Nanostructure-Based Electrochemical Immunosensors as Diagnostic Tools
Abstract
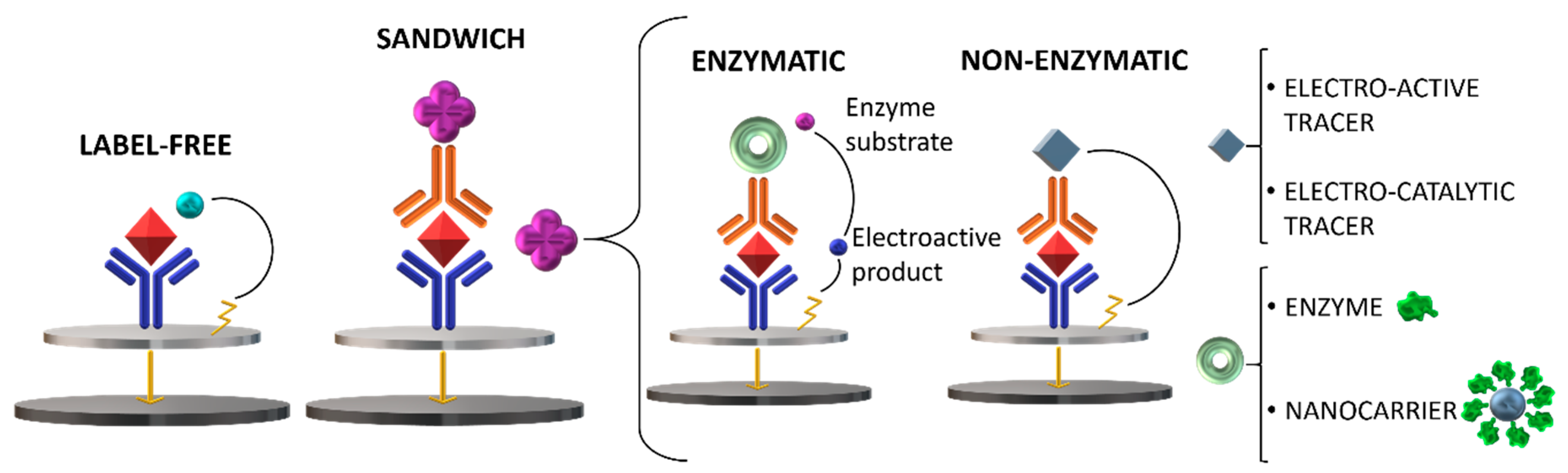
:1. Introduction
2. The Role of Nanomaterials in Electrochemical Immunosensors
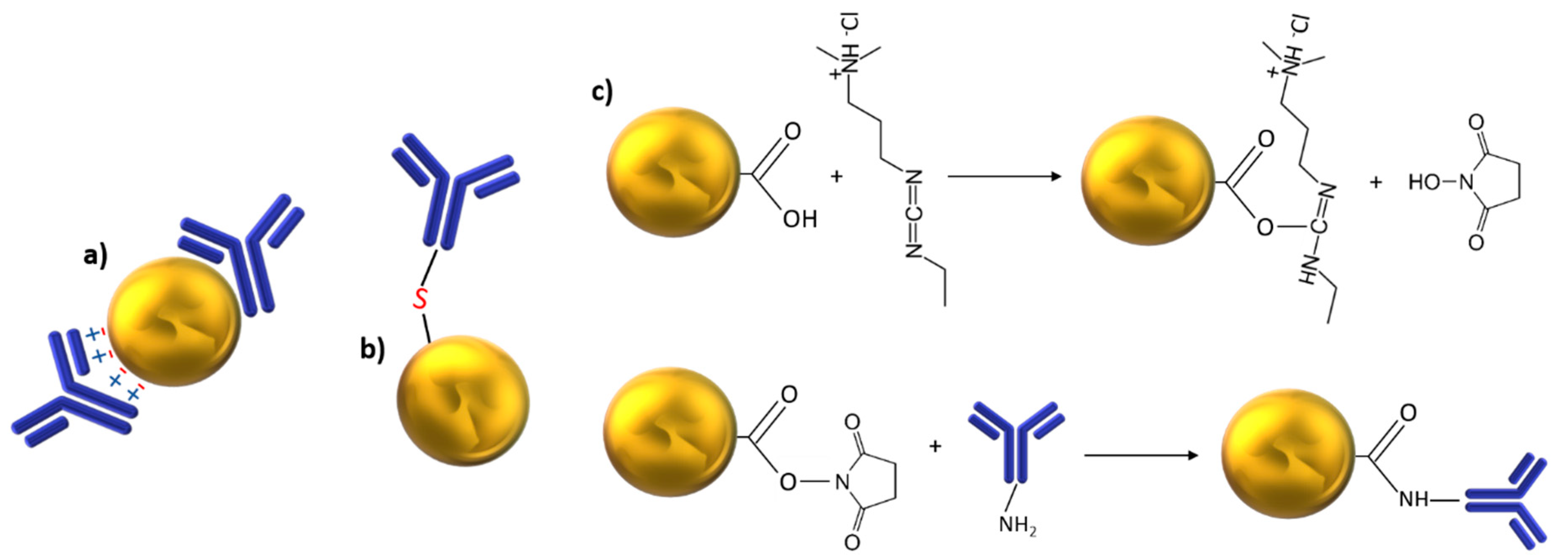
2.1. Metallic Nanoparticles
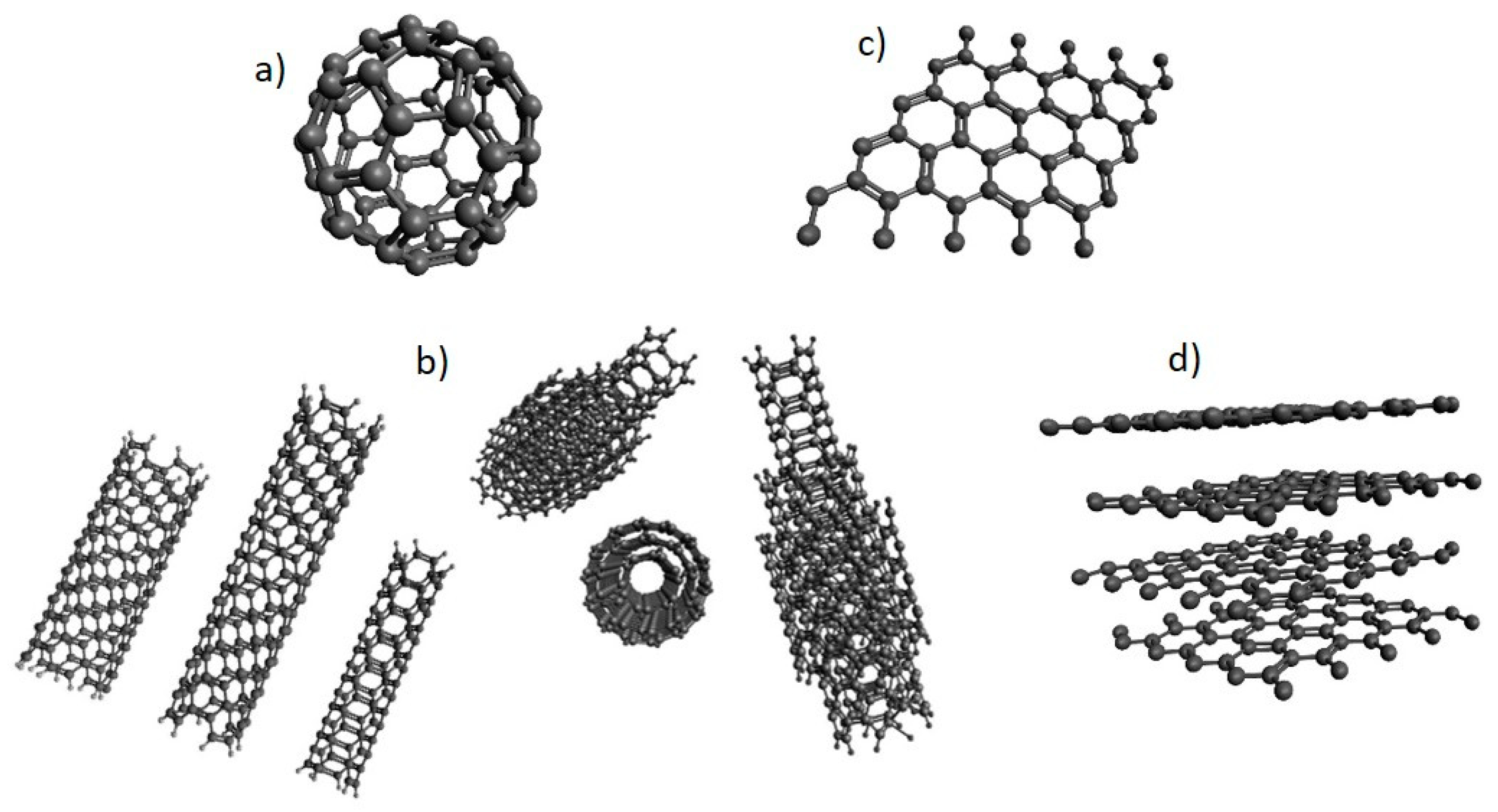
2.2. Carbon-Based Nanomaterials (NMs)
3. Nano-Immunosensors for Tumor Biomarkers Detection
3.1. Mucin Associated Antigens as Tumor Markers: Metallic Nanoparticles/Carbon Nanostructured Based-Electrochemical Immunosensors
3.2. Oncofetal Proteins as Tumor Markers: Carbon-Based Nanostructures, Nanodots and Nanocages in Electrochemical Immunosensors
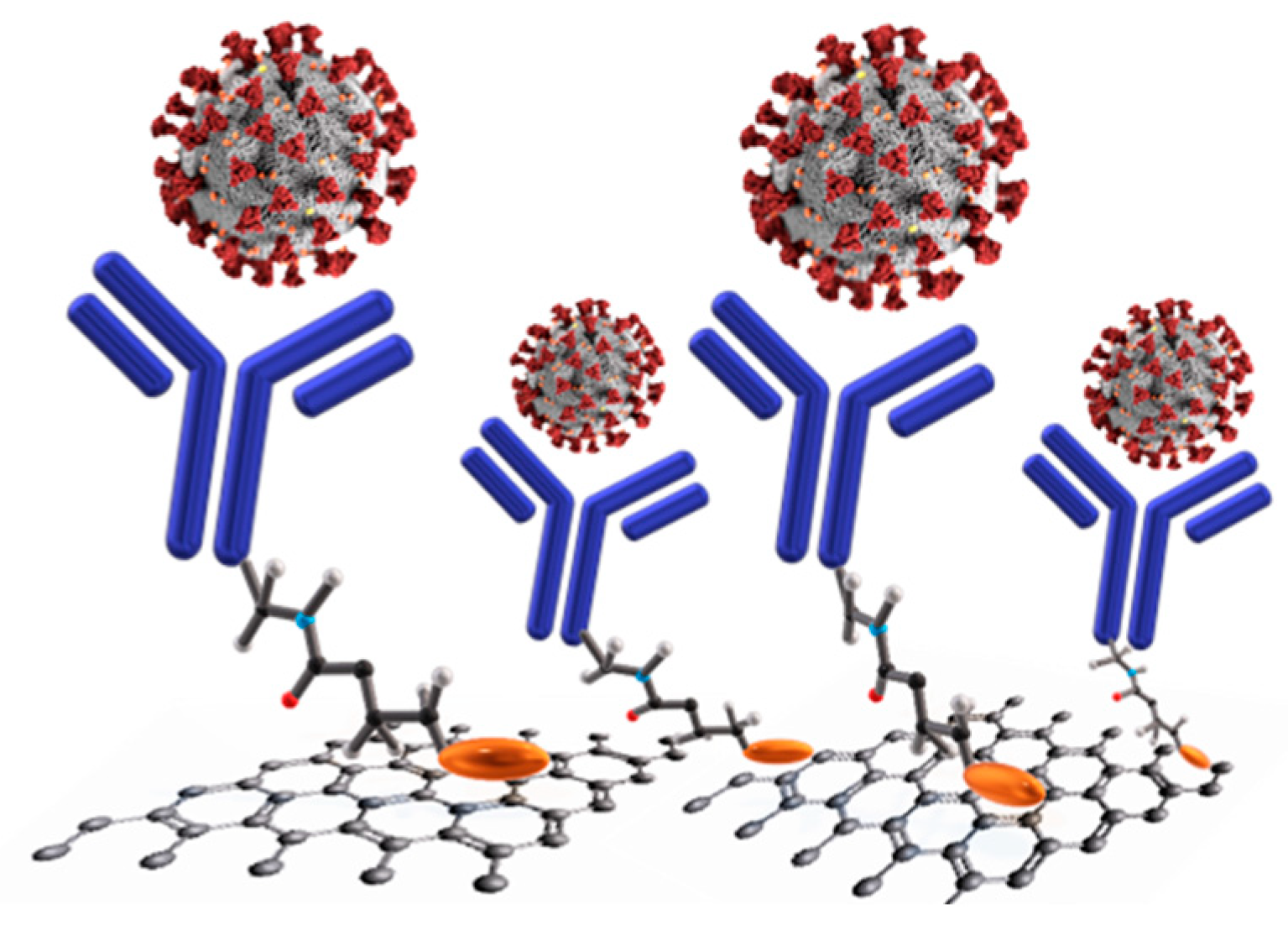
4. Nano-Immunosensors for Virus Detection
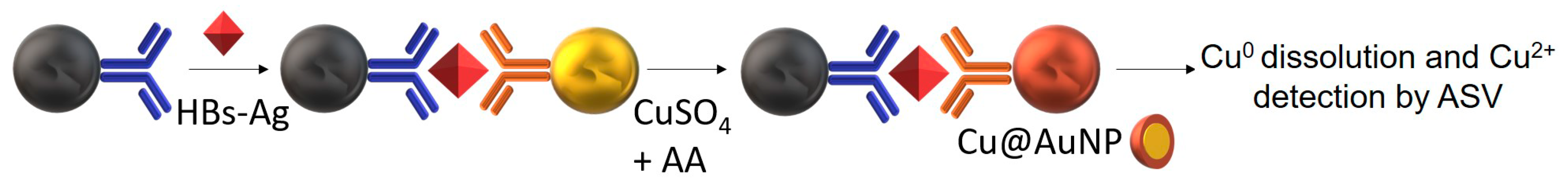
4.1. HBV (Hepatitis B Virus)
4.2. CoVs (Human Coronaviruses)
4.3. H1N1 (Influenza A)
4.4. HIV (Human Immunodeficiency Virus)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Justino, C.I.; Duarte, A.C.; Rocha-Santos, T.A. Chapter Three—Immunosensors in clinical laboratory diagnostics. In Advances in Clinical Chemistry; Makowski, C.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 73, pp. 65–108. [Google Scholar] [CrossRef]
- Králík, P.; Ricchi, M. A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything. Front. Microbiol. 2017, 8, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mascini, M.; Tombelli, S. Biosensors for biomarkers in medical diagnostics. Biomarkers 2008, 13, 637–657. [Google Scholar] [CrossRef] [PubMed]
