Crystal Structure and Preparation of Li7La3Zr2O12 (LLZO) Solid-State Electrolyte and Doping Impacts on the Conductivity: An Overview
Abstract
:1. Introduction
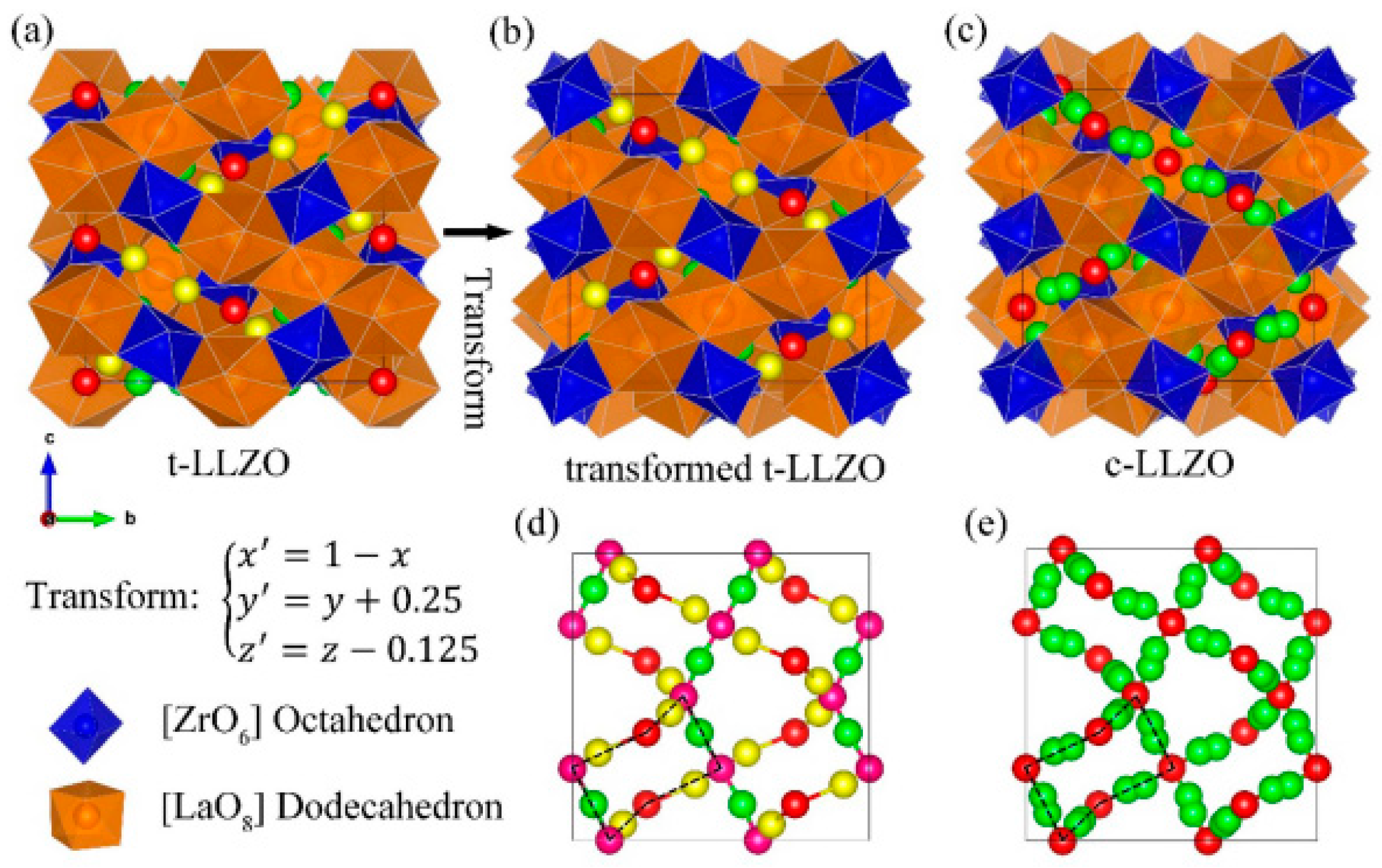
2. Structural Analysis of LLZO
Transition to Cubic Phase from Tetragonal Phase and Vice Versa
3. Synthesis Techniques of LLZO (Li7La3Zr2O12)
3.1. Solid-State Reaction Method
3.2. Sol-Gel Method
3.3. Pechini Method
3.4. Radio Frequency (RF) Magnetron Sputtering
3.5. Pulsed Laser Deposition
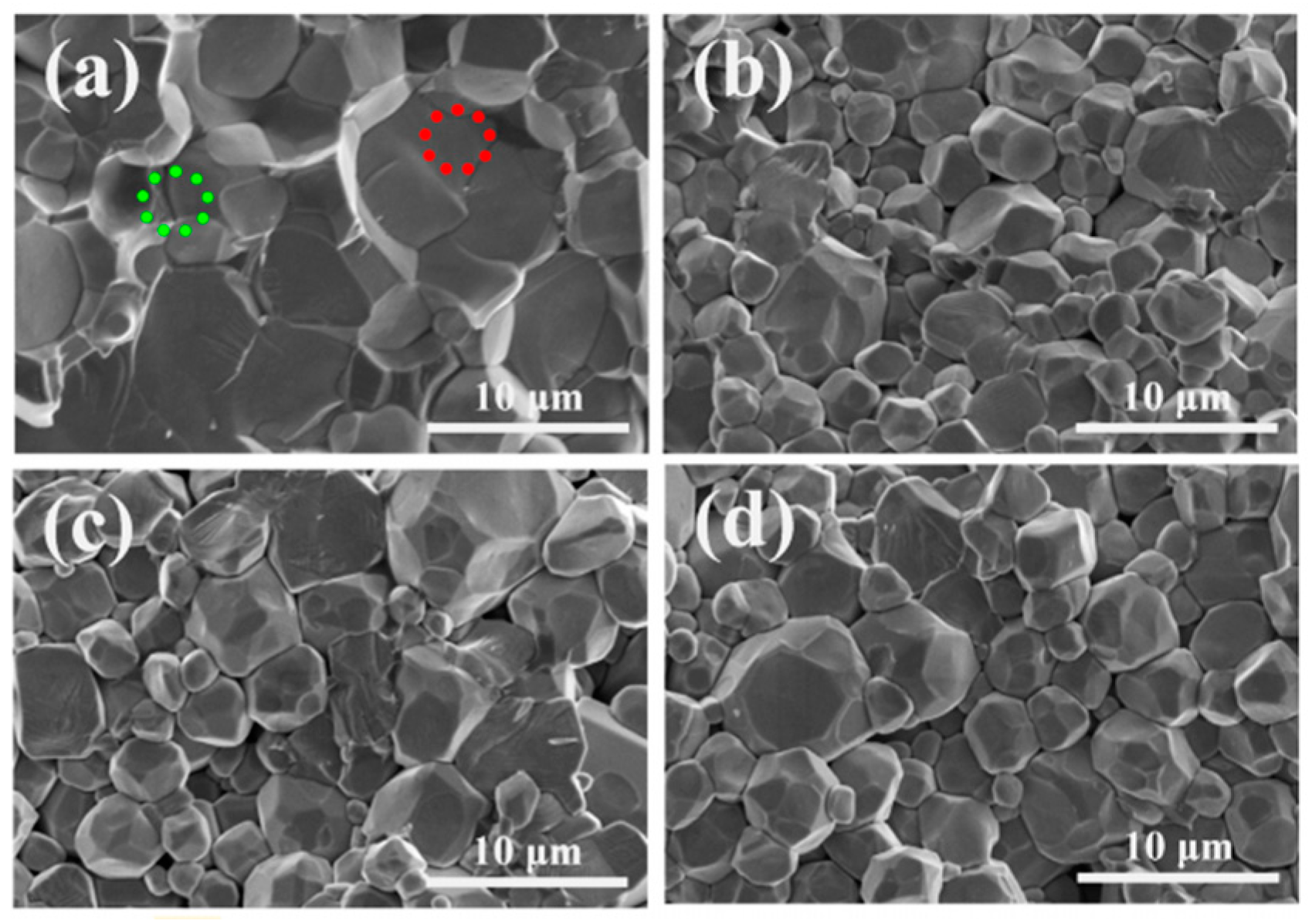
4. Sintering Techniques of LLZO
4.1. Furnace Sintering
4.2. Hot Pressing
4.3. Field-Assisted Sintering Technology (FAST)
4.4. Spark Plasma Sintering (SPS)
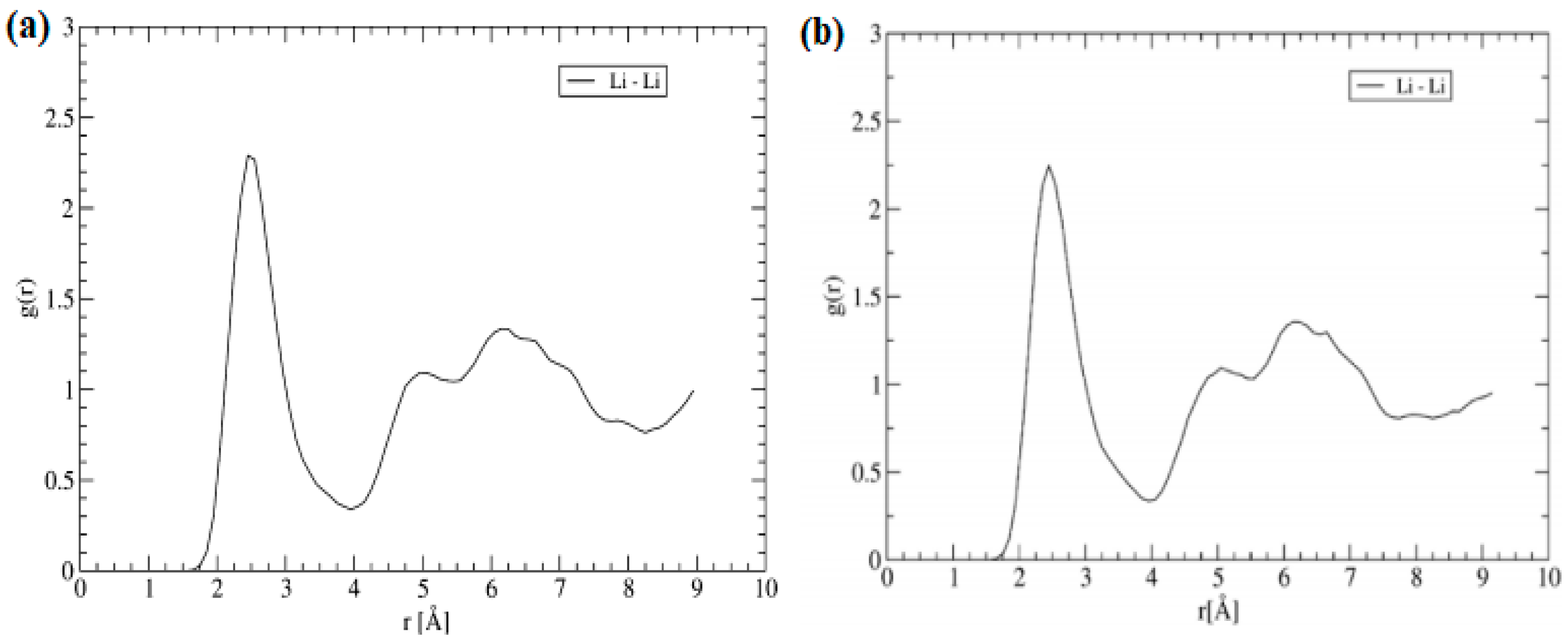
5. Li Ion Diffusion Mechanism
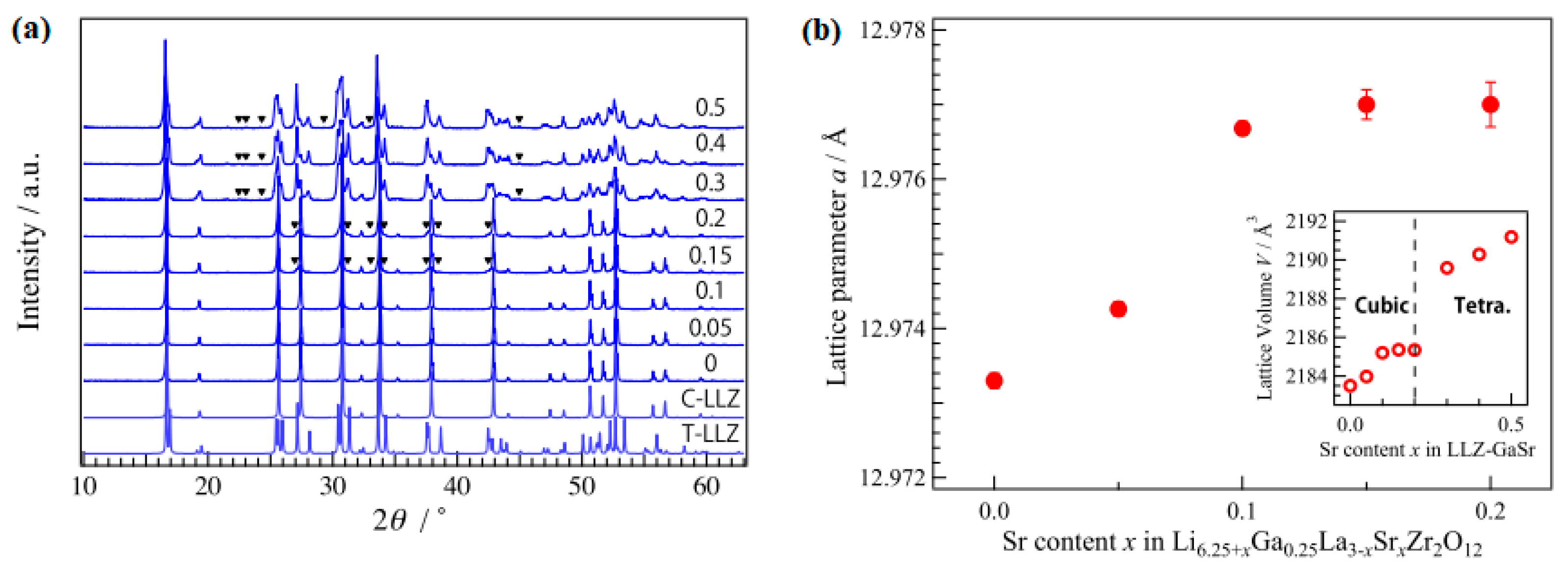
6. Doping and Li Ionic Conductivity
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Mater. Sustain. Energy 2010, 171–179. [Google Scholar] [CrossRef]
- Scrosati, B.; Hassoun, J.; Sun, Y.-K. Lithium-ion batteries. A look into the future. Energy Environ. Sci. 2011, 4, 3287–3295. [Google Scholar] [CrossRef]
- Sloop, S.E.; Pugh, J.K.; Wang, S.; Kerr, J.B.; Kinoshita, K. Chemical Reactivity of PF5 and LiPF6 in Ethylene Carbonate/Dimethyl Carbonate Solutions. Electrochem. Solid-State Lett. 2001, 4, A42–A44. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.; Wan, C. Review of gel-type polymer electrolytes for lithium-ion batteries. J. Power Sources 1999, 77, 183–197. [Google Scholar] [CrossRef]
- Vashishta, P.; Mundy, J.N. Fast Ion Transport in Solids: Electrodes and Electrolytes; Elsevier North Holland Inc.: Amsterdam, The Netherlands, 1979. [Google Scholar]
- Gray, F.M. Solid Polymer Electrolytes-Fundamentals and Technological Applications; VCH: New York, NY, USA, 1991. [Google Scholar]
- Rogers, R.D. Chemistry: Ionic Liquids—Solvents of the Future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef]
- Gabriel, S.; Weiner, J. Ueber einige Abkömmlinge des Propylamins. Eur. J. Inorg. Chem. 1888, 21, 2669–2679. [Google Scholar] [CrossRef] [Green Version]
- Sato, T.; Maruo, T.; Marukane, S.; Takagi, K. Ionic liquids containing carbonate solvent as electrolytes for lithium ion cells. J. Power Sources 2004, 138, 253–261. [Google Scholar] [CrossRef]
- Gregory, D.H.; O’Meara, P.M.; Gordon, A.G.; Hodges, J.P.; Short, S.; Jorgensen, J.D. Structure of Lithium Nitride and Transition-Metal-Doped Derivatives, Li3-x-yMxN (M = Ni, Cu): A Powder Neutron Diffraction Study. Chem. Mater. 2002, 14, 2063–2070. [Google Scholar] [CrossRef]
- Zintl, E.; Schneider, A. Röntgenanalyse der Lithium-Zink-Legierungen (15. Mitteilung über Metalle und Legierungen). Z. Elektrochem. Angew. Phys. Chem. 1935, 41, 764–767. [Google Scholar] [CrossRef]
- Alpen, U.V.; Rabenau, A.; Talat, G.H. Ionic conductivity in Li3N single crystals. Appl. Phys. Lett. 1977, 30, 621–623. [Google Scholar] [CrossRef]
- Kanno, R.; Murayama, M. Lithium Ionic Conductor Thio-LISICON: The Li2S-GeS2-P2S5 System. J. Electrochem. Soc. 2001, 148, A742–A746. [Google Scholar] [CrossRef]
- Abrahams, I.; Bruce, P.; West, A.; David, W. Structure determination of LISICON solid solutions by powder neutron diffraction. J. Solid State Chem. 1988, 75, 390–396. [Google Scholar] [CrossRef]
- Bruce, P.; West, A. Ionic conductivity of LISICON solid solutions, Li2+2xZn1−xGeO4. J. Solid State Chem. 1982, 44, 354–365. [Google Scholar] [CrossRef]
- Von Alpen, U.; Bell, M.F.; Höfer, H.H. Ionic conductivity in Na4ZrSi3O10. Solid State Ionics 1982, 7, 345–348. [Google Scholar] [CrossRef]
- Hong, H.-P. Crystal structures and crystal chemistry in the system Na1+xZr2SixP3−xO12. Mater. Res. Bull. 1976, 11, 173–182. [Google Scholar] [CrossRef]
- Golub, A.; Shumilova, I.; Zubavichus, Y.; Slovokhotov, Y.; Novikov, Y.; Marie, A.; Danot, M. From single-layer dispersions of molybdenum disulfide towards ternary metal sulfides: Incorporating copper and silver into a MoS2 matrix. Solid State Ionics 1999, 122, 137–144. [Google Scholar] [CrossRef]
- Aono, H.; Sugimoto, E.; Sadaoka, Y.; Imanaka, N.; Adachi, G. ChemInform Abstract: The Electrical Properties of Ceramic Electrolytes for LiMxTi2-x(PO4)3 + yLi2O, M: Ge, Sn, Hf, and Zr Systems. J. Electrochem. Soc. 1993, 140, 1827. [Google Scholar] [CrossRef]
- Ivanov-Schitz, A. Ionic conductivity of the NaZr2(PO4)3 single crystals. Solid State Ionics 1997, 100, 153–155. [Google Scholar] [CrossRef]
- Knauth, P. Inorganic solid Li ion conductors: An overview. Solid State Ionics 2009, 180, 911–916. [Google Scholar] [CrossRef]
- Alamo, J.; Roy, R. Crystal chemistry of the NaZr2(PO4)3, NZP or CTP, structure family. J. Mater. Sci. 1986, 21, 444–450. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Hong, H.-P.; Kafalas, J. Fast Na+-ion transport in skeleton structures. Mater. Res. Bull. 1976, 11, 203–220. [Google Scholar] [CrossRef]
- Sudworth, J. The sodium/sulphur battery. J. Power Sources 1984, 11, 143–154. [Google Scholar] [CrossRef]
- Beevers, C.A.; Ross, Μ.A.S. The Crystal Structure of “Beta Alumina” Na2O·11Al2O3. Z. Krist. Cryst. Mater. 1937, 97, 59–66. [Google Scholar] [CrossRef]
- Farrington, G.; Dunn, B.; Briant, J. Li+ and divalent ion conductivity in beta and beta″ alumina. Solid State Ionics 1981, 3–4, 405–408. [Google Scholar] [CrossRef]
- Bettman, M.; Peters, C.R. Crystal structure of Na2O.MgO.5Al2O3 [sodium oxide-magnesia-alumina] with reference to Na2O.5Al2O3 and other isotypal compounds. J. Phys. Chem. 1969, 73, 1774–1780. [Google Scholar] [CrossRef]
- Bragg, W.L.; Gottfried, C.; West, J. The Structure of β Alumina. Z. Krist. Cryst. Mater. 1931, 77, 255–274. [Google Scholar] [CrossRef]
- Wolfenstine, J.; Allen, J.; Sumner, J.; Sakamoto, J. Electrical and mechanical properties of hot-pressed versus sintered LiTi2(PO4)3. Solid State Ionics 2009, 180, 961–967. [Google Scholar] [CrossRef]
- Wang, B.; Chakoumakos, B.C.; Sales, B.; Kwak, B.; Bates, J. Synthesis, Crystal Structure, and Ionic Conductivity of a Polycrystalline Lithium Phosphorus Oxynitride with the γ-Li3PO4 Structure. J. Solid State Chem. 1995, 115, 313–323. [Google Scholar] [CrossRef]
- Bates, J.B.; Dudney, N.J.; Neudecker, B.; Ueda, A.; Evans, C.D. Thin-film lithium and lithium-ion batteries. Solid State Ionics 2000, 135, 33–45. [Google Scholar] [CrossRef]
- Catti, M. Local structure of the Li1/8La5/8TiO3(LLTO) ionic conductor by theoretical simulations. J. Phys. Conf. Ser. 2008, 117, 12008. [Google Scholar] [CrossRef]
