Structural and Electrochemical Properties of the High Ni Content Spinel LiNiMnO4
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of LiNiyMn1−yO4 Samples
2.2. Material Characterization
3. Results and Discussion
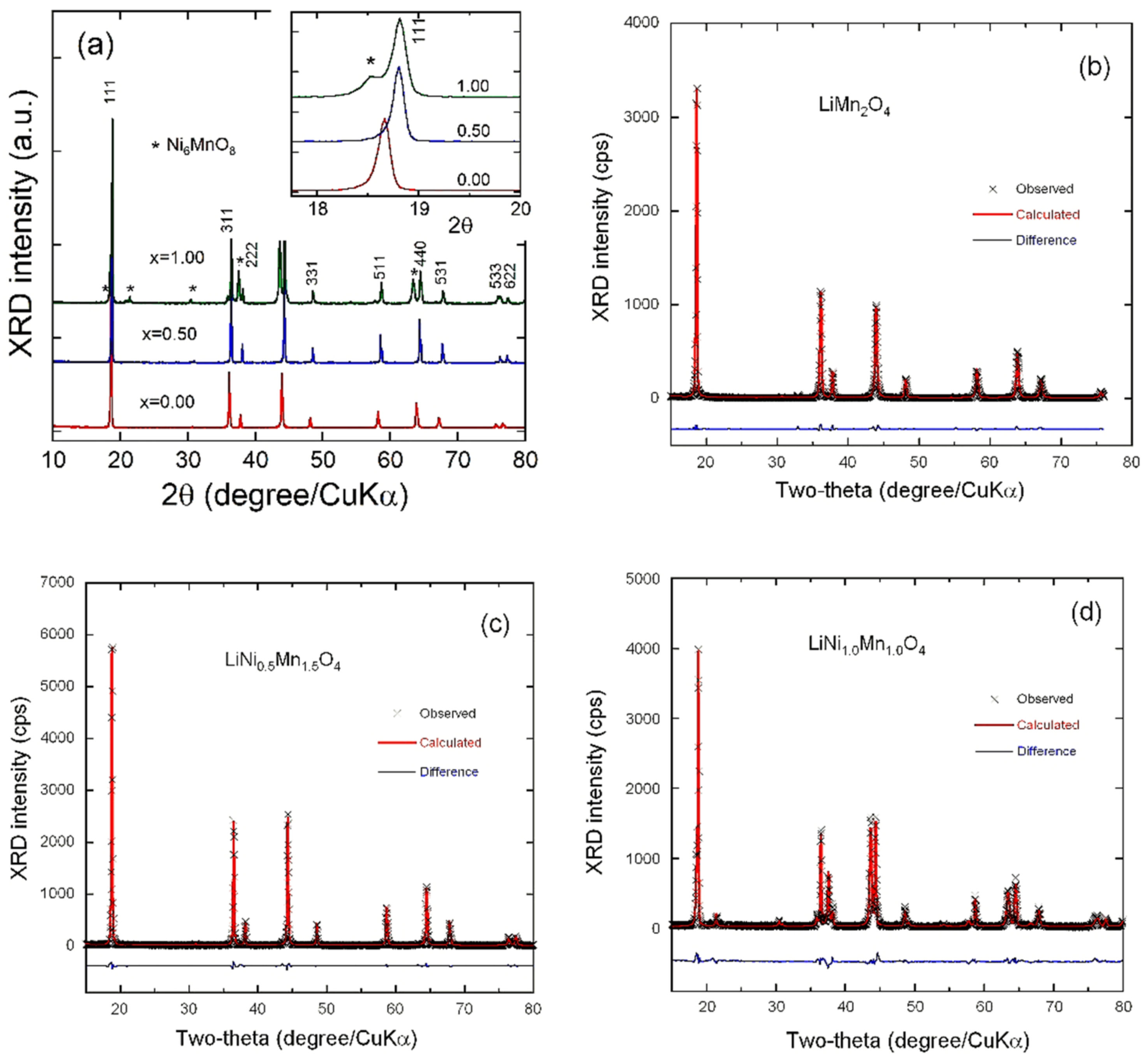
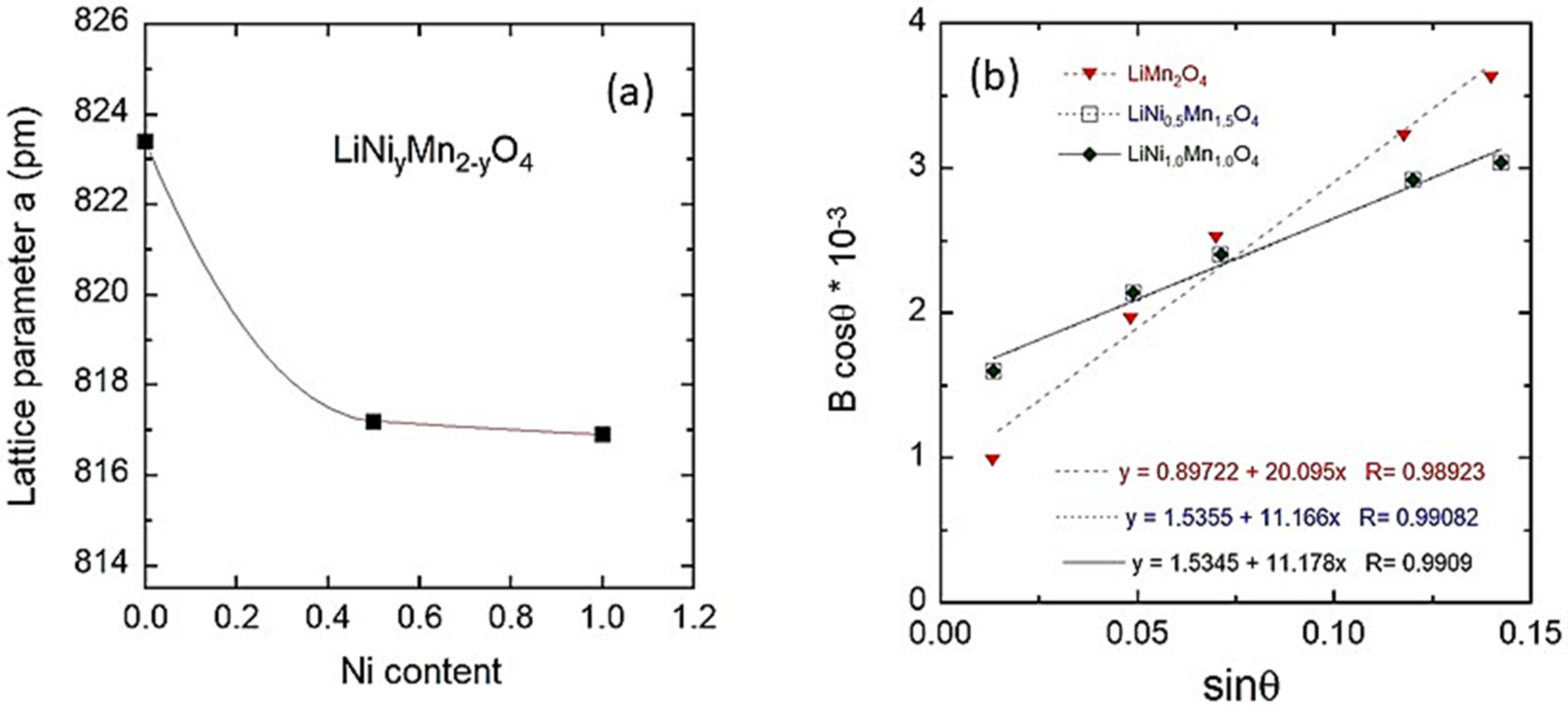
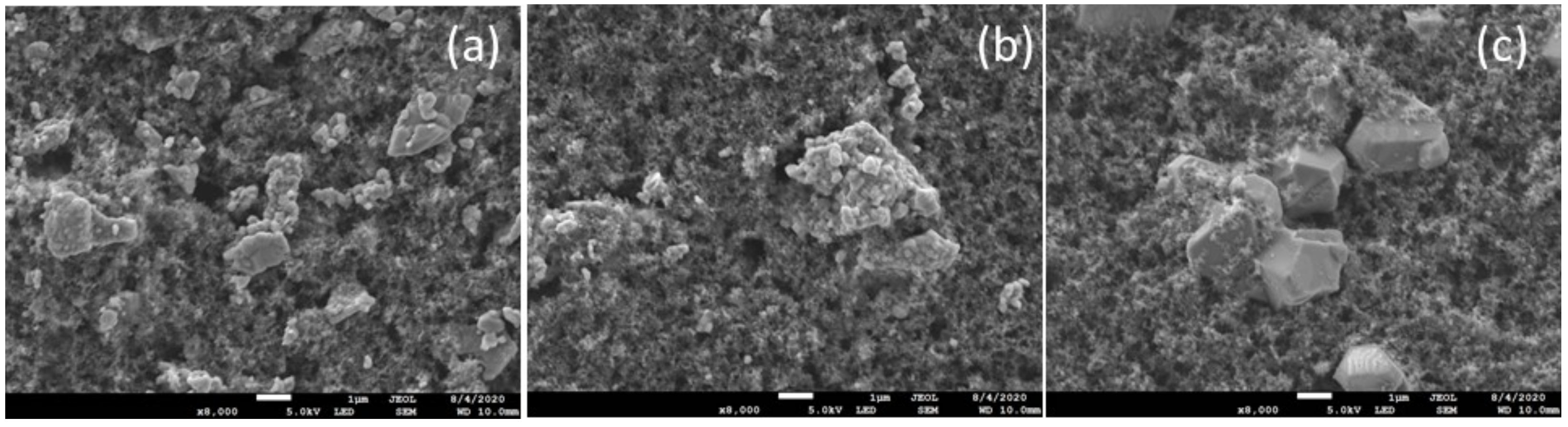
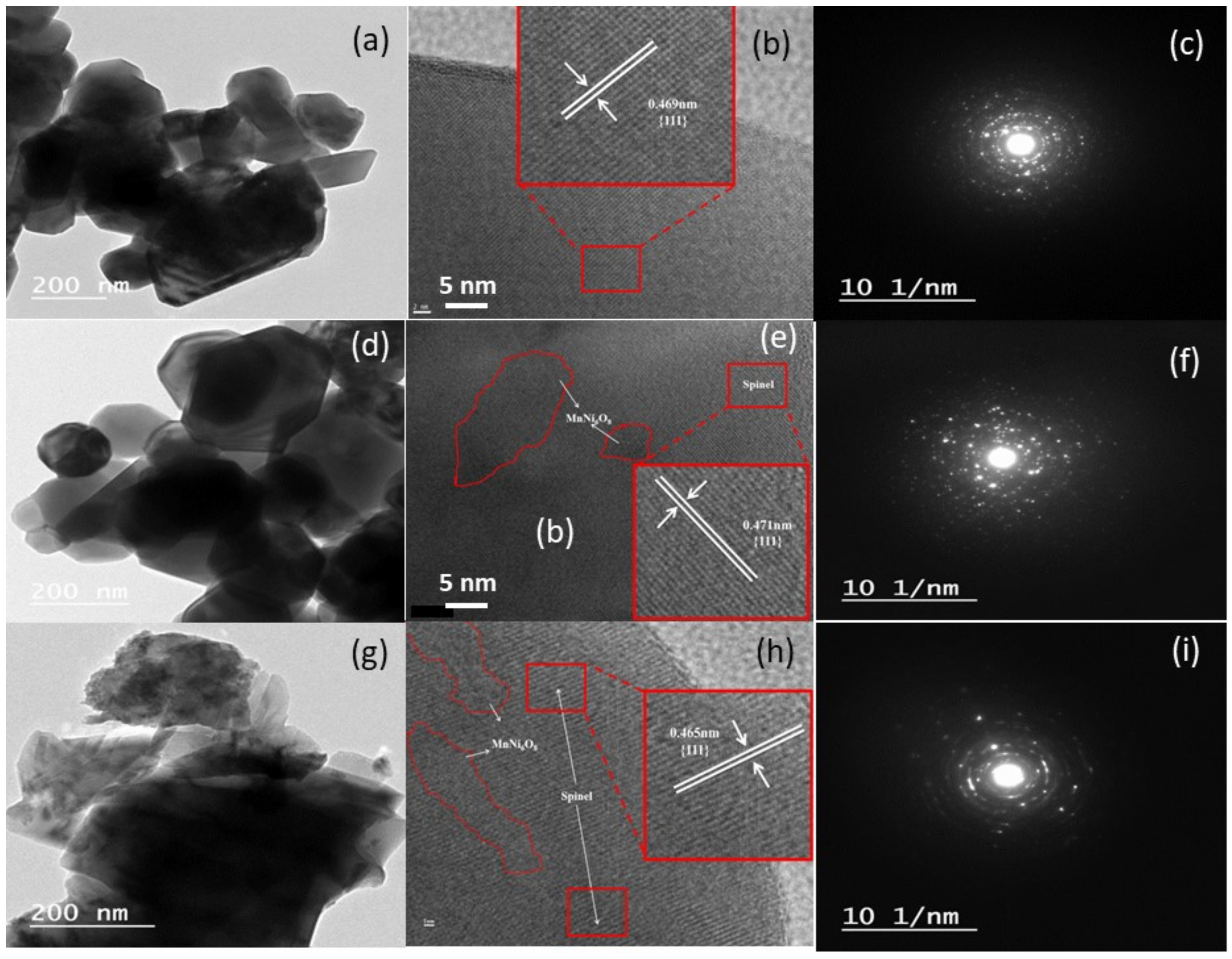
3.1. Structure and Morphology
3.2. Electrochemical Properties
3.2.1. Cyclic Voltammetry and Charge-Discharge Profiles
3.2.2. Electrochemical Impedance Spectroscopy (EIS)
3.2.3. Area-Specific Impedance (ASI)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zaghib, K.; Dontigny, M.; Guerfi, A.; Charest, P.; Rodrigues, I.; Mauger, A.; Julien, C.M. Safe and fast-charging Li-ion battery with long shelf life for power applications. J. Power Sources 2011, 196, 3949–3954. [Google Scholar] [CrossRef]
- Berckmans, G.; Messagie, M.; Smekens, J.; Omar, N.; Vanhaverbeke, L.; Van Mierlo, J. Cost projection of state of the art lithium-ion batteries for electric vehicles up to 2030. Energies 2017, 10, 1314. [Google Scholar] [CrossRef]
- Perner, A.; Vetter, J. Lithium-ion batteries for hybrid electric vehicles and battery electric vehicles. In Advances in Battery Technology for Electric Vehicles; Scrosati, B., Garche, J., Tillmetz, W., Eds.; Woodhead Publishing: Oxford, UK, 2015; pp. 173–190. [Google Scholar]
- Julien, C.M.; Mauger, A. Review of 5-V electrodes for Li-ion batteries: Status and trends. Ionics 2013, 19, 951–988. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A.; Zaghib, K.; Groult, H. Comparative issues of cathode materials for Li-ion batteries. Inorganics 2014, 2, 132–154. [Google Scholar] [CrossRef]
- Gummow, R.J.; De Kock, A.; Thackeray, M.M. Improved capacity retention in rechargeable 4 V lithium/lithium manganese oxide (spinel) cells. Solid State Ion. 1994, 69, 59–67. [Google Scholar] [CrossRef]
- Lu, J.; Zhou, C.; Liu, Z.; Lee, K.S.; Lu, L. LiMn2O4 cathode materials with large porous structure and radial interior channels for lithium ion batteries. Electrochim. Acta 2016, 212, 553–560. [Google Scholar] [CrossRef]
- Ebin, B.; Battaglia, V.; Gürmen, S. Comparison of 4 V and 3 V electrochemical properties of nanocrystalline LiMn2O4 cathode particles in lithium ion batteries prepared by ultrasonic spray pyrolysis. Ceram. Int. 2014, 40, 7029–7035. [Google Scholar] [CrossRef]
- Zhan, C.; Lu, J.; Kropf, A.J.; Wu, T.; Jansen, A.N.; Sun, Y.; Qiu, X.; Amine, K. Mn(II) deposition on anodes and its effects on capacity fade in spinel lithium manganate–carbon systems. Nat. Commun. 2013, 4, 2437. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Wang, C. Suppression of Jahn-Teller distortion of spinel LiMn2O4 cathode. J. Alloys Compd. 2009, 479, 310–313. [Google Scholar] [CrossRef]
- Xu, G.J.; Liu, Z.H.; Zhang, C.J.; Cui, G.L.; Chen, L.Q. Strategies for improving the cyclability and thermo-stability of LiMn2O4-based batteries at elevated temperatures. J. Mater. Chem. A 2015, 3, 4092–4123. [Google Scholar] [CrossRef]
