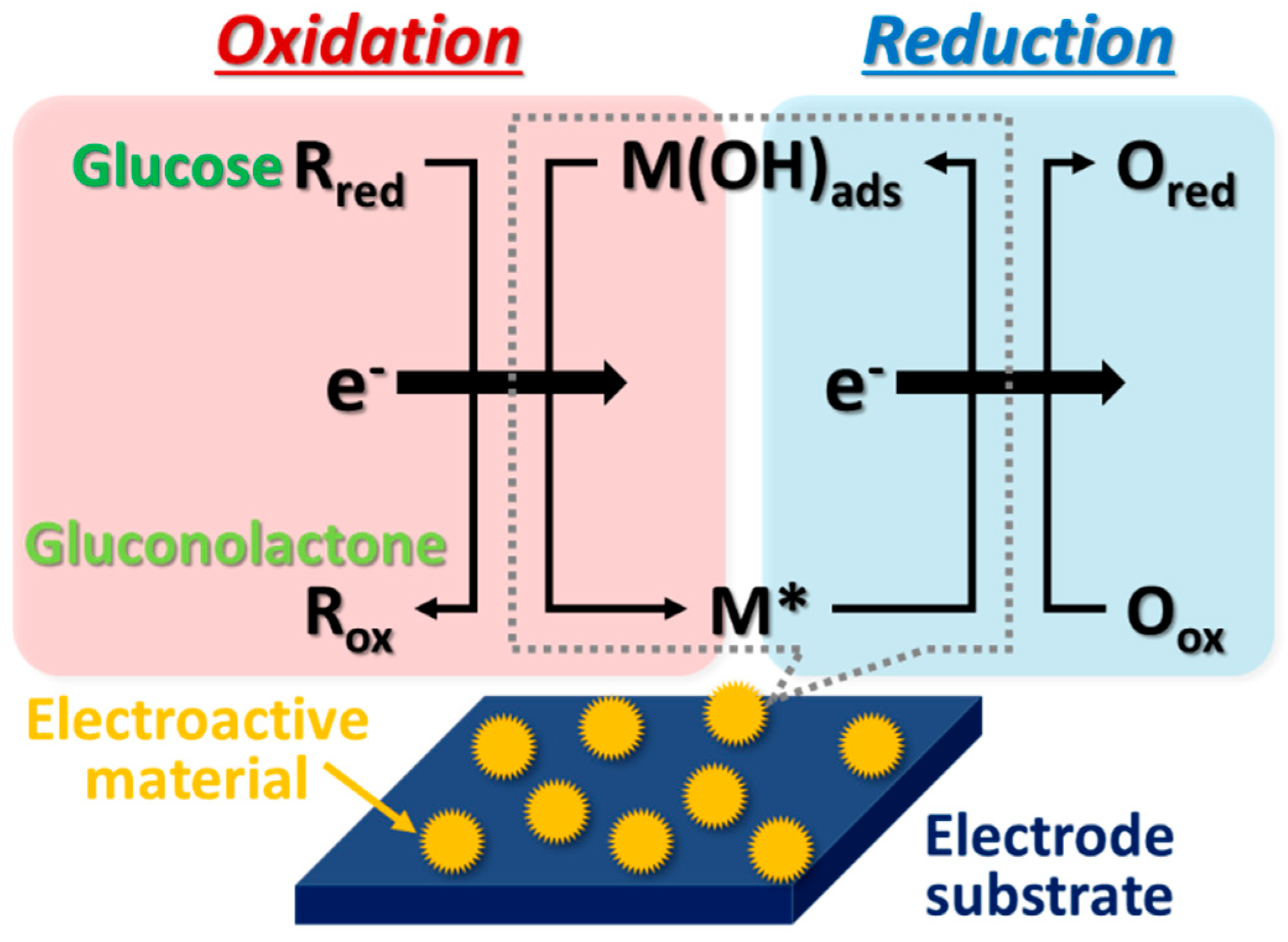
4. Mechanisms for Glucose-Sensing in Non-Enzymatic Electrodes
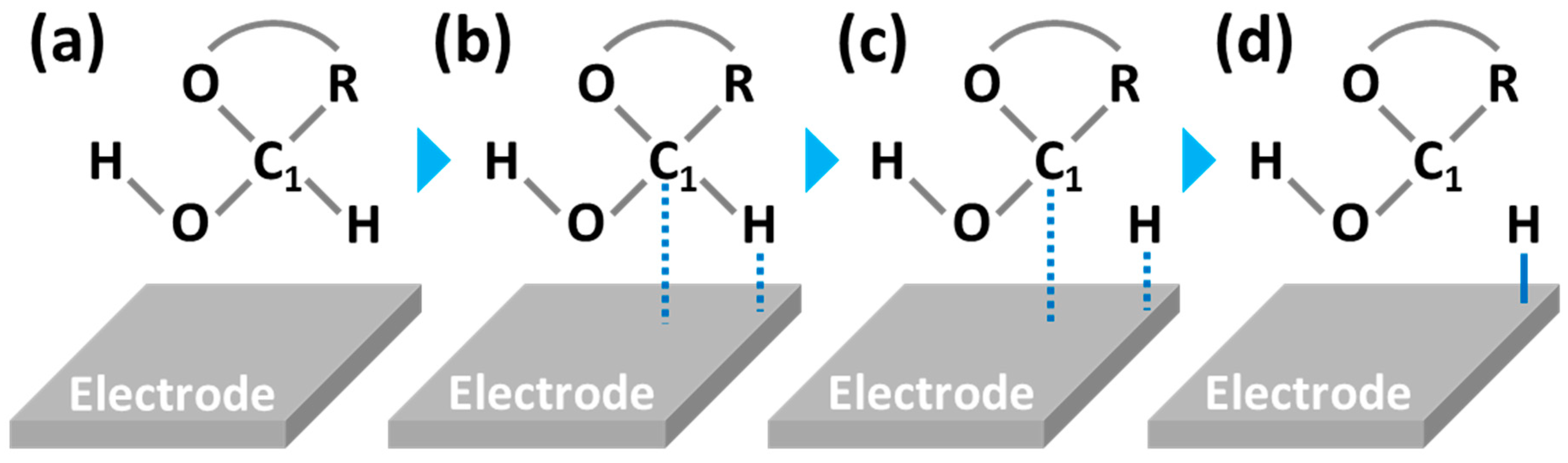
4.1. Activated Chemisorption Model (First Model)
4.2. Incipient Hydrous Oxide Adatom Mediator (IHOAM) (Second Model)
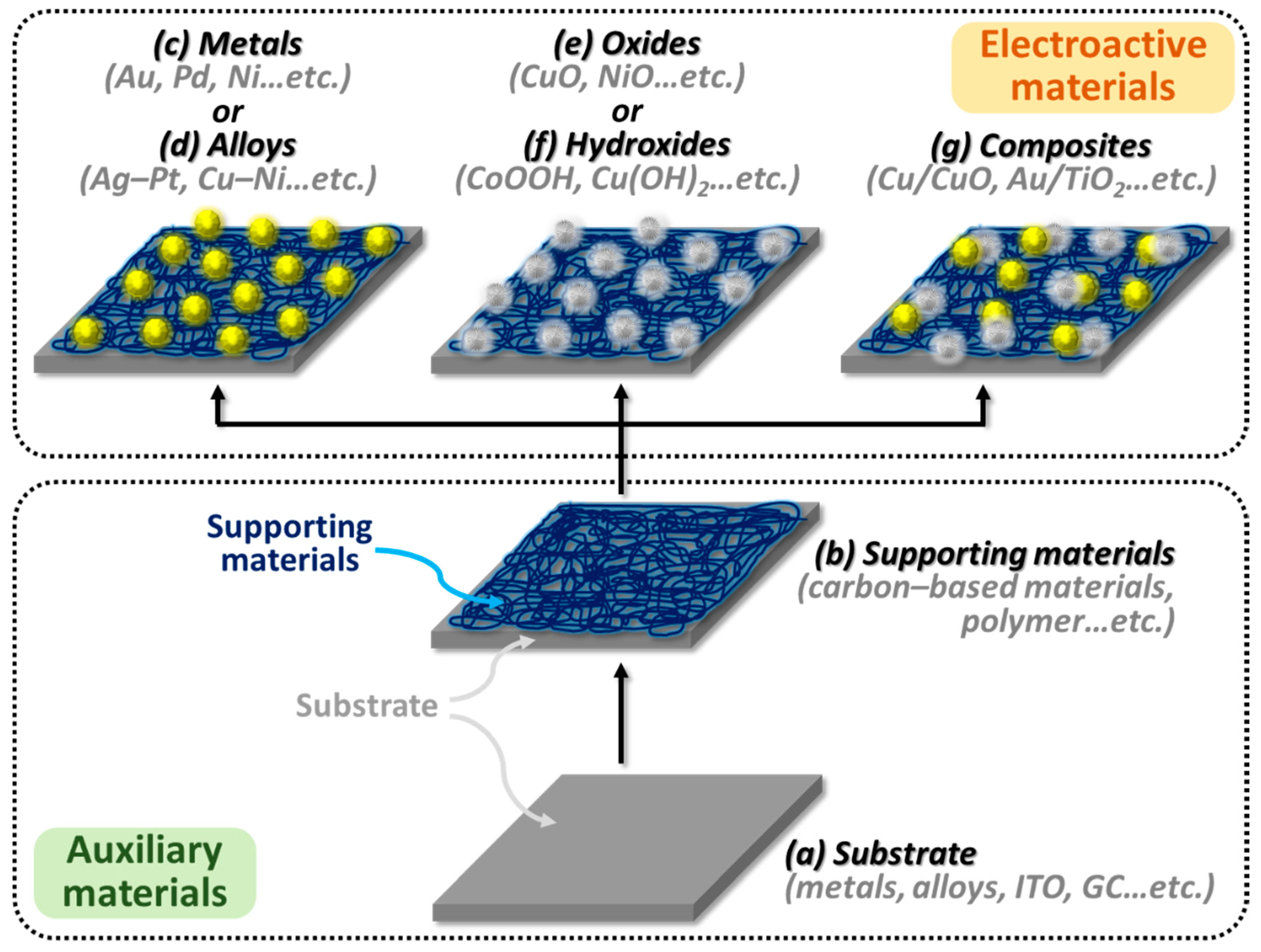
5. Systems of Electroactive Materials for the Sensing of Glucose
6. Electroactive Materials of Mono-Metallic Materials
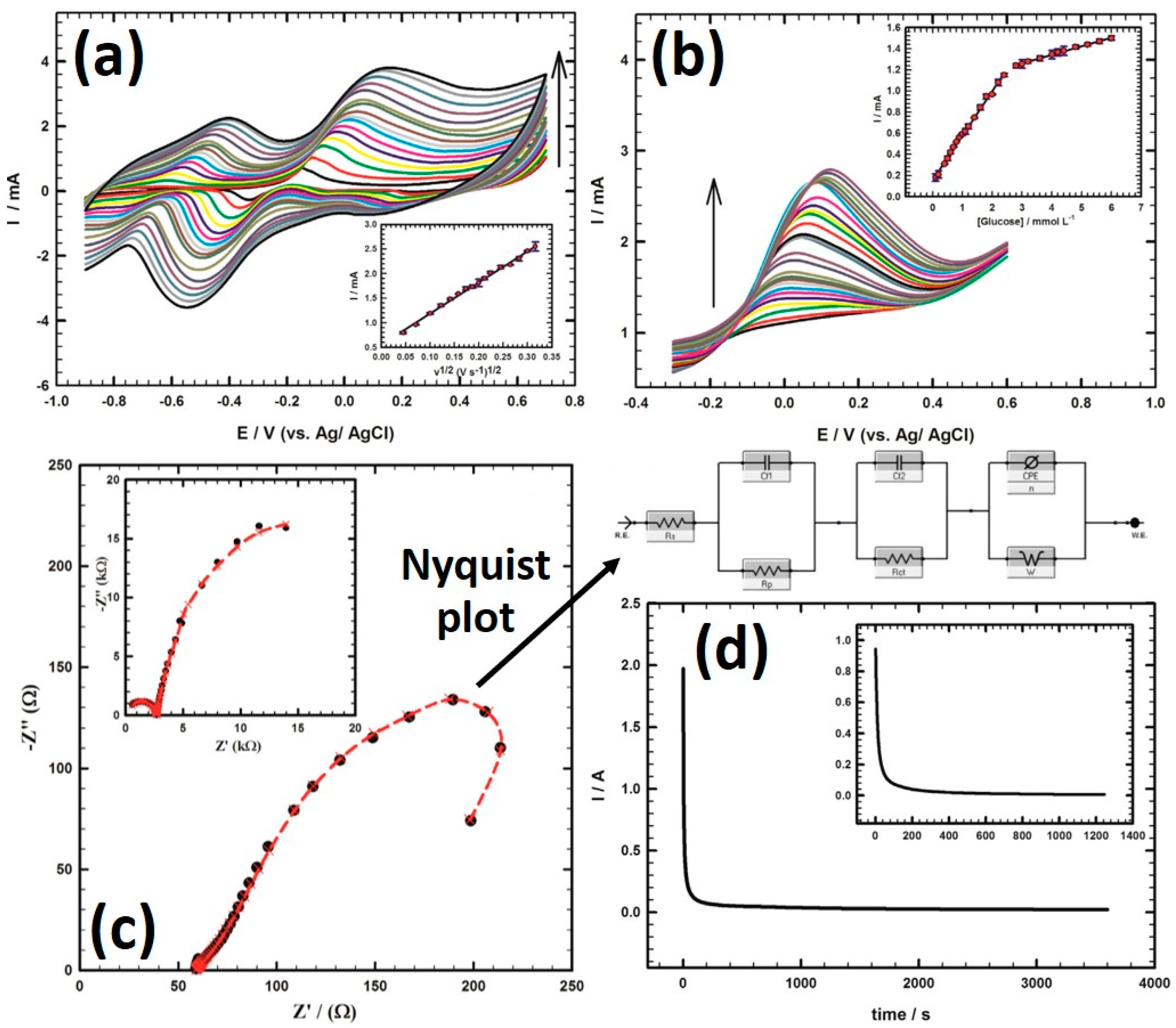
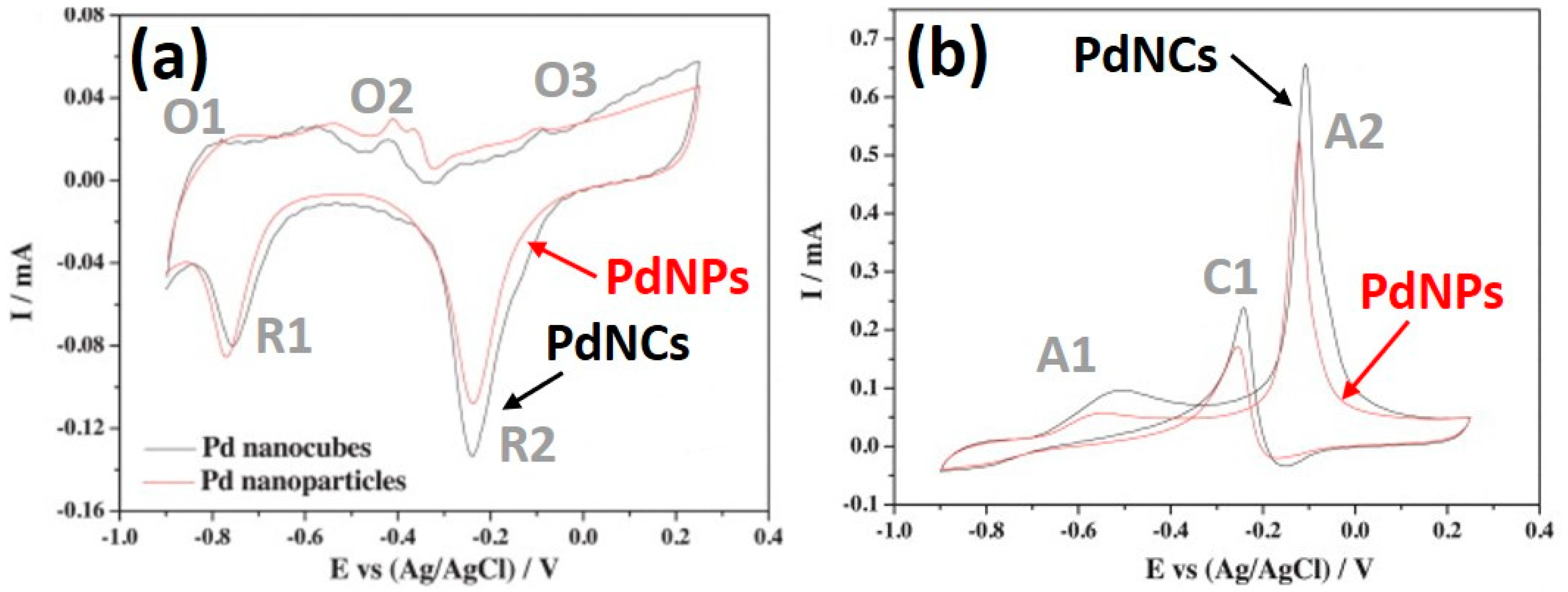
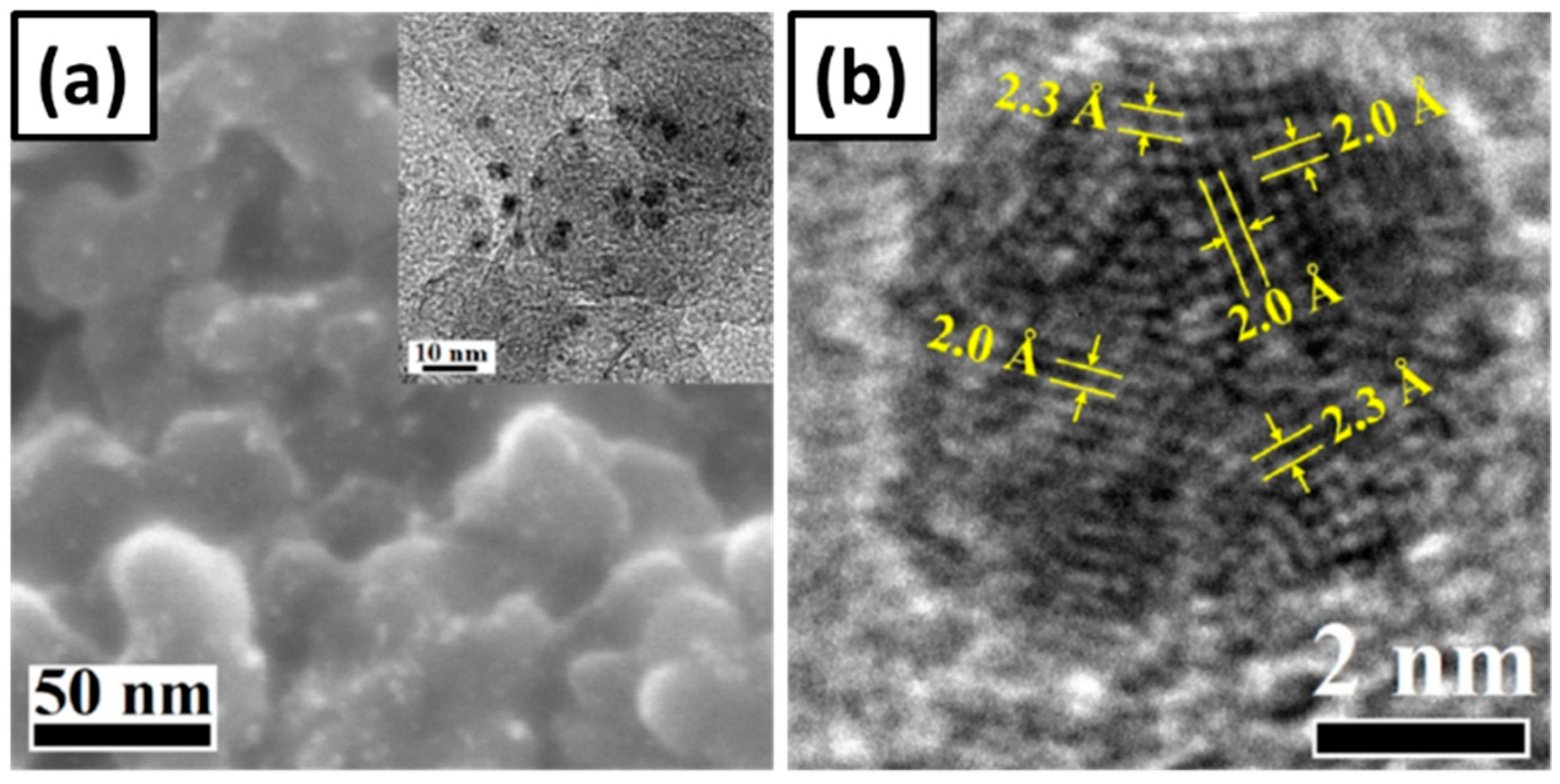
6.1. Crystal Structure Controlled Pd Nanocubes
6.2. Mechanisms and Comparisons of the Mono-Metallic Materials
7. Electroactive Materials of Bi-Metallic Materials (Alloys)
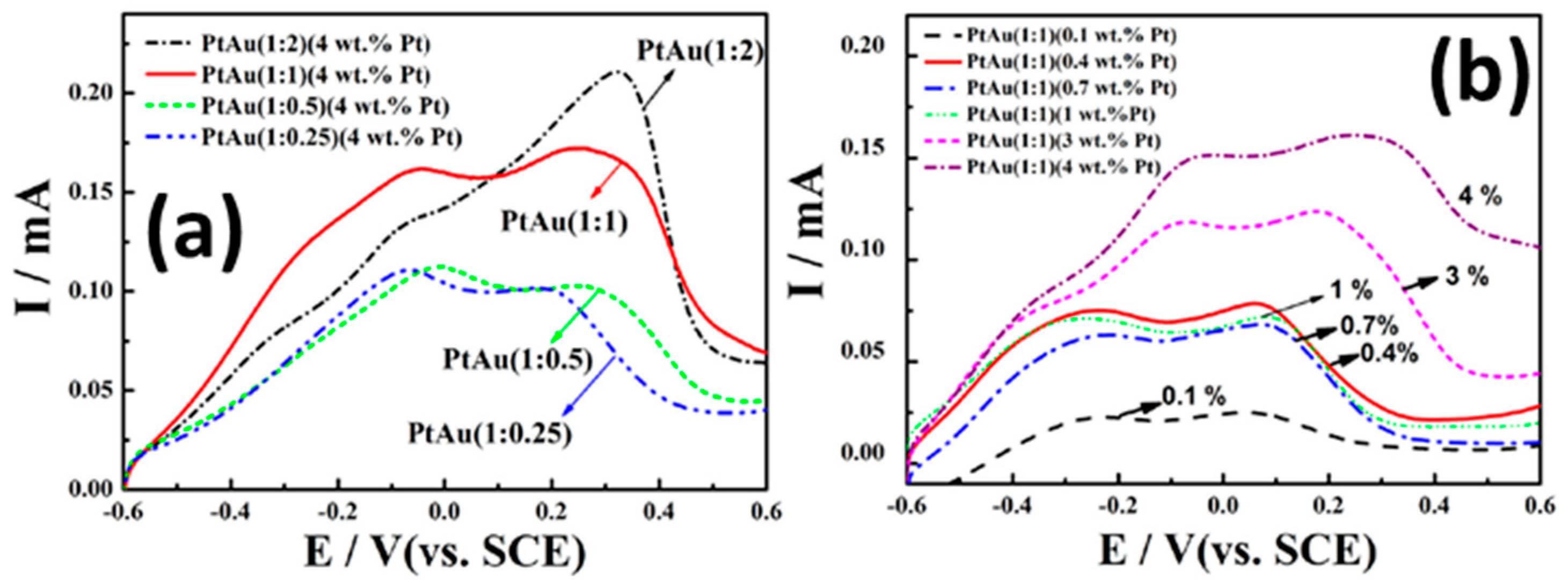
7.1. Bi-Metallic Pt-Au Alloy Nanomaterials
7.2. Mechanisms of Electro-oxidation of Glucose by Pt and Pt-Au Electrocatalysts
7.3. Characterizations of the Bi-Metallic Pt-Au Electrocatalysts
7.4. Comparison of the Mono-Metallic and the Bi-Metallic Electrodes
7.5. A Prospective Approach to the Bi-Metallic Electrodes
8. Electroactive Materials of Oxide Compounds
9. Electroactive Materials of Hydroxide Compounds
10. Electroactive Materials of Metals and Their Derivatives
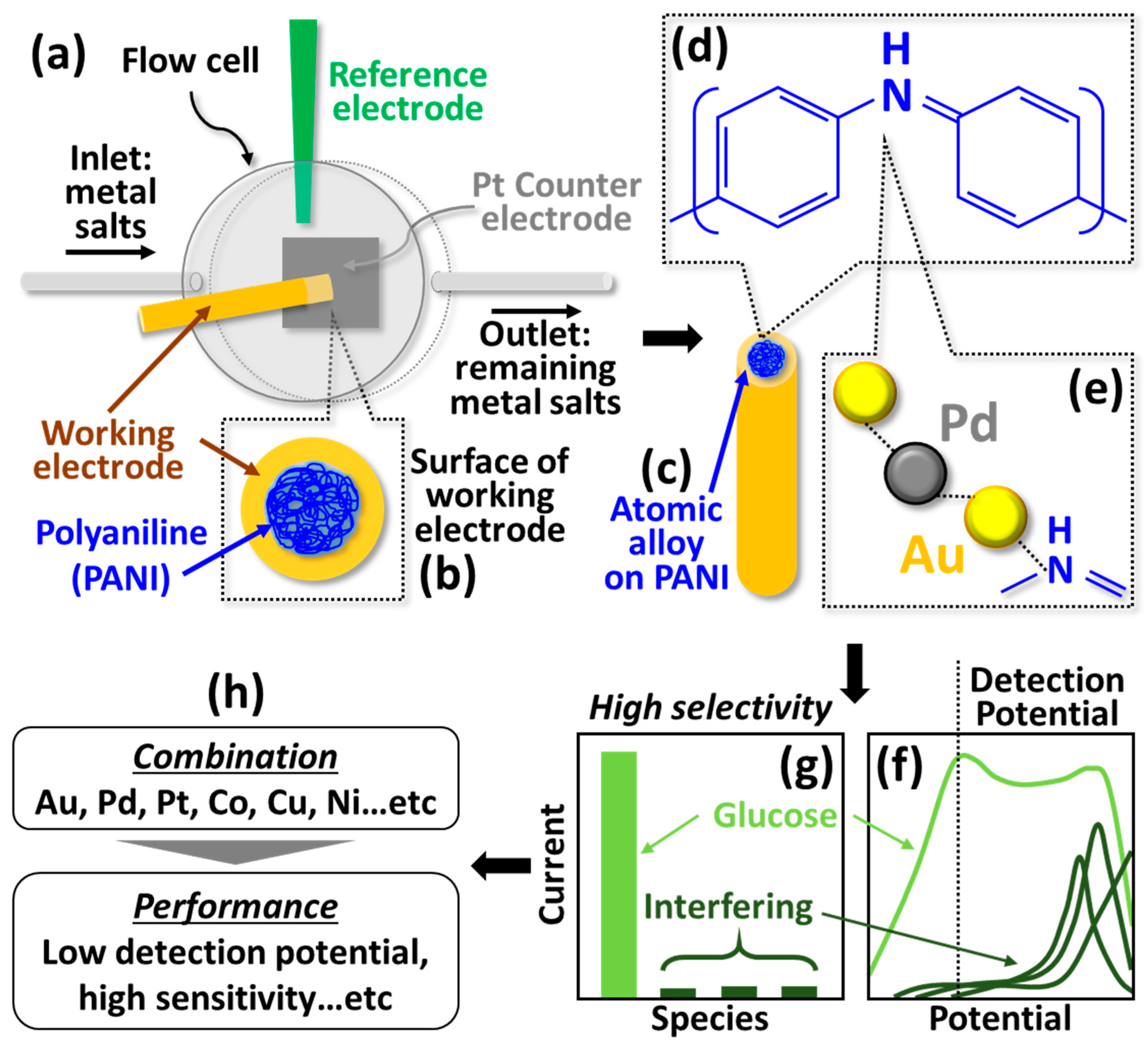
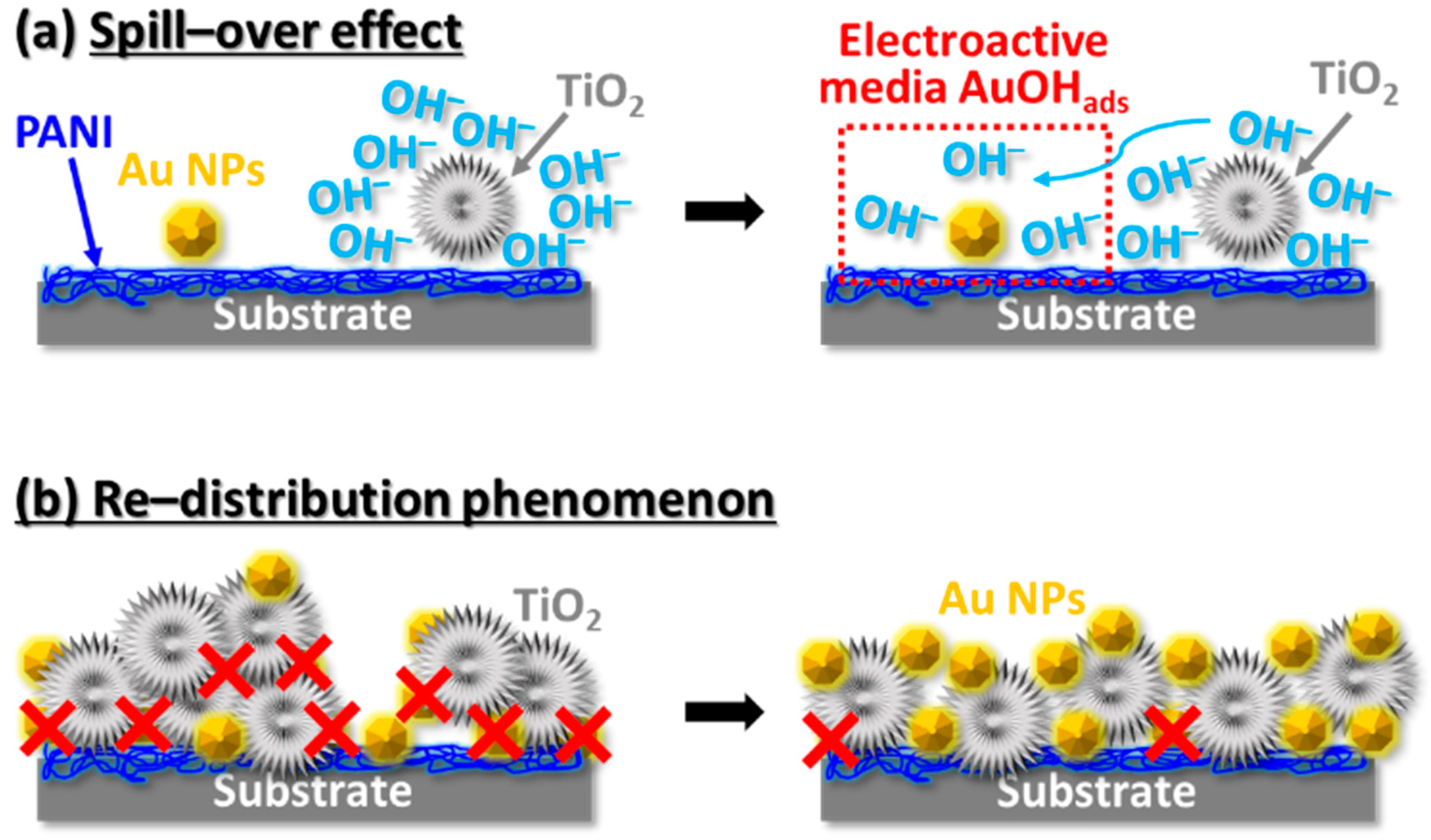
10.1. Electrocatalysts of the Au Nanoparticles-TiO2
10.2. Strategies for Enhancement of Performances of the Electrodes
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 8th ed.; International Diabetes Federation: Brussels, Belgium, 2017. [Google Scholar]
- Papatheodorou, K.; Banach, M.; Bekiari, E.; Rizzo, M.; Edmonds, M. Complications of Diabetes 2017. J. Diabetes Res. 2018, 2018, 3086167. [Google Scholar] [CrossRef]
- Fox, C.S.; Coady, S.; Sorlie, P.D.; Levy, D.; Meigs, J.B.; D’Agostino, R.B.; Wilson, P.W.F.; Savage, P.J. Trends in cardiovascular complications of diabetes. JAMA 2004, 292, 2495–2499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nathan, D.M. Long-term complications of diabetes mellitus. N. Engl. J. Med. 1993, 328, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, H.; Akerlund, A.C.; Mosbach, K. Determination of glucose, urea and penicillin using enzyme-pH-electrodes. Biochem. Biophys. Acta 1973, 320, 529. [Google Scholar] [CrossRef]
- Updike, S.J.; Hicks, G.P. The Enzyme Electrode. Nature 1067, 214, 986. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29. [Google Scholar] [CrossRef]
- Hwang, D.W.; Lee, S.; Seo, M.; Chung, T.D. Recent advances in electrochemical non-enzymatic glucose sensors—A review. Anal. Chim. Acta 2018, 1033, 1–34. [Google Scholar] [CrossRef]
- Wang, G.; He, X.; Wang, L.; Gu, A.; Huang, Y.; Fang, B.; Geng, B.; Zhang, X. Non-enzymatic electrochemical sensing of glucose. Microchim. Acta 2013, 180, 161–186. [Google Scholar] [CrossRef]
- Park, S.; Boo, H.; Chung, T.D. Electrochemical non-enzymatic glucose sensors. Anal. Chim. Acta 2006, 556, 46–57. [Google Scholar] [CrossRef]
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Glucose oxidase—An overview. Biotechnol. Adv. 2009, 27, 489–501. [Google Scholar] [CrossRef]
- Cao, L.; Ye, J.; Tong, L.; Tang, B. A New Route to the Considerable Enhancement of Glucose Oxidase (GOx) Activity: The Simple Assembly of a Complex from CdTe Quantum Dots and GOx, and Its Glucose Sensing. Chem. Eur. J. 2008, 14, 9633–9640. [Google Scholar] [CrossRef]
- Mala Ekanayake, E.M.I.; Preethichandra, D.M.G.; Kaneto, K. Polypyrrole nanotube array sensor for enhanced adsorption of glucose oxidase in glucose biosensors. Biosens. Bioelectron. 2007, 23, 107–113. [Google Scholar] [CrossRef]
- Holt, R.E.; Cotton, T.M. Surface-Enhanced Resonance Raman and Electrochemical Investigation of Glucose Oxidase Catalysis at a Silver Electrode. J. Am. Chem. Soc. 1989, 111, 2815–2821. [Google Scholar] [CrossRef]
- Foulds, N.C.; Lowe, C.R. Immobilization of Glucose Oxidase in Ferrocene-Modified Pyrrole Polymers. Anal. Cham. 1998, 60, 2473–2478. [Google Scholar] [CrossRef] [PubMed]
- Degani, Y.; Heller, A. Direct Electrical Communication between Chemically Modified Enzymes and Metal Electrodes. 1. Electron Transfer from Glucose Oxidase to Metal Electrodes via Electron Relays, Bound Covalently to the Enzyme. J. Phys. Chem. 1987, 91, 1285–1289. [Google Scholar] [CrossRef]
- Abellán-Llobregat, A.; Jeerapan, I.; Bandodkar, A.; Vidal, L.; Canals, A.; Wang, J.; Morallón, E. A stretchable and screen-printed electrochemical sensor for glucose determination in human perspiration. Biosens. Bioelectron. 2017, 91, 885–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferri, S.; Kojima, K.; Sode, K. Review of Glucose Oxidases and Glucose Dehydrogenases: A Bird’s Eye View of Glucose Sensing Enzymes. J. Diabetes Sci. Technol. 2011, 5, 1068–1076. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.M.; Wong, K.H.; Chen, X.D. Glucose oxidase: Natural occurrence, function, properties and industrial applications. Appl. Microbiol. Biotechnol. 2008, 78, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Malitesta, C.; Palmisano, F.; Torsi, L.; Zambonin, P.G. Glucose Fast-Response Amperometric Sensor Based on Glucose Oxidase Immobilized in an Electropolymerized Poly (o-phenyienediamine) Film. Anal. Chem. 1990, 62, 2735–2740. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D.-H. Enzyme-Based Glucose Sensor: From Invasive to Wearable Device. Adv. Healthc. Mater. 2018, 7, 1701150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, A.F.; Nieman, T.A. Glucose quantitation using an immobilized glucose dehydrogenase enzyme reactor and a tris (2,2′-bipyridyl) ruthenium (II) chemiluminescent sensor. Anal. Chim. Acta 1993, 281, 475–481. [Google Scholar] [CrossRef]
- Ye, L.; Hámmerle, M.; Olsthoorn, A.J.J.; Schuhmann, W.; Schmidt, H.-L.; Duine, J.A.; Heller, A. High Current Density “Wired” Quinoprotein Glucose Dehydrogenase Electrode. Anal. Chem. 1993, 65, 238–241. [Google Scholar] [CrossRef]
- D’Costa, E.J.; Higgins, I.J.; Turner, A.P.F. Quinoprotein glucose dehydrogenase and its application in an amperometric glucose sensor. Biosensors 1986, 2, 71–87. [Google Scholar] [CrossRef]
- Wilson, R.; Turner, A.P.F. Glucose oxidase: An ideal enzyme. Biosens. Bioelectron. 1992, 7, 165–185. [Google Scholar] [CrossRef]
- Harris, J.M.; Reyes, C.; Lopez, G.P. Causes of Glucose Oxidase Instability in In Vivo Biosensing: A Brief Review. J. Diabetes Sci. Technol. 2013, 7, 1030–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umaña, M.; Waller, J. Protein-Modified Electrodes. The Glucose Oxidase/Polypyrrole System. Anal. Chem. 1986, 58, 2979–2983. [Google Scholar] [CrossRef]
- Zdarta, J.; Pinelo, M.; Jesionowski, T.; Meyer, A. Upgrading of biomass monosaccharides by immobilized glucose dehydrogenase and xylose dehydrogenase. ChemCatChem 2018, 10, 5164–5173. [Google Scholar] [CrossRef] [Green Version]
- Sode, K.; Tsugawa, W.; Yamazaki, T.; Watanabe, M.; Ogasawara, N.; Tanaka, M. A novel thermostable glucose dehydrogenase varying temperature properties by altering its quaternary structures. Enzyme Microb. Technol. 1996, 19, 82–85. [Google Scholar] [CrossRef]
- Deep, A.; Tiwari, U.; Kumar, P.; Mishra, V.; Jain, S.C.; Singh, N.; Kapur, P.; Bharadwaj, L.M. Immobilization of enzyme on long period grating fibers for sensitive glucose detection. Biosens. Bioelectron. 2012, 33, 190–195. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Deng, J. A Glucose Biosensor Based on Immobilization of Glucose Oxidase in Electropolymerized o-Aminophenol Film on Platinized Glassy Carbon Electrode. Anal. Chem. 1996, 68, 1632–1638. [Google Scholar] [CrossRef]
- Demura, M.; Asakura, T.; Kuroo, T. Immobilization of biocatalysts with bombyx mori silk fibroin by several kinds of physical treatment and its application to glucose sensors. Biosensors 1989, 4, 361–372. [Google Scholar] [CrossRef]
- Jönsson, G.; Gorton, L. An amperometric glucose sensor made by modification of a graphite electrode surface with immobilized glucose oxidase and adsorbed mediator. Biosensors 1985, 1, 355–368. [Google Scholar] [CrossRef]
- Ye, J.-S.; Chen, C.-W.; Lee, C.-L. Pd nanocube as non-enzymatic glucose sensor. Sens. Actuators B Chem. 2015, 208, 569–574. [Google Scholar] [CrossRef]
- Luo, J.; Jiang, S.; Zhang, H.; Jiang, J.; Liu, X. A novel non-enzymatic glucose sensor based on Cu nanoparticle modified graphene sheets electrode. Anal. Chim. Acta 2012, 709, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Cellat, K.; Arıkan, K.; Savk, A.; Karimi, F.; Şen, F. Palladium-Nickel nanoparticles decorated on Functionalized-MWCNT for high precision non-enzymatic glucose sensing. Mater. Chem. Phys. 2020, 250, 123042. [Google Scholar] [CrossRef]
- Li, H.; Guo, C.-Y.; Xu, C.-L. A highly sensitive non-enzymatic glucose sensor based on bimetallic Cu-Ag superstructures. Biosens. Bioelectron. 2015, 63, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Su, X.; Yuan, H.; Sun, Q.; Xiao, D.; Choi, M.M.F. An improved sensitivity non-enzymatic glucose sensor based on a CuO nanowire modified Cu electrode. Analyst 2008, 133, 126–132. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B.; Zhang, T. A novel non-enzymatic glucose sensor based on NiO hollow spheres. Electrochim. Acta 2013, 102, 104–107. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, X.; Huo, H.; Xu, C.; Han, X. Co3O4 microspheres with free-standing nanofibers for high performance non-enzymatic glucose sensor. Analyst 2013, 138, 6727–6731. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, D.; Liu, S.; Qin, S.; Sun, X.; Wang, Z.; Qin, C.; Li, Y.; Zhou, J. Flexible porous Ni (OH)2 nanopetals sandwiches for wearable non-enzyme glucose sensors. Appl. Surf. Sci. 2021, 552, 149529. [Google Scholar] [CrossRef]
- Chiu, W.-T.; Chang, T.-F.M.; Sone, M.; Tixier-Mita, A.; Toshiyoshi, H. Electrocatalytic activity enhancement of Au NPs-TiO2 electrode via a facile redistribution process towards the non-enzymatic glucose sensors. Sens. Actuators B Chem. 2020, 319, 128279. [Google Scholar] [CrossRef]
- Su, Y.; Guo, H.; Wang, Z.; Long, Y.; Li, W.; Tu, Y. Au@Cu2O core-shell structure for high sensitive non-enzymatic glucose sensor. Sens. Actuators B Chem. 2018, 255, 2510–2519. [Google Scholar] [CrossRef]
- Zheng, B.; Liu, G.; Yao, A.; Xiao, Y.; Du, J.; Guo, Y.; Xiao, D.; Hu, Q.; Choi, M.M.F. A sensitive AgNPs/CuO nanofibers non-enzymatic glucose sensor based on electrospinning technology. Sens. Actuators B Chem. 2014, 195, 431–438. [Google Scholar] [CrossRef]
- Yoon, S.S.; Ramadoss, A.; Saravanakumar, B.; Kim, S.J. Novel Cu/CuO/ZnO hybrid hierarchical nanostructures for non-enzymatic glucose sensor application. J. Electroanal. Chem. 2014, 717–718, 90–95. [Google Scholar]
- Ding, Y.; Wang, Y.; Su, L.; Zhang, H.; Lei, Y. Preparation and characterization of NiO-Ag nanofibers, NiO nanofibers, and porous Ag: Towards the development of a highly sensitive and selective non-enzymatic glucose sensor. J. Mater. Chem. 2010, 20, 9918–9926. [Google Scholar] [CrossRef]
- Chiu, W.-T.; Chang, T.-F.M.; Sone, M.; Tixier-Mita, A.; Toshiyoshi, H. Roles of TiO2 in the highly robust Au nanoparticles-TiO2 modified polyaniline electrode towards non-enzymatic sensing of glucose. Talanta 2020, 212, 120780. [Google Scholar] [CrossRef]