- Harada, Y.; Ishigaki, T.; Kawai, H.; Kuwano, J. Lithium ion conductivity of polycrystalline perovskite La0.67−xLi3xTiO3 with ordered and disordered arrangements of the A-site ions. Solid State Ionics 1998, 108, 407–413. [Google Scholar] [CrossRef]
- Thangadurai, V.; Kaack, H.; Weppner, W.J.F. Novel Fast Lithium Ion Conduction in Garnet-Type Li5La3M2O12(M = Nb, Ta). J. Am. Ceram. Soc. 2003, 86, 437–440. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast Lithium Ion Conduction in Garnet-Type Li7La3Zr2O12. Angew. Chem. Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef] [PubMed]
- Ramakumar, S.; Janani, N.; Murugan, R. Influence of lithium concentration on the structure and Li+ transport properties of cubic phase lithium garnets. Dalton Trans. 2014, 44, 539–552. [Google Scholar] [CrossRef]
- Awaka, J.; Kijima, N.; Hayakawa, H.; Akimoto, J. Synthesis and structure analysis of tetragonal Li7La3Zr2O12 with the garnet-related type structure. J. Solid State Chem. 2009, 182, 2046–2052. [Google Scholar] [CrossRef]
- Awaka, J.; Takashima, A.; Kataoka, K.; Kijima, N.; Idemoto, Y.; Akimoto, J. Crystal Structure of Fast Lithium-ion-conducting Cubic Li7La3Zr2O12. Chem. Lett. 2011, 40, 60–62. [Google Scholar] [CrossRef]
- Bernstein, N.; Johannes, M.D.; Hoang, K. Origin of the Structural Phase Transition inLi7La3Zr2O12. Phys. Rev. Lett. 2012, 109, 205702. [Google Scholar] [CrossRef] [Green Version]
- Meier, K.; Laino, T.; Curioni, A. Solid-State Electrolytes: Revealing the Mechanisms of Li-Ion Conduction in Tetragonal and Cubic LLZO by First-Principles Calculations. J. Phys. Chem. C 2014, 118, 6668–6679. [Google Scholar] [CrossRef]
- Kühne, T.D.; Iannuzzi, M.; Del Ben, M.; Rybkin, V.V.; Seewald, P.; Stein, F.; Laino, T.; Khaliullin, R.Z.; Schütt, O.; Schiffmann, F.; et al. CP2K: An electronic structure and molecular dynamics software package—Quickstep: Efficient and accurate electronic structure calculations. J. Chem. Phys. 2020, 152, 194103. [Google Scholar] [CrossRef]
- Geiger, C.A.; Alekseev, E.; Lazic, B.; Fisch, M.; Armbruster, T.; Langner, R.; Fechtelkord, M.; Kim, N.; Pettke, T.; Weppner, W. Crystal Chemistry and Stability of “Li7La3Zr2O12” Garnet: A Fast Lithium-Ion Conductor. Inorg. Chem. 2011, 50, 1089–1097. [Google Scholar] [CrossRef]
- Klenk, M.J.; Lai, W. Finite-size effects on the molecular dynamics simulation of fast-ion conductors: A case study of lithium garnet oxide Li7La3Zr2O12. Solid State Ionics 2016, 289, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, A.; Narayanan, S.; Spencer, L.; Goward, G.; Thangadurai, V.; Wilkening, M. Li self-diffusion in garnet-type Li7La3Zr2O12 as probed directly by diffusion-inducedLi7spin-lattice relaxation NMR spectroscopy. Phys. Rev. B 2011, 83, 094302. [Google Scholar] [CrossRef]
- Klenk, M.; Lai, W. Local structure and dynamics of lithium garnet ionic conductors: Tetragonal and cubic Li7La3Zr2O7. Phys. Chem. Chem. Phys. 2015, 17, 8758–8768. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, H.; Dölle, J.; Berendts, S.; Kuhn, A.; Bottke, P.; Wilkening, M.; Heitjans, P.; Senyshyn, A.; Ehrenberg, H.; Lotnyk, A.; et al. Structure and dynamics of the fast lithium ion conductor “Li7La3Zr2O12”. Phys. Chem. Chem. Phys. 2011, 13, 19378–19392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, S.; Rao, R.P. Ion transport and phase transition in Li7−xLa3(Zr2−xMx)O12(M = Ta5+, Nb5+, x = 0, 0.25). J. Mater. Chem. 2012, 22, 1426–1434. [Google Scholar] [CrossRef]
- Cao, S.; Song, S.; Xiang, X.; Hu, Q.; Zhang, C.; Xia, Z.; Xu, Y.; Zha, W.; Li, J.; Gonzale, P.M.; et al. Modeling, Preparation, and Elemental Doping of Li7La3Zr2O12 Garnet-Type Solid Electrolytes: A Review. J. Korean Ceram. Soc. 2019, 56, 111–129. [Google Scholar] [CrossRef] [Green Version]
- Mori, D.; Sugimoto, K.; Matsuda, Y.; Ohmori, K.; Katsumata, T.; Taminato, S.; Takeda, Y.; Yamamoto, O.; Imanishi, N. Synthesis, Structure and Ionic Conductivity of Garnet Like Lithium Ion Conductor Li6.25+xGa0.25La3-xSrxZr2O12. J. Electrochem. Soc. 2018, 166, A5168–A5173. [Google Scholar] [CrossRef]
- Dumon, A.; Huang, M.; Shen, Y.; Nan, C.-W. High Li ion conductivity in strontium doped Li7La3Zr2O12 garnet. Solid State Ionics 2013, 243, 36–41. [Google Scholar] [CrossRef]
- Matsui, M.; Sakamoto, K.; Takahashi, K.; Hirano, A.; Takeda, Y.; Yamamoto, O.; Imanishi, N. Phase transformation of the garnet structured lithium ion conductor: Li7La3Zr2O12. Solid State Ionics 2014, 262, 155–159. [Google Scholar] [CrossRef]
- O’Callaghan, M.P.; Lynham, D.R.; Cussen, E.J.; Chen, G. Structure and Ionic-Transport Properties of Lithium-Containing Garnets Li3Ln3Te2O12(Ln = Y, Pr, Nd, Sm−Lu). Chem. Mater. 2006, 18, 4681–4689. [Google Scholar] [CrossRef]
- Liu, K.; Ma, J.-T.; Wang, C.-A. Excess lithium salt functions more than compensating for lithium loss when synthesizing Li6.5La3Ta0.5Zr1.5O12 in alumina crucible. J. Power Sources 2014, 260, 109–114. [Google Scholar] [CrossRef]
- Rangasamy, E.; Wolfenstine, J.; Sakamoto, J. The role of Al and Li concentration on the formation of cubic garnet solid electrolyte of nominal composition Li7La3Zr2O12. Solid State Ionics 2012, 206, 28–32. [Google Scholar] [CrossRef]
- Yoo, A.R.; A Yoon, S.; Kim, Y.S.; Sakamoto, J.; Lee, H.C. A Comparative Study on the Synthesis of Al-Doped Li6.2La3Zr2O12 Powder as a Solid Electrolyte Using Sol–Gel Synthesis and Solid-State Processing. J. Nanosci. Nanotechnol. 2016, 16, 11662–11668. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Wang, W.; Fang, Q. Sol–gel synthesis and electrical properties of Li5La3Ta2O12 lithium ionic conductors. Solid State Ionics 2010, 181, 33–36. [Google Scholar] [CrossRef]
- Kokal, I.; Somer, M.; Notten, P.; Hintzen, H. Sol–gel synthesis and lithium ion conductivity of Li7La3Zr2O12 with garnet-related type structure. Solid State Ionics 2011, 185, 42–46. [Google Scholar] [CrossRef]
- Shimonishi, Y.; Toda, A.; Zhang, T.; Hirano, A.; Imanishi, N.; Yamamoto, O.; Takeda, Y. Synthesis of garnet-type Li7−xLa3Zr2O12−1/2x and its stability in aqueous solutions. Solid State Ionics 2011, 183, 48–53. [Google Scholar] [CrossRef]
- Xie, H.; Li, Y.; Goodenough, J.B. Low-temperature synthesis of Li7La3Zr2O12 with cubic garnet-type structure. Mater. Res. Bull. 2012, 47, 1229–1232. [Google Scholar] [CrossRef]
- Janani, N.; Deviannapoorani, C.; Dhivya, L.; Murugan, R. Influence of sintering additives on densification and Li+ conductivity of Al doped Li7La3Zr2O12 lithium garnet. RSC Adv. 2014, 4, 51228–51238. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Wang, W.; Zhuang, Z.; Zhang, D.; Fang, Q. Synthesis, ionic conductivity, and chemical compatibility of garnet-like lithium ionic conductor Li5La3Bi2O12. Solid State Ionics 2010, 181, 1415–1419. [Google Scholar] [CrossRef]