- Mollarasouli, F.; Kurbanoglu, S.; Özkan, S.A. The Role of Electrochemical Immunosensors in Clinical Analysis. Biosensors 2019, 9, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antiochia, R.; Favero, G.; Conti, M.E.; Mazzei, F.; Tortolini, C. Affinity-based biosensors for pathogenic bacteria detection. Int. J. Environ. Technol. Manag. 2015, 18, 185. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Q.; Cui, D. Recent Advances in Nanotechnology Applied to Biosensors. Sensors 2009, 9, 1033–1053. [Google Scholar] [CrossRef] [Green Version]
- Mokhtarzadeh, A.; Eivazzadeh-Keihan, R.; Pashazadeh, P.; Hejazi, M.; Gharaatifar, N.; Hasanzadeh, M.; Baradaran, B.; De La Guardia, M. Nanomaterial-based biosensors for detection of pathogenic virus. TrAC Trends Anal. Chem. 2017, 97, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2, 63. [Google Scholar] [CrossRef] [Green Version]
- Sanvicens, N.; Pastells, C.; Pascual, N.; Marco, M.-P. Nanoparticle-based biosensors for detection of pathogenic bacteria. TrAC Trends Anal. Chem. 2009, 28, 1243–1252. [Google Scholar] [CrossRef]
- Ju, H.; Zhang, X.; Wang, J. Signal Amplification for Nanobiosensing: Principles, Development and Application; Springer: New York, NY, USA, 2011; pp. 39–84. [Google Scholar] [CrossRef]
- Lei, J.; Ju, H. Signal amplification using functional nanomaterials for biosensing. Chem. Soc. Rev. 2012, 41, 2122–2134. [Google Scholar] [CrossRef]
- Lee, S.H.; Sung, J.H.; Park, T.H. Nanomaterial-Based Biosensor as an Emerging Tool for Biomedical Applications. Ann. Biomed. Eng. 2011, 40, 1384–1397. [Google Scholar] [CrossRef]