- Baghayeri, M.; Amiri, A.; Farhadi, S. Development of non-enzymatic glucose sensor based on efficient loading Ag nanoparticles on functionalized carbon nanotubes. Sens. Actuators B Chem. 2016, 225, 354–362. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, X.; Chen, J.; Zheng, X.; Liu, C.; Xue, T.; Wang, H.; Jin, Z.; Qiao, L.; Zheng, W. Well-dispersed palladium nanoparticles on graphene oxide as a non-enzymatic glucose sensor. RSC Adv. 2012, 2, 6245–6249. [Google Scholar] [CrossRef]
- Wu, H.-X.; Cao, W.-M.; Li, Y.; Liu, G.; Wen, Y.; Yang, H.-F.; Yang, S.-P. In situ growth of copper nanoparticles on multiwalled carbon nanotubes and their application as non-enzymatic glucose sensor materials. Electrochim. Acta 2010, 55, 3734–3740. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, L.-C.; Zhang, W.-D.; Gunasekaran, S. A highly sensitive non-enzymatic glucose sensor based on a simple two-step electrodeposition of cupric oxide (CuO) nanoparticles onto multi-walled carbon nanotube arrays. Talanta 2010, 82, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Ahn, M.-S.; Hahn, Y.-B. Fabrication of a non-enzymatic glucose sensor field-effect transistor based on vertically-oriented ZnO nanorods modified with Fe2O3. Electrochem. Commun. 2017, 77, 107–111. [Google Scholar] [CrossRef]
- Iwu, K.O.; Lombardo, A.; Sanz, R.; Scirè, S.; Mirabella, S. Facile synthesis of Ni nanofoam for flexible and low-cost non-enzymatic glucose sensing. Sens. Actuators B Chem. 2016, 224, 764–771. [Google Scholar] [CrossRef]
- Zeng, G.; Li, W.; Ci, S.; Jia, J.; Wen, Z. Highly Dispersed NiO Nanoparticles Decorating graphene Nanosheets for Non-enzymatic Glucose Sensor and Biofuel Cell. Sci. Rep. 2016, 6, 36454. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Fan, G.; Yang, L.; Li, F. Novel ultrasensitive non-enzymatic glucose sensors based on controlled flower-like CuO hierarchical films. Sens. Actuators B Chem. 2014, 199, 175–182. [Google Scholar] [CrossRef]
- Xu, H.; Xia, C.; Wang, S.; Han, F.; Akbari, M.K.; Hai, Z.; Zhuiykov, S. Electrochemical non-enzymatic glucose sensor based on hierarchical 3D Co3O4/Ni heterostructure electrode for pushing sensitivity boundary to a new limit. Sens. Actuators B Chem. 2018, 267, 93–103. [Google Scholar] [CrossRef]
- Ngo, Y.-L.T.; Hoa, L.T.; Chung, J.S.; Hur, S.H. Multi-dimensional Ag/NiO/reduced graphene oxide nanostructures for a highly sensitive non-enzymatic glucose sensor. J. Alloys Compd. 2017, 712, 742–751. [Google Scholar] [CrossRef]
- Sedighi, A.; Montazer, M.; Mazinani, S. Synthesis of wearable and flexible NiP0.1-SnOx/PANI/CuO/cotton towards a non-enzymatic glucose sensor. Biosens. Bioelectron. 2019, 135, 192–199. [Google Scholar] [CrossRef]
- Stepniowski, W.J.; Misiolek, W.Z. Review of Fabrication Methods, Physical Properties, and Applications of Nanostructured Copper Oxides Formed via Electrochemical Oxidation. Nanomaterials 2018, 8, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Mugabo, Y.; Iglesias, J.; Xie, L.; Delghingaro-Augusto, V.; Lussier, R.; Peyot, M.L.; Joly, E.; Taïb, B.; Davis, M.A.; et al. α/β-Hydrolase domain-6-accessible monoacylglycerol controls glucose-stimulated insulin secretion. Cell Metab. 2014, 19, 993–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalaf, N.; Ahamad, T.; Naushad, M.; Al-hokbany, N.; Al-Saeedi, S.I.; Almotairi, S.; Alshehri, S.M. Chitosan polymer complex derived nanocomposite (AgNPs/NSC) for electrochemical non-enzymatic glucose sensor. Int. J. Biol. Macromol. 2020, 146, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, S.; Lee, H.N.; Park, Y.M.; Bae, Y.-S.; Kim, H.-J. Electrochemically derived CuO nanorod from copper-based metal-organic framework for non-enzymatic detection of glucose. Appl. Surf. Sci. 2019, 479, 720–726. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Karakocak, B.B.; Kavadiya, S.; Soundappan, T.; Biswas, P. A highly sensitive non-enzymatic glucose sensor based on Cu/Cu2O/CuO ternary composite hollow spheres prepared in a furnace aerosol reactor. Sens. Actuators B Chem. 2018, 259, 745–752. [Google Scholar] [CrossRef]
- Xu, J.; Sun, Y.; Zhang, J. Solvothermal synthesis of Fe3O4 nanospheres for high-performance electrochemical non-enzymatic glucose sensor. Sci. Rep. 2020, 10, 16026. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Li, X.; Xu, J. Fabrication of NiCo2O4 nanobelt by a chemical co-precipitation method for non-enzymatic glucose electrochemical sensor application. J. Alloys Compd. 2020, 831, 154796. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, C.; Yang, M.; Zhou, Y.; Bi, C.; Lv, Q.; Ma, N. A facile and sensitive electrochemical sensor for non-enzymatic glucose detection based on three-dimensional flexible polyurethane sponge decorated with nickel hydroxide. Anal. Chim. Acta 2020, 1109, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Sridara, T.; Upan, J.; Saianand, G.; Tuantranont, A.; Karuwan, C.; Jakmunee, J. Non-Enzymatic Amperometric Glucose Sensor Based on Carbon Nanodots and Copper Oxide Nanocomposites Electrode. Sensors 2020, 20, 808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villena Gonzales, W.; Mobashsher, A.T.; Abbosh, A. The Progress of Glucose Monitoring—A Review of Invasive to Minimally and Non-Invasive Techniques, Devices and Sensors. Sensors 2019, 19, 800. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.; Ju, Y.; West, L.; Moussy, Y.; Moussy, F. An Investigation of Long-Term Performance of Minimally Invasive Glucose Biosensors. Diabetes Technol. Ther. 2007, 9, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, B.H. An Overview of Minimally Invasive Technologies. Clin. Chem. 1992, 38, 1596–1600. [Google Scholar] [CrossRef] [PubMed]
- Dungel, P.; Long, N.; Yu, B.; Moussy, Y.; Moussy, F. Study of the effects of tissue reactions on the function of implanted glucose sensors. J. Biomed. Mater. Res. 2008, 85, 699–706. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Kim, S.-Y.; Cheong, W.H.; Jang, J.; Park, Y.-G.; Na, K.; Kim, Y.-T.; Heo, J.H.; Lee, C.Y.; et al. Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays. Sci. Adv. 2018, 4, 9841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, H.; Liao, Y.; Lingley, A.R.; Afanasiev, A.; Lähdesmäki, I.; Otis, B.P.; Parviz, B.A. A contact lens with integrated telecommunication circuit and sensors for wireless and continuous tear glucose monitoring. J. Micromech. Microeng. 2012, 22, 075007. [Google Scholar] [CrossRef]
- Liao, Y.-T.; Yao, H.; Lingley, A.; Parviz, B.; Otis, B.P. A 3-μW CMOS Glucose Sensor for Wireless Contact-Lens Tear Glucose Monitoring. IEEE J. Solid State Circuits 2011, 47, 335–344. [Google Scholar] [CrossRef]
- Diouf, A.; Bouchikhi, B.; El Bari, N. A nonenzymatic electrochemical glucose sensor based on molecularly imprinted polymer and its application in measuring saliva glucose. Mater. Sci. Eng. C 2019, 98, 1196–1209. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Kuroki, Y.; Nitta, H.; Chouhan, P.; Toma, K.; Sawada, S.; Takeuchi, S.; Sekita, T.; Akiyoshi, K.; Minakuchi, S.; et al. Mouthguard biosensor with telemetry system for monitoring of saliva glucose: A novel cavitas sensor. Biosens. Bioelectron. 2016, 84, 106–111. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Zhang, W.; Wang, M.L. An On-Chip Disposable Salivary Glucose Sensor for Diabetes Control. J. Diabetes Sci. Technol. 2016, 10, 1344–1352. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Du, Y.; Wang, M.L. Noninvasive glucose monitoring using saliva nano-biosensor. Sens. Bio-Sens. Res. 2015, 4, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, M.; Mitsumori, M.; Kano, Y. Noninvasively measuring blood glucose using saliva. IEEE Eng. Med. Biol. Mag. 1998, 17, 59–63. [Google Scholar] [CrossRef]
- Samant, P.P.; Prausnitz, M.R. Mechanisms of sampling interstitial fluid from skin using a microneedle patch. Proc. Natl. Acad. Sci. USA 2018, 115, 4583–4588. [Google Scholar] [CrossRef] [Green Version]
- Schmelzeisen-Redeker, G.; Staib, A.; Strasser, M.; Müller, U.; Schoemaker, M. Overview of a Novel Sensor for Continuous Glucose Monitoring. J. Diabetes Sci. Technol. 2013, 7, 808–814. [Google Scholar] [CrossRef]
- Wang, P.M.; Cornwell, M.; Prausnitz, M.R. Minimally Invasive Extraction of Dermal Interstitial Fluid for Glucose Monitoring Using Microneedles. Diabetes Technol. Ther. 2005, 7, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Thennadil, S.N.; Rennert, J.L.; Wenzel, B.J.; Hazen, K.H.; Ruchti, T.L.; Block, M.B. Comparison of Glucose Concentration in Interstitial Fluid, and Capillary and Venous Blood During Rapid Changes in Blood Glucose Levels. Diabetes Technol. Ther. 2001, 3, 357–365. [Google Scholar] [CrossRef] [Green Version]
- Ginsberg, B.H. The FDA Panel Advises Approval of the First Continuous Glucose Sensor. Diabetes Technol. Ther. 1999, 1, 203–204. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhai, Q.; Dong, D.; An, T.; Gong, S.; Shi, Q.; Cheng, W. Highly Stretchable and Strain-Insensitive Fiber-Based Wearable Electrochemical Biosensor to Monitor Glucose in the Sweat. Anal. Chem. 2019, 91, 6569–6576. [Google Scholar] [CrossRef]
- Toi, P.T.; Trung, T.Q.; Dang, T.M.L.; Bae, C.W.; Lee, N.-E. Highly Electrocatalytic, Durable, and Stretchable Nanohybrid Fiber for On-Body Sweat Glucose Detection. ACS Appl. Mater. Interfaces 2019, 11, 10707–10717. [Google Scholar] [CrossRef]
- Alizadeh, A.; Burns, A.; Lenigk, R.; Gettings, R.; Ashe, J.; Porter, A.; McCaul, M.; Barrett, R.; Diamond, D.; White, P.; et al. A wearable patch for continuous monitoring of sweat electrolytes during exertion. Lab Chip 2018, 18, 2632–2641. [Google Scholar] [CrossRef]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.Y.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.; Hyeon, T.; et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 2016, 11, 566–572. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, W.-D.; Ye, J.-S. Nonenzymatic electrochemical glucose sensor based on MnO2/MWNTs nanocomposite. Electrochem. Commun. 2008, 10, 1268–1271. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2007, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Gernet, S.; Koudelka, M.; De Rooij, N.F. Fabrication and characterization of a planar electrochemical cell and its application as a glucose sensor. Sens. Actuators 1989, 18, 59–70. [Google Scholar] [CrossRef]
- El-Ads, E.H.; Galal, A.; Atta, N.F. Electrochemistry of glucose at gold nanoparticles modified graphite/SrPdO3 electrode–Towards a novel non-enzymatic glucose sensor. J. Electroanal. Chem. 2015, 749, 42–52. [Google Scholar] [CrossRef]
- Zanello, P.; Nervi, C.; De Biani, F.F. Inorganic Electrochemistry: Theory, Practice and Application; Royal Society of Chemistry: London, UK, 2019. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods Fundamentals and Applications; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Liu, S.; Zeng, W.; Guo, Q.; Li, Y. Metal oxide-based composite for non-enzymatic glucose sensors. J. Mater. Sci. Mater. Electron. 2020, 31, 16111–16136. [Google Scholar] [CrossRef]