- Jin, Y.; McGinn, P.J. Al-doped Li7La3Zr2O12 synthesized by a polymerized complex method. J. Power Sources 2011, 196, 8683–8687. [Google Scholar] [CrossRef]
- Lobe, S.; Dellen, C.; Finsterbusch, M.; Gehrke, H.-G.; Sebold, D.; Tsai, C.-L.; Uhlenbruck, S.; Guillon, O. Radio frequency magnetron sputtering of Li7La3Zr2O12 thin films for solid-state batteries. J. Power Sources 2016, 307, 684–689. [Google Scholar] [CrossRef] [Green Version]
- Kalita, D.; Lee, S.; Lee, K.; Ko, D.; Yoon, Y. Ionic conductivity properties of amorphous Li–La–Zr–O solid electrolyte for thin film batteries. Solid State Ionics 2012, 229, 14–19. [Google Scholar] [CrossRef]
- Park, J.S.; Cheng, L.; Zorba, V.; Mehta, A.; Cabana, J.; Chen, G.; Doeff, M.M.; Richardson, T.J.; Park, J.H.; Son, J.-W.; et al. Effects of crystallinity and impurities on the electrical conductivity of Li–La–Zr–O thin films. Thin Solid Films 2015, 576, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Tiwari, A. Fabrication and Characterization of Li7La3Zr2O12 Thin Films for Lithium Ion Battery. ECS Solid State Lett. 2012, 1, Q57–Q60. [Google Scholar] [CrossRef]
- Kim, S.; Hirayama, M.; Taminato, S.; Kanno, R. Epitaxial growth and lithium ion conductivity of lithium-oxide garnet for an all solid-state battery electrolyte. Dalton Trans. 2013, 42, 13112–13117. [Google Scholar] [CrossRef] [PubMed]
- Afyon, S.; Krumeich, F.; Rupp, J.L.M. A shortcut to garnet-type fast Li-ion conductors for all-solid state batteries. J. Mater. Chem. A 2015, 3, 18636–18648. [Google Scholar] [CrossRef]
- Dhivya, L.; Karthik, K.; Ramakumar, S.; Murugan, R. Facile synthesis of high lithium ion conductive cubic phase lithium garnets for electrochemical energy storage devices. RSC Adv. 2015, 5, 96042–96051. [Google Scholar] [CrossRef]
- Chen, R.-J.; Huang, M.; Huang, W.-Z.; Shen, Y.; Lin, Y.-H.; Nan, C.-W. Sol–gel derived Li–La–Zr–O thin films as solid electrolytes for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 13277–13282. [Google Scholar] [CrossRef]
- Quinlan, F.T.; Vidu, R.; Predoana, L.; Zaharescu, M.; Gartrner, M.; Groza, J.; Stroeve, P. Lithium Cobalt Oxide (LiCoO2) Nanocoatings by Sol−Gel Methods. Ind. Eng. Chem. Res. 2004, 43, 2468–2477. [Google Scholar] [CrossRef]
- Yi, E.; Wang, W.; Kieffer, J.; Laine, R.M. Flame made nanoparticles permit processing of dense, flexible, Li+ conducting ceramic electrolyte thin films of cubic-Li7La3Zr2O12 (c-LLZO). J. Mater. Chem. A 2016, 4, 12947–12954. [Google Scholar] [CrossRef]
- Gai, J.; Zhao, E.; Ma, F.; Sun, D.; Ma, X.; Jin, Y.; Wu, Q.; Cui, Y. Improving the Li-ion conductivity and air stability of cubic Li7La3Zr2O12 by the co-doping of Nb, Y on the Zr site. J. Eur. Ceram. Soc. 2018, 38, 1673–1678. [Google Scholar] [CrossRef]
- Rettenwander, D.; Wagner, R.; Reyer, A.; Bonta, M.; Cheng, L.; Doeff, M.M.; Limbeck, A.; Wilkening, M.; Amthauer, G. Interface Instability of Fe-Stabilized Li7La3Zr2O12 versus Li Metal. J. Phys. Chem. C 2018, 122, 3780–3785. [Google Scholar] [CrossRef]
- Wu, J.-F.; Chen, E.-Y.; Yu, Y.; Liu, L.; Wu, Y.; Pang, W.K.; Peterson, V.K.; Guo, X. Gallium-Doped Li7La3Zr2O12 Garnet-Type Electrolytes with High Lithium-Ion Conductivity. ACS Appl. Mater. Interfaces 2017, 9, 1542–1552. [Google Scholar] [CrossRef] [Green Version]
- Tong, X.; Thangadurai, V.; Wachsman, E.D. Highly Conductive Li Garnets by a Multielement Doping Strategy. Inorg. Chem. 2015, 54, 3600–3607. [Google Scholar] [CrossRef]
- Liu, C.; Wen, Z.Y.; Rui, K. High Ion conductivity in garnet-type F-doped Li7La3Zr2O12. Wuji Cailiao Xuebao J. Inorgnanic Materails 2015. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Cao, Y.; Du, F.; Chen, C.; Cui, Z.; Guo, X. W-Doped Li7La3Zr2O12 Ceramic Electrolytes for Solid State Li-ion Batteries. Electrochimica Acta 2015, 180, 37–42. [Google Scholar] [CrossRef]
- Narayanan, S.; Ramezanipour, F.; Thangadurai, V. Dopant Concentration–Porosity–Li-Ion Conductivity Relationship in Garnet-Type Li5+2xLa3Ta2–xYxO12 (0.05 ≤ x ≤ 0.75) and Their Stability in Water and 1 M LiCl. Inorg. Chem. 2015, 54, 6968–6977. [Google Scholar] [CrossRef] [PubMed]
- Nemori, H.; Matsuda, Y.; Mitsuoka, S.; Matsui, M.; Yamamoto, O.; Takeda, Y.; Imanishi, N. Stability of garnet-type solid electrolyte LixLa3A2-yByO12 (A=Nb or Ta, B=Sc or Zr). Solid State Ionics 2015, 282, 7–12. [Google Scholar] [CrossRef]
- Deviannapoorani, C.; Ramakumar, S.; Janani, N.; Murugan, R. Synthesis of lithium garnets from La2Zr2O7 pyrochlore. Solid State Ionics 2015, 283, 123–130. [Google Scholar] [CrossRef]
- Janani, N.; Ramakumar, S.; Kannan, S.; Murugan, R. Optimization of Lithium Content and Sintering Aid for Maximized Li+ Conductivity and Density in Ta-Doped Li7 La3 Zr2 O12. J. Am. Ceram. Soc. 2015, 98, 2039–2046. [Google Scholar] [CrossRef]
- Wang, D.; Zhong, G.; Pang, W.K.; Guo, Z.; Li, Y.; McDonald, M.J.; Fu, R.; Mi, J.-X.; Yang, Y. Toward Understanding the Lithium Transport Mechanism in Garnet-type Solid Electrolytes: Li+ Ion Exchanges and Their Mobility at Octahedral/Tetrahedral Sites. Chem. Mater. 2015, 27, 6650–6659. [Google Scholar] [CrossRef]
- Inada, R.; Kusakabe, K.; Tanaka, T.; Kudo, S.; Sakurai, Y. Synthesis and properties of Al-free Li7−xLa3Zr2−xTaxO12 garnet related oxides. Solid State Ionics 2014, 262, 568–572. [Google Scholar] [CrossRef]
- Cao, Z.-Z.; Ren, W.; Liu, J.-R.; Li, G.-R.; Gao, Y.-F.; Fang, M.-H.; He, W.-Y. Microstructure and Ionic Conductivity of Sb-doped Li7La3Zr2O12 Ceramics. J. Inorg. Mater. 2014, 29, 220–224. [Google Scholar] [CrossRef]
- Nemori, H.; Matsuda, Y.; Matsui, M.; Yamamoto, O.; Takeda, Y.; Imanishi, N. Relationship between lithium content and ionic conductivity in the Li5+2xLa3Nb2−xScxO12 system. Solid State Ionics 2014, 266, 9–12. [Google Scholar] [CrossRef] [Green Version]
- Baral, A.K.; Narayanan, S.; Ramezanipour, F.; Thangadurai, V. Evaluation of fundamental transport properties of Li-excess garnet-type Li5+2xLa3Ta2−xYxO12 (x = 0.25, 0.5 and 0.75) electrolytes using AC impedance and dielectric spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 11356–11365. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Li, C.; Cao, Y.; Guo, X. Densification and ionic-conduction improvement of lithium garnet solid electrolytes by flowing oxygen sintering. J. Power Sources 2014, 248, 642–646. [Google Scholar] [CrossRef]
- Wang, D.; Zhong, G.; Dolotko, O.; Li, Y.; McDonald, M.J.; Mi, J.; Fu, R.; Yang, Y. The synergistic effects of Al and Te on the structure and Li+-mobility of garnet-type solid electrolytes. J. Mater. Chem. A 2014, 2, 20271–20279. [Google Scholar] [CrossRef]