- Yi, T.F.; Mei, J.; Zhu, Y.R. Key strategies for enhancing the cycling stability and rate capacity of LiNi0.5Mn1.5O4 as high-voltage cathode materials for high power lithium-ion batteries. J. Power Sources 2016, 316, 85–105. [Google Scholar] [CrossRef]
- Amarilla, J.M.; Petrov, K.; Picó, F.; Avdeev, G.; Rojo, J.M.; Rojas, R.M. Sucrose-aided combustion synthesis of nanosized LiMn1.99-yLiyM0.01O4 (M = Al3+, Ni2+, Cr3+, Co3+, y = 0.01 and 0.06) spinels: Characterization and electrochemical behavior at 25 and 55 °C in rechargeable lithium cells. J. Power Sources 2009, 191, 591–600. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, D.; Trottier, J.; Gagnon, C.; Howe, J.; Mauger, A.; Julien, C.M.; Zaghib, K. In-situ Raman spectroscopic investigation of LiMn1.45Ni0.45M0.1O4 (M = Cr, Co) 5-V cathode materials. J. Power Sources 2015, 298, 341–348. [Google Scholar] [CrossRef]
- Cai, Z.; Ma, Y.; Huang, X.; Yan, X.; Yu, Z.; Zhang, S.; Song, G.; Xu, Y.; Wen, C.; Yang, W. High electrochemical stability Al-doped spinel LiMn2O4 cathode material for Li-ion batteries. J. Energy Storage 2020, 27, 101036. [Google Scholar] [CrossRef]
- Ohzuku, T.; Takeda, S.; Iwanaga, M. Solid-state redox potentials for Li[Me1/2Mn3/2]O4 (Me: 3d-transition metal) having spinel-framework structures: A series of 5 volt materials for advanced lithium-ion batteries. J. Power Sources 1999, 81–82, 90–94. [Google Scholar] [CrossRef]
- Amdouni, N.; Zaghib, K.; Gendron, F.; Mauger, A.; Julien, C.M. Structure and insertion properties of disordered and ordered LiNi0.5Mn1.5O4 spinels prepared by wet chemistry. Ionics 2006, 12, 117–126. [Google Scholar] [CrossRef]
- Amdouni, N.; Zaghib, K.; Gendron, F.; Mauger, A.; Julien, C.M. Magnetic properties of LiNi0.5Mn1.5O4 spinels prepared by wet-chemical methods. J. Magn. Magn. Mater. 2007, 309, 100–105. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, W.; Trottier, J.; Gagnon, C.; Barray, F.; Guerfi, A.; Mauger, A.; Groult, H.; Julien, C.M.; Goodenough, J.B.; et al. Spinel materials for high-voltage cathodes in Li-ion batteries. RSC Adv. 2014, 4, 154–167. [Google Scholar] [CrossRef]
- Hashem, A.M.; Abdel-Ghany, A.E.; Abuzeid, H.M.; El-Tawil, R.S.; Indris, S.; Ehrenberg, H.; Mauger, A.; Julien, C.M. EDTA as chelating agent for sol-gel synthesis of spinel LiMn2O4 cathode material for lithium batteries. J. Alloys Compd. 2018, 737, 758–766. [Google Scholar] [CrossRef]
- Rodriguez-Carjaval, J. Recent developments of the program FULLPROF. Commission on Powder Diffraction (IUCr). Newsletter 2001, 26, 12–19. [Google Scholar]
- Park, S.H.; Oh, S.-W.; Kang, S.H.; Belharouak, I.; Amine, K.; Sun, Y.-K. Comparative study of different crystallographic structure of LiNi0.5Mn1.5O4-d cathodes with wide operation voltage (2.0–5.0 V). Electrochim. Acta 2007, 52, 7226–7230. [Google Scholar] [CrossRef]