- Singh, P.; Pandey, S.K.; Singh, J.; Srivastava, S.; Sachan, S.; Singh, S.K. Biomedical Perspective of Electrochemical Nanobiosensor. Nano-Micro Lett. 2016, 8, 193–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghafar-Zadeh, E. Wireless Integrated Biosensors for Point-of-Care Diagnostic Applications. Sensors 2015, 15, 3236–3261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.A.; Ahmed, M.U. Electrochemical immunosensors and their recent nanomaterial-based signal amplification strategies: A review. RSC Adv. 2016, 6, 24995–25014. [Google Scholar] [CrossRef]
- Khashayar, P.; Amoabediny, G.; Larijani, B.; Hosseini, M.; Vanfleteren, J. Fabrication and Verification of Conjugated AuNP-Antibody Nanoprobe for Sensitivity Improvement in Electrochemical Biosensors. Sci. Rep. 2017, 7, 16070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siti, R.M.; Khairunisak, A.R.; Aziz, A.A.; Noordin, R.; Makhsin, S.R.; Razak, K.A.; Rahmah, N. Study on Controlled Size, Shape and Dispersity of Gold Nanoparticles (AuNPs) Synthesized via Seeded-Growth Technique for Immunoassay Labeling. Adv. Mater. Res. 2011, 364, 504–509. [Google Scholar] [CrossRef]
- Wang, X.; Mei, Z.; Wang, Y.; Tang, L. Comparison of four methods for the biofunctionalization of gold nanorods by the introduction of sulfhydryl groups to antibodies. Beilstein J. Nanotechnol. 2017, 8, 372–380. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Zhu, Z.; Jin, L.; Peng, L.; Guo, W.; Hu, S. Detection of HIV-1 p24 antigen using streptavidin–biotin and gold nanoparticles based immunoassay by inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2014, 29, 1477–1482. [Google Scholar] [CrossRef]
- Fan, Y.; Shi, S.; Ma, J.; Guo, Y. A paper-based electrochemical immunosensor with reduced graphene oxide/thionine/gold nanoparticles nanocomposites modification for the detection of cancer antigen 125. Biosens. Bioelectron. 2019, 135, 1–7. [Google Scholar] [CrossRef]