- Zeier, W.G.; Zhou, S.; López-Bermúdez, B.; Page, K.; Melot, B.C. Dependence of the Li-Ion Conductivity and Activation Energies on the Crystal Structure and Ionic Radii in Li6MLa2Ta2O12. ACS Appl. Mater. Interfaces 2014, 6, 10900–10907. [Google Scholar] [CrossRef]
- Rangasamy, E.; Wolfenstine, J.; Allen, J.; Sakamoto, J. The effect of 24c-site (A) cation substitution on the tetragonal–cubic phase transition in Li7−xLa3−xAxZr2O12 garnet-based ceramic electrolyte. J. Power Sources 2013, 230, 261–266. [Google Scholar] [CrossRef]
- Hitz, G.T.; Wachsman, E.D.; Thangadurai, V. Highly Li-Stuffed Garnet-Type Li7+xLa3Zr2-xYxO12. J. Electrochem. Soc. 2013, 160, A1248–A1255. [Google Scholar] [CrossRef]
- Howard, M.A.; Clemens, O.; Knight, K.S.; Anderson, P.A.; Hafiz, S.; Panchmatia, P.M.; Slater, P.R. Synthesis, conductivity and structural aspects of Nd3Zr2Li7−3xAlxO12. J. Mater. Chem. A 2013, 1, 14013. [Google Scholar] [CrossRef] [Green Version]
- Truong, L.; Colter, J.; Thangadurai, V. Chemical stability of Li-stuffed garnet-type Li5+xBaxLa3−xTa2O12 (x = 0, 0.5, 1) in water: A comparative analysis with the Nb analogue. Solid State Ionics 2013, 247–248, 1–7. [Google Scholar] [CrossRef]
- Mariappan, C.R.; Gnanasekar, K.I.; Jayaraman, V.; Gnanasekaran, T. Lithium ion conduction in Li5La3Ta2O12 and Li7La3Ta2O13 garnet-type materials. J. Electroceramics 2013, 30, 258–265. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Y.; Guo, X. Influence of lithium oxide additives on densification and ionic conductivity of garnet-type Li6.75La3Zr1.75Ta0.25O12 solid electrolytes. Solid State Ionics 2013, 253, 76–80. [Google Scholar] [CrossRef]
- Deviannapoorani, C.; Dhivya, L.; Ramakumar, S.; Murugan, R. Lithium ion transport properties of high conductive tellurium substituted Li7La3Zr2O12 cubic lithium garnets. J. Power Sources 2013, 240, 18–25. [Google Scholar] [CrossRef]
- Ramakumar, S.; Satyanarayana, L.; Manorama, S.V.; Murugan, R. Structure and Li+ dynamics of Sb-doped Li7La3Zr2O12 fast lithium ion conductors. Phys. Chem. Chem. Phys. 2013, 15, 11327. [Google Scholar] [CrossRef]
- Dhivya, L.; Janani, N.; Palanivel, B.; Murugan, R. Li+ transport properties of W substituted Li7La3Zr2O12 cubic lithium garnets. AIP Adv. 2013, 3, 082115. [Google Scholar] [CrossRef] [Green Version]
- Buschmann, H.; Berendts, S.; Mogwitz, B.; Janek, J. Lithium metal electrode kinetics and ionic conductivity of the solid lithium ion conductors “Li7La3Zr2O12” and Li7−xLa3Zr2−xTaxO12 with garnet-type structure. J. Power Sources 2012, 206, 236–244. [Google Scholar] [CrossRef]
- Il’Ina, E.; Andreev, O.; Antonov, B.; Batalov, N. Morphology and transport properties of the solid electrolyte Li7La3Zr2O12 prepared by the solid-state and citrate–nitrate methods. J. Power Sources 2012, 201, 169–173. [Google Scholar] [CrossRef]
- Tan, J.; Tiwari, A. Synthesis of Cubic Phase Li7La3Zr2O12 Electrolyte for Solid-State Lithium-Ion Batteries. Electrochem. Solid-State Lett. 2012, 15, A37. [Google Scholar] [CrossRef]
- Huang, M.; Dumon, A.; Nan, C.-W. Effect of Si, In and Ge doping on high ionic conductivity of Li7La3Zr2O12. Electrochem. Commun. 2012, 21, 62–64. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, W. High Ionic Conductivity Lithium Garnet Oxides of Li7−xLa3Zr2−xTaxO12 Compositions. Electrochem. Solid-State Lett. 2012, 15, A68–A71. [Google Scholar] [CrossRef]
- Li, Y.; Han, J.-T.; Wang, C.-A.; Xie, H.; Goodenough, J.B. Optimizing Li+ conductivity in a garnet framework. J. Mater. Chem. 2012, 22, 15357–15361. [Google Scholar] [CrossRef]
- Narayanan, S.; Epp, V.; Wilkening, M.; Thangadurai, V. Macroscopic and microscopic Li+ transport parameters in cubic garnet-type “Li6.5La2.5Ba0.5ZrTaO12” as probed by impedance spectroscopy and NMR. RSC Adv. 2012, 2, 2553–2561. [Google Scholar] [CrossRef]
- Murugan, R.; Ramakumar, S.; Janani, N. High conductive yttrium doped Li7La3Zr2O12 cubic lithium garnet. Electrochem. Commun. 2011, 13, 1373–1375. [Google Scholar] [CrossRef]
- Ohta, S.; Kobayashi, T.; Asaoka, T. High lithium ionic conductivity in the garnet-type oxide Li7−X La3(Zr2−X, NbX)O12 (X = 0–2). J. Power Sources 2011, 196, 3342–3345. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.-A.; Xie, H.; Cheng, J.; Goodenough, J.B. High lithium ion conduction in garnet-type Li6La3ZrTaO12. Electrochem. Commun. 2011, 13, 1289–1292. [Google Scholar] [CrossRef]
- Kotobuki, M.; Munakata, H.; Kanamura, K.; Sato, Y.; Yoshida, T. Compatibility of Li7La3Zr2O12 Solid Electrolyte to All-Solid-State Battery Using Li Metal Anode. J. Electrochem. Soc. 2010, 157, A1076. [Google Scholar] [CrossRef]
- Zaiß, T.; Ortner, M.; Murugan, R.; Weppner, W. Fast ionic conduction in cubic hafnium garnet Li7La3Hf2O12. Ionics 2010, 16, 855–858. [Google Scholar] [CrossRef]
- Awaka, J.; Kijima, N.; Kataoka, K.; Hayakawa, H.; Ohshima, K.-I.; Akimoto, J. Neutron powder diffraction study of tetragonal Li7La3Hf2O12 with the garnet-related type structure. J. Solid State Chem. 2010, 183, 180–185. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Gao, Y.; Fang, Q. Lithium-ionic diffusion and electrical conduction in the Li7La3Ta2O13 compounds. Solid State Ionics 2009, 180, 1252–1256. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Effect of lithium ion content on the lithium ion conductivity of the garnet-like structure Li5+xBaLa2Ta2O11.5+0.5x (x = 0–2). Appl. Phys. A 2008, 91, 615–620. [Google Scholar] [CrossRef]
- Lee, C.-H.; Park, G.-J.; Choi, J.; Doh, C.-H.; Bae, D.-S.; Kim, J.-S.; Lee, S.-M. Low temperature synthesis of garnet type solid electrolyte by modified polymer complex process and its characterization. Mater. Res. Bull. 2016, 83, 309–315. [Google Scholar] [CrossRef]
- Sakamoto, J.; Rangasamy, E.; Kim, H.; Kim, Y.; Wolfenstine, J. Synthesis of nano-scale fast ion conducting cubic Li7La3Zr2O12. Nanotechnology 2013, 24, 424005. [Google Scholar] [CrossRef] [PubMed]
- Raskovalov, A.; Il’Ina, E.; Antonov, B. Structure and transport properties of Li7La3Zr2−0.75xAlxO12 superionic solid electrolytes. J. Power Sources 2013, 238, 48–52. [Google Scholar] [CrossRef]