- Nakamura, T.; Yamada, Y.; Tabuchi, M. Magnetic and electrochemical studies on Ni2+-substituted Li-Mn spinel oxides. J. Appl. Phys. 2005, 98, 093905. [Google Scholar] [CrossRef]
- Shao-Horn, Y.; Middaugh, R.L. Redox reactions of cobalt, aluminum and titanium substituted lithium manganese spinel compounds in lithium cells. Solid State Ion. 2001, 139, 13–25. [Google Scholar] [CrossRef]
- Raja, M.W.; Mahanty, S.; Basu, R.N. Influence of S and Ni co-doping on structure, band gap and electrochemical properties of lithium manganese oxide synthesized by soft chemical method. J. Power Sources 2009, 192, 618–626. [Google Scholar] [CrossRef]
- Xiong, L.; Xua, Y.; Taoa, T.; Goodenough, J.B. Synthesis and electrochemical characterization of multi-cations doped spinel LiMn2O4 used for lithium ion batteries. J. Power Sources 2012, 199, 214–219. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Kumada, N.; Yoshio, M. Synthesis and characterization of lithium aluminum-doped spinel (LiAlxMn2-xO4) for lithium secondary battery. J. Power Sources 2001, 96, 376–384. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- McCalla, E.; Dahn, J.R. The spinel and cubic rocksalt solid-solutions in the Li–Mn–Ni oxide pseudo-ternary system. Solid State Ion. 2013, 242, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Gu, Y.-J.; Chen, Y.-B.; Liu, H.-Q.; Ding, J.-X.; Wang, Y.-M. Phase transformation during synthesis of LiNi0.5Mn1.5O4 by oxalate co-precipitation. Mater. Lett. 2016, 180, 105–108. [Google Scholar] [CrossRef]
- Qin, X.; Gong, J.; Guo, J.; Zhou, B.; Zhou, M.; Wang, L.; Liang, G. Synthesis and performance of LiNi0.5Mn1.5O4 cathode materials with different particle morphologies and sizes for lithium-ion battery. J. Alloys Compd. 2019, 786, 240–249. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Arrebola, J.C.; Caballero, A.; Cruz, M.; Hernán, L.; Morales, J.; Castellón, E.R. Crystallinity control of a nanostructured LiNi0.5Mn1.5O4 spinel via polymer-assisted synthesis: A method for improving Its rate capability and performance in 5 V lithium batteries. Adv. Funct. Mater. 2006, 16, 1904–1912. [Google Scholar] [CrossRef]
- Hashem, A.M.; Abbas, S.M.; Hou, X.; Eid, A.E.; Abdel-Ghany, A.E. Facile one step synthesis method of spinel LiMn2O4 cathode material for lithium batteries. Heliyon 2019, 5, e02027. [Google Scholar] [CrossRef]
- Yang, S.; Schmidt, D.O.; Khetan, A.; Schrader, F.; Jakobi, S.; Homberger, M.; Noyong, M.; Paulus, A.; Kungl, H.; Eichel, R.-A.; et al. Electrochemical and electronic charge transport properties of Ni-doped LiMn2O4 spinel obtained from polyol-mediated synthesis. Materials 2018, 11, 806. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, F.; Zhang, K.; Liang, Y.; Yang, S.; Liang, J.; Chen, J. Facile polymer-assisted synthesis of LiNi0.5Mn1.5O4 with a hierarchical micro–nano structure and high rate capability. RSC Adv. 2012, 2, 5669–5675. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, R.; Dai, P.; Bai, Z.; Yu, X.; Wu, M.; Li, G. Synthesis of 3D porous flower-like NiO/Ni6MnO8 composites for supercapacitor with enhanced performance. J. Mater. Sci. Mater. Electron. 2018, 29, 7510–7518. [Google Scholar] [CrossRef]