- Ren, X.; Wu, D.; Wang, Y.; Zhang, Y.; Fan, D.; Pang, X.; Li, Y.; Du, B.; Wei, Q. An ultrasensitive squamous cell carcinoma antigen biosensing platform utilizing double-antibody single-channel amplification strategy. Biosens. Bioelectron. 2015, 72, 156–159. [Google Scholar] [CrossRef]
- Qi, T.; Liao, J.; Li, Y.; Peng, J.; Li, W.; Chu, B.; Li, H.; Wei, Y.; Qiana, Z. Label-free alpha fetoprotein immunosensor established by the facile synthesis of a palladium–graphene nanocomposite. Biosens. Bioelectron. 2014, 61, 245–250. [Google Scholar] [CrossRef]
- Suresh, L.; Bondili, J.S.; Brahman, P. Fabrication of Immunosensor Based on Polyaniline, Fullerene-C 60 and Palladium Nanoparticles Nanocomposite: An Electrochemical Detection Tool for Prostate Cancer. Electroanalysis 2020, 32, 1439–1448. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, H.; Ma, Z. A nanocomposite containing Prussian Blue, platinum nanoparticles and polyaniline for multi-amplification of the signal of voltammetric immunosensors: Highly sensitive detection of carcinoma antigen 125. Microchim. Acta 2017, 184, 4269–4277. [Google Scholar] [CrossRef]
- Lan, Q.; Ren, C.; Lambert, A.; Zhang, G.; Li, J.; Cheng, Q.; Hu, X.; Yang, Z. Platinum Nanoparticle-decorated Graphene Oxide@Polystyrene Nanospheres for Label-free Electrochemical Immunosensing of Tumor Markers. ACS Sustain. Chem. Eng. 2020, 8, 4392–4399. [Google Scholar] [CrossRef]
- Piguillem, S.V.; Ortega, F.G.; Raba, J.; Messina, G.A.; Fernández-Baldo, M.A. Development of a nanostructured electrochemical immunosensor applied to the early detection of invasive aspergillosis. Microchem. J. 2018, 139, 394–400. [Google Scholar] [CrossRef] [Green Version]