- Janani, N.; Ramakumar, S.; Dhivya, L.; Deviannapoorani, C.; Saranya, K.; Murugan, R. Synthesis of cubic Li7La3Zr2O12 by modified sol–gel process. Ionics 2011, 17, 575–580. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, F.; Tu, R.; Shen, Q.; Zhang, L. Field assisted sintering of dense Al-substituted cubic phase Li7La3Zr2O12 solid electrolytes. J. Power Sources 2014, 268, 960–964. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Miyashiro, H.; Takeuchi, T.; Shigemura, H.; Balakrishnan, N.; Tabuchi, M.; Kageyama, H.; Iwahori, T. All-solid-state lithium secondary battery with ceramic/polymer composite electrolyte. Solid State Ionics 2002, 152–153, 137–142. [Google Scholar] [CrossRef]
- Kumazaki, S.; Iriyama, Y.; Kim, K.-H.; Murugan, R.; Tanabe, K.; Yamamoto, K.; Hirayama, T.; Ogumi, Z. High lithium ion conductive Li7La3Zr2O12 by inclusion of both Al and Si. Electrochem. Commun. 2011, 13, 509–512. [Google Scholar] [CrossRef]
- Li, Y.; Han, J.; Wang, C.-A.; Vogel, S.C.; Xie, H.; Xu, M.; Goodenough, J.B. Ionic distribution and conductivity in lithium garnet Li7La3Zr2O12. J. Power Sources 2012, 209, 278–281. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Y.-Q.; Guo, X.-X.; Yang, C.; Yi-Qiu, L.; Xiang-Xin, G. Densification and lithium ion conductivity of garnet-type Li7−xLa3Zr2−xTaxO12(x= 0.25) solid electrolytes. Chin. Phys. B 2013, 22, 078201. [Google Scholar] [CrossRef]
- Rosenkiewitz, N.; Schuhmacher, J.; Bockmeyer, M.; Deubener, J. Nitrogen-free sol–gel synthesis of Al-substituted cubic garnet Li7La3Zr2O12 (LLZO). J. Power Sources 2015, 278, 104–108. [Google Scholar] [CrossRef]
- Takano, R.; Tadanaga, K.; Hayashi, A.; Tatsumisago, M. Low temperature synthesis of Al-doped Li7La3Zr2O12 solid electrolyte by a sol–gel process. Solid State Ionics 2014, 255, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, Y.; Kami, K.; Watanabe, K.; Saito, N.; Ohnishi, T.; Takada, K.; Sudo, R.; Imanishi, N. Transparent cubic garnet-type solid electrolyte of Al2O3-doped Li7La3Zr2O12. Solid State Ionics 2015, 278, 172–176. [Google Scholar] [CrossRef]
- Qin, S.; Zhu, X.; Jiang, Y.; Ling, M.; Hu, Z.; Zhu, J. Extremely dense microstructure and enhanced ionic conductivity in hot-isostatic pressing treated cubic garnet-type solid electrolyte of Ga2O3-doped Li7La3Zr2O12. Funct. Mater. Lett. 2018, 11, 1850029. [Google Scholar] [CrossRef]
- Wolfenstine, J.; Sakamoto, J.; Allen, J.L. Electron microscopy characterization of hot-pressed Al substituted Li7La3Zr2O12. J. Mater. Sci. 2012, 47, 4428–4431. [Google Scholar] [CrossRef]
- Wolfenstine, J.; Ratchford, J.; Rangasamy, E.; Sakamoto, J.; Allen, J.L. Synthesis and high Li-ion conductivity of Ga-stabilized cubic Li7La3Zr2O12. Mater. Chem. Phys. 2012, 134, 571–575. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, J.; Chen, F.; Tu, R.; Shen, Q.; Zhang, X.; Zhang, L. Preparation of cubic Li7La3Zr2O12 solid electrolyte using a nano-sized core–shell structured precursor. J. Alloy. Compd. 2015, 644, 793–798. [Google Scholar] [CrossRef]
- Botros, M.; Djenadic, R.; Clemens, O.; Möller, M.; Hahn, H. Field assisted sintering of fine-grained Li7−3La3Zr2Al O12 solid electrolyte and the influence of the microstructure on the electrochemical performance. J. Power Sources 2016, 309, 108–115. [Google Scholar] [CrossRef]
- Baek, S.-W.; Lee, J.-M.; Kim, T.Y.; Song, M.-S.; Park, Y. Garnet related lithium ion conductor processed by spark plasma sintering for all solid state batteries. J. Power Sources 2014, 249, 197–206. [Google Scholar] [CrossRef]
- Mei, A.; Jiang, Q.-H.; Lin, Y.-H.; Nan, C.-W. Lithium lanthanum titanium oxide solid-state electrolyte by spark plasma sintering. J. Alloy. Compd. 2009, 486, 871–875. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, F.; Li, J.; Zhang, L.; Gu, J.; Zhang, D.; Saito, K.; Guo, Q.; Luo, P.; Dong, S. Regulation mechanism of bottleneck size on Li+ migration activation energy in garnet-type Li7La3Zr2O12. Electrochim. Acta 2018, 261, 137–142. [Google Scholar] [CrossRef]
- Wu, J.-F.; Pang, W.K.; Peterson, V.K.; Wei, L.; Guo, X. Garnet-Type Fast Li-Ion Conductors with High Ionic Conductivities for All-Solid-State Batteries. ACS Appl. Mater. Interfaces 2017, 9, 12461–12468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miara, L.J.; Ong, S.P.; Mo, Y.; Richards, W.D.; Park, Y.; Lee, J.-M.; Lee, H.S.; Ceder, G. Effect of Rb and Ta Doping on the Ionic Conductivity and Stability of the Garnet Li7+2x–y(La3–xRbx)(Zr2–yTay)O12 (0 ≤ x ≤ 0.375, 0 ≤ y ≤ 1) Superionic Conductor: A First Principles Investigation. Chem. Mater. 2013, 25, 3048–3055. [Google Scholar] [CrossRef]
- Kotobuki, M.; Kanamura, K.; Sato, Y.; Yoshida, T. Fabrication of all-solid-state lithium battery with lithium metal anode using Al2O3-added Li7La3Zr2O12 solid electrolyte. J. Power Sources 2011, 196, 7750–7754. [Google Scholar] [CrossRef]
- Düvel, A.; Kuhn, A.; Robben, L.; Wilkening, M.; Heitjans, P. Mechanosynthesis of Solid Electrolytes: Preparation, Characterization, and Li Ion Transport Properties of Garnet-Type Al-Doped Li7La3Zr2O12 Crystallizing with Cubic Symmetry. J. Phys. Chem. C 2012, 116, 15192–15202. [Google Scholar] [CrossRef]
- Allen, J.; Wolfenstine, J.; Rangasamy, E.; Sakamoto, J. Effect of substitution (Ta, Al, Ga) on the conductivity of Li7La3Zr2O12. J. Power Sources 2012, 206, 315–319. [Google Scholar] [CrossRef]
- Rettenwander, D.; Geiger, C.A.; Amthauer, G. Synthesis and Crystal Chemistry of the Fast Li-Ion Conductor Li7La3Zr2O12Doped with Fe. Inorg. Chem. 2013, 52, 8005–8009. [Google Scholar] [CrossRef]
- Hubaud, A.A.; Schroeder, D.J.; Key, B.; Ingram, B.J.; Dogan, F.; Vaughey, J.T. Low temperature stabilization of cubic (Li7−xAlx/3)La3Zr2O12: Role of aluminum during formation. J. Mater. Chem. A 2013, 1, 8813. [Google Scholar] [CrossRef]
- Galven, C.; Fourquet, J.-L.; Crosnier-Lopez, M.-P.; Le Berre, F. Instability of the Lithium Garnet Li7La3Sn2O12: Li+/H+Exchange and Structural Study. Chem. Mater. 2011, 23, 1892–1900. [Google Scholar] [CrossRef]
- Thangadurai, V.; Weppner, W. Investigations on electrical conductivity and chemical compatibility between fast lithium ion conducting garnet-like Li6BaLa2Ta2O12 and lithium battery cathodes. J. Power Sources 2005, 142, 339–344. [Google Scholar] [CrossRef]
- Gupta, A.; Murugan, R.; Paranthaman, M.; Bi, Z.; Bridges, C.A.; Nakanishi, M.; Sokolov, A.P.; Han, K.S.; Hagaman, E.; Xie, H.; et al. Optimum lithium-ion conductivity in cubic Li7−xLa3Hf2−xTaxO12. J. Power Sources 2012, 209, 184–188. [Google Scholar] [CrossRef]
- Miara, L.J.; Richards, W.D.; Wang, Y.; Ceder, G. First-Principles Studies on Cation Dopants and Electrolyte|Cathode Interphases for Lithium Garnets. Chem. Mater. 2015, 27, 4040–4047. [Google Scholar] [CrossRef]
- Alpen, U.V.; Bell, M.F.; Wichelhaus, W.; Cheung, K.Y.; Dudley, G.J. Ionic conductivity of Li14Zn(GeO44(Lisicon). Electrochimica Acta 1978, 23, 1395–1397. [Google Scholar] [CrossRef]