- Edstrom, K.; Gustafsson, T.; Thomas, J.O. The cathode-electrolyte interface in the Li-ion battery. Electrochim. Acta 2004, 50, 397–403. [Google Scholar] [CrossRef]
- Xu, M.Q.; Lu, D.S.; Garsuch, A.; Lucht, B.L. Improved performance of LiNi0.5Mn1.5O4 cathodes with electrolytes containing dimethylmethylphosphonate (DMMP). J. Electrochem. Soc. 2012, 159, A2130–A2134. [Google Scholar] [CrossRef]
- Li, L.; Sui, J.; Chen, J.; Lu, Y. LiNi0.5Mn1.5O4 microrod with ultrahigh Mn3+ content: A high performance cathode material for lithium ion battery. Electrochim. Acta 2019, 305, 433–442. [Google Scholar] [CrossRef]
- Yang, S.; Chen, J.; Liu, Y.; Yi, B. Preparing LiNi0.5Mn1.5O4 nanoplates with superior properties in lithium-ion batteries using bimetal– organic coordination-polymers as precursors. J. Mater. Chem. A 2014, 2, 9322–9330. [Google Scholar] [CrossRef]
- Wan, L.N.; Deng, Y.F.; Yang, C.X.; Xu, H.; Qin, X.S.; Chen, G.H. Ni/Mn ratio and morphology-dependent crystallographic facet structure and electrochemical properties of the high-voltage spinel LiNi0.5Mn1.5O4 cathode material. RSC Adv. 2015, 5, 25988–25997. [Google Scholar] [CrossRef]
- Cai, Y.; Huang, S.-Z.; She, F.-S.; Liu, J.; Zhang, R.-L.; Huang, Z.-H.; Wang, F.-Y.; Wang, H.-E. Facile synthesis of well-shaped spinel LiNi0.5Mn1.5O4 nanoparticles as cathode materials for lithium ion batteries. RSC Adv. 2016, 6, 2785–2792. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Z.; Deng, C.; Xu, W.; Hu, T.; Yan, B.; Yang, G. Preparation and electrochemical properties of high-voltage spinel LiNi0.5Mn1.5O4 synthesized by using different manganese sources. ChemElectroChem 2017, 4, 1205–1213. [Google Scholar] [CrossRef]
- Chen, Z.; Qiu, S.; Cao, Y.; Ai, X.; Xie, K.; Hong, X.; Yang, H. Surface-oriented and nanoflake-stacked LiNi0.5Mn 1.5O4 spinel for high-rate and long-cycle-life lithium ion batteries. J. Mater. Chem. 2012, 22, 17768–17772. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, F.; Yang, J.; Chen, J. LiNi0.5Mn 1.5O4 porous nanorods as high-rate and long-life cathodes for Li-ion batteries. Nano Lett. 2013, 13, 2822–2825. [Google Scholar] [CrossRef]
- Wang, L.; Chen, D.; Wang, J.; Liu, G.; Wu, W.; Liang, G. Synthesis of LiNi0.5Mn 1.5O4 cathode material with improved electrochemical performances through a modified solid-state method. Powder Technol. 2016, 292, 203–209. [Google Scholar] [CrossRef]
- Julien, C.; Mauger, A.; Zaghib, K.; Groult, H. Optimization of layered cathode materials for lithium-ion batteries. Materials 2016, 9, 595. [Google Scholar] [CrossRef]