- Sureshab, L.; Bondili, J.; Brahman, P. Development of proof of concept for prostate cancer detection: An electrochemical immunosensor based on fullerene-C60 and copper nanoparticles composite film as diagnostic tool. Mater. Today Chem. 2020, 16, 100257. [Google Scholar] [CrossRef]
- Upan, J.; Banet, P.; Aubert, P.-H.; Ounnunkad, K.; Jakmunee, J. Sequential injection-differential pulse voltammetric immunosensor for hepatitis B surface antigen using the modified screen-printed carbon electrode. Electrochim. Acta 2020, 349, 136335. [Google Scholar] [CrossRef]
- Valipour, A.; Roushani, M. Fabrication of an electrochemical immunosensor for determination of human chorionic gonadotropin based on PtNPs/cysteamine/AgNPs as an efficient interface. Anal. Bioanal. Chem. Res. 2017, 4, 341–352. [Google Scholar] [CrossRef]
- Cho, I.-H.; Lee, J.; Kim, J.; Kang, M.-S.; Paik, J.K.; Ku, S.; Cho, H.-M.; Irudayaraj, J.; Kim, D.-H. Current Technologies of Electrochemical Immunosensors: Perspective on Signal Amplification. Sensors 2018, 18, 207. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Tanga, D. Non-enzymatic electrochemical immunoassay using noble metal nanoparticles: A review. Microchim. Acta 2015, 182, 2077–2089. [Google Scholar] [CrossRef]
- Doron, A.; Katz, E.; Willner, I. Organization of Au Colloids as Monolayer Films onto ITO Glass Surfaces: Application of the Metal Colloid Films as Base Interfaces To Construct Redox-Active Monolayers. Langmuir 1995, 11, 1313–1317. [Google Scholar] [CrossRef]