- Bruce, P.G.; West, A.R. The A-C Conductivity of Polycrystalline LISICON, Li2+2x Zn1−x GeO4, and a Model for Intergranular Constriction Resistances. J. Electrochem. Soc. 1983, 130, 662–669. [Google Scholar] [CrossRef]
- Shao, C.; Liu, H.; Yu, Z.; Zheng, Z.; Sun, N.; Diao, C. Structure and ionic conductivity of cubic Li7La3Zr2O12 solid electrolyte prepared by chemical co-precipitation method. Solid State Ionics 2016, 287, 13–16. [Google Scholar] [CrossRef]
- Jonson, R.A.; McGinn, P.J. Tape casting and sintering of Li7La3Zr1.75Nb0.25Al0.1O12 with Li3BO3 additions. Solid State Ionics 2018, 323, 49–55. [Google Scholar] [CrossRef]
- Hayamizu, K.; Matsuda, Y.; Matsui, M.; Imanishi, N. Lithium ion diffusion measurements on a garnet-type solid conductor Li6.6La3Zr1.6Ta0.4O12 by using a pulsed-gradient spin-echo NMR method. Solid State Nucl. Magn. Reson. 2015, 70, 21–27. [Google Scholar] [CrossRef]
- A Yoon, S.; Oh, N.R.; Yoo, A.R.; Lee, H.G. Preparation and Characterization of Ta-substituted Li7La3Zr2−xO12 Garnet Solid Electrolyte by Sol-Gel Processing. J. Korean Ceram. Soc. 2017, 54, 278–284. [Google Scholar] [CrossRef]
- Cheng, S.H.-S.; He, K.-Q.; Liu, Y.; Zha, J.-W.; Kamruzzaman; Ma, L.W.; Dang, Z.-M.; Li, R.K.; Chung, C. Electrochemical performance of all-solid-state lithium batteries using inorganic lithium garnets particulate reinforced PEO/LiClO4 electrolyte. Electrochim. Acta 2017, 253, 430–438. [Google Scholar] [CrossRef]
- Stanje, B.; Rettenwander, D.; Breuer, S.; Uitz, M.; Berendts, S.; Lerch, M.; Uecker, R.; Redhammer, G.; Hanzu, I.; Wilkening, M. Solid Electrolytes: Extremely Fast Charge Carriers in Garnet-Type Li6La3ZrTaO12 Single Crystals. Ann. Phys. 2017, 529, 1700140. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Liu, T.; Shen, Y.; Lin, Y.; Nan, C.-W. Garnet-type oxide electrolyte with novel porous-dense bilayer configuration for rechargeable all-solid-state lithium batteries. Ionics 2017, 23, 2521–2527. [Google Scholar] [CrossRef]
- El-Shinawi, H.; Cussen, E.J.; Corr, S.A. Enhancement of the lithium ion conductivity of Ta-doped Li7La3Zr2O12 by incorporation of calcium. Dalton Trans. 2017, 46, 9415–9419. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Li, Y.; Yang, T.; Cao, Z.; He, W.; Gao, Y. High lithium ionic conductivity in the garnet-type oxide Li7−2xLa3Zr2−xMoxO12 (x = 0–0.3) ceramics by sol-gel method. J. Am. Ceram. Soc. 2017, 100, 1527–1533. [Google Scholar] [CrossRef]
- Liu, C.; Rui, K.; Shen, C.; Badding, M.E.; Zhang, G.; Wen, Z. Reversible ion exchange and structural stability of garnet-type Nb-doped Li7La3Zr2O12 in water for applications in lithium batteries. J. Power Sources 2015, 282, 286–293. [Google Scholar] [CrossRef]
- Tang, Y.; Luo, Z.; Liu, T.; Liu, P.; Li, Z.; Lu, A. Effects of B2O3 on microstructure and ionic conductivity of Li6.5La3Zr1.5Nb0.5O12 solid electrolyte. Ceram. Int. 2017, 43, 11879–11884. [Google Scholar] [CrossRef]
- Hu, S.; Li, Y.-F.; Yang, R.; Yang, Z.; Wang, L. Structure and ionic conductivity of Li7La3Zr2−xGexO12 garnet-like solid electrolyte for all solid state lithium ion batteries. Ceram. Int. 2018, 44, 6614–6618. [Google Scholar] [CrossRef]
- Gu, W.; Ezbiri, M.; Rao, R.P.; Avdeev, M.; Adams, S. Effects of penta- and trivalent dopants on structure and conductivity of Li7La3Zr2O12. Solid State Ionics 2015, 274, 100–105. [Google Scholar] [CrossRef]
- Rettenwander, D.; Redhammer, G.; Preishuber-Pflügl, F.; Cheng, L.; Miara, L.; Wagner, R.; Welzl, A.; Suard, E.; Doeff, M.; Wilkening, M.; et al. Structural and Electrochemical Consequences of Al and Ga Cosubstitution in Li7La3Zr2O12 Solid Electrolytes. Chem. Mater. 2016, 28, 2384–2392. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, S.; Ramezanipour, F.; Thangadurai, V. Enhancing Li Ion Conductivity of Garnet-Type Li5La3Nb2O12 by Y- and Li-Codoping: Synthesis, Structure, Chemical Stability, and Transport Properties. J. Phys. Chem. C 2012, 116, 20154–20162. [Google Scholar] [CrossRef]
- Dhivya, L.; Murugan, R. Effect of Simultaneous Substitution of Y and Ta on the Stabilization of Cubic Phase, Microstructure, and Li+ Conductivity of Li7La3Zr2O12 Lithium Garnet. ACS Appl. Mater. Interfaces 2014, 6, 17606–17615. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Redhammer, G.J.; Rettenwander, D.; Senyshyn, A.; Schmidt, W.; Wilkening, M.; Amthauer, G. Crystal Structure of Garnet-Related Li-Ion Conductor Li7–3xGaxLa3Zr2O12: Fast Li-Ion Conduction Caused by a Different Cubic Modification? Chem. Mater. 2016, 28, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Itami, Y.; Hayamizu, K.; Ishigaki, T.; Matsui, M.; Takeda, Y.; Yamamoto, O.; Imanishi, N. Phase relation, structure and ionic conductivity of Li7−x−3yAlyLa3Zr2−xTaxO12. RSC Adv. 2016, 6, 78210–78218. [Google Scholar] [CrossRef]
- Matsuda, Y.; Sakaida, A.; Sugimoto, K.; Mori, D.; Takeda, Y.; Yamamoto, O.; Imanishi, N. Sintering behavior and electrochemical properties of garnet-like lithium conductor Li6.25M0.25La3Zr2O12 (M: Al3+ and Ga3+). Solid State Ionics 2017, 311, 69–74. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Schnelle Lithiumionenleitung in granatartigem Li7La3Zr2O12. Angew. Chem. 2007, 119, 7925–7928. [Google Scholar] [CrossRef]
- Xia, W.; Xu, B.; Duan, H.; Guo, Y.; Kang, H.; Li, H.; Liu, H. Ionic Conductivity and Air Stability of Al-Doped Li7La3Zr2O12 Sintered in Alumina and Pt Crucibles. ACS Appl. Mater. Interfaces 2016, 8, 5335–5342. [Google Scholar] [CrossRef]
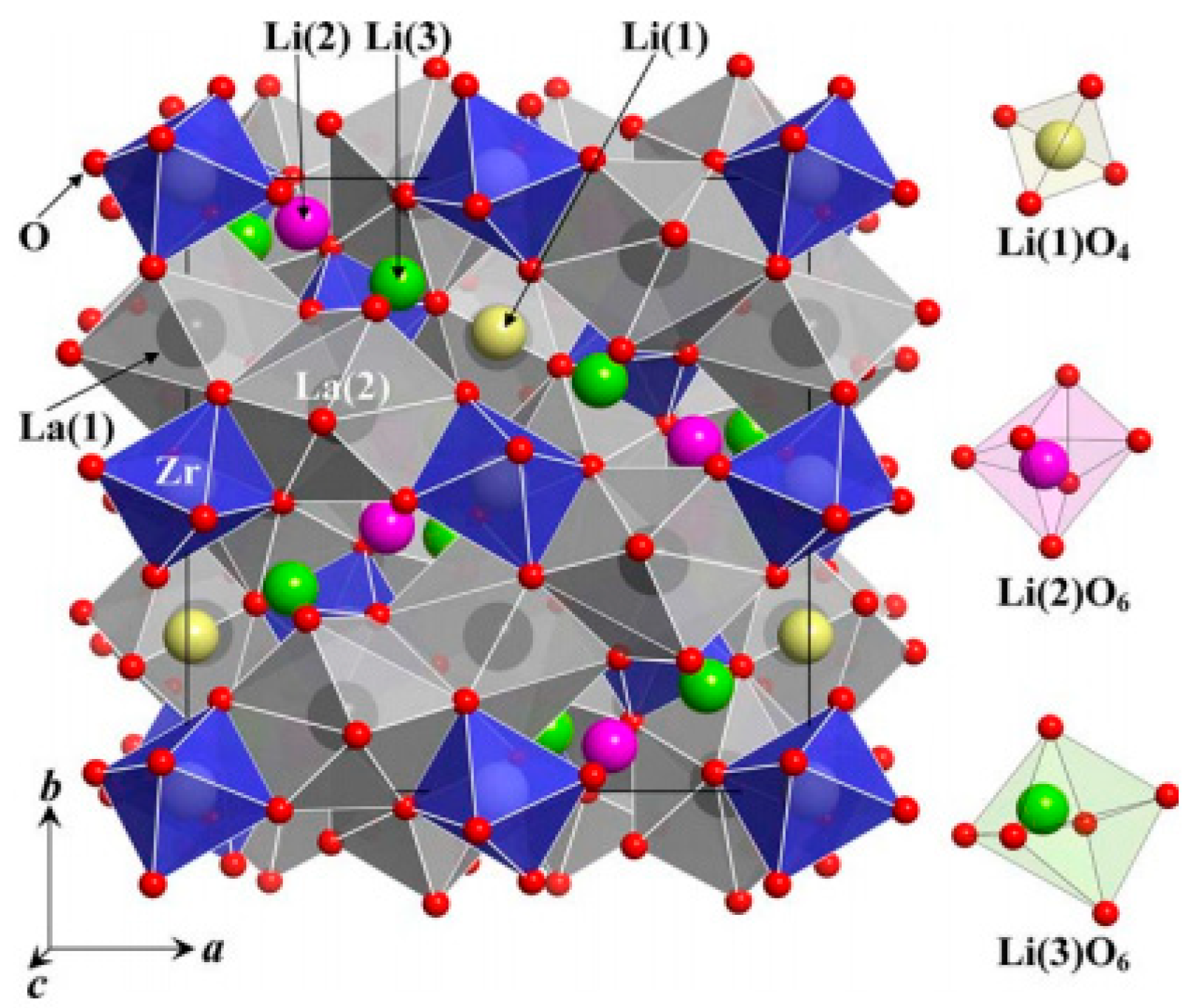

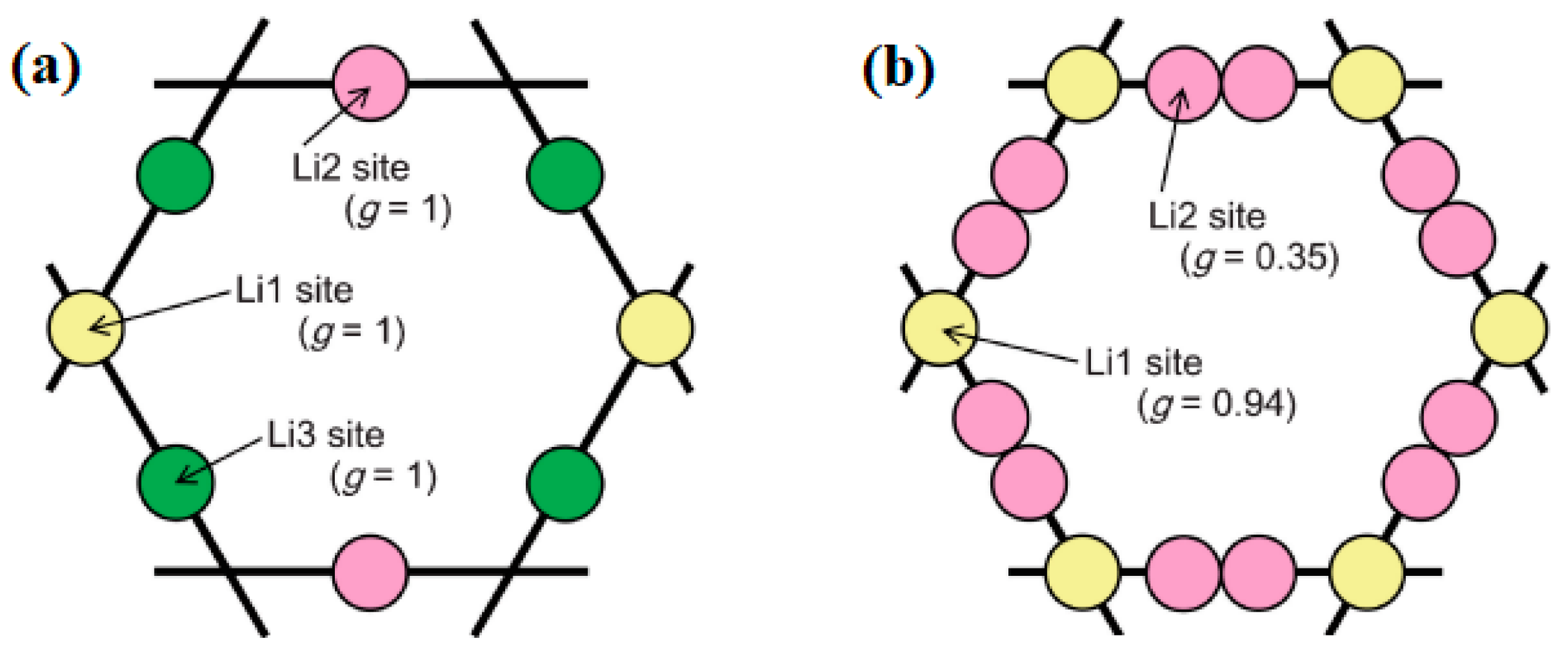
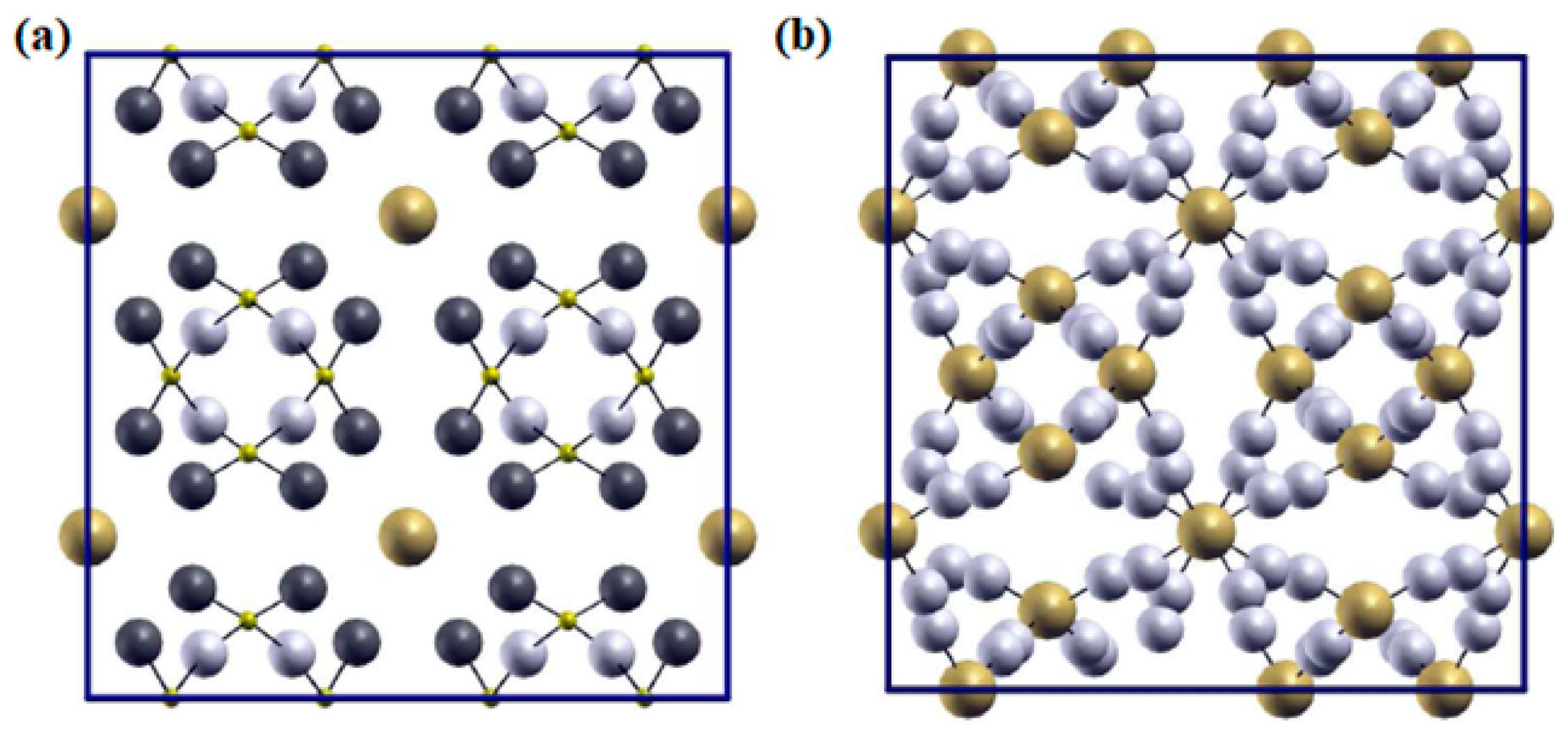
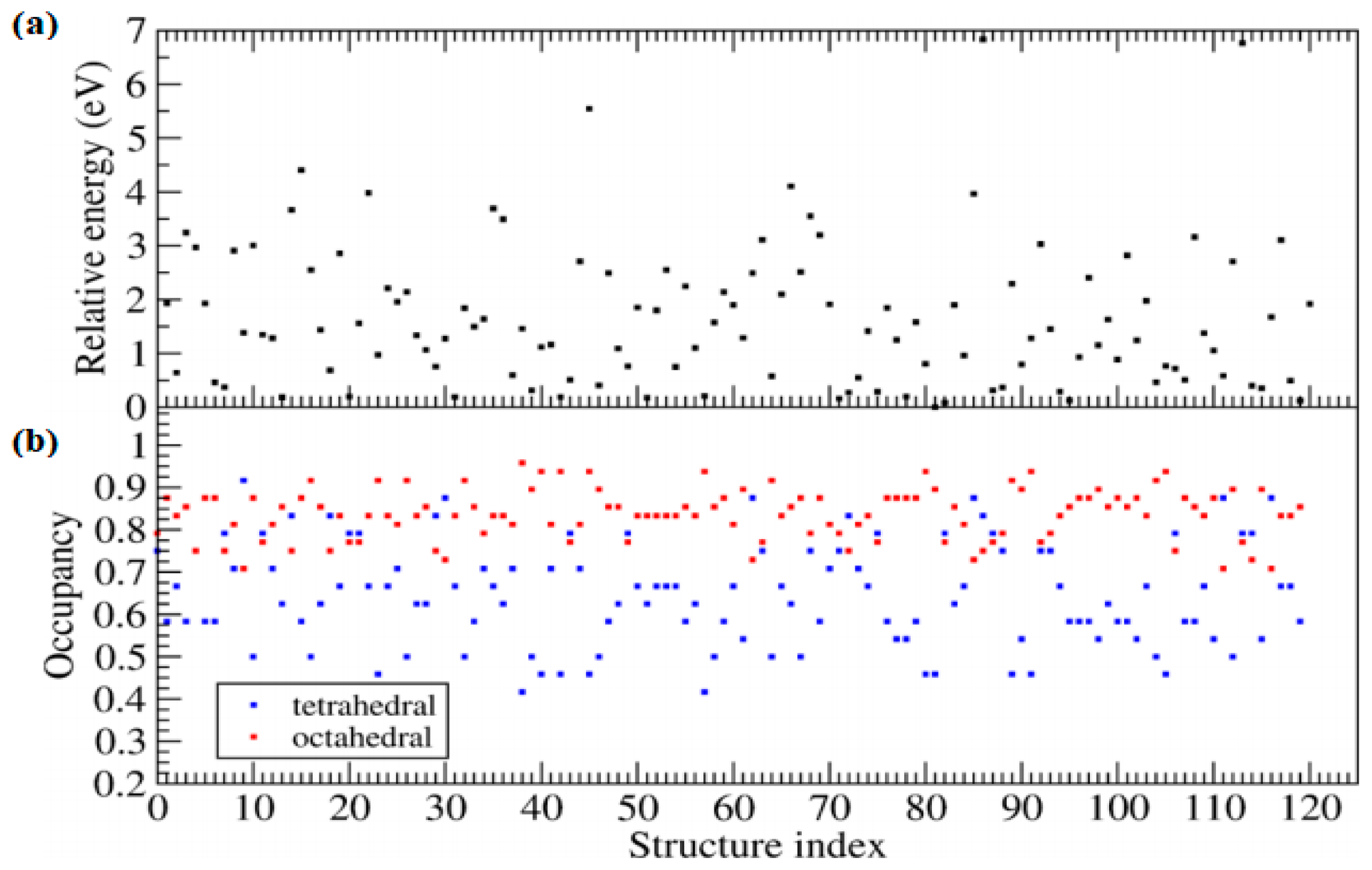
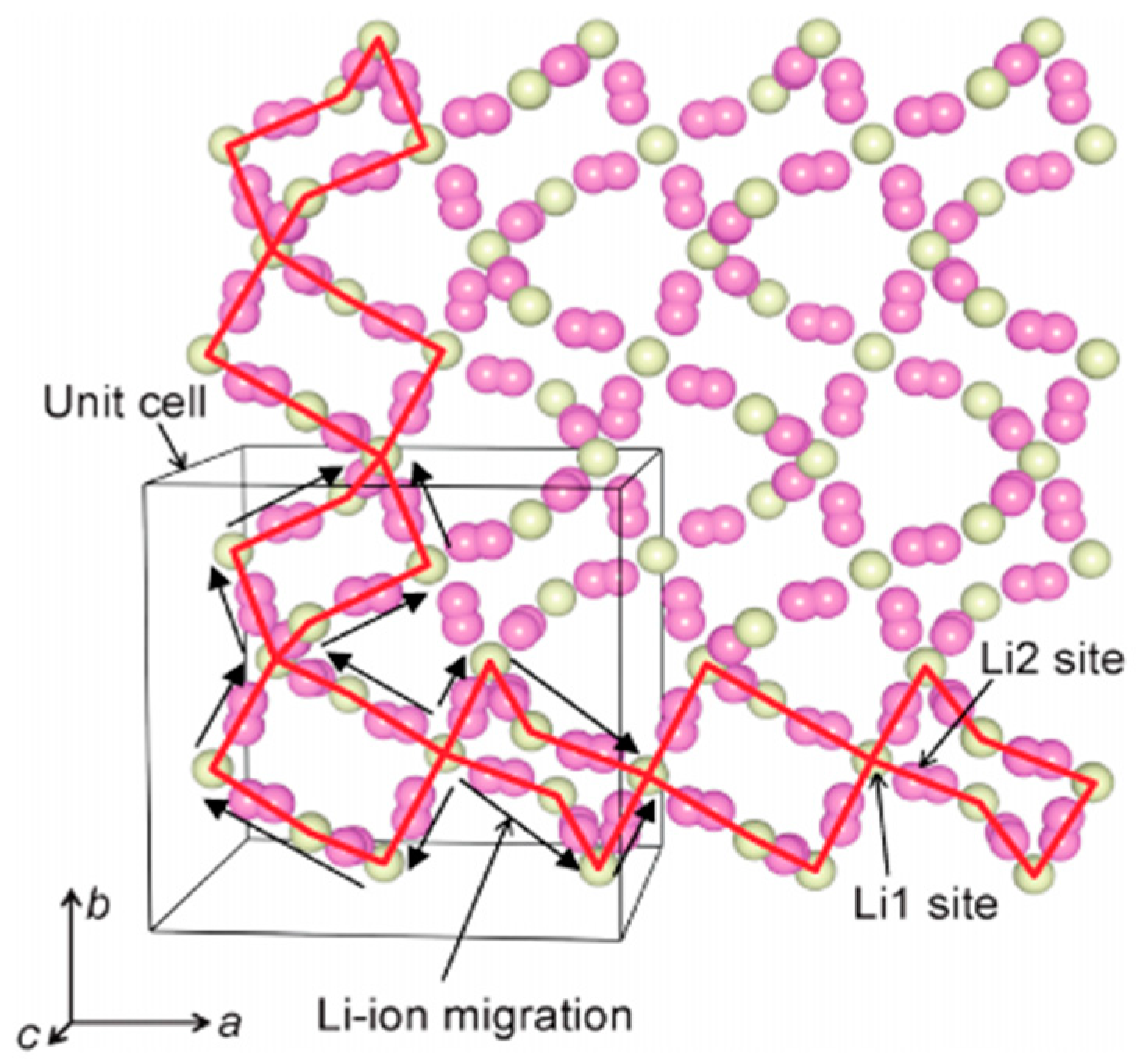
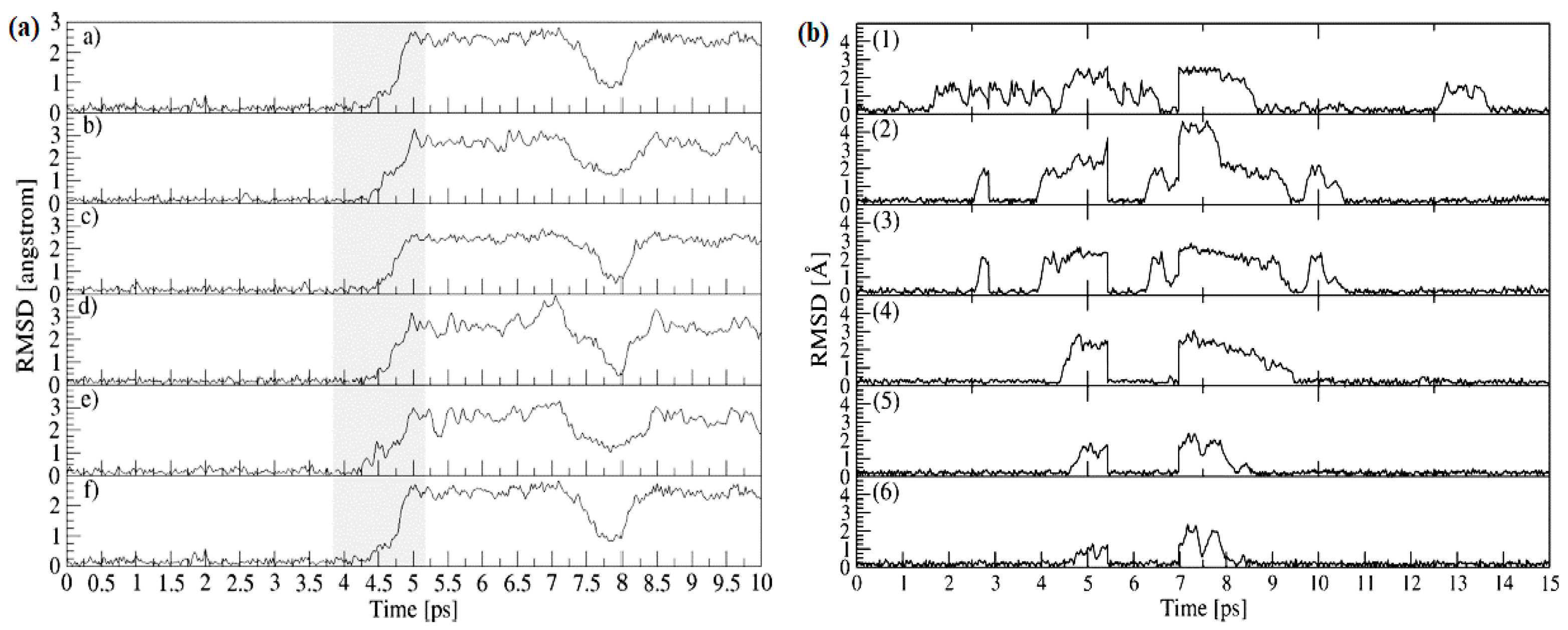
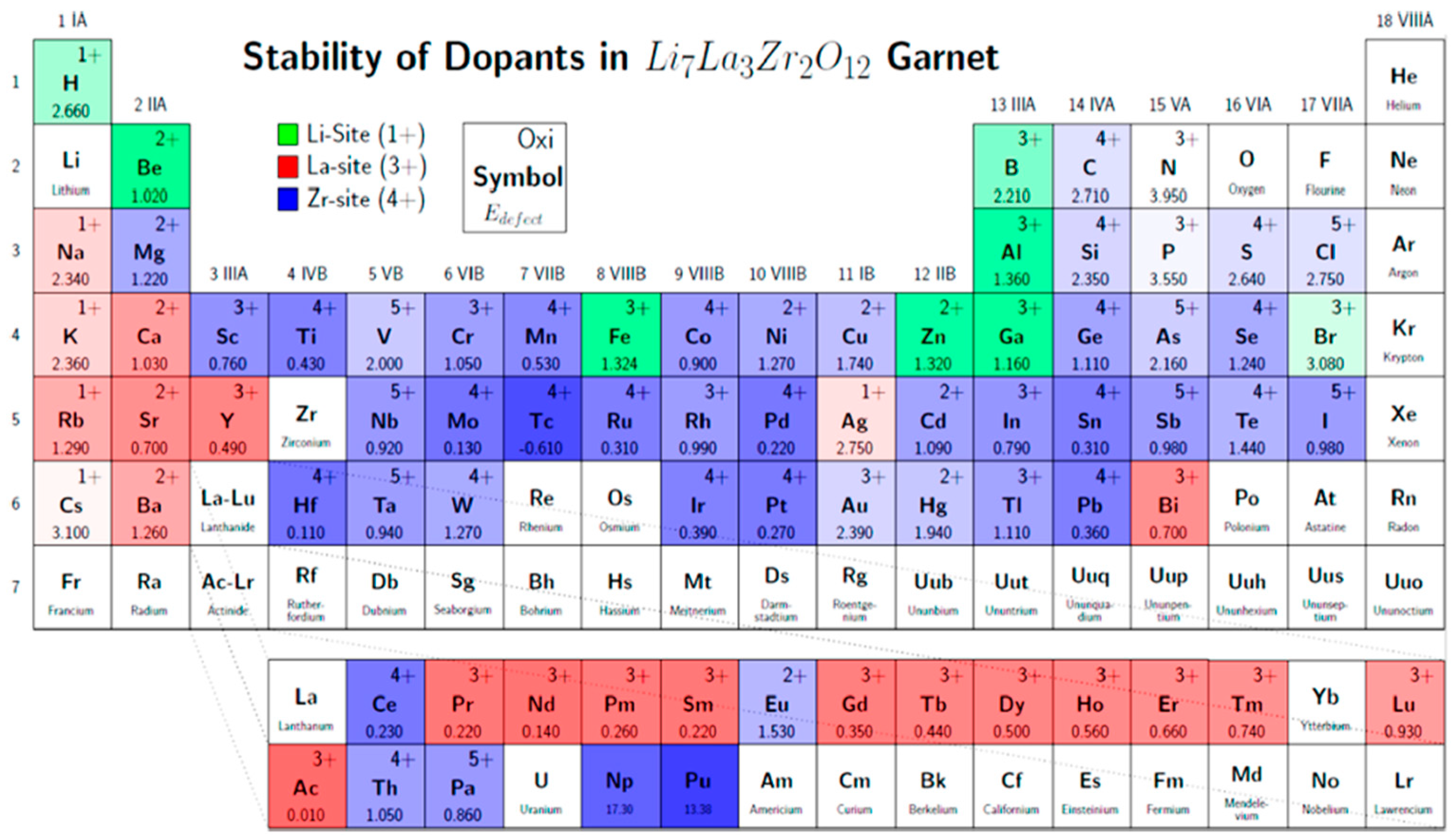
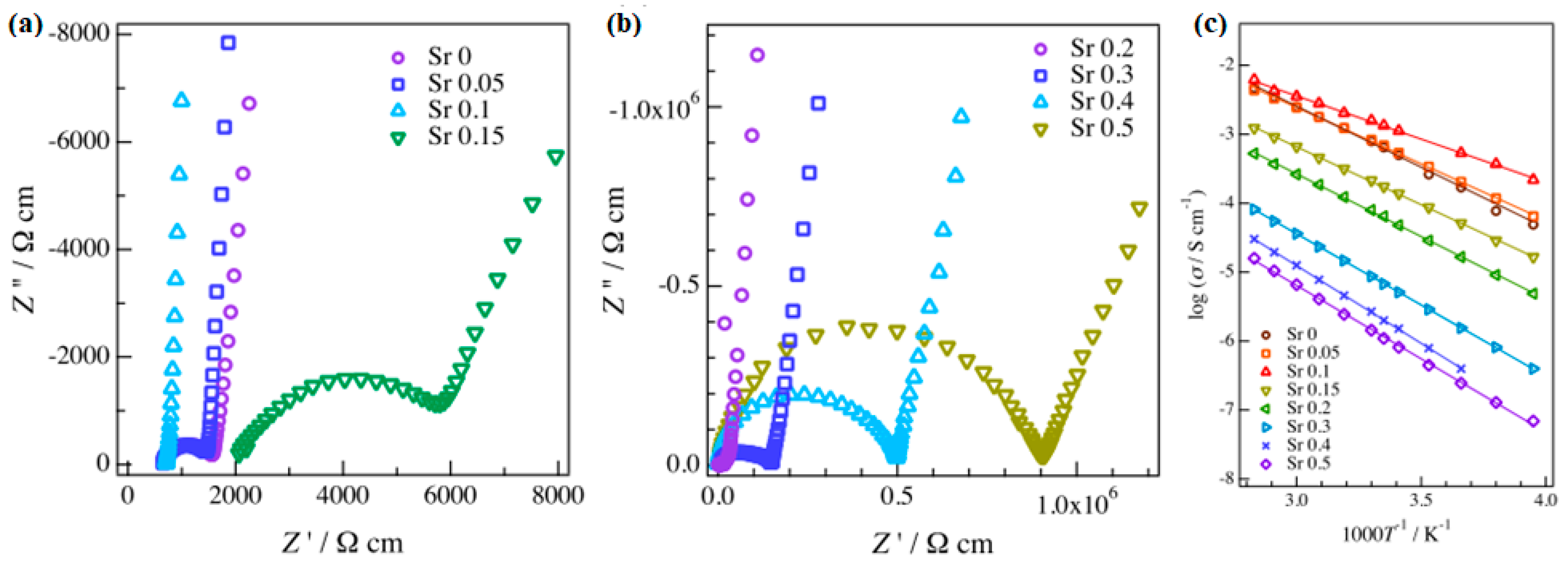
| Chemical Composition | Year Reported | Synthesis Process | Space Group | Ionic Conductivity, σ (mS/cm) | Activation Energy, Ea (eV) | σtotal Measuring Temperature (°C) | Ref. |
|---|---|---|---|---|---|---|---|
| Li7La3ZrNb0.5Y0.5O12 | 2018 | SSR | Iad | 0.830 (T) | 0.31 | 30 | [74] |
| Li6.4Fe0.2La3Zr2O12 | 2018 | SSR | Iad | 1.100 (T) | - | RT | [75] |
| Li6.25Ga0.25La3Zr2O12 | 2017 | SSR | Iad | 1.460 (T) | 0.25 | RT | [76] |
| Li7La3Zr2O12 | 2015 | SSR | Iad | 0.174 (B) | 0.26 (Bulk) | 25 | [77] |
| Li6.4La3Zr1.4Ta0.3Nb0.3O12 | 2015 | SSR | Iad | 0.606 (B) | 0.26 (Bulk) | 25 | [77] |
| Li6.4La3Zr1.4Ta0.4Nb0.2O12 | 2015 | SSR | Iad | 0.455 (B) | 0.28 (Bulk) | 25 | [77] |
| Li6.4La3Zr1.4Ta0.5Nb0.1O12 | 2015 | SSR | Iad | 0.444 (B) | 0.27 (Bulk) | 25 | [77] |