- Qiao, R.M.; Wang, Y.S.; Olalde-Velasco, P.; Li, H.; Hu, Y.S.; Yang, W.L. Direct evidence of gradient Mn(II) evolution at charged states in LiNi0.5Mn1.5O4 electrodes with capacity fading. J. Power Sources 2015, 273, 1120–1126. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, W.J.; Mauger, A.; Gendron, F.; Julien, C.M.; Qilu, R. Minimization of the cation mixing in Li1+x(NMC)1-xO2 as cathode material. J. Power Sources 2010, 195, 1292–1301. [Google Scholar] [CrossRef]
- Abbas, S.M.; Hashem, A.M.; Abdel-Ghany, A.E.; Ismail, E.H.; Kotlar, M.; Winter, M.; Li, J.; Julien, C.M. Ag-modified LiMn2O4 cathode for lithium-ion batteries: Coating functionalization. Energies 2020, 13, 5194. [Google Scholar] [CrossRef]
- Li, L.; Zhao, R.; Xu, T.; Wang, D.; Pan, D.; Zhang, K.; Yu, C.; Lu, X.; He, G.; Bai, Y. Stabilizing a high-voltage LiNi0.5Mn1.5O4 cathode towards all solid state batteries: A Li–Al–Ti–P–O solid electrolyte nano-shell with a host material. Nanoscale 2019, 11, 8967–8977. [Google Scholar] [CrossRef]
- Spence, S.L.; Xu, Z.; Sainio, S.; Nordlund, D.; Lin, F. Tuning the morphology and electronic properties of single-crystal LiNi0.5Mn1.5O4−δ: Exploring the influence of LiCl−KCl molten salt flux composition and synthesis temperature. Inorg. Chem. 2020, 59, 10591–10603. [Google Scholar] [CrossRef]
- Ho, C.; Raistrick, I.D.; Huggins, R.A. Application of a-c techniques to the study of lithium diffusion in tungsten trioxide thin films. J. Electrochem. Soc. 1980, 127, 343–350. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications; Wiley & Sons, Inc.: New York, NY, USA, 2001; pp. 226–260. [Google Scholar]
- Raju, K.; Nkosi, F.P.; Viswanathan, E.; Mathe, M.K.; Damodaran, K.; Ozoemena, K.I. Microwave-enhanced electrochemical cycling performance of the LiNi0.2Mn1.8O4 spinel cathode material at elevated temperature. Phys. Chem. Chem. Phys. 2016, 18, 13074–13083. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Guo, J.; Xiang, M.; Zhu, J.; Su, C.; Bai, H.; Liu, X.; Bai, W.; Wang, R. Single crystalline polyhedral LiNixM 2-xO4 as high-performance cathodes for ultralong cycling lithium-ion batteries. Solid State Ion. 2018, 326, 100–109. [Google Scholar] [CrossRef]
- Amine, K.; Liu, J.; Kang, S.; Belharouak, I.; Hyung, Y.; Vissers, D.; Henriksen, G. Improved lithium manganese oxide spinel/graphite Li-ion cells for high-power applications. J. Power Sources 2004, 129, 14–19. [Google Scholar] [CrossRef]
- Kim, J.-H.; Myung, S.-T.; Yoon, C.S.; Kang, S.G.; Sun, Y.-K. Comparative study of LiNi0.5Mn1.5O4-d and LiNi0.5Mn1.5O4 cathodes having two crystallographic structures: Fd-3m and P4332. Chem. Mater. 2004, 16, 906–914. [Google Scholar] [CrossRef]
- Zhang, X.; Porras-Gutierrez, A.-G.; Mauger, A.; Groult, H.; Julien, C.M. Nanotechnology of positive electrodes for Li-ion batteries. Inorganics 2017, 5, 25. [Google Scholar] [CrossRef]
- Liu, J.; Wen, Z.; Gu, Z.; Wu, M.; Lin, Z. Synthesis by an EDTA-based soft-chemistry route and characterization of nanosized LiCoO2 cathode materials. J. Electrochem. Soc. 2002, 149, A1405–A1408. [Google Scholar] [CrossRef]