- Zhu, H.; Li, L.; Zhou, W.; Shao, Z.; Chen, X. Advances in non-enzymatic glucose sensors based on metal oxides. J. Mater. Chem. B 2016, 4, 7333–7349. [Google Scholar] [CrossRef]
- Toghill, K.E.; Compton, R.G. Electrochemical Non-enzymatic Glucose Sensors: A Perspective and an Evaluation. Int. J. Electrochem. Sci. 2010, 5, 1246–1301. [Google Scholar]
- Wei, M.; Qiao, Y.; Zhao, H.; Liang, J.; Li, T.; Luo, Y.; Lu, S.; Shi, X.; Lu, W.; Sun, X. Electrochemical non-enzymatic glucose sensors: Recent progress and perspectives. Chem. Commun. 2020, 56, 14553. [Google Scholar] [CrossRef]
- Suzuki, N.; Lee, J.; Loew, N.; Takahashi-Inose, Y.; Okuda-Shimazaki, J.; Kojima, K.; Mori, K.; Tsugawa, W.; Sode, K. Engineered Glucose Oxidase Capable of Quasi-Direct Electron Transfer after a Quick-and-Easy Modification with a Mediator. Int. J. Mol. Sci. 2020, 21, 1137. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.; Loew, N.; Tsugawa, W.; Lin, C.-E.; Probst, D.; La Belle, J.T.; Sode, K. The electrochemical behavior of a FAD dependent glucose dehydrogenase with direct electron transfer subunit by immobilization on self-assembled monolayers. Bioelectrochemistry 2018, 121, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pletcher, D. Electrocatalysis: Present and future. J. Appl. Electrochem. 1984, 14, 403–415. [Google Scholar] [CrossRef]
- Burke, L.D. Premonolayer oxidation and its role in electrocatalysis. Electrochim. Acta 1994, 39, 1841. [Google Scholar] [CrossRef]
- Wang, J. Amperometric biosensors for clinical and therapeutic drug monitoring: A review. J. Pharm. Biomed. Anal. 1999, 19, 47–53. [Google Scholar] [CrossRef]
- Ernst, S.; Heitbaum, J.; Hamann, C.H. The electrooxidation of glucose in phosphate buffer solutions: Part I. Reactivity and kinetics below 350 mV/RHE. J. Electroanal. Chem. 1979, 100, 173–183. [Google Scholar] [CrossRef]
- Luo, P.; Zhang, F.; Baldwin, R.P. Comparison of metallic electrodes for constant-potential amperometric detection of carbohydrates, amino acids and related compounds in flow systems. Anal. Chim. Acta 1991, 244, 169–178. [Google Scholar] [CrossRef]
- Mello, G.A.B.; Cheuquepán, W.; Briega-Martos, V.; Feliu, J.M. Glucose electro-oxidation on Pt(100) in phosphate buffer solution (pH 7): A mechanistic study. Electrochim. Acta 2020, 354, 136765. [Google Scholar] [CrossRef]
- Li, Y.; Song, Y.-Y.; Yang, C.; Xia, X.-H. Hydrogen bubble dynamic template synthesis of porous gold for nonenzymatic electrochemical detection of glucose. Electrochem. Commun. 2007, 9, 981–988. [Google Scholar] [CrossRef]
- Sun, Y.; Buck, H.; Mallouk, T.E. Combinatorial Discovery of Alloy Electrocatalysts for Amperometric Glucose Sensors. Anal. Chem. 2001, 73, 1599–1604. [Google Scholar] [CrossRef]
- Wang, T.; Yu, Y.; Tian, H.; Hu, J. A Novel Non-Enzymatic Glucose Sensor Based on Cobalt Nanoparticles Implantation-Modified Indium Tin Oxide Electrode. Electroanalysis 2014, 26, 2693–2700. [Google Scholar] [CrossRef]
- Chang, G.; Shu, H.; Huang, Q.; Oyama, M.; Ji, K.; Liu, X.; He, Y. Synthesis of highly dispersed Pt nanoclusters anchored graphene composites and their application for non-enzymatic glucose sensing. Electrochim. Acta 2015, 157, 149–157. [Google Scholar] [CrossRef]
- Wu, G.-H.; Song, X.-H.; Wu, Y.-F.; Chen, X.-M.; Luo, F.; Chen, X. Non-enzymatic electrochemical glucose sensor based on platinum nanoflowers supported on graphene oxide. Talanta 2013, 105, 379–385. [Google Scholar] [CrossRef]
- Geng, D.; Bo, X.; Guo, L. Ni-doped molybdenum disulfide nanoparticles anchored on reduced graphene oxide as novel electroactive material for a non-enzymatic glucose sensor. Sens. Actuators B Chem. 2017, 244, 131–141. [Google Scholar] [CrossRef]
- Karikalan, N.; Karthik, R.; Chen, S.-M.; Karuppiah, C.; Elangovan, A. Sonochemical Synthesis of Sulfur Doped Reduced Graphene Oxide Supported CuS Nanoparticles for the Non-Enzymatic Glucose Sensor Applications. Sci. Rep. 2017, 7, 2494. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Li, Y.; Hu, L.; Lee, L.Y.S. Use of carbon supports with copper ion as a highly sensitive non-enzymatic glucose sensor. Sens. Actuators B Chem. 2019, 282, 187–196. [Google Scholar] [CrossRef]
- Kangkamano, T.; Numnuam, A.; Limbut, W.; Kanatharana, P.; Thavarungkul, P. Chitosan cryogel with embedded gold nanoparticles decorated multiwalled carbon nanotubes modified electrode for highly sensitive flow based non-enzymatic glucose sensor. Sens. Actuators B Chem. 2017, 246, 854–863. [Google Scholar] [CrossRef]
- Rathod, D.; Dickinson, C.; Egan, D.; Dempsey, E. Platinum nanoparticle decoration of carbon materials with applications in non-enzymatic glucose sensing. Sens. Actuators B Chem. 2010, 143, 547–554. [Google Scholar] [CrossRef]
- Chen, X.-M.; Cai, Z.-M.; Lin, Z.-J.; Jia, T.-T.; Liu, H.-Z.; Jiang, Y.-Q.; Chen, X. A novel non-enzymatic ECL sensor for glucose using palladium nanoparticles supported on functional carbon nanotubes. Biosens. Bioelectron. 2009, 24, 3475–3480. [Google Scholar] [CrossRef]
- Kailasa, S.; Geeta, B.; Jayarambabu, N.; Reddy, R.K.K.; Sharma, S.; Rao, K.V. Conductive Polyaniline Nanosheets (CPANINS) for a non-enzymatic glucose sensor. Mat. Lett. 2019, 245, 118–121. [Google Scholar] [CrossRef]
- Esmaeeli, A.; Ghaffarinejad, A.; Zahedi, A.; Vahidi, O. Copper oxide-polyaniline nanofiber modified fluorine doped tin oxide (FTO) electrode as non-enzymatic glucose sensor. Sens. Actuators B Chem. 2018, 266, 294–301. [Google Scholar] [CrossRef]
- Emir, G.; Dilgin, Y.; Ramanaviciene, A.; Ramanavicius, A. Amperometric nonenzymatic glucose biosensor based on graphite rod electrode modified by Ni-nanoparticle/polypyrrole composite. Microchem. J. 2021, 161, 105751. [Google Scholar] [CrossRef]
- Quintero-Jaime, A.F.; Conzuelo, F.; Schuhmann, W.; Cazorla-Amorós, D.; Morallón, E. Multi-wall carbon nanotubes electrochemically modified with phosphorus and nitrogen functionalities as a basis for bioelectrodes with improved performance. Electrochim. Acta 2021, 387, 138530. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Zhou, C.; Zhou, L.; Kong, Y.; Long, H.; Zhong, S. A novel self-cleaning, non-enzymatic glucose sensor working under a very low applied potential based on a Pt nanoparticle-decorated TiO2 nanotube array electrode. Electrochim. Acta 2014, 115, 269–276. [Google Scholar] [CrossRef]
- Lee, K.K.; Loh, P.Y.; Sow, C.H.; Chin, W.S. CoOOH nanosheets on cobalt substrate as a non-enzymatic glucose sensor. Electrochem. Commun. 2012, 20, 128–132. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Xiang, Y.; Wang, D.; Zhang, P.; Wang, Y.; Lu, S.; Xu, R.; Zhao, J. A flexible non-enzymatic glucose sensor based on copper nanoparticles anchored on laser-induced graphene. Carbon 2020, 156, 506–513. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, C.; Li, X.; Ding, Y.; Liang, H.; Zhao, G.; Wang, Y. A CuNi/C Nanosheet Array Based on a Metal–Organic Framework Derivate as a Supersensitive Non-Enzymatic Glucose Sensor. Nano Micro Lett. 2018, 10, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marini, S.; Mansour, N.B.; Hjiri, M.; Dhahri, R.; El Mir, L.; Espro, C.; Bonavita, A.; Galvagno, S.; Neri, G.; Leonardi, S.G. Non-enzymatic Glucose Sensor Based on Nickel/Carbon Composite. Electroanalysis 2018, 30, 727–733. [Google Scholar] [CrossRef]
- Shabnam, L.; Faisal, S.N.; Roy, A.K.; Haque, E.; Minett, A.I.; Gomes, V.G. Doped graphene/Cu nanocomposite: A high sensitivity non-enzymatic glucose sensor for food. Food Chem. 2017, 221, 751–759. [Google Scholar] [CrossRef]
- Ramachandran, K.; Rajkumar, T.; Babu, K.J.; Kumar, G.G. Ni-Co bimetal nanowires filled multiwalled carbon nanotubes for the highly sensitive and selective non-enzymatic glucose sensor applications. Sci. Rep. 2016, 6, 36583. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.; Su, J.; Li, X.; Luo, J.; Yang, M. Electrochemical sensing platform based on Pd–Au bimetallic cluster for non-enzymatic detection of glucose. Sens. Actuators B Chem. 2015, 209, 695–700. [Google Scholar] [CrossRef]
- Hoa, L.T.; Sun, K.G.; Hur, S.H. Highly sensitive non-enzymatic glucose sensor based on Pt nanoparticle decorated graphene oxide hydrogel. Sens. Actuators B Chem. 2015, 210, 618–623. [Google Scholar] [CrossRef]
- Sun, A.; Zheng, J.; Sheng, Q. A highly sensitive non-enzymatic glucose sensor based on nickel and multi-walled carbon nanotubes nanohybrid films fabricated by one-step co-electrodeposition in ionic liquids. Electrochim. Acta 2012, 65, 64–69. [Google Scholar] [CrossRef]
- Ren, Z.; Mao, H.; Luo, H.; Deng, X.; Liu, Y. One-step formation of a hybrid material of graphene and porous Ni with highly active Ni (OH)2 used for glucose detection. Nanotechnology 2020, 31, 185501. [Google Scholar] [CrossRef]
- Heyser, C.; Schrebler, R.; Grez, P. New route for the synthesis of nickel (II) oxide nanostructures and its application as non-enzymatic glucose sensor. J. Electroanal. Chem. 2019, 832, 189–195. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Ahn, M.-S.; Bhat, K.S.; Mahmoudi, T.; Wang, Y.; Yoo, J.-Y.; Kwon, D.-W.; Yang, W.-Y.; Hahn, Y.-B. Highly Efficient Non-Enzymatic Glucose Sensor Based on CuO Modified Vertically-Grown ZnO Nanorods on Electrode. Sci. Rep. 2017, 7, 5715. [Google Scholar] [CrossRef] [Green Version]
- Chung, R.-J.; Wang, A.-N.; Liao, Q.-L.; Chuang, K.-Y. Non-Enzymatic Glucose Sensor Composed of Carbon-Coated Nano-Zinc Oxide. Nanomaterials 2017, 7, 36. [Google Scholar] [CrossRef]
- Mani, S.; Vediyappan, V.; Chen, S.-M.; Madhu, R.; Pitchaimani, V.; Chang, J.Y.; Liu, S.-B. Hydrothermal synthesis of NiWO4 crystals for high performance non-enzymatic glucose biosensors. Sci. Rep. 2016, 6, 24128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanbari, K.H.; Babaei, Z. Fabrication and characterization of non-enzymatic glucose sensor based on ternary NiO/CuO/polyaniline nanocomposite. Anal. Biochem. 2016, 498, 37–46. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, L.; Manuzzi, D.; de los Monteros, H.V.E.; Jia, W.; Huo, D.; Hou, C.; Lei, Y. Ultrasensitive and selective non-enzymatic glucose detection using copper nanowires. Biosens. Bioelectron. 2012, 31, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, W.; Li, X.; Liu, J.; Han, Y.; Wu, J.; Zhang, X.; Xu, Y. Hierarchical α-Fe2O3 microcubes supported on Ni foam as non-enzymatic glucose sensor. Appl. Surf. Sci. 2020, 512, 145710. [Google Scholar] [CrossRef]
- Liu, X.; Yang, W.; Chen, L.; Jia, J. Three-Dimensional Copper Foam Supported CuO Nanowire Arrays: An Efficient Non-enzymatic Glucose Sensor. Electrochim. Acta 2017, 235, 219–526. [Google Scholar] [CrossRef]
- Ranjani, M.; Sathishkumar, Y.; Lee, Y.S.; Yoo, D.J.; Kim, A.R.; Kumar, G.G. Ni-Co alloy nanostructures anchored on mesoporous silica nanoparticles for non-enzymatic glucose sensor applications. RSC Adv. 2015, 5, 57804–57814. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Abarghoui, M.M.; Rezaei, B. A new non-enzymatic glucose sensor based on copper/porous silicon nanocomposite. Electrochim. Acta 2014, 123, 219–226. [Google Scholar] [CrossRef]
- Huo, H.; Guo, C.; Li, G.; Han, X.; Xu, C. Reticular-vein-like Cu@Cu2O/reduced graphene oxide nanocomposites for a non-enzymatic glucose sensor. RSC Adv. 2014, 4, 20459–20465. [Google Scholar] [CrossRef]