| Li6.4La3Zr1.4Ta0.6O12 | 2015 | SSR | Iad | 0.724 (B) | 0.24 (Bulk) | 25 | [77] |
| Li6.65La2.75Ba0.25Zr1.4Ta0.5Nb0.1O12 | 2015 | SSR | Iad | 0.527 (B) | 0.26 (Bulk) | 25 | [77] |
| 0.5F-LLZO | 2015 | SSR | Iad | 0.450 (T) | - | RT | [78] |
| 1.0F-LLZO | 2015 | SSR | Iad | 0.500 (T) | 0.26 | RT | [78] |
| 1.5CF-LLZO | 2015 | SSR | Iad | 0.050 (T) | - | RT | [78] |
| 2.0Al-LLZO | 2015 | SSR | Iad | 0.200 (T) | - | RT | [78] |
| Li7La3Zr2O12 | 2015 | SSR | I41/acd | 0.017 (T) | 0.47 | 25 | [79] |
| Li5.9La3Zr1.45W0.55O12 | 2015 | SSR | Iad | 0.440 (T) | 0.43 | 25 | [79] |
| Li6.5La3Zr1.75W0.25O12 | 2015 | SSR | Iad | 0.490 (T) | 0.42 | 25 | [79] |
| Li6.30La3Zr1.65W0.35O12 | 2015 | SSR | Iad | 0.660 (T) | 0.42 | 25 | [79] |
| Li6.1La3Zr1.55W0.45O12 | 2015 | SSR | Iad | 0.640 (T) | 0.43 | [79] | |
| Li6La3Ta1.5Y0.50O12 | 2015 | SSR | Iad | 0.126 (T) | 0.37 | 23 | [80] |
| Li6.5La3Ta1.25Y0.75O12 | 2015 | SSR | Iad | 0.183 (T) | 0.33 | 23 | [80] |
| Li6.25La3Nb1.375Sc0.625O12 | 2015 | SSR | Iad | 0.102 (T) | 0.48 | 25 | [81] |
| Li6.25La3Nb0.75Zr1.25O12 | 2015 | SSR | Iad | 0.203 (T) | 0.42 | 25 | [81] |
| Li6.5La1.5Ba1.5Ta2O12 | 2015 | SSR | Iad | 0.062 (T) | 0.40 | 33 | [37] |
| Li6.28Al0.24La3Zr2O12 | 2015 | SSR | Iad | 0.443 (T) | 0.37 | 30 | [82] |
| Li6.2La3Zr1.2Ta0.8O12 | 2015 | SSR | Iad | 2.3 10−3 (T) | 0.53 | 33 | [83] |
| Li5.5La3Zr1.25W0.75O12 | 2015 | SSR | Iad | 0.053 (T) | 0.43 | 25 | [84] |
| Li7La3Zr2O12 | 2014 | SSR | I41/acd | 9.90 10−3 (T) | 0.43 | 27 | [85] |
| Li6.75La3Zr1.75Ta0.25O12 | 2014 | SSR | Iad | 0.410 (T) | 0.42 | 27 | [85] |
| Li6.5La3Zr1.5Ta0.5O12 | 2014 | SSR | Iad | 0.610 (T) | 0.40 | 27 | [85] |
| Li6La3ZrTaO12 | 2014 | SSR | Iad | 0.210 (T) | 0.42 | 27 | [85] |
| Li6.925La3Zr1.925Sb0.075O12 | 2014 | SSR | Iad | 0.340 (T) | 0.37 | 30 | [86] |
| Li7La3Zr2O12 | 2014 | SSR | Iad | 0.120 (T) | 0.41 | 30 | [86] |
| Li7La3Nb2ScO12 | 2014 | SSR | Iad | 0.068 (T) | 0.40 | 25 | [87] |
| Li6La3Nb1.5Sc0.5O12 | 2014 | SSR | Iad | 0.102 (T) | 0.40 | 25 | [87] |
| Li6.25La3Nb1.375Sc0.625O12 | 2014 | SSR | Iad | 0.138 (T) | 0.36 | 25 | [87] |
| Li6.5La3Nb1.25Sc0.75O12 | 2014 | SSR | Iad | 0.126 (T) | 0.39 | 25 | [87] |
| Li5La3Nb2O12 | 2014 | SSR | Iad | 0.022 (T) | 0.45 | 25 | [87] |
| Li5.5La3Nb1.75Sc0.25O12 | 2014 | SSR | Iad | 0.052 (T) | 0.40 | 25 | [87] |
| Li6.5La3Ta1.25Y0.75O12 | 2014 | SSR | Iad | 0.183 (T) | 0.36 | 23 | [88] |
| Li6.75La3Zr1.75Ta0.25O12 with flow of O2 | 2014 | SSR | Iad | 0.740 (T) | 0.33 | 25 | [89] |
| Li6.75La3Zr1.75Ta0.25O12 with flow of Air | 2014 | SSR | Iad | 0.240 (T) | 0.39 | 25 | [89] |
| Li6.5La3Ta0.5Zr1.5O12 30 mol.% excess Li | 2014 | SSR | Iad | 0.433 (T) | - | RT | [54] |
| Li6La3Zr1.5Te0.5O12 | 2014 | SSR | Iad | 2.19 10−3 (T) | 0.37 | RT | [90] |
| Li6Ba0.5Sr0.5La2Ta2O12 | 2014 | SSR | Iad | 7.19 10−3 (T) | 0.45 | 18 | [91] |
| Li7La3Zr2O12 | 2013 | SSR | I41/acd | 5.77 10−3 (T) | 0.40 | RT | [92] |
| Li6.6La2.6Ce0.4Zr2O12 | 2013 | SSR | Iad | 0.0144 (T) | 0.48 | RT | [92] |
| Li6.4La2.4Ce0.6Zr2O12 | 2013 | SSR | Iad | 0.0126 (T) | 0.50 | RT | [92] |
| Li7.06La3Zr1.94Y0.06O12 | 2013 | SSR | Iad | 1.0 10−3 (B) | 0.47 | 23 | [93] |
| Li7.16La3Zr1.84Y0.16O12 | 2013 | SSR | Iad | 1.0 10−3 (B) | 0.47 | 23 | [93] |
| Li7Nd3Zr2O12 | 2013 | SSR | I41/acd | - | 0.66 | - | [94] |
| Li7La3Zr2O12 | 2013 | SSR | Iad | 0.500 (T) | 0.31 | 24 | [51] |
| Li6BaLa2Ta2O12 | 2013 | SSR | Iad | 0.10 (T) | 0.39 | 25 | [95] |
| Li7La3Ta2O13 | 2013 | SSR | Iad | 3.21 10−3 (B) | 0.55 | 40 | [96] |
| Li6.75La3Zr1.75Ta0.25O12 | 2013 | SSR | Iad | 0.640 (T) | 0.30 | 28 | [97] |
| Li6.75La3Zr1.875Te0.125O12 | 2013 | SSR | Iad | 0.330 (T) | 0.41 | 30 | [98] |
| Li6.6La3Zr1.6Sb0.4O12 | 2013 | SSR | Iad | 0.770 (T) | 0.34 | 30 | [99] |
| Li6.4La3Zr1.6W0.3O12 | 2013 | SSR | Iad | 0.789 (T) | 0.45 | 30 | [100] |
| 0.28Al-Li7La3Zr2O12 | 2012 | SSR | Iad | 0.350 (T) | 0.36 | 25 | [101] |
| Li6La3ZrTaO12 | 2012 | SSR | Iad | 0.260 (T) | 0.46 | 25 | [101] |
| Li6.5La3Zr1.5Ta0.5O12 | 2012 | SSR | Iad | 2.0 10−3 (T) | 0.49 | 25 | [101] |
| Li6.625La3Zr1.625Ta0.375O12 | 2012 | SSR | Iad | 0.520 (T) | 0.41 | 25 | [101] |
| Li7La3Zr2O12 | 2012 | SSR | I41/acd | 1.3 10−3 (B) | 0.46 | 20 | [102] |
| 0.204Al-Li7La3Zr2O12 | 2012 | SSR | Iad | 0.400 (T) | 0.26 | RT | [55] |
| Li7La3Zr2O12 | 2012 | SSR | Iad | 0.160 (T) | - | RT | [103] |
| Ge-Li7La3Zr2O12 | 2012 | SSR | Iad | 0.763 (T) | - | 25 | [104] |
| Li6.7La3Zr1.7Ta0.3O12 | 2012 | SSR | Iad | 0.690 (T) | 0.36 | RT | [105] |
| Li6.4La3Zr1.4Ta0.6O12 | 2012 | SSR | Iad | 1.0 (T) | 0.35 | 25 | [106] |
| Li6.5La2.5Ba0.5ZrTaO12 | 2012 | SSR | Iad | 0.090 (T) | 0.57 | 24 | [107] |
| Li7La3Zr2O12 | 2011 | SSR | I41/acd | 2.0 10−3 (T) | 0.49 | 25 | [47] |
| Li7La3Zr2O12 | 2011 | SSR | Iad | 0.400 (T) | 0.34 | 25 | [47] |
| Li7.06La3Y0.06Zr1.94O12 | 2011 | SSR | Iad | 0.810 (T) | 0.26 | 25 | [108] |
| Li6.75La3Zr1.75Nb0.25O12 | 2011 | SSR | Iad | 0.80 (T) | 0.31 | 25 | [109] |
| Li6La3ZrTaO12 | 2011 | SSR | Iad | 0.180 (T) | 0.42 | 25 | [110] |
| Li7La3Zr2O12 | 2010 | SSR | Iad | 0.180 (B) | - | 25 | [111] |
| Li7La3Hf2O12 | 2010 | SSR | Iad | 0.240 (B) | 0.29 | 25 | [112] |
| Li7La3Hf2O12 | 2010 | SSR | I41/acd | 9.85 10−4 (B) | 0.59 | 27 | [113] |
| Li7La3Ta2O12 | 2009 | SSR | Iad | 2.2 10−3 (T) | 0.38 | 27 | [114] |
| Li7La3Zr2O12 | 2009 | SSR | I41/acd | 1.63 10−3 (T) | 0.54 | 27 | [38] |
| Li7BaLa2Ta2O12.5 | 2008 | SSR | Iad | 0.097 (T) | 0.45 | 50 | [115] |
| Li5BaLa2Ta2O11.5 | 2008 | SSR | Iad | 4.9 10−3 (T) | 0.51 | 50 | [115] |
| Li5.5BaLa2Ta2O11.75 | 2008 | SSR | Iad | 0.031 (T) | 0.47 | 50 | [115] |
| Li5.75BaLa2Ta2O11.875 | 2008 | SSR | Iad | 0.089 (T) | 0.44 | 50 | [115] |
| Li7La3Zr2O12 | 2007 | SSR | Iad | 0.774 (T) | 0.30 | 25 | [36] |
| Li7La3Zr2O12 | 2016 | Sol-gel | Iad | 0.140 (T) | - | RT | [116] |
| Li6.4Ga0.2La3Zr2O12 | 2015 | Sol-gel | Iad | 0.024 (T) | 0.32 | RT | [69] |
| Li6.16Al0.28La3Zr2O12 | 2014 | Sol-gel | I41/acd | 3.0 10−4 (T) | - | 33 | [61] |
| Li6.16Al0.28La3Zr2O12 | 2014 | Sol-gel | Iad | 0.110 (T) | 0.38 | 33 | [61] |
| Li7La3Zr2O12 | 2013 | Sol-gel | Iad | 0.400 (T) | 0.41 | RT | [117] |
| Li7La3Zr1.89Al0.15O12 | 2013 | Sol-gel | Iad | 0.340 (T) | 0.33 | RT | [118] |
| Li6La3Zr2O11.5 | 2011 | Sol-gel | Iad | 0.139 (T) | - | 25 | [59] |
| Li7La3Zr2O12 | 2011 | Sol-gel | Iad | - | - | - | [119] |
| Li5La3Ta2O12 | 2010 | Sol-gel | Iad | 1.54 10−3 (T) | 0.57 | 25 | [57] |
| Al-Li7La3Zr2O12 | 2011 | Pechini | Iad | 0.200 (T) | - | 25 | [63] |
| Li7La3Zr2O12 | 2011 | Sol-gel and Pechini | Iad | - | - | - | [58] |