- Abdel-Ghany, A.E.; Hashem, A.M.; Mauger, A.; Julien, C.M. Effects of chelators on the structure and electrochemical properties of Li-rich Li1.2Ni0.13Co0.13Mn0.54O2 cathode materials. J. Solid State Electrochem. 2020, 24, 3157–3172. [Google Scholar] [CrossRef]
- Zhong, Q.; Bonakdarpour, A.; Zhang, M.; Gao, Y.; Dahn, J.R. Synthesis and electrochemistry of LiNixMn2-xO4. J. Electrochem. Soc. 1997, 144, 205–213. [Google Scholar] [CrossRef]
- Patoux, S.; Daniel, L.; Bourbon, C.; Lignier, H.; Pagano, C.; Le Cras, F.; Jouanneau, S.; Martinet, S. High voltage spinel oxides for Li-ion batteries: From the material research to the application. J. Power Sources 2009, 189, 344–352. [Google Scholar] [CrossRef]
- Lee, Y.S.; Sun, Y.K.; Ota, S.; Miyashita, T.; Yoshio, M. Preparation and characterization of nano-crystalline LiNi0.5Mn1.5O4 for 5 V cathode material by composite carbonate process. Electrochem. Commun. 2002, 4, 989–994. [Google Scholar] [CrossRef]
- Oh, S.H.; Chung, K.Y.; Jeon, S.H.; Kim, C.S.; Cho, W.I.; Cho, B.W. Structural and electrochemical investigations on the LiNi0.5−xMn1.5−yMx+yO4 (M = Cr, Al, Zr) compound for 5 V cathode material. J. Alloys Compd. 2009, 469, 244–250. [Google Scholar] [CrossRef]
- Wu, H.M.; Rao, C.V.; Rambabu, B. Electrochemical performance of LiNi0.5Mn1.5O4 prepared by improved solid state method as cathode in hybrid supercapacitor. Mater. Chem. Phys. 2009, 116, 532–535. [Google Scholar] [CrossRef]
- Liu, G.Q.; Wen, L.; Wang, X.; Ma, B.Y. Effect of the impurity LixNi1-xO on the electrochemical properties of 5 V cathode material LiNi0.5Mn1.5O4. J. Alloys Compd. 2011, 509, 9377–9381. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, X.; Sushko, P.V.; Sushko, M.L.; Kovarik, L.; Feng, J.; Deng, Z.; Zheng, J.; Graff, G.L.; Nie, Z.; et al. High-performance LiNi0.5Mn1.5O4 spinel controlled by Mn3+ concentration and site disorder. Adv. Mater. 2012, 24, 2109–2116. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, Z.; Zheng, L.; Yu, F.; Liu, B.; Zhang, Y.; Ke, K. Investigation on preparation and performance of spinel LiNi0.5Mn1.5O4 with different microstructures for lithium-ion batteries. Sci. Rep. 2015, 5, 13299. [Google Scholar] [CrossRef]
- Duncan, H.; Abu-Lebdeh, Y.; Davidson, I.J. Study of the cathode–electrolyte interface of LiNi0.5Mn1.5O4 synthesized by a sol–gel Method for Li-ion batteries. J. Electrochem. Soc. 2010, 157, A528–A535. [Google Scholar] [CrossRef]
- Duncan, H.; Duguay, D.; Abu-Lebdeh, Y.; Davidson, I.J. Study of the LiNi0.5Mn1.5O4/electrolyte interface at room temperature and 60 °C. J. Electrochem. Soc. 2011, 158, A537–A545. [Google Scholar] [CrossRef]
- Börner, M.; Niehoff, P.; Vortmann, B.; Nowak, S.; Winter, M.; Schappacher, F.M. Comparison of different synthesis methods for LiNi0.5Mn1.5O4—Influence on battery cycling performance, degradation, and aging. Energy Technol. 2016, 4, 1631–1640. [Google Scholar]