- Mello, G.A.B.; Cheuquepán, W.; Feliu, J.M. Investigation of reactivity of Pt basal planes towards glucose electro-oxidation in neutral solution (pH 7): Structure-sensitivity dependence and mechanistic study. J. Electroanal. Chem. 2020, 878, 114549. [Google Scholar] [CrossRef]
- Housmans, T.H.M.; Wonders, A.H.; Koper, M.T.M. Structure sensitivity of methanol electrooxidation pathways on platinum: An on-line electrochemical mass spectrometry study. J. Phys. Chem. B 2006, 110, 10021–10031. [Google Scholar] [CrossRef]
- Solla–Gullon, J.; Vidal-Iglesias, F.J.; Lopez–Cudero, A.; Garnier, E.; Feliu, J.M.; Aldaza, A. Shape-dependent electrocatalysis: Methanol and formic acid electrooxidation on preferentially oriented Pt nanoparticles. Phys. Chem. Chem. Phys. 2008, 10, 3689–3698. [Google Scholar] [CrossRef]
- Ren, J.; Shi, W.T.; Li, K.; Ma, Z.F. Ultrasensitive platinum nanocubes enhanced amperometric glucose biosensor based on chitosan and nafion film. Sens. Actuators B Chem. 2012, 163, 115–120. [Google Scholar] [CrossRef]
- Khan, Z.; Singh, T.; Hussain, J.I.; Hashmi, A.A. Au (III)-CTAB reduction by ascorbic acid: Preparation and characterization of gold nanoparticles. Colloids Surf. B Biointerfaces 2013, 104, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Dong, S.; Wang, E. Synthesis and Self-Assembly of Cetyltrimethylammonium Bromide-Capped Gold Nanoparticles. Langmuir 2003, 19, 9434–9439. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Chen, J.; Pang, H. Copper metal-organic framework nanocrystal for plane effect nonenzymatic electro-catalytic activity of glucose. Nanoscale 2014, 6, 10989–10994. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.-S.; Hsu, S.-Y.; Lee, C.-L. Sequential and Transient Electrocatalysis of Glucose Oxidation Reactions by Octahedral, Rhombic Dodecahedral, and Cubic Palladium Nanocrystals. Electrochim. Acta 2016, 211, 1024–1032. [Google Scholar] [CrossRef]
- Liang, Z.X.; Zhao, T.S.; Xu, J.B.; Zhu, L.D. Mechanism study of the ethanol oxidation reaction on palladium in alkaline media. Electrochim. Acta 2009, 54, 2203–2208. [Google Scholar] [CrossRef]
- Hoshi, N.; Nakamura, M.; Maki, N.; Yamaguchi, S.; Kitajima, A. Structural effects on voltammograms of the low index planes of palladium and Pd(S)-[n(100) × (111)] surfaces in alkaline solution. J. Electroanal. Chem. 2008, 624, 134–138. [Google Scholar] [CrossRef]
- Shao, M.H.; Odell, J.; Humbert, M.; Yu, T.Y.; Xia, Y.N. Electrocatalysis on shape-controlled palladium nanocrystals: Oxygen reduction reaction and formic acid oxidation. J. Phys. Chem. C 2013, 117, 4172–4180. [Google Scholar] [CrossRef]
- Li, Z.; Qian, W.; Guo, H.; Song, X.; Yan, H.; Jin, R.; Zheng, J. Facile preparation of novel Pd nanowire networks on a polyaniline hydrogel for sensitive determination of glucose. Anal. Bioanal. Chem. 2020, 412, 6849–6858. [Google Scholar] [CrossRef]
- Niu, X.; Lan, M.; Chen, C.; Zhao, H. Nonenzymatic electrochemical glucose sensor based on novel Pt-Pd nanoflakes. Talanta 2012, 99, 1062–1067. [Google Scholar] [CrossRef]
- Meng, L.; Jin, J.; Yang, G.X.; Lu, T.H.; Zhang, H.; Cai, C.X. Nonenzymatic electrochemical detection of glucose based on palladium-single-walled carbon nanotube hybrid nanostructures. Anal. Chem. 2009, 81, 7271–7280. [Google Scholar] [CrossRef]
- Kuang, Y.J.; Wu, B.H.; Hu, D.; Zhang, X.H.; Chen, J.H. One-pot synthesis of highly dispersed palladium nanoparticles on acetylenic ionic liquid polymer functionalized carbon nanotubes for electrocatalytic oxidation of glucose. J. Solid State Electrochem. 2012, 16, 759–766. [Google Scholar] [CrossRef]
- Lu, L.M.; Li, H.B.; Qu, F.L.; Zhang, X.B.; Shen, G.L.; Yu, R.Q. In situ synthesis of palladium nanoparticle-graphene nanohybrids and their application in nonenzymatic glucose biosensors. Biosens. Bioelectron. 2011, 26, 3500–3504. [Google Scholar] [CrossRef]
- Becerik, I.; Kadirgan, F. The electrocatalytic properties of palladium electrodes for the oxidation of d-glucose in alkaline medium. Electrochim. Acta 1992, 37, 2651–2657. [Google Scholar] [CrossRef]
- Prehn, R.; Cortina-Puig, M.; Muñoz, F.X. A Non-Enzymatic Glucose Sensor Based on the Use of Gold Micropillar Array Electrodes. J. Electrochem. Soc. 2012, 159, F134–F139. [Google Scholar] [CrossRef]
- Xiang, X.; Feng, S.; Chen, J.; Feng, J.; Hou, Y.; Ruan, Y.; Weng, X.; Milcovich, G. Gold nanoparticles/electrochemically expanded graphite composite: A bifunctional platform toward glucose sensing and SERS applications. J. Electroanal. Chem. 2019, 851, 113471. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, X.; Sun, J.; Li, M.; Lin, Y.; Kang, K.; Meng, Y.; Feng, Z.; Wang, J. A non-enzymatic amperometric glucose sensor based on the use of graphene frameworks-promoted ultrafine platinum nanoparticles. Microchim. Acta 2019, 186, 538. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, N.; Cao, P.; Lin, M.; Xu, L.; Ma, H. Electrochemical non-enzymatic glucose sensor using ionic liquid incorporated cobalt-based metal-organic framework. Microchem. J. 2020, 159, 105343. [Google Scholar] [CrossRef]
- Tomanin, P.P.; Cherepanov, P.V.; Besford, Q.A.; Christofferson, A.J.; Amodio, A.; McConville, C.F.; Yarovsky, I.; Caruso, F.; Cavalieri, F. Cobalt Phosphate Nanostructures for Non-Enzymatic Glucose Sensing at Physiological pH. ACS Appl. Mater. Interfaces 2018, 10, 42786–42795. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Wang, N.; Xu, Q.Q.; Xu, L.; Lin, M. Copper-based Metal-organic Framework for Non-enzymatic Electrochemical Detection of Glucose. Electroanalysis 2018, 30, 474–478. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Wu, Y.; Chen, Y.; Weng, B.; Li, C. Flexible paper sensor fabricated via in situ growth of Cu nanoflower on RGO sheets towards amperometrically non-enzymatic detection of glucose. Sens. Actuators B Chem. 2017, 238, 802–808. [Google Scholar] [CrossRef]
- Kung, C.-W.; Cheng, Y.-H.; Ho, K.-C. Single layer of nickel hydroxide nanoparticles covered on a porous Ni foam and its application for highly sensitive non-enzymatic glucose sensor. Sens. Actuators B Chem. 2014, 204, 159–166. [Google Scholar] [CrossRef]
- Lu, L.-M.; Zhang, L.; Qu, F.-L.; Lu, H.-X.; Zhang, X.-B.; Wu, Z.-S.; Huan, S.-Y.; Wang, Q.-A.; Shen, G.-L.; Yu, R.-Q. A nano-Ni based ultrasensitive nonenzymatic electrochemical sensor for glucose: Enhancing sensitivity through a nanowire array strategy. Biosens. Bioelectron. 2009, 25, 218–223. [Google Scholar] [CrossRef]
- Samoson, K.; Thavarungkul, P.; Kanatharana, P.; Limbut, W. A Nonenzymatic Glucose Sensor Based on the Excellent Dispersion of a Graphene Oxide-Poly (acrylic acid)-Palladium Nanoparticle-Modified Screen-Printed Carbon Electrode. J. Electrochem. Soc. 2019, 166, B1079. [Google Scholar] [CrossRef]
- Rafatmah, E.; Hemmateenejad, B. Dendrite gold nanostructures electrodeposited on paper fibers: Application to electrochemical non-enzymatic determination of glucose. Sens. Actuators B Chem. 2020, 304, 127335. [Google Scholar] [CrossRef]
- Jeong, H.; Nguyen, D.M.; Lee, M.S.; Kim, H.G.; Ko, S.C.; Kwac, L.K. N-doped graphene-carbon nanotube hybrid networks attaching with gold nanoparticles for glucose non-enzymatic sensor. Mater. Sci. Eng. C 2018, 90, 38–45. [Google Scholar] [CrossRef]
- Nugraha, A.S.; Li, C.; Bo, J.; Iqbal, M.; Alshehri, S.M.; Ahamad, T.; Malgras, V.; Yamauchi, Y.; Asahi, T. Block-Copolymer-Assisted Electrochemical Synthesis of Mesoporous Gold Electrodes: Towards a Non-Enzymatic Glucose Sensor. ChemElectroChem 2017, 4, 2571–2576. [Google Scholar] [CrossRef] [Green Version]
- Soomro, R.A.; Akyuz, O.P.; Ozturk, R.; Ibupoto, Z.H. Highly sensitive non-enzymatic glucose sensing using gold nanocages as efficient electrode material. Sens. Actuators B Chem. 2016, 233, 230–236. [Google Scholar] [CrossRef]
- Heli, H.; Amirizadeh, O. Non-enzymatic glucose biosensor based on hyperbranched pine-like gold nanostructure. Mater. Sci. Eng. C 2016, 63, 150–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, G.; Shu, H.; Ji, K.; Oyama, M.; Liu, X.; He, Y. Gold nanoparticles directly modified glassy carbon electrode for non-enzymatic detection of glucose. Appl. Surf. Sci. 2014, 288, 524–529. [Google Scholar] [CrossRef]
- Ismail, N.S.; Le, Q.H.; Yoshikawa, H.; Saito, M.; Tamiya, E. Development of Non-enzymatic Electrochemical Glucose Sensor Based on Graphene Oxide Nanoribbon—Gold Nanoparticle Hybrid. Electrochim. Acta 2014, 146, 98–105. [Google Scholar] [CrossRef]
- Gougis, M.; Tabet-Aoul, A.; Ma, D.; Mohamedi, M. Laser synthesis and tailor-design of nanosized gold onto carbon nanotubes for non-enzymatic electrochemical glucose sensor. Sens. Actuators B Chem. 2014, 193, 363–369. [Google Scholar] [CrossRef]
- Lamiri, L.; Belgherbi, O.; Dehchar, C.; Laidoudi, S.; Tounsi, A.; Nessark, B.; Habelhames, F.; Hamam, A.; Gourari, B. Performance of polybithiophene-palladium particles modified electrode for non-enzymatic glucose detection. Synth. Met. 2020, 266, 116437. [Google Scholar] [CrossRef]
- Promsuwan, K.; Kachatong, N.; Limbut, W. Simple flow injection system for non-enzymatic glucose sensing based on an electrode modified with palladium nanoparticles-graphene nanoplatelets/mullti-walled carbon nanotubes. Electrochim. Acta 2019, 320, 134621. [Google Scholar] [CrossRef]
- Wang, F.; Niu, X.; Wang, W.; Jing, W.; Huang, Y.; Zhang, J. Green synthesis of Pd nanoparticles via extracted polysaccharide applied to glucose detection. J. Taiwan Inst. Chem. Eng. 2018, 93, 87–93. [Google Scholar] [CrossRef]
- Wu, Q.; Sheng, Q.; Zheng, J. Nonenzymatic sensing of glucose using a glassy carbon electrode modified with halloysite nanotubes heavily loaded with palladium nanoparticles. J. Electroanal. Chem. 2016, 762, 51–58. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Q.; Qi, K.; Xue, T.; Liu, C.; Zheng, W.; Cui, X. In situ preparation of porous Pd nanotubes on a GCE for non-enzymatic electrochemical glucose sensors. Anal. Methods 2015, 7, 8605–8610. [Google Scholar] [CrossRef]
- Haghighi, B.; Karimi, B.; Tavahodi, M.; Behzadneia, H. Fabrication of a nonenzymatic glucose sensor using Pd-nanoparticles decorated ionic liquid derived fibrillated mesoporous carbon. Mater. Sci. Eng. C 2015, 52, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Bhardwaj, N.; Jain, V.K.; Bhatia, V. Palladium nanoparticles decorated electrostatically functionalized MWCNTs as a non enzymatic glucose sensor. Sens. Actuator A Phys. 2014, 220, 126–133. [Google Scholar] [CrossRef]
- Cai, Z.-X.; Liu, C.-C.; Wu, G.-H.; Chen, X.-M.; Chen, X. Palladium nanoparticles deposit on multi-walled carbon nanotubes and their catalytic applications for electrooxidation of ethanol and glucose. Electrochim. Acta 2013, 112, 756–762. [Google Scholar] [CrossRef]
- Wei, Y.H.; Hsieh, C.K.; Tseng, F.G. Highly-Sensitive Non-Enzymatic Glucose Sensor via Nano Platinum Crystals Fabricated by Phase-Controlled Electrochemical Deposition. J. Electrochem. Soc. 2018, 165, B48. [Google Scholar] [CrossRef]