| Li7La3Zr2O12 | 2011 | Sol-gel and Pechini | I41/acd | 3.12 10−4 (T) | 0.67 | 25 | [58] |
| Li5La3Bi2O12 | 2010 | Pechini | Iad | 0.024 (T) | 0.40 | 26 | [62] |
| Al-LLZO | 2016 | RFMS | Iad | 0.120 (T) | 0.47 | 25 | [64] |
| Li7La3Zr2O12 | 2012 | RFMS | I41/acd | 4.0 10−4 (T) | 0.70 | 25 | [65] |
| Li7La3Zr2O12 | 2013 | PLD | Iad | 2.50 10−3 (T) | 0.52 | 25 | [68] |
| Li7La3Zr2O12 | 2013 | PLD | Iad | 0.010 (T) | 0.55 | 25 | [68] |
| Laser annealed Li7La3Zr2O12 | 2012 | PLD | I41/acd | 7.36 10−4 (T) | 0.32 | RT | [67] |
| Deposited on As Li7La3Zr2O12 | 2012 | PLD | I41/acd | 3.35 10−4 (T) | 0.36 | RT | [67] |
| Annealed at 800 °C Li7La3Zr2O12 | 2012 | PLD | I41/acd | 1.78 10−4 (T) | 0.41 | RT | [67] |
| Chemical Composition | Sintering Temperature (°C) | Sintering Duration (Hours) | Total Li Ionic Conductivity (mS/cm) at (Temperature) | Reference |
|---|---|---|---|---|
| Li7La3ZrNb0.5Y0.5O12 | 1230 | 15 | 0.830 (30 °C) | [74] |
| Li7La3Zr2O12 | 1230 | 36 | 0.30 (RT) | [36] |
| Li6.75La3Zr1.75Ta0.25O12 (0 wt.% Li2O) | 1230 | 6 | 0.220 (28 °C) | [97] |
| Li7La3Zr2O12 (1.7 wt.% Al + 0.1 wt.% Si) | 1230 | 36 | 0.680 (25 °C) | [122] |
| Li7La3Zr2O12 (1.3 wt.% Al) | 1230 | 36 | 0.240 (25 °C) | [122] |
| Li6.75La3Zr1.75Ta0.25O12 (4 wt.% Li2O) | 1200 | 6 | 0.440 (28 °C) | [97] |
| Al-Li7La3Zr2O12 | 1200 | 36 | 0.014 (RT) | [123] |
| Al-Li7La3Zr2O12 | 1200 | 6 | 0.020 (RT) | [63] |
| Li6.75La3Zr1.75Nb0.25O12 | 1200 | 36 | 0.80 (RT) | [109] |
| Li6La3Zr2O11.5 | 1180 | 32 | 0.0140 (RT) | [59] |
| Li6.75La3Zr1.75Ta0.25O12 (1 wt.% Li3PO4) | 1175 | 6 | 0.720 (25 °C) | [124] |
| Li6.75La3Zr1.75Ta0.25O12 (6 wt.% Li2O) | 1170 | 6 | 0.640 (28 °C) | [97] |
| Li5.9Al0.2La3Zr1.75W0.25O12 | 1150 | 12 | 0.490 (RT) | [84] |
| Li7La3Zr1.89Al0.15O12 | 1150 | 1 | 0.0340 (RT) | [118] |
| Li6.75La3Zr1.75Ta0.25O12 (in Oxygen) | 1140 | 9 | 0.740 (25 °C) | [89] |
| Li6.75La3Zr1.75Ta0.25O12 (in Air) | 1140 | 9 | 0.240 (25 °C) | [89] |
| Li6.75La3Zr1.75Ta0.25O12 (in Nitrogen) | 1140 | 9 | 0.210 (25 °C) | [89] |
| Li6.75La3Zr1.75Ta0.25O12 (in Argon) | 1140 | 9 | 0.180 (25 °C) | [89] |
| Li6.4La3Zr1.75Ta0.6O12 | 1140 | 16 | 1.00 (RT) | [106] |
| Li7La3Zr2O12 | 1100 | 5 | 0.0140 (RT) | [116] |
| Li6.42Al0.32La3Zr1.91O12.02 | 1000 | 7 | 0.0015 (RT) | [125] |
| Li7La3Zr2O12 | 1000 | 4 | 0.040 (RT) | [117] |
| Al-Li7La3Zr2O12 | 900 | 5 | 0.0019 (RT) | [126] |
| Li7La3Zr2O12 | 900 | 5 | 0.0003 (RT) | [58] |
| Chemical Composition | Sintering Temperature (°C) | Sintering Duration (Hours) | Total Li Ionic Conductivity (mS/cm) at (Temperature) | Relative Density in % | Reference |
|---|---|---|---|---|---|
| Al2O3-doped Li7La3Zr2O12 | 1180 | 127 | 0.990 (25 °C) | 99.1 | [127] |
| Li6.55Ga0.15La3Zr2O12 | 1160 | 120 | 1.130 (25 °C) | 97.5 | [128] |
| Li6.6La2.6Ce0.4Zr2O12 | 1050 | 40 | 0.014 | 96.0 | [92] |
| Li6.24La3Zr2Al0.24O11.98 | 1000 | - | - | 97.0 | [129] |
| Li6.25La3Zr2Ga0.25O12 | 1000 | 40 | 0.350 (RT) | 91.0 | [130] |
| Li6.24La3Zr2Al0.24O11.98 | 1000 | 40 | 0.400 (RT) | 98.0 | [55] |
| Chemical Composition | Sintering Temperature (°C) | Sintering Duration (Hours) | Total Li Ionic Conductivity (mS/cm) at (Temperature) | Relative Density in % | Reference |
|---|---|---|---|---|---|
| Al doped LLZO | 1150 | 10 | 0.570 (RT) | 99.8 | [120] |
| Al doped LLZO | 1000 | 10 | 0.332 (RT) | 96.5 | [131] |
| Li6.49La3Zr2Al0.17O12 | 950 | 50 | 0.330 (25 °C) | 96.0 | [132] |
| Al doped LLZO | 900 | 10 | 0.034 (RT) | 88.9 | [131] |
| Chemical Composition | Sintering Temperature (°C) | Sintering Duration (Hours) | Total Li Ionic Conductivity (mS/cm) at (Temperature) | Relative Density in % | Reference |
|---|---|---|---|---|---|
| Li7−xLa3Zr1.5Ta0.5O12 | 1100 | 50 | 1.35 (25 °C) | - | [133] |
| Li3xLa2/3-3xTiO3 | 1100 | 39 | 1.00 (22 °C) | 100 | [121] |
| Li3xLa2/3-3xTiO3 | 1050 | 40 | 5.8 × 10−3 (RT) | 98.5 | [134] |
| Chemical Composition | Total Ionic Conductivity, σtotal (mS/cm) | Activation Energy, Ea (eV) | Reference |
|---|---|---|---|
| Li site substitution | |||
| Li6.28Al0.24La3Zr2O12 | 0.44 (30 °C) | 0.37 | [82] |
| Li6.22Al0.26La3Zr2O12 | 0.01 (25 °C) | 0.40 | [84] |
| Li6.25Ga0.25La3Zr2O12 | 1.46 (RT) | 0.25 | [76] |
| Li6.4Fe0.2La3Zr2O12 | 1.10 (RT) | - | [75] |
| Li6.4Al0.05Ga0.15La3Zr2O12 | 0.88 | 0.26 | [162] |
| La site substitution | |||
| Li6.6La2.6Ce0.4Zr2O12 | 0.01 (RT) | 0.48 | [92] |
| Li7Nd3Zr2O12 | - | 0.66 | [94] |
| Sr doped LLZO | 0.50 (25 °C) | 0.31 | [51] |
| Zr site substitution | |||
| Li6La3ZrTaO12 | 0.18 (25 °C) | 0.42 | [110] |
| Li6.75La3Zr1.75Nb0.25O12 | 0.80 (25 °C) | 0.31 | [109] |
| Li6.5La3Nb1.25Y0.75O12 | 0.27 (25 °C) (Bulk) | 0.36 | [163] |
| Li6.75La3Zr1.875Te0.125O12 | 0.33 (30 °C) | 0.41 | [98] |
| Li6.55La3Hf1.55Ta0.45O12 | 0.35 (22 °C) | 0.44 | [145] |
| Li6.6La3Zr1.6Sb0.4O12 | 0.77 (30 °C) | 0.34 | [99] |
| Li6.7La3Zr1.7Ta0.3O12 | 0.69 (RT) | 0.36 | [105] |
| Li6.4La3Zr1.6W0.3O12 | 0.79 (30 °C) | 0.45 | [100] |
| Li6.5La3Ta1.25Y0.75O12 | 0.18 (23 °C) | 0.36 | [88] |
| Li6.5La3Zr1.75W0.25O12 | 0.49 (25 °C) | 0.42 | [79] |
| Co-doping: substitution to two or more sites | |||
| Li6.6La2.5Y0.5Zr1.6Ta0.4O12 | 0.23 (27 °C) | 0.39 | [164] |
| Li6BaLa2Ta2O12 | 0.09 (50 °C) | 0.42 | [115] |
| Li6Ba0.5Sr0.5La2Ta2O12 | 7.1 10−3 (18 °C) | 0.45 | [91] |
| Li6Sr0.5Ca0.5La2Ta2O12 | 3.2 10−3 (18 °C) | 0.50 | [91] |
| Li6.5La2.5Ba0.5ZrTaO12 | 0.09 (24 °C) | 0.57 | [107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raju, M.M.; Altayran, F.; Johnson, M.; Wang, D.; Zhang, Q. Crystal Structure and Preparation of Li7La3Zr2O12 (LLZO) Solid-State Electrolyte and Doping Impacts on the Conductivity: An Overview. Electrochem 2021, 2, 390-414. https://doi.org/10.3390/electrochem2030026
Raju MM, Altayran F, Johnson M, Wang D, Zhang Q. Crystal Structure and Preparation of Li7La3Zr2O12 (LLZO) Solid-State Electrolyte and Doping Impacts on the Conductivity: An Overview. Electrochem. 2021; 2(3):390-414. https://doi.org/10.3390/electrochem2030026
Chicago/Turabian StyleRaju, Md Mozammal, Fadhilah Altayran, Michael Johnson, Danling Wang, and Qifeng Zhang. 2021. "Crystal Structure and Preparation of Li7La3Zr2O12 (LLZO) Solid-State Electrolyte and Doping Impacts on the Conductivity: An Overview" Electrochem 2, no. 3: 390-414. https://doi.org/10.3390/electrochem2030026
APA StyleRaju, M. M., Altayran, F., Johnson, M., Wang, D., & Zhang, Q. (2021). Crystal Structure and Preparation of Li7La3Zr2O12 (LLZO) Solid-State Electrolyte and Doping Impacts on the Conductivity: An Overview. Electrochem, 2(3), 390-414. https://doi.org/10.3390/electrochem2030026