- Yoon, J.; Kim, D.; Um, J.H.; Jeong, M.; Oh, W.; Yoon, W.-S. Effect of local structural changes on rate capability of LiNi0.5Mn1.5O4-δ cathode material for lithium ion batteries. J. Alloys Compd. 2016, 686, 593–600. [Google Scholar] [CrossRef]
- Jin, Y.-C.; Lin, C.-Y.; Duh, J.-G. Improving rate capability of high potential LiNi0.5Mn1.5O4−x cathode materials via increasing oxygen non-stoichiometries. Electrochim. Acta 2012, 69, 45–50. [Google Scholar] [CrossRef]
- Song, J.; Shin, D.W.; Lu, Y.H.; Amos, C.D.; Manthiram, A.; Goodenough, J.B. Role of oxygen vacancies on the performance of Li[Ni0.5-xMn1.5+x]O4 (x = 0, 0.05, and 0.08) spinel cathodes for lithium-ion batteries. Chem. Mater. 2012, 24, 3101–3109. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Ma, B.; Li, Y. Study of the intrinsic electrochemical properties of spinel LiNi0.5Mn1.5O4. Electrochim. Acta 2013, 112, 557–561. [Google Scholar] [CrossRef]
- Poyraz, A.S.; Kuo, C.-H.; Biswas, S.; King’ondu, C.K.; Suib, S.L. A general approach to crystalline and monomodal pore size mesoporous materials. Nat. Commun. 2013, 4, 2952. [Google Scholar] [CrossRef]
- Jiang, M.; Key, B.; Meng, Y.S.; Grey, C.P. Electrochemical and structural study of the layered, “Li-excess” lithium-ion battery electrode material Li[Li1/9Ni1/3Mn5/9]O2. Chem. Mater. 2009, 21, 2733–2745. [Google Scholar] [CrossRef]
- Fell, C.R.; Carroll, K.J.; Chi, M.; Meng, Y.S. Synthesis−structure−property relations in layered, “Li-excess” oxides electrode materials Li[Li1/3−2x/3NixMn2/3−x/3]O2 (x = 1/3, 1/4, and 1/5). J. Electrochem. Soc. 2010, 157, A1202–A1211. [Google Scholar] [CrossRef]
- Gao, Y.; Patel, R.L.; Shen, K.-Y.; Wang, X.; Axelbaum, R.L.; Liang, X. Boosting the electrochemical performance of Li1.2Mn 0.54Ni0.13Co0.13O2 by atomic layer-deposited CeO2 coating. ACS Omega 2018, 3, 906–916. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Wang, X.; Sun, X.; Zhang, J.-N.; Yu, X.; Li, H. Investigations on the fundamental process of cathode electrolyte interphase formation and evolution of high-voltage cathodes. ACS Appl. Mater. Interfaces 2020, 12, 2319–2326. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, P.; Wang, L.; Sun, F.; Zhao, L.; Tian, C.; Zhou, W.; Fu, H. Self-supported Ni6MnO8 3D mesoporous nanosheet arrays with ultrahigh lithium storage properties and conversion mechanism by in-situ XAFS. Nano Res. 2016, 10, 1–13. [Google Scholar] [CrossRef]
- Sharma, P.; Das, C.; Indris, S.; Bergfeldt, T.; Mereacre, L.; Knapp, M.; Gekle, U.; Ehrenberg, H.; Dewi Darma, M.S. Synthesis and characterization of a multication doped Mn spinel, LiNi0.3Cu0.1Fe0.2Mn1.4O4, as 5 V positive electrode material. ACS Omega 2020, 5, 22861–22873. [Google Scholar] [CrossRef] [PubMed]

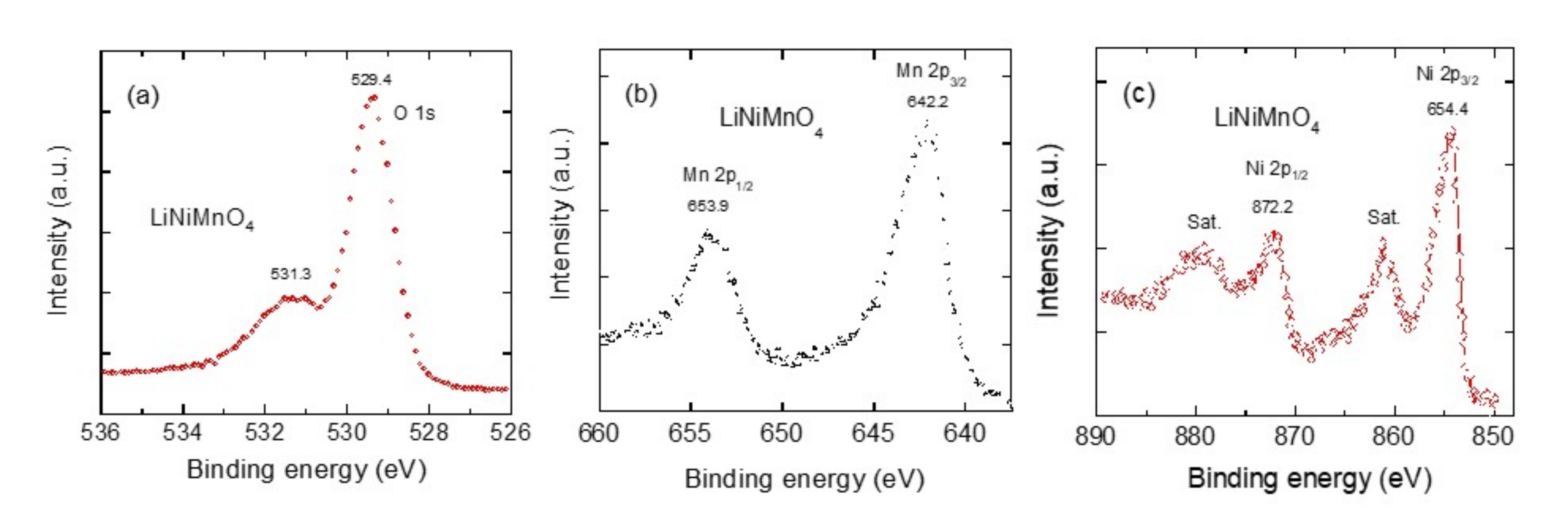
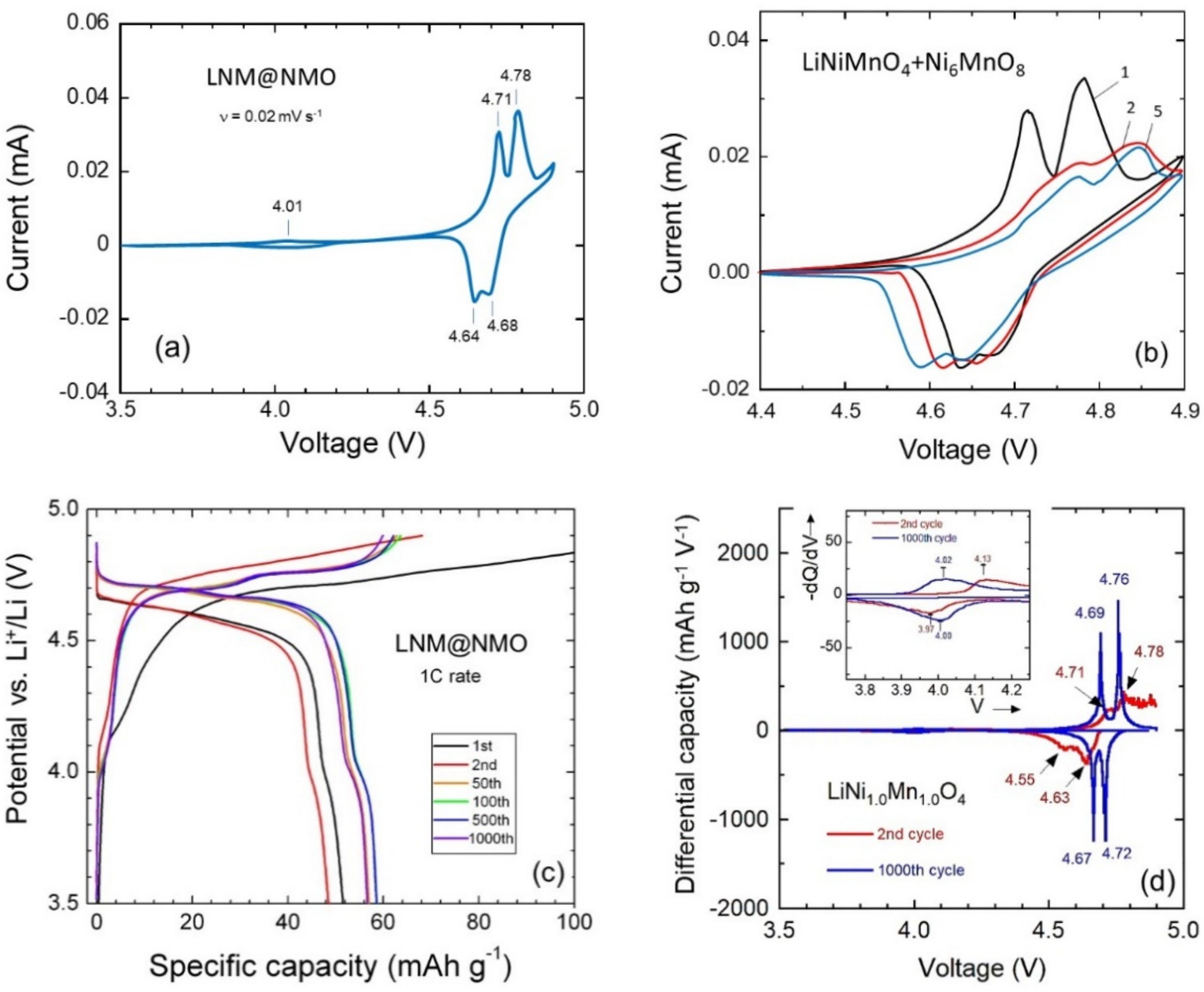
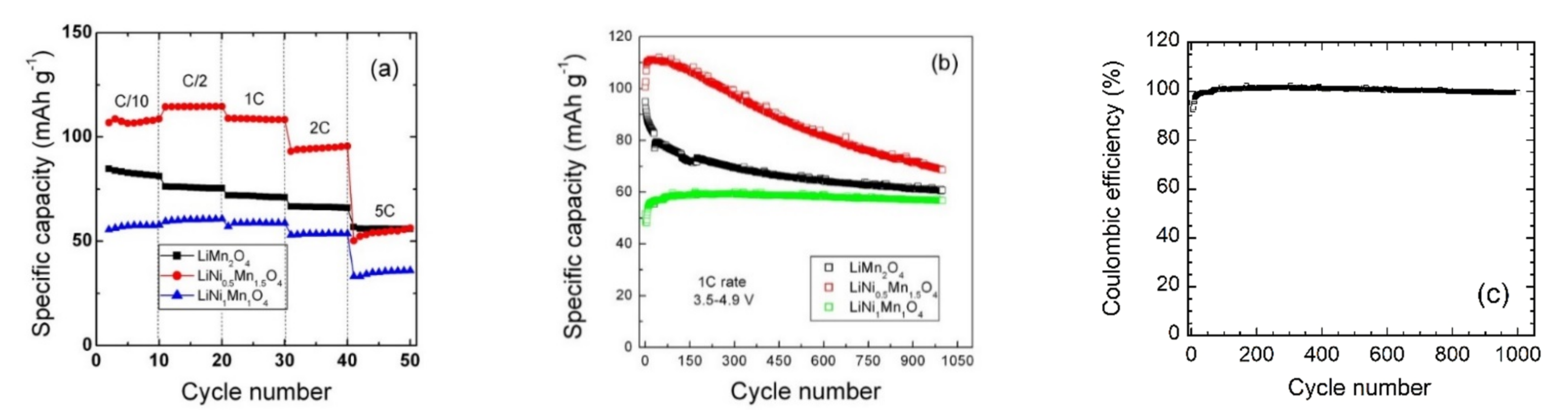
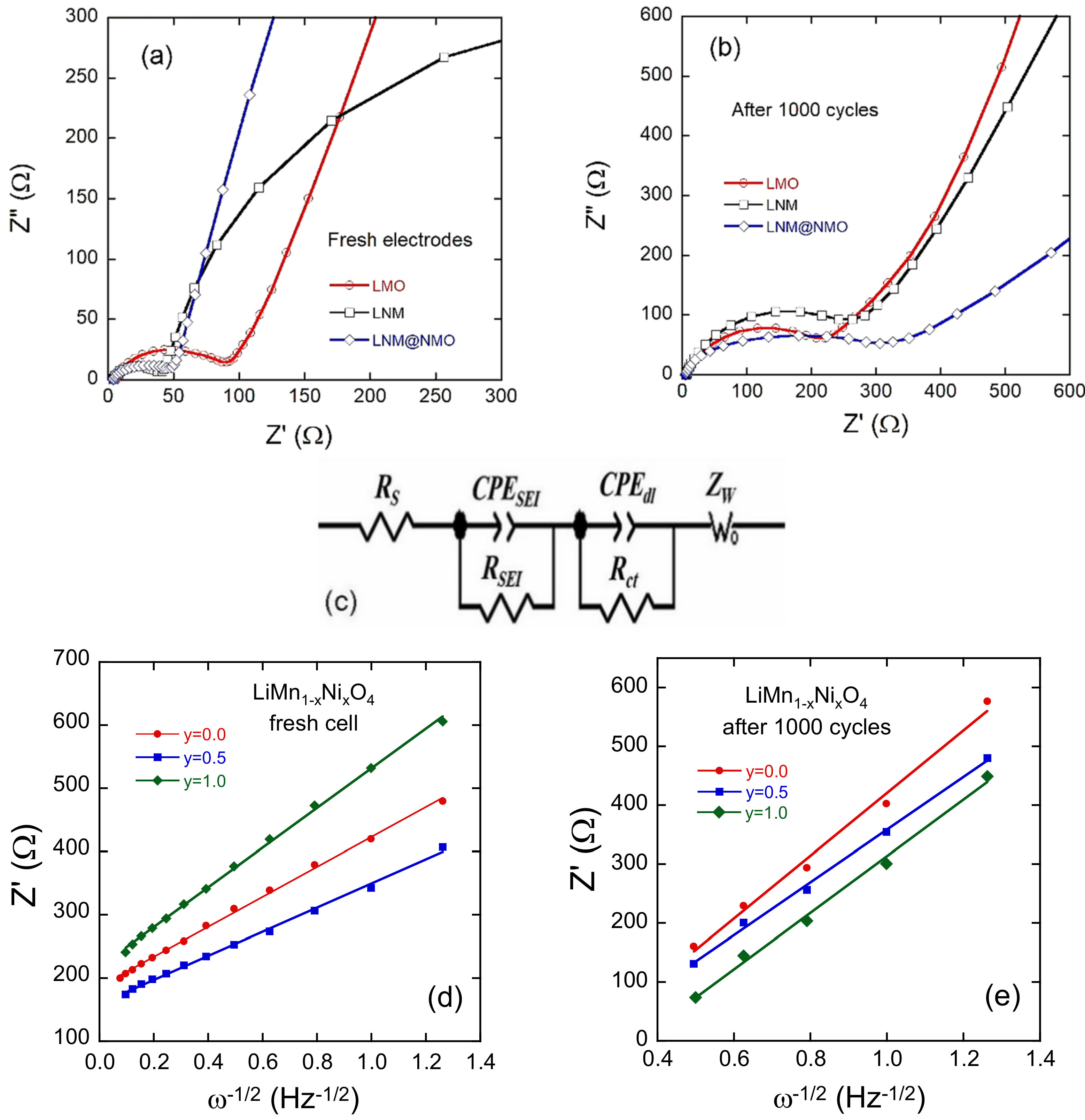