- Malhotra, S.; Tang, Y.; Varshney, P.K. Non-Enzymatic Glucose Sensor based on Electrodeposition of Platinum Particles on Polyaniline Modified Pt Electrode. Anal. Bioanal. Electrochem. 2018, 10, 699–715. [Google Scholar]
- Weremfo, A.; Fong, S.T.C.; Khan, A.; Hibbert, D.B.; Zhao, C. Electrochemically roughened nanoporous platinum electrodes for non-enzymatic glucose sensors. Electrochim. Acta 2017, 231, 20–26. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, X.; Lv, R.; Kong, D.; Li, Q.L. Electrodeposition of platinum on poly (glutamic acid) modified glassy carbon electrode for non-enzymatic amperometric glucose detection. Electrochim. Acta 2013, 107, 164–169. [Google Scholar] [CrossRef]
- Chang, H.-W.; Tsai, Y.-C.; Cheng, C.-W.; Lin, C.-Y.; Wu, P.-H. Preparation of platinum/carbon nanotube in aqueous solution by femtosecond laser for non-enzymatic glucose determination. Sens. Actuators B Chem. 2013, 183, 34–39. [Google Scholar] [CrossRef]
- Guo, M.Q.; Hong, H.S.; Tang, X.N.; Fang, H.D.; Xu, X.H. Ultrasonic electrodeposition of platinum nanoflowers and their application in nonenzymatic glucose sensors. Electrochim. Acta 2012, 63, 1–8. [Google Scholar] [CrossRef]
- Sun, Q.; Ding, J.; Chen, D.; Han, C.; Jiang, M.; Li, T.-T.; Hu, Y.; Qian, J.; Huang, S. Silica-Templated Metal Organic Framework-Derived Hierarchically Porous Cobalt Oxide in Nitrogen-Doped Carbon Nanomaterials for Electrochemical Glucose Sensing. ChemElectroChem 2021, 8, 812–818. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Feng, S.; He, D.; Jiang, P. Controlled synthesis of flower-like cobalt phosphate microsheet arrays supported on Ni foam as a highly efficient 3D integrated anode for non-enzymatic glucose sensing. Inorg. Chem. Front. 2020, 7, 108–116. [Google Scholar] [CrossRef]
- Chaiyo, S.; Mehmeti, E.; Siangproh, W.; Hoang, T.L.; Nguyen, H.P.; Chailapakul, O.; Kalcher, K. Non-enzymatic electrochemical detection of glucose with a disposable paper-based sensor using a cobalt phthalocyanine-ionic liquid-graphene composite. Biosens. Bioelectron. 2018, 102, 113–120. [Google Scholar] [CrossRef]
- Xie, F.; Cao, X.; Qu, F.; Asiri, A.M.; Sun, X. Cobalt nitride nanowire array as an efficient electrochemical sensor for glucose and H2O2 detection. Sens. Actuators B Chem. 2018, 255, 1254–1261. [Google Scholar] [CrossRef]
- Liu, T.; Li, M.; Guo, L. Designing and facilely synthesizing a series of cobalt nitride (Co4N) nanocatalysts as non-enzymatic glucose sensors: A comparative study toward the influences of material structures on electrocatalytic activities. Talanta 2018, 181, 154–164. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X.; Kong, R.; Du, G.; Asiri, A.M.; Lu, Q.; Sun, X. Cobalt phosphide nanowire array as an effective electrocatalyst for non-enzymatic glucose sensing. J. Mater. Chem. B 2017, 5, 1901–1904. [Google Scholar] [CrossRef] [PubMed]
- Premlatha, S.; Sivasakthi, P.; Bapu, G.N.K.R. Electrodeposition of a 3D hierarchical porous flower-like cobalt-MWCNT nanocomposite electrode for non-enzymatic glucose sensing. RSC Adv. 2015, 5, 74374–74380. [Google Scholar] [CrossRef]
- Ci, S.; Wen, Z.; Mao, S.; Hou, Y.; Cui, S.; He, Z.; Chen, J. One-pot synthesis of high-performance Co/graphene electrocatalysts for glucose fuel cells free of enzymes and precious metals. Chem. Commun. 2015, 51, 9354–9357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, L.; Zhao, H.; Bi, S.; Xu, Y.; Lu, Y. Ultrasensitive and highly selective sandpaper-supported copper framework for non-enzymatic glucose sensor. Electrochim. Acta 2017, 248, 281–291. [Google Scholar] [CrossRef]
- Ju, L.; Wu, G.; Lu, B.; Li, X.; Wu, H.; Liu, A. Non-enzymatic Amperometric Glucose Sensor Based on Copper Nanowires Decorated Reduced Graphene Oxide. Electroanalysis 2016, 28, 2543–2551. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.; Liu, Q.; Wang, K.; Qian, J.; Dong, X.; Yang, X.; Du, X.; Qiu, B. Enhanced non-enzymatic glucose sensing based on copper nanoparticles decorated nitrogen-doped graphene. Biosens. Bioelectron. 2014, 54, 273–278. [Google Scholar] [CrossRef]
- Huang, J.; Dong, Z.; Li, Y.; Li, J.; Wang, J.; Yang, H.; Li, S.; Guo, S.; Jin, J.; Li, R. High performance non-enzymatic glucose biosensor based on copper nanowires–carbon nanotubes hybrid for intracellular glucose study. Sens. Actuators B Chem. 2013, 182, 618–624. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, H.; Jiang, S.; Jiang, J.; Liu, X. Facile one-step electrochemical fabrication of a non-enzymatic glucose-selective glassy carbon electrode modified with copper nanoparticles and graphene. Microchim. Acta 2012, 177, 485–490. [Google Scholar] [CrossRef]
- Wu, H.; Yu, Y.; Gao, W.; Gao, A.; Qasim, A.M.; Zhang, F.; Wang, J.; Ding, K.; Wu, G.; Chu, P.K. Nickel plasma modification of graphene for high-performance non-enzymatic glucose sensing. Sens. Actuators B Chem. 2017, 251, 842–850. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.H.; Cho, M.; Lee, Y. Solvent-assisted morphology confinement of a nickel sulfide nanostructure and its application for non-enzymatic glucose sensor. Biosens. Bioelectron. 2016, 85, 587–595. [Google Scholar] [CrossRef]
- Kannan, P.K.; Rout, C.S. High Performance Non-enzymatic Glucose Sensor Based on One-Step Electrodeposited Nickel Sulfide. Chem. Eur. J. 2015, 21, 9355–9359. [Google Scholar] [CrossRef]
- Choi, T.; Kim, S.H.; Lee, C.W.; Kim, H.; Choi, S.-K.; Kim, S.-H.; Kim, E.; Park, J.; Kin, H. Synthesis of carbon nanotube-nickel nanocomposites using atomic layer deposition for high-performance non-enzymatic glucose sensing. Biosens. Bioelectron. 2015, 63, 325–330. [Google Scholar] [CrossRef]
- Huo, H.; Zhao, Y.; Xu, C. 3D Ni3S2 nanosheet arrays supported on Ni foam for high-performance supercapacitor and non-enzymatic glucose detection. J. Mater. Chem. A 2014, 2, 15111–15117. [Google Scholar] [CrossRef]
- Lin, T.-W.; Liu, C.-J.; Dai, C.-S. Ni3S2/carbon nanotube nanocomposite as electrode material for hydrogen evolution reaction in alkaline electrolyte and enzyme-free glucose detection. Appl. Catal. B 2014, 154–155, 213–220. [Google Scholar] [CrossRef]
- Niu, X.; Lan, M.; Zhao, H.; Chen, C. Highly Sensitive and Selective Nonenzymatic Detection of Glucose Using Three-Dimensional Porous Nickel Nanostructures. Anal. Chem. 2013, 85, 3561–3569. [Google Scholar] [CrossRef]
- Nie, H.G.; Yao, Z.; Zhou, X.M.; Yang, Z.; Huang, S.M. Nonenzymatic electrochemical detection of glucose using well-distributed nickel nanoparticles on straight multi-walled carbon nanotubes. Biosens. Bioelectron. 2011, 30, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Si, P.; Huang, Y.; Wang, T.; Ma, J. Nanomaterials for electrochemical non-enzymatic glucose biosensors. RSC Adv. 2013, 3, 3487–3502. [Google Scholar] [CrossRef]
- Song, Y.Y.; Zhang, D.; Gao, W.; Xia, X.-H. Nonenzymatic Glucose Detection by Using a Three-Dimensionally Ordered, Macroporous Platinum Template. Chem. Eur. J. 2005, 11, 2177–2182. [Google Scholar] [CrossRef]
- Chawla, M.; Pramanick, B.; Randhawa, J.K.; Siril, P.F. Effect of composition and calcination on the enzymeless glucose detection of Cu-Ag bimetallic nanocomposites. Mater. Today Commun. 2020, 26, 101815. [Google Scholar] [CrossRef]
- Nodehi, Z.; Rafati, A.A.; Ghaffarinejad, A. Palladium-silver polyaniline composite as an efficient catalyst for ethanol oxidation. Appl. Catal. A Gen. 2018, 554, 24–34. [Google Scholar] [CrossRef]
- Ma, D.; Tang, X.; Guo, M.; Lu, H.; Xu, X. Fabrication and characterization of non-enzymatic glucose sensor based on bimetallic hollow Ag/Pt nanoparticles prepared by galvanic replacement reaction. Ionics 2015, 21, 1417–1426. [Google Scholar] [CrossRef]
- Singh, B.; Laffir, F.; McCormac, T.; Dempsey, E. PtAu/C based bimetallic nanocomposites for non-enzymatic electrochemical glucose detection. Sens. Actuators B Chem. 2010, 150, 80–92. [Google Scholar] [CrossRef]
- Sheng, Q.; Mei, H.; Wu, H.; Zhang, X.; Wang, S. A highly sensitive non-enzymatic glucose sensor based on PtxCo1−x/C nanostructured composites. Sens. Actuators B Chem. 2015, 207, 51–58. [Google Scholar] [CrossRef]
- Bilal, S.; Ullah, W.; Shah, A.H.A. Polyaniline@CuNi nanocomposite: A highly selective, stable and efficient electrode material for binder free non-enzymatic glucose sensor. Electrochim. Acta 2018, 284, 382–391. [Google Scholar] [CrossRef]
- Chawla, M.; Randhawa, J.K.; Siril, P.F. Calcination temperature as a probe to tune the non-enzymatic glucose sensing activity of Cu–Ni bimetallic nanocomposites. New J. Chem. 2017, 41, 4582–4591. [Google Scholar] [CrossRef]
- Lakhdari, D.; Guittoum, A.; Benbrahim, N.; Belgherbi, O.; Berkani, M.; Vasseghian, Y.; Lakhdari, N. A novel non-enzymatic glucose sensor based on NiFe (NPs)-polyaniline hybrid materials. Food Chem. Toxicol. 2021, 151, 112099. [Google Scholar] [CrossRef]
- Mahshid, S.S.; Mahshid, S.; Dolati, A.; Ghorbani, M.; Yang, L.; Luo, S.; Cai, Q. Template-based electrodeposition of Pt/Ni nanowires and its catalytic activity towards glucose oxidation. Electrochim. Acta 2011, 58, 551–555. [Google Scholar] [CrossRef]
- Ma, L.; Wang, X.; Zhang, Q.; Tong, X.; Zhang, Y.; Li, Z. Pt catalyzed formation of a Ni@Pt/reduced graphene oxide nanocomposite: Preparation and electrochemical sensing application for glucose detection. Anal. Methods 2018, 10, 3845–3850. [Google Scholar] [CrossRef]
- Chen, X.; Tian, X.; Zhao, L.; Huang, Z.; Oyama, M. Nonenzymatic sensing of glucose at neutral pH values using a glassy carbon electrode modified with graphene nanosheets and Pt-Pd bimetallic nanocubes. Microchim. Acta 2014, 181, 783–789. [Google Scholar] [CrossRef]
- Yang, J.; Liang, X.; Cui, L.; Liu, H.; Xie, J.; Liu, W. A novel non-enzymatic glucose sensor based on Pt3Ru1 alloy nanoparticles with high density of surface defects. Biosens. Bioelectron. 2016, 80, 171–174. [Google Scholar] [CrossRef]
- Lin, L.; Weng, S.; Zheng, Y.; Liu, X.; Ying, S.; Chen, F.; You, D. Bimetallic PtAu alloy nanomaterials for nonenzymatic selective glucose sensing at low potential. J. Electroanal. Chem. 2020, 865, 114147. [Google Scholar] [CrossRef]
- Park, S.; Chung, T.D.; Kim, H.C. Nonenzymatic glucose detection using mesoporous platinum. Anal. Chem. 2003, 75, 3046–3049. [Google Scholar] [CrossRef]
- Beden, B.; Largeaud, F.; Kokoh, K.B.; Lamy, C. Fourier transform infrared reflectance spectroscopic investigation of the electrocatalytic oxidation of d-glucose: Identification of reactive intermediates and reaction products. Electrochim. Acta 1996, 41, 701–709. [Google Scholar] [CrossRef]
- Nantaphol, S.; Watanabe, T.; Nomura, N.; Siangproh, W.; Chailapakul, O.; Einaga, Y. Bimetallic Pt-Au nanocatalysts electrochemically deposited on borondoped diamond electrodes for nonenzymatic glucose detection. Biosens. Bioelectron. 2017, 98, 76–82. [Google Scholar] [CrossRef]
- Shim, K.; Lee, W.C.; Park, M.S.; Shahabuddin, M.; Yamauchi, Y.; Hossain, M.S.A.; Shim, Y.B.; Kim, J.H. Au decorated core-shell structured Au@Pt for the glucose oxidation reaction. Sens. Actuators B Chem. 2019, 278, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Shim, K.; Lee, W.C.; Heo, Y.U.; Shahabuddin, M.; Park, M.S.; Hossain, M.S.A.; Kim, J.H. Rationally designed bimetallic Au@Pt nanoparticles for glucose oxidation. Sci. Rep. 2019, 9, 894. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wang, L.; Lu, L.; Toshima, N. Preparation and catalytic activity for aerobic glucose oxidation of crown jewel structured Pt/Au bimetallic nanoclusters. Sci. Rep. 2016, 6, 30752. [Google Scholar] [CrossRef]
- Tee, S.Y.; Teng, C.P.; Ye, E. Metal nanostructures for non-enzymatic glucose sensing. Mater. Sci. Eng. C 2017, 70, 1018–1030. [Google Scholar] [CrossRef]
- Li, Z.; Qian, W.; Guo, H.; Long, X.; Tang, Y.; Zheng, J. Electrostatic Self-Assembled Bracelet-Like Au@Pt Nanoparticles: An Efficient Electrocatalyst for Highly Sensitive Non-Enzymatic Hydrogen Peroxide Sensing. ChemElectroChem 2020, 7, 1581–1589. [Google Scholar] [CrossRef]
- Habrioux, A.; Sibert, E.; Servat, K.; Vogel, W.; Kokoh, K.B.; Alonso-Vante, N. Activity of platinum−gold alloys for glucose electrooxidation in biofuel cells. J. Phys. Chem. B 2007, 111, 10329–10333. [Google Scholar] [CrossRef]
- Grochowska, K.; Ryl, J.; Karczewski, J.; Śliwiński, G.; Cenian, A.; Siuzdak, K. Non-enzymatic flexible glucose sensing platform based on nanostructured TiO2-Au composite. J. Electroanal. Chem. 2019, 837, 230–239. [Google Scholar] [CrossRef]
- Parsons, R.; VanderNoot, T. The oxidation of small organic molecules: A survey of recent fuel cell related research. J. Electroanal. Chem. Interfacial Electrochem. 1988, 257, 9–45. [Google Scholar] [CrossRef]
- Khawaji, M.; Zhang, Y.; Loh, M.; Graça, I.; Ware, E.; Chadwick, D. Composition dependent selectivity of bimetallic Au-Pd NPs immobilised on titanate nanotubes in catalytic oxidation of glucose. Appl. Catal. B Environ. 2019, 256, 117799. [Google Scholar] [CrossRef]
- Zhang, H.; Toshima, N. Synthesis of Au/Pt bimetallic nanoparticles with a Pt-rich shell and their high catalytic activities for aerobic glucose oxidation. J. Colloid. Interface Sci. 2013, 394, 166–176. [Google Scholar] [CrossRef]
- Dai, L.; Zhao, Y.; Chi, Q.; Liu, H.; Li, J.; Huang, T. Morphological control and evolution of octahedral and truncated trisoctahedral Pt-Au alloy nanocrystals under microwave irradiation. Nanoscale 2014, 6, 9944–9950. [Google Scholar] [CrossRef]
- Fan, Z.; Zhu, Y.; Huang, X.; Han, Y.; Wang, Q.; Liu, Q.; Huang, Y.; Gan, C.L.; Zhang, H. Synthesis of Ultrathin Face-Centered-Cubic Au@Pt and Au@Pd Core–Shell Nanoplates from Hexagonal-Close-Packed Au Square Sheets. Angew. Chem. 2015, 127, 5764–5768. [Google Scholar] [CrossRef]
- Da Silva, S.G.; Silva, J.C.M.; Buzzo, G.S.; De Souza, R.F.B.; Spinacé, E.V.; Neto, A.O.; Assumpção, M.H.M.T. Electrochemical and fuel cell evaluation of PtAu/C electrocatalysts for ethanol electro-oxidation in alkaline media. Int. J. Hydrogen Energy 2014, 39, 10121–10127. [Google Scholar] [CrossRef]
- Zhang, J.; Sasaki, K.; Sutter, E.; Adzic, R.R. Stabilization of platinum oxygen-reduction electrocatalysts using gold clusters. Science 2007, 315, 220–222. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.C.; Chen, Z.D. Electrocatalytic oxidation of glucose on gold–platinum nanocomposite electrodes and platinum-modified gold electrodes. Synth. Met. 2007, 157, 592–596. [Google Scholar] [CrossRef]
- Moller, H.; Pistorius, P.C. The electrochemistry of gold–platinum alloys. J. Electroanal. Chem. 2004, 570, 243–255. [Google Scholar] [CrossRef]
- Wu, Y.-S.; Wu, Z.-W.; Lee, C.-L. Concave Pd core/island Pt shell nanoparticles: Synthesis and their promising activities toward neutral glucose oxidation. Sens. Actuators B Chem. 2019, 281, 1–7. [Google Scholar] [CrossRef]
- Coyle, V.E.; Oppedisano, D.K.J.; Jones, L.A.; Kandjani, A.E.; Sabri, Y.M.; Bhargava, S.K. Hydrogen Bubble Templated Growth of Honeycomb-Like Au-Pt Alloy Films for Non-Enzymatic Glucose Sensing. J. Electrochem. Soc. 2016, 163, B689. [Google Scholar] [CrossRef]
- Wang, C.; Sun, Y.; Yu, X.; Ma, D.; Zheng, J.; Dou, P.; Cao, Z.; Xu, X. Ag–Pt hollow nanoparticles anchored reduced graphene oxide composites for non-enzymatic glucose biosensor. J. Mater. Sci. Mater. Electron. 2016, 27, 9370–9378. [Google Scholar] [CrossRef]
- Ye, J.-S.; Hong, B.-D.; Wu, Y.-S.; Chen, H.-R.; Lee, C.-L. Heterostructured palladium-platinum core-shell nanocubes for use in a nonenzymatic amperometric glucose sensor. Microchim. Acta 2016, 183, 3311–3320. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, C.; Li, H.; Dua, D.; Lin, Y. A nonenzymatic electrochemical glucose sensor based on mesoporous Au/Pt nanodendrites. RSC Adv. 2015, 5, 82617–82622. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, G.; Cai, Z.; Zhao, T.; Yao, Q.; Chen, X. Ultrasensitive non-enzymatic glucose sensing at near-neutral pH values via anodic stripping voltammetry using a glassy carbon electrode modified with Pt3Pd nanoparticles and reduced graphene oxide. Microchim. Acta 2015, 182, 2055–2060. [Google Scholar] [CrossRef]
- Li, Y.; Niu, X.; Tang, J.; Lan, M.; Zhao, H. A Comparative Study of Nonenzymatic Electrochemical Glucose Sensors Based on Pt-Pd Nanotube and Nanowire Arrays. Electrochim. Acta 2014, 130, 1–8. [Google Scholar] [CrossRef]
- Waqas, M.; Lan, J.; Zhang, X.; Fan, Y.; Zhang, P.; Liu, C.; Jiang, Z.; Wang, X.; Zeng, J.; Chen, W. Fabrication of Non-enzymatic Electrochemical Glucose Sensor Based on Pd−Mn Alloy Nanoparticles Supported on Reduced Graphene Oxide. Electroanalysis 2020, 32, 1226–1236. [Google Scholar] [CrossRef]
- Şavk, A.; Aydın, H.; Cellat, K.; Şen, F. A novel high performance non-enzymatic electrochemical glucose biosensor based on activated carbon-supported Pt-Ni nanocomposite. J. Mol. Liq. 2020, 300, 112355. [Google Scholar] [CrossRef]
- Şavk, A.; Cellat, K.; Arıkan, K.; Tezcan, F.; Gülbay, S.K.; Kızıldağ, S.; Işgın, E.S.; Şen, F. Highly monodisperse Pd-Ni nanoparticles supported on rGO as a rapid, sensitive, reusable and selective enzyme-free glucose sensor. Sci. Rep. 2019, 9, 19228. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Liang, X.; Liu, H.; Cui, L.; Zhang, X.; Liu, C. Non-enzymatic electrochemical glucose sensor based on monodispersed stone-like PtNi alloy nanoparticles. Microchim. Acta 2018, 185, 339. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; He, L.; Zhao, J.; Zhao, B.; Yin, Y.; Yang, Y. Synthesis of Ni/Au multilayer nanowire arrays for ultrasensitive non-enzymatic sensing of glucose. Sens. Actuators B Chem. 2017, 240, 779–784. [Google Scholar] [CrossRef]
- Mei, H.; Wu, W.; Yu, B.; Li, Y.; Wu, H.; Wang, S.; Xia, Q. Non-enzymatic sensing of glucose at neutral pH values using a glassy carbon electrode modified with carbon supported Co@Pt core-shell nanoparticles. Microchim. Acta 2015, 182, 1869–1875. [Google Scholar] [CrossRef]
- Xue, Z.; Jia, L.; Zhu, R.-R.; Du, L.; Zhao, Q.-H. High-performance non-enzymatic glucose electrochemical sensor constructed by transition nickel modified Ni@Cu-MOF. J. Electroanal. Chem. 2020, 858, 113783. [Google Scholar] [CrossRef]
- Cui, D.; Su, L.; Li, H.; Li, M.; Li, C.; Xu, S.; Qian, L.; Yang, B. Non-enzymatic glucose sensor based on micro-/nanostructured Cu/Ni deposited on graphene sheets. J. Electroanal. Chem. 2019, 838, 154–162. [Google Scholar] [CrossRef]
- Li, W.; Lv, S.; Wang, Y.; Zhang, L.; Cui, X. Nanoporous gold induced vertically standing 2D NiCo bimetal-organic framework nanosheets for non-enzymatic glucose biosensing. Sens. Actuators B Chem. 2019, 281, 652–658. [Google Scholar] [CrossRef]
- Pötzelberger, I.; Mardare, A.I.; Hassel, A.W. Non-enzymatic glucose sensing on copper-nickel thin film alloy. Appl. Surf. Sci. 2017, 417, 48–53. [Google Scholar] [CrossRef]
- Jonke, A.P.; Josowicz, M.; Janata, J. Polyaniline Electrodes Containing Tri-Atomic Au/Pd Clusters: Effect of Ordering. Catal. Lett. 2013, 143, 1261–1265. [Google Scholar] [CrossRef]
- Jonke, A.P.; Steeb, J.L.; Josowicz, M.; Janata, J. Atomic Clusters of Pd and AuNPdM in Polyaniline. Catal. Lett. 2013, 143, 531–538. [Google Scholar] [CrossRef]
- Schwartz, I.T.; Jonke, A.P.; Josowicz, M.; Janata, J. Polyaniline Electrodes with Atomic AunPd1 Alloys: Oxidation of Methanol and Ethanol. Catal. Lett. 2013, 143, 636–641. [Google Scholar] [CrossRef]
- Chakraborty, P.; Chien, Y.-A.; Chiu, W.-T.; Chang, T.-F.M.; Sone, M.; Nakamoto, T.; Josowicz, M.; Janata, J. Design and development of amperometric gas sensor with atomic Au–Polyaniline/Pt composite. IEEE Sens. J. 2020, 20, 12479–12487. [Google Scholar] [CrossRef]
- Schwartz, I.T.; Jonke, A.P.; Josowicz, M.; Janata, J. Effect of Structured Atomic Gold on Electrooxidation of Alcohols in Alkaline Medium. Catal. Lett. 2013, 143, 777–782. [Google Scholar] [CrossRef]
- Jonke, A.P.; Josowicz, M.; Janata, J. Odd-Even Pattern Observed in Polyaniline/(Au0–Au8) Composites. J. Electrochem. Soc. 2012, 159, P40–P43. [Google Scholar] [CrossRef]
- Jonke, A.P.; Josowicz, M.; Janata, J. Polyaniline Doped with Atomic Gold. J. Electrochem. Soc. 2011, 158, E147–E151. [Google Scholar] [CrossRef]
- Tian, L.; He, G.; Cai, Y.; Wu, S.; Su, Y.; Yan, H.; Yang, C.; Chen, Y.; Li, L. Co3O4 based non-enzymatic glucose sensor with high sensitivity and reliable stability derived from hollow hierarchical architecture. Nanotechnology 2018, 29, 075502. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Dinesh, B.; Rethinasabapathy, M.; Hwang, S.-K.; Jin, C.-S.; Huh, Y.S.; Han, Y.-K. Hexagonal Co3O4 anchored reduced graphene oxide sheets for high-performance supercapacitors and non-enzymatic glucose sensing. J. Mater. Chem. A 2018, 6, 14367–14379. [Google Scholar] [CrossRef]
- Yang, M.H.; Jeong, J.-M.; Lee, K.G.; Kim, D.H.; Lee, S.J.; Choi, B.G. Hierarchical porous microspheres of the Co3O4@graphene with enhanced electrocatalytic performance for electrochemical biosensors. Biosens. Bioelectron. 2017, 89, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Zhao, M.; Ding, L.; Liang, J.; Chen, J.; Li, Y.; Chen, S. Synthesis of 3D hierarchical porous Co3O4 film by eggshell membrane for non-enzymatic glucose detection. J. Electroanal. Chem. 2016, 775, 52–57. [Google Scholar] [CrossRef]
- Zhang, E.; Xie, Y.; Ci, S.; Jia, J.; Wen, Z. Porous Co3O4 hollow nanododecahedra for nonenzymatic glucose biosensor and biofuel cell. Biosens. Bioelectron. 2016, 81, 46–53. [Google Scholar] [CrossRef]
- Hoa, L.T.; Chung, J.S.; Hur, S.H. A highly sensitive enzyme-free glucose sensor based on Co3O4 nanoflowers and 3D graphene oxide hydrogel fabricated via hydrothermal synthesis. Sens. Actuators B Chem. 2016, 223, 76–82. [Google Scholar] [CrossRef]
- Balouch, Q.; Ibupoto, Z.H.; Khaskheli, G.Q.; Soomro, R.A.; Sirajuddin; Samoon, M.K.; Deewani, V.K. Cobalt oxide nanoflowers for electrochemical determination of glucose. J. Electron. Mater. 2015, 44, 3724–3732. [Google Scholar] [CrossRef]
- Li, M.; Han, C.; Zhang, Y.; Bo, X.; Guo, L. Facile synthesis of ultrafine Co3O4 nanocrystals embedded carbon matrices with specific skeletal structures as efficient non-enzymatic glucose sensors. Anal. Chim. Acta 2015, 861, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Khun, K.; Ibupoto, Z.H.; Liu, X.; Beni, V.; Willander, M. The ethylene glycol template assisted hydrothermal synthesis of Co3O4 nanowires; structural characterization and their application as glucose non-enzymatic sensor. Mater. Sci. Eng. B 2015, 194, 94–100. [Google Scholar] [CrossRef]