| Crystal Data | LiNiMnO4 | LiMn2O4 | LiNi0.5Mn1.5O4 |
|---|---|---|---|
| Lattice parameters | |||
| a (Å) | 8.168(1) | 8.234(0) | 8.172(6) |
| V (Å3) | 544.96 | 558.25 | 545.74 |
| I(311)/I(400) | 0.887 | 1.005 | 1.046 |
| fwhm(400) (°) | 0.24 | 0.13 | 0.19 |
| Lc (nm) | 67.9 | 62.8 | 64.0 |
| ε × 10−3 (rd) | 2.79 | 5.02 | 2.79 |
| Reliability | |||
| Rp(%) | 9.2 | 6.2 | 8.5 |
| Rw(%) | 8.6 | 5.8 | 7.5 |
| Rexp | 11.1 | 9.2 | 10.1 |
| χ2 | 2.21 | 1.47 | 1.96 |
| Material fraction (%) | |||
| Fdm | 62.8 | 100 | 97.3 |
| Fm3m | 37.2 | - | 2.7 |
| Ni in 8a site | 0.026 | 0.000 | 0.024 |
| Sample | Specific Surface Area (m2 g−1) | Pore Width (nm) | Pore Volume (cm3 g−1) |
|---|---|---|---|
| LMO | 1.79 | 12.5 | 0.04 |
| LNM | 2.04 | 12.8 | 0.04 |
| LNM@NMO | 2.31 | 13.9 | 0.05 |
| Sample | Binding Energy (eV) | |||
|---|---|---|---|---|
| Mn 2p3/2 | Mn 2p1/2 | Ni 2p3/2 | Ni 2p1/2 | |
| LiNiMnO4 | 642.2 | 653.9 | 855.2 | 873.3 |
| LiNi0.5Mn1.5O4 | 642.5 | 654.2 | 855.3 | 873.1 |
| LiMn2O4 | 643.2 | 654.6 | - | - |
| Electrode | Redox Reaction | Φanodic (V) | Φcathodic (V) | ΔΦ (mV) |
|---|---|---|---|---|
| LNM@NMO | Ni2+/Ni3+ | 4.71 | 4.64 | 70 |
| Ni3+/Ni4+ | 4.78 | 4.68 | 100 | |
| Mn3+/Mn4+ | 4.01 | 3.97 | 40 | |
| LNM | Ni2+/Ni3+ | 4.69 | 4.67 | 20 |
| Ni3+/Ni4+ | 4.76 | 4.72 | 40 | |
| Mn3+/Mn4+ | 4.02 | 3.99 | 30 |
| Electrode | Rs (Ω) | RSEI (Ω) | Rct (Ω) | DLi+(10−12 cm2 s−1) | ||||
|---|---|---|---|---|---|---|---|---|
| Fresh | 1000th | Fresh | 1000th | Fresh | 1000th | Fresh | 1000th | |
| LMO | 8.2 | 6.9 | 32 | 801 | 65 | 250 | 7.7 | 1.6 |
| LNM | 4.1 | 4.9 | 10 | 97 | 41 | 295 | 8.1 | 2.3 |
| LNM@NMO | 4.2 | 4.8 | 11 | 102 | 39 | 302 | 4.3 | 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Chang, K.; Hashem, A.M.; Abdel-Ghany, A.E.; El-Tawil, R.S.; Wang, H.; El-Mounayri, H.; Tovar, A.; Zhu, L.; Julien, C.M. Structural and Electrochemical Properties of the High Ni Content Spinel LiNiMnO4. Electrochem 2021, 2, 95-117. https://doi.org/10.3390/electrochem2010009
Li T, Chang K, Hashem AM, Abdel-Ghany AE, El-Tawil RS, Wang H, El-Mounayri H, Tovar A, Zhu L, Julien CM. Structural and Electrochemical Properties of the High Ni Content Spinel LiNiMnO4. Electrochem. 2021; 2(1):95-117. https://doi.org/10.3390/electrochem2010009
Chicago/Turabian StyleLi, Tianyi, Kai Chang, Ahmed M. Hashem, Ashraf E. Abdel-Ghany, Rasha S. El-Tawil, Hua Wang, Hazim El-Mounayri, Andres Tovar, Likun Zhu, and Christian M. Julien. 2021. "Structural and Electrochemical Properties of the High Ni Content Spinel LiNiMnO4" Electrochem 2, no. 1: 95-117. https://doi.org/10.3390/electrochem2010009
APA StyleLi, T., Chang, K., Hashem, A. M., Abdel-Ghany, A. E., El-Tawil, R. S., Wang, H., El-Mounayri, H., Tovar, A., Zhu, L., & Julien, C. M. (2021). Structural and Electrochemical Properties of the High Ni Content Spinel LiNiMnO4. Electrochem, 2(1), 95-117. https://doi.org/10.3390/electrochem2010009