- Yang, P.; Wang, X.; Ge, C.-Y.; Fu, X.; Liu, X.Y.; Chai, H.; Guo, X.; Yao, H.-C.; Zhang, Y.X.; Chen, K. Fabrication of CuO nanosheets-built microtubes via Kirkendall effect for non-enzymatic glucose sensor. Appl. Surf. Sci. 2019, 494, 484–491. [Google Scholar] [CrossRef]
- Jagadeesan, M.S.; Movlaee, K.; Krishnakumar, T.; Leonardi, S.G.; Neri, G. One-step microwave-assisted synthesis and characterization of novel CuO nanodisks for non-enzymatic glucose sensing. J. Electroanal. Chem. 2019, 835, 161–168. [Google Scholar] [CrossRef]
- Wang, X.; Ge, C.-Y.; Chen, K.; Zhang, Y.X. An ultrasensitive non-enzymatic glucose sensors based on controlled petal-like CuO nanostructure. Electrochim. Acta 2018, 259, 225–232. [Google Scholar] [CrossRef]
- Velmurugan, M.; Karikalan, N.; Chen, S.-M. Synthesis and characterizations of biscuit-like copper oxide for the non-enzymatic glucose sensor applications. J. Colloid Interface Sci. 2017, 493, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Su, L.; Zhang, Z.; Huo, D.; Hou, C.; Lei, Y. CuO nanowires based sensitive and selective non-enzymatic glucose detection. Sens. Actuators B Chem. 2014, 191, 86–93. [Google Scholar] [CrossRef]
- Lu, N.; Shao, C.; Li, X.; Shen, T.; Zhang, M.; Miao, F.; Zhang, P.; Zhang, X.; Wang, K.; Zhang, Y.; et al. CuO/Cu2O nanofibers as electrode materials for non-enzymatic glucose sensors with improved sensitivity. RSC Adv. 2014, 4, 31056–31061. [Google Scholar] [CrossRef]
- Ibupoto, Z.H.; Khun, K.; Beni, V.; Liu, X.; Willander, M. Synthesis of Novel CuO Nanosheets and Their Non-Enzymatic Glucose Sensing Applications. Sensors 2013, 13, 7926–7938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, Y.; Xu, J.; Liang, Q.; Shao, S. Enzyme-free glucose sensor based on heteroatom-enriched activated carbon (HAC) decorated with hedgehog-like NiO nanostructures. Sens. Actuators B Chem. 2017, 250, 491–498. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, T.; Jiang, L.; Zhang, K.; Yuen, M.M.F.; Xu, J.B.; Fu, X.Z.; Sun, R.; Wong, C.P. NiO mesoporous nanowalls grown on RGO coated nickel foam as high performance electrodes for supercapacitors and biosensors. Electrochim. Acta 2016, 192, 205–215. [Google Scholar] [CrossRef]
- Soomro, R.A.; Ibupoto, Z.H.; Sirajuddin; Abro, M.I.; Willander, M. Controlled synthesis and electrochemical application of skein-shaped NiO nanostructures. J. Solid State Electrochem. 2015, 19, 913–922. [Google Scholar] [CrossRef]
- Ibupoto, Z.H.; Nafady, A.; Soomro, R.A.; Sirajuddin; Sherazi, S.T.H.; Abroe, M.I.; Willander, M. Glycine-assisted synthesis of NiO hollow cage-like nanostructures for sensitive non-enzymatic glucose sensing. RSC Adv. 2015, 5, 18773–18781. [Google Scholar] [CrossRef]
- Guo, C.; Wang, Y.; Zhao, Y.; Xu, C. Non-enzymatic glucose sensor based on three dimensional nickel oxide for enhanced sensitivity. Anal. Methods 2013, 5, 1644–1647. [Google Scholar] [CrossRef]
- Dung, N.Q.; Patil, D.; Jung, H.; Kim, J.; Kim, D. NiO-decorated single-walled carbon nanotubes for high-performance nonenzymatic glucose sensing. Sens. Actuators B 2013, 183, 381–387. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Wang, Y.Z.; Jia, J.B.; Wang, J.G. Nonenzymatic glucose sensor based on graphene oxide and electrospun NiO nanofibers. Sens. Actuators B 2012, 171–172, 580–587. [Google Scholar] [CrossRef]
- Zeid, E.F.A.; Nassar, A.M.; Hussein, M.A.; Alam, M.M.; Asiri, A.M.; Hegazy, H.H.; Rahman, M.M. Mixed oxides CuO-NiO fabricated for selective detection of 2-Aminophenol by electrochemical approach. J. Mater. Res. Technol. 2020, 9, 1457–1467. [Google Scholar] [CrossRef]
- Guo, Q.; Zeng, W.; Liu, S.; Li, Y. In situ formation of Co3O4 hollow nanocubes on carbon cloth-supported NiCo2O4 nanowires and their enhanced performance in non-enzymatic glucose sensing. Nanotechnology 2020, 31, 265501. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Luo, X.; He, D.; Jiang, P. Hierarchical Co (OH)2 nanotube arrays grown on carbon cloth for use in non-enzymatic glucose sensing. Anal. Methods 2017, 9, 5903–5909. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, C.; Zhao, G.; Mu, J.; Wang, Y. Self-supported porous CoOOH nanosheet arrays as a non-enzymatic glucose sensor with good reproducibility. Sens. Actuators B Chem. 2015, 210, 190–196. [Google Scholar] [CrossRef]
- Zhou, S.; Feng, X.; Shi, H.; Chen, J.; Zhang, F.; Song, W. Direct growth of vertically aligned arrays of Cu (OH)2 nanotubes for the electrochemical sensing of glucose. Sens. Actuators B Chem. 2013, 177, 445–452. [Google Scholar] [CrossRef]
- Sim, H.; Kim, J.-H.; Lee, S.-K.; Song, M.-J.; Yoon, D.-H.; Lim, D.-S.; Hong, S.-I. High-sensitivity non-enzymatic glucose biosensor based on Cu (OH)2 nanoflower electrode covered with boron-doped nanocrystalline diamond layer. Thin Solid Films 2012, 520, 7219–7223. [Google Scholar] [CrossRef]
- Mao, W.; He, H.; Ye, X.; Huang, J. Three-dimensional graphene foam integrated with Ni (OH)2 nanosheets as a hierarchical structure for non-enzymatic glucose sensing. J. Electroanal. Chem. 2019, 832, 275–283. [Google Scholar] [CrossRef]
- Zhao, Y.; Gu, G.; You, S.; Ji, R.; Suo, H.; Zhao, C.; Liu, F. Preparation of Ni (OH)2 nanosheets on Ni foam via a direct precipitation method for a highly sensitive non-enzymatic glucose sensor. RSC Adv. 2015, 5, 53665–53670. [Google Scholar] [CrossRef]
- Yang, J.; Cho, M.; Pang, C.; Lee, Y. Highly sensitive non-enzymatic glucose sensor based on over-oxidized polypyrrole nanowires modified with Ni (OH)2 nanoflakes. Sens. Actuators B Chem. 2015, 211, 93–101. [Google Scholar] [CrossRef]
- Kiani, M.A.; Tehrani, M.A.; Sayahi, H. Reusable and robust high sensitive non-enzymatic glucose sensor based on Ni (OH)2 nanoparticles. Anal. Chim. Acta 2014, 839, 26–33. [Google Scholar] [CrossRef]
- Pal, N.; Banerjee, S.; Bhaumik, A. A facile route for the syntheses of Ni (OH)2 and NiO nanostructures as potential candidates for non-enzymatic glucose sensor. J. Colloid Interface Sci. 2018, 516, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Zhang, X.; Peng, X.; Wu, H.; Bai, L.; Jin, W.; Wu, G.; Hang, R.; Chu, P.K. In situ synthesis of Ni (OH)2/TiO2 composite film on NiTi alloy for non-enzymatic glucose sensing. Sens. Actuators B Chem. 2016, 232, 150–157. [Google Scholar] [CrossRef]
- Sun, S.; Shi, N.; Liao, X.; Zhang, B.; Yin, G.; Huang, Z.; Chen, X.; Pu, X. Facile synthesis of CuO/Ni (OH)2 on carbon cloth for non-enzymatic glucose sensing. Appl. Surf. Sci. 2020, 529, 147067. [Google Scholar] [CrossRef]
- Mahmoudian, M.R.; Basirun, W.J.; Woi, P.M.; Sookhakian, M.; Yousefi, R.; Ghadimi, H.; Alias, Y. Synthesis and characterization of Co3O4 ultra-nanosheets and Co3O4 ultra-nanosheet-Ni (OH)2 as non-enzymatic electrochemical sensors for glucose detection. Mater. Sci. Eng. C 2016, 59, 500–508. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, X.; Zhao, Q.; Wang, G. Preparation and characterization of super-hydrophilic porous TiO2 coating films. Mater. Chem. Phys. 2001, 68, 253–259. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Lee, H.Y.; Park, Y.H.; Ko, K.H. Correlation between Surface Morphology and Hydrophilic/Hydrophobic Conversion of MOCVD−TiO2 Films. Langmuir 2000, 16, 7289–7293. [Google Scholar] [CrossRef]
- Sakai, N.; Wang, R.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Effect of Ultrasonic Treatment on Highly Hydrophilic TiO2 Surfaces. Langmuir 1998, 14, 5918–5920. [Google Scholar] [CrossRef]
- Chiu, W.-T.; Chen, C.-Y.; Chang, T.-F.M.; Tahara, Y.; Hashimoto, T.; Kurosu, H.; Sone, M. Fabrication and Photocatalytic Performance of Au/ZnO Layered Structure on Silk Textile for Flexible Device Applications. Electrochim. Acta 2017, 253, 39–46. [Google Scholar] [CrossRef]





| Electrodes | Chemical Reactions and Mechanisms |
|---|---|
| (a) Au [162,163] | • Formation of electroactive intermediate |
| • Electro-oxidation of glucose | |
| (b) Pd [34,171] | • Formation of electroactive intermediate: step 1 (Pd(0)) → Pd(I)) • Electrooxidation reaction of Pd: step 2 (Pd(I)) → Pd(II)) |
| • Electro-oxidation of glucose | |
| (c) Pt [164] | • Electro-oxidation reaction of Pt: step 1 (Pt(0) → Pt(II)) • Formation of electroactive intermediate: step 2 (Pt(II) → Pt(IV)) |
| • Electro-oxidation of glucose | |
| (d) Co [110,165,166] | • Electro-oxidation reaction of Co: step 1 (Co(0) → Co(II)) • Formation of electroactive intermediate: step 2 (Co(II) → Co(III)) • Formation of electroactive intermediate: step 3 (Co(III) → Co(IV)) |
| • Electro-oxidation of glucose or | |
| (e) Cu [167,168] | • Electro-oxidation reaction of Cu: step 1 (Cu(0) → Cu(II)) or • Formation of electroactive intermediates: step 2 (Cu(II) → Cu(III)) or |
| • Electro-oxidation of glucose | |
| (f) Ni [169,170] | • Electro-oxidation reaction of Ni: step 1 (Ni(0) → Ni(II)) or • Formation of electroactive intermediates: step 2 (Ni(II) → Ni(III)) or |
| • Electro-oxidation of glucose | |
| (g) General | • Formation of electroactive intermediates and/or |
| • Electro-oxidation of glucose where M = Mono-metal LOS = Low oxidation state MOS = Medium oxidation state HOS = High oxidation state Products = Glucose derivatives |
| Electrode [Electrolyte] | Sensitivity (μA mM−1 cm−2) | Linear Range (mM) | LOD (μM) | Working Potential (V) * | Year [Reference] |
|---|---|---|---|---|---|
| (a) Mono-metallic Au electrodes | |||||
| Dendrite Au/paper fiber [0.1 M NaOH] | 30.0 | 0.01–15 | 0.6 | +0.60 | 2020 [172] |
| Au NP/N-doped GCNTs/GCE [0.1 M NaOH] | 0.98 | 0.002–19.6 | 0.5 | +0.20 | 2018 [173] |
| Mesoporous Au/Au-Si electrode [0.1 M NaOH] | 291.6 | 0.0–10 | 4.13 | +0.20 | 2017 [174] |
| Au NPs-MWCNTs/AuE [0.05 M NaOH] | 27.7 | 0.001–1.0 | 0.5 | +0.20 | 2017 [116] |
| Au nanocages/GCE [0.2 M NaOH] | 2131 | 1–9 | 100 | -- | 2016 [175] |
| Pine-like nano-Au/AuE [0.1 M NaOH] | 776.9 | 0.02–0.24 | 3.39 | +0.07 | 2016 [176] |
| Au NPs/GCE [1 M NaOH] | 87.5 | 0.1–25 | 50 | +0.24 | 2014 [177] |
| Au NPs-GO nanoribbons/Carbon sheet [0.1 M PBS] | 59.1 31.4 | 0.005–4.92 4.92–10 | 5 | +0.20 | 2014 [178] |
| Nano Au-CNTs/GCE [0.01 M PBS] | 25 | 0–50 | 100 | +0.39 | 2014 [179] |
| Au MPA/Si [0.01 M PBS] | 13.2 | 0.5–9 | 60 | -- | 2012 [162] |
| (b) Mono-metallic Pd electrodes | |||||
| PBTh-Pd particles/ITO [0.1 M NaOH] | 5620 | 0.04–0.4 | 7 | +0.60 | 2020 [180] |
| Pd NPs-GN-MWCNTs/GCE [0.1 M NaOH] | 83.0 52.9 | 0.025–10 10–100 | 8 | −0.1 | 2019 [181] |
| GO-PAA-Pd NPs/SPCE [0.1 M NaOH] | 75 37 | 0.05–15 15–60 | 22 | −0.1 | 2019 [171] |
| Pd NPs-CSP/GCE [0.1 M NaOH] | 17.7 | 1–8 | 237 | −0.05 | 2018 [182] |
| Pd NPs-halloysite nanotubes/GCE [0.1 M NaOH] | 362.9 86.3 | 0.0005–2 2–15 | 0.43 | +0.45 | 2016 [183] |
| Porous Pd NTs/GCE [0.1 M NaOH] | 6.58 | 0.005–10 | 1 | +0.45 | 2015 [184] |
| Pd NPs-IFMC/GCE [0.15 M NaOH] | -- | 1–55 | 200 | +0.40 | 2015 [185] |
| Pd nanocubes/GCE [0.1 M NaOH] | 34 | 1–10 | -- | −0.05 | 2015 [34] |
| Pd NPs-MWCNTs/GCE [0.1 M NaOH] | 1275 | 1–22 | 0.2 | −0.4 | 2014 [186] |
| Pd NPs-MWCNTs/GCE [1 M NaOH] | 11 | 1–10 | -- | +0.025 | 2013 [187] |
| (c) Mono-metallic Pt electrodes | |||||
| GFs Pt/GCE [0.1 M NaOH] | 46,060.9 205.2 | 0.0001–0.01 0.01–20 | 0.03 | −0.15 | 2019 [164] |
| Pt CNs/FTO [0.1 M NaOH] | 20.75 | 0.33–12.5 | 0.7 | +0.2 | 2018 [188] |
| Pt particles-PANI/PtE [0.1 M NaOH] | 215.8 | 0.1–12 | 10 | +0.02 | 2018 [189] |
| Nanoporous Pt/PtE [0.5 M H2SO4] | 5.67 | 1–10 | 800 | +0.4 | 2017 [190] |
| Pt NPs-GOH/GCE [0.1 M NaOH] | 137.4 | 5–20 | -- | +0.1 | 2015 [131] |
| Pt nanoclusters-graphene/GCE [0.1 M PBS] | 1.21 | 1–25 | 30 | +0.05 | 2015 [111] |
| Pt NFs-GO/GCE [0.05 M PBS] | 1.26 0.64 | 0.002–10.3 10.3–20.3 | 2 | +0.52 | 2013 [112] |
| Pt-PGA/GCE [0.2 M PBS] | 0.88 | 0.05–5.95 | 11 | +0.35 | 2013 [191] |
| Pt-MWCNTs/GCE [0.1 M NaOH] | 106 | Up to 2.4 | -- | −0.25 | 2013 [192] |
| Pt NFs-MWCNT/AuE [0.2 M PBS] | 1.87 | 1–16 | 48 | -- | 2012 [193] |
| Electrode [Electrolyte] | Sensitivity (μA mM−1 cm−2) | Linear Range (mM) | LOD (μM) | Working Potential (V) * | Year [Reference] |
|---|---|---|---|---|---|
| (a) Mono-metallic Co electrodes | |||||
| Co-MOF/GCE [0.1 M NaOH] | 2860 | 1.0–1300.0 | 0.19 | -- | 2021 [194] |
| Co-PO-MA/NF [0.1 M NaOH] | 3550 | 0.001–1.16 | 1 | +0.55 | 2020 [195] |
| CoPc-graphene-IL/SPCE [0.1 M NaOH] | -- | 0.01–1.3 | 0.67 | -- | 2018 [196] |
| Co3N NA/TM [0.1 M NaOH] | 3325.6 | 0.0001–2.5 | 0.05 | +0.54 | 2018 [197] |
| Co-phosphate nanostructures/GCE [0.1 M PBS] | 7900 | 1–30 | 3 × 10−4 | +0.65 | 2018 [166] |
| Co4N-NSs/GCE [0.1 M NaOH] | 1137.2 | 0.6–10 | 0.1 | +0.55 | 2018 [198] |
| Co-phosphide NA/TM [0.1 M NaOH] | 5168.6 | 5 × 10−4–1.5 | 0.1 | +0.54 | 2017 [199] |
| Co-MWCNTs nanocomposite/MSPs [0.1 M NaOH] | 727.4 | 0.005–0.1 0.2–3.6 | 0.009 0.3 | +0.43 | 2015 [200] |
| Co NPs/graphene [0.1 M NaOH] | 4700 | 0.00167–0.47 | 0.05 | +0.54 | 2015 [201] |
| Co NPs/ITO [1 M NaOH] | 1720 | 0.005–0.18 | 0.25 | +0.59 | 2014 [110] |
| (b) Mono-metallic Cu electrodes | |||||
| Cu NPs-LIG/PIFs [0.1 M NaOH] | 495 | 0.001–6 | 0.39 | +0.50 | 2020 [125] |
| Cu-MOF/GCE [0.01 M NaOH] | 89 | 0.01–3.5 | 0.0105 | +0.55 | 2018 [167] |
| Cu NPs-N-graphene/GCE [0.1 M NaOH] | 4846.9 | 1 × 10−5–0.1 | 0.01 | +0.60 | 2017 [128] |
| Cu framework/Sandpaper [0.1 M NaOH] | 2165.5 | 0.001–4.6 | 0.03 | +0.45 | 2017 [202] |
| Cu NWs/rGO [0.1 M NaOH] | 1625 | Up to 11 | 0.2 | +0.58 | 2016 [203] |
| Cu-PSi/CPE [0.1 M NaOH] | -- | 0.001–0.19 0.19–2.3 | 0.2 | +0.55 | 2014 [143] |
| Cu-N-graphene/GCE [0.1 M NaOH] | 48.1 | 0.004–4.5 | 1.3 | +0.55 | 2014 [204] |
| Cu NWs-MWCNTs/GCE [0.1 M NaOH] | 1995 | Up to 3 | 0.26 | +0.55 | 2013 [205] |
| Cu NWs-Nafion/GCE [0.05 M NaOH] | 420.3 | Up to 3 | 0.035 | +0.60 | 2012 [139] |
| Cu NPs-graphene/GCE [0.1 M NaOH] | 607 | 0.005–1.4 | 0.2 | +0.55 | 2012 [206] |
| (c) Mono-metallic Ni electrodes | |||||
| Ni-C/SPCE [0.1 M KOH] | 670 | 0.02–0.5 | 8 | -- | 2018 [127] |
| Ni plasma-modified graphene/GCE [0.1 M NaOH] | 2213 | 0.1–3 | 1 | +0.60 | 2017 [207] |
| Ni3S2/NF [0.5 M NaOH] | 16,460 | 5 × 10−4–3 | 0.82 | +0.55 | 2016 [208] |
| NiS/ITO [0.1 M NaOH] | 7430 | 0.005–0.045 | 0.32 | +0.50 | 2015 [209] |
| CNT-Ni/SiO2 [0.1 M NaOH] | 813 | 0.001–0.11 | 1 | +0.50 | 2015 [210] |
| 3D Ni3S2 NSs arrays/NF [0.5 M NaOH] | 6148 | 0.005–3 | 1.2 | +0.49 | 2014 [211] |
| Ni3S2/MWCNTs [0.1 M NaOH] | 3345 | 0.03–0.5 | 1 | +0.54 | 2014 [212] |
| 3D porous Ni-SPCE/ITO [0.1 M NaOH] | 2900 | 5 × 10−4–4 | 0.07 | +0.50 | 2013 [213] |
| Ni-MWCNTs/GCE [0.1 M NaOH] | 67.2 | 0.0032–17.5 | 0.89 | +0.60 | 2012 [132] |
| Ni NPs-MWCNTs/GCE [0.1 M NaOH] | 1438 | 0.001–1 | 0.5 | +0.45 | 2011 [214] |
| Electrode [Electrolyte] | Sensitivity (μA mM−1 cm−2) | Linear Range (mM) | LOD (μM) | Working Potential (V) * | Year [Reference] |
|---|---|---|---|---|---|
| (a) Noble-noble bi-metallic electrodes | |||||
| Pt-Au-Carbon/GCE [0.08 M NaOH] | -- | 0.01–10 | 3 | −0.28 | 2020 [229] |
| Pd@Pt CINPs [0.1 M PB] | 15.14 | 1–8.5 | 1.92 | −0.10 | 2019 [249] |
| Honeycomb-like Au-Pt films/Si [0.5 M KOH] | 109.3 | 0.02–10 | 12.9 | −0.01 | 2016 [250] |
| Ag-Pt hollow NPs/rGO [0.2 M PBS] | 129.3 | 0.003–7.72 | 1.8 | +0.30 | 2016 [251] |
| Pd-Pt NCbs-rGO/GCE [0.1 M NaOH] | 170 | 0.3–6.8 | 41.1 | −0.05 | 2016 [252] |
| Hollow Ag-Pt NPs/Carbon [0.2 M PBS] | 7 | 1–12 | 13 | +0.30 | 2015 [219] |
| Mesoporous Au-Pt NDs/GCE [0.1 M NaOH] | -- | 1 × 10−5–0.1 | 0.001 | −0.35 | 2015 [253] |
| Pt3Pd NPs-rGO/GCE [0.5 M H2SO4] | 1.52 | 0.03–3 | 0.002 | +0.11 | 2015 [254] |
| Pt-Pd NCbs-GNSs/GCE [0.05 M PBS] | 1.4 | 0.5–24.5 | -- | +0.30 | 2014 [227] |
| Pt-Pd NTAs/Au electrode [0.1 M PBS] | 41.5 | Up to 10 | -- | +0.20 | 2014 [255] |
| (b) Noble-transition bi-metallic electrodes | |||||
| Cu-Ag NCs/GCE [0.1 M NaOH] | 1340 | 0.01–30 | 0.6 | +0.40 | 2020 [217] |
| Pd-Mn NPs-rGO/GCE [0.1 M NaOH] | 52.2 22.6 | 0.02–1.15 1.15–4.88 | 1.25 | −0.05 | 2020 [256] |
| Pt-Ni@AC/GCE [0.1 M NaOH] | 40,900 | 0.025–12 | 0.052 | -- | 2020 [257] |
| Pd-Ni NPs-rGO/GCE [0.1 M NaOH] | 37,500 | 0.05–1.1 | 0.15 | -- | 2019 [258] |
| Ni@Pt-rGO/GCE [0.1 M NaOH] | -- | 0.008–10 | 8 | +0.60 | 2018 [226] |
| Stone-like Pt-Ni NPs/GCE [0.01 M PB] | 40.17 | 0.5–40 | 0.35 | +0.48 | 2018 [259] |
| Au-Ni multilayer NWA/ITO [0.2 M NaOH] | 3372 1906 | 2.5 × 10−4–2 2–5.5 | 0.1 | +0.60 | 2017 [260] |
| Cu-Ag/NF [0.5 M NaOH] | 7745.7 | 0.005–3.5 | 0.08 | +0.49 | 2015 [37] |
| Co@Pt core-shell NPs/GCE [0.1 M PB] | 2.26 | 1–30 | 300 | 0 | 2015 [261] |
| Pt-Ni NWs-PC/GCE [0.1 M NaOH] | 920 | 0.002–2 | 1.5 | +0.50 | 2011 [225] |
| (c) Transition-transition bi-metallic electrodes | |||||
| Ni-Fe NPs-PANI/FTO [0.1 M NaOH] | 1050 | 0.02–1 | 0.5 | +0.55 | 2021 [224] |
| Ni@Cu-MOF/GCE [0.1 M NaOH] | 1703.3 | 0.005–2.5 | 1.67 | -- | 2020 [262] |
| Cu-Ni/graphene sheets [0.1 M NaOH] | 314,285 17,857 1678 | 5 × 10−5–2.4 × 10−4 2.4 × 10−4–0.00233 0.00233–2.174 | 0.003 | +0.66 | 2019 [263] |
| Ni-Co-MOF-NSA/Au electrode [0.1 M NaOH] | 684.4 | 0.001–8 | 0.29 | +0.55 | 2019 [264] |
| Cu-Ni-NF/CNSA [0.1 M NaOH] | 17,120 | 0.2–2.72 | 0.067 | +0.54 | 2018 [126] |
| PANI@Cu-Ni NCs/GCE [0.1 M NaOH] | 1030 | 0.1–5.6 | 0.2 | +0.55 | 2018 [222] |
| Cu-Ni bi-metallic NCs/GCE [0.1 M NaOH] | 63.87 | 0.01–18 | 8 | +0.51 | 2017 [223] |
| Cu-Ni thin film/Cu substrate [0.1 M NaOH] | 240.1 | 0–10 | -- | +0.50 | 2017 [265] |
| Ni-Co NWs-MWCNTs/GCE [0.1 M NaOH] | 695 | 0.005–10 | 1.2 | +0.45 | 2016 [129] |
| Ni-Co-MSN/GCE [0.1 M NaOH] | 536.6 | 0.001–5 | 0.39 | +0.50 | 2015 [142] |
| Electrode [Electrolyte] | Sensitivity (μA mM−1 cm−2) | Linear Range (mM) | LOD (μM) | Working Potential (V) * | Year [Reference] |
|---|---|---|---|---|---|
| (a) Electrodes containing Co oxides | |||||
| Co3O4 HAA/GCE [0.1 M NaOH] | 839.3 | 5.3 × 10−4–19 | 0.53 | +0.60 | 2018 [273] |
| Co3O4-rGO/SPCE [0.1 M KOH] | 1315 | 0.001–0.5 | 0.4 | +0.35 | 2018 [274] |
| Co3O4@graphene/GCE [0.1 M NaOH] | 628 | 0.02–8 | 0.04 | +0.55 | 2017 [275] |
| Co3O4 porous film/GCE [0.1 M NaOH] | 366 | Up to 3 | 1.0 | +0.60 | 2016 [276] |
| Co3O4-HND/GCE [0.1 M KOH] | 708.4 | 2–6.06 | 0.58 | +0.55 | 2016 [277] |
| Co3O4 NFs-GOH/GCE [0.1 M NaOH] | 492.8 | 0.25–10 | -- | +0.62 | 2016 [278] |
| Co3O4/GCE-Nafion [0.1 M NaOH] | 1618.7 | 0.1–50 | 0.1 | +0.5 | 2015 [279] |
| Co3O4 OMC/GCE [0.1 M NaOH] | 955.9 | 0.9–7 | 1.0 | +0.55 | 2015 [280] |
| Co3O4 NWs/GCE [0.1 M NaOH] | 45.8 | 0.001–12 | 0.27 | +0.20 | 2015 [281] |
| Co3O4 NFs/GCE [0.5 M NaOH] | 1440 | 0.005–12 | 0.08 | +0.47 | 2013 [40] |
| (b) Electrodes containing Cu oxides | |||||
| CuO MTs/GCE [0.05 M NaOH] | 992.1 541.8 | 0.001–1.164 1.164–5.664 | 0.31 | +0.70 | 2019 [282] |
| CuO nanodisk/SPCE [0.1 M KOH] | 627.3 | 0.002–2.5 | 0.2 | +0.60 | 2019 [283] |
| Cu3(BTC)2-derived CuO nanorod/GCE [0.1 M NaOH] | 1523.5 | Up to 1.25 | 1 | +0.60 | 2019 [62] |
| Petal-like nano CuO/GCE [0.05 M NaOH] | 2634.4 | 5 × 10−4–2.67 | 0.26 | +0.65 | 2018 [284] |
| CuO-PANI-NF/FTO [0.1 M NaOH] | 2800 1359 | 2.5 × 10−4–0.28 0.28–4.6 | 0.24 | +0.6 | 2018 [120] |
| CuO biscuits/SPCE [0.1 M NaOH] | 308.7 | Up to 4.03 | 0.1 | +0.50 | 2017 [285] |
| CuO NWs/GCE [0.05 M NaOH] | 648.2 | -- | 2 | +0.55 | 2014 [286] |
| CuO-Cu2O/GCE [0.1 M NaOH] | 830 | 0.5–10 | 0.7 | +0.60 | 2014 [287] |
| Dandelion-like CuO film/Cu foils [0.1 M NaOH] | 5368 | 0.005–1.6 | 1.2 | +0.60 | 2014 [55] |
| CuO NSs/Au-coated glass [0.1 M NaOH] | 520 | 0.1–10 | -- | +0.50 | 2013 [288] |
| (c) Electrodes containing Ni oxides | |||||
| NiO nanostructures/Ni sheets [0.1 M KCl + 0.5 M NaOH] | 206.9 | 0.1–10 | 1.16 | +0.55 | 2019 [134] |
| NiO-HAC/GCE [0.1 M NaOH] | 199.9 | 0.01–3.3 | 1.0 | +0.55 | 2017 [289] |
| NiO NPs graphene NSs/GCE [0.1 M NaOH] | 666.7 | 0.005–4.2 | 5 | +0.50 | 2016 [54] |
| Mesoporous NWas NiO/3D NF-G [0.1 M NaOH] | 3230 | 0.01–0.2 | 1 × 10−4 | +0.55 | 2016 [290] |
| NiO-PPy/GCE [0.1 M PBS] | 1094.8 62.9 | 0.01–0.5 1–20 | 5.8 | +0.58 | 2015 [122] |
| NiO NSks/GCE [0.1 M NaOH] | 1915 | 0.1–5.0 | 0.7 | +0.48 | 2015 [291] |
| NiO HCs/GCE [0.1 M NaOH] | 2476.4 | 0.1–5.0 | 0.1 | -- | 2015 [292] |
| 3D NiO/NF [0.5 M NaOH] | 6657.5 | 0.005–5.5 | 0.46 | +0.47 | 2013 [293] |
| NiO-SWCNT/ITO [0.1 M NaOH] | 907 | 0.001–0.9 | 0.3 | +0.55 | 2013 [294] |
| NiO NF-GO/GCE [0.1 M NaOH] | 1100 | 0.002–0.6 | 0.77 | +0.60 | 2012 [295] |
| Electrode [Electrolyte] | Sensitivity (μA mM−1 cm−2) | Linear Range (mM) | LOD (μM) | Working Potential (V) * | Year [Reference] |
|---|---|---|---|---|---|
| Co(OH)2 NTAs/CC [0.1 M NaOH] | 2770 | 0.001–0.6 | 0.5 | +0.50 | 2017 [298] |
| CoOOH NSAs/GCE [0.1 M NaOH] | 526.8 | 0.003–1.11 | 1.37 | +0.52 | 2015 [299] |
| CoOOH NSs/Co foil [0.1 M NaOH] | 967 341 | 0.01–0.5 0.03–0.7 | 10.6 30.9 | +0.50 +0.40 | 2012 [124] |
| Cu(OH)2 NTs [0.1 M NaOH] | 418 | Up to 3.0 | 0.5 | +0.45 | 2013 [300] |
| Cu(OH)2 NFs/Cu foil [0.1 M NaOH] | 2159.2 | 0–6.0 | 9.0 | +0.50 | 2012 [301] |
| Ni(OH)2/3DGF [0.2 M NaOH] | 2366 | Up to 2.2 | 0.32 | +0.46 | 2019 [302] |
| Ni(OH)2 NSs-NF/GCE [0.2 M NaOH] | 1097 1130 | 0.1–2.5 2–40 | 1.0 | +0.51 | 2015 [303] |
| Ni(OH)2 NFks@oPPyNW/Graphite [0.1 M NaOH] | 1049 | 0.001–3.86 | 0.3 | +0.54 | 2015 [304] |
| Ni(OH)2 NPs/graphene [0.1 M NaOH] | 2400 | 0.001–15 | 0.53 | +0.53 | 2014 [305] |
| Ni(OH)2 NPs/NF [0.2 M NaOH] | 1950.3 | Up to 6.0 | 0.16 | +0.45 | 2014 [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, W.-T.; Chang, T.-F.M.; Sone, M.; Hosoda, H.; Tixier-Mita, A.; Toshiyoshi, H. Developments of the Electroactive Materials for Non-Enzymatic Glucose Sensing and Their Mechanisms. Electrochem 2021, 2, 347-389. https://doi.org/10.3390/electrochem2020025
Chiu W-T, Chang T-FM, Sone M, Hosoda H, Tixier-Mita A, Toshiyoshi H. Developments of the Electroactive Materials for Non-Enzymatic Glucose Sensing and Their Mechanisms. Electrochem. 2021; 2(2):347-389. https://doi.org/10.3390/electrochem2020025
Chicago/Turabian StyleChiu, Wan-Ting, Tso-Fu Mark Chang, Masato Sone, Hideki Hosoda, Agnès Tixier-Mita, and Hiroshi Toshiyoshi. 2021. "Developments of the Electroactive Materials for Non-Enzymatic Glucose Sensing and Their Mechanisms" Electrochem 2, no. 2: 347-389. https://doi.org/10.3390/electrochem2020025
APA StyleChiu, W.-T., Chang, T.-F. M., Sone, M., Hosoda, H., Tixier-Mita, A., & Toshiyoshi, H. (2021). Developments of the Electroactive Materials for Non-Enzymatic Glucose Sensing and Their Mechanisms. Electrochem, 2(2), 347-389. https://doi.org/10.3390/electrochem2020025