- Iglesias-Mayor, A.; Amor-Gutiérrez, O.; Costa-García, A.; De La Escosura-Muñiz, A. Nanoparticles as Emerging Labels in Electrochemical Immunosensors. Sensors 2019, 19, 5137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, P.; Teng, X.; Chen, M.; Zhang, Y.; Wang, H.; Yang, C.; Yangc, W.; Barrow, C.J. Ultrasensitive enzyme-free electrochemical immunosensor for microcystin-LR using molybdenum disulfide/gold nanoclusters nanocomposites as platform and Au@Pt core-shell nanoparticles as signal enhancer. Sens. Actuators B Chem. 2018, 266, 400–407. [Google Scholar] [CrossRef]
- Li, N.; Ma, H.; Cao, W.; Wu, D.; Yan, T.; Du, B.; Wei, Q. Highly sensitive electrochemical immunosensor for the detection of alpha fetoprotein based on PdNi nanoparticles and N-doped graphene nanoribbons. Biosens. Bioelectron. 2015, 74, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, D.; Sheng, S.; Yang, J.; Chen, X.; Xie, G.; Xiang, H. Electrochemical immunoassay for the cancer marker LMP-1 (Epstein-Barr virus-derived latent membrane protein 1) using a glassy carbon electrode modified with Pd@Pt nanoparticles and a nanocomposite consisting of graphene sheets and MWCNTs. Microchim. Acta 2016, 183, 2055–2062. [Google Scholar] [CrossRef]
- Zhang, J.; Xiong, Z.; Chen, Z. Ultrasensitive electrochemical microcystin-LR immunosensor using gold nanoparticle functional polypyrrole microsphere catalyzed silver deposition for signal amplification. Sens. Actuators B Chem. 2017, 246, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Ho, J.-A.A.; Chang, H.-C.; Shih, N.-Y.; Wu, L.-C.; Chang, Y.-F.; Chen, C.-C.; Chou, C. Diagnostic Detection of Human Lung Cancer-Associated Antigen Using a Gold Nanoparticle-Based Electrochemical Immunosensor. Anal. Chem. 2010, 82, 5944–5950. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xie, Z.; Xie, Z.; Luo, S.; Xie, L.; Huang, L.; Fan, Q.; Zhang, Y.; Wang, S.; Zeng, T. Silver nanoparticles coated graphene electrochemical sensor for the ultrasensitive analysis of avian influenza virus H7. Anal. Chim. Acta 2016, 913, 121–127. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, M.; Wu, X.; Dong, S.; Zhu, N.; Gyimah, E.; Wang, K.; Li, Y. A competitive immunosensor for ultrasensitive detection of sulphonamides from environmental waters using silver nanoparticles decorated single-walled carbon nanohorns as labels. Chemosphere 2019, 225, 282–287. [Google Scholar] [CrossRef]
- Ning, S.; Zhou, M.; Liu, C.; Waterhouse, G.I.; Dong, J.; Ai, S. Ultrasensitive electrochemical immunosensor for avian leukosis virus detection based on a β-cyclodextrin-nanogold-ferrocene host-guest label for signal amplification. Anal. Chim. Acta 2019, 1062, 87–93. [Google Scholar] [CrossRef]
- Alizadeh, N.; Hallaj, R.; Salimi, A. A highly sensitive electrochemical immunosensor for hepatitis B virus surface antigen detection based on Hemin/G-quadruplex horseradish peroxidase-mimicking DNAzyme-signal amplification. Biosens. Bioelectron. 2017, 94, 184–192. [Google Scholar] [CrossRef]
- Fan, L.; Yan, Y.; Guoa, B.; Zhaoa, M.; Lia, J.; Biana, X.; Wua, H.; Cheng, W.; Ding, S. Trimetallic hybrid nanodendrites and magnetic nanocomposites-based electrochemical immunosensor for ultrasensitive detection of serum human epididymis protein 4. Sens. Actuators B Chem. 2019, 296, 126697. [Google Scholar] [CrossRef]
- Azmi, U.Z.M.; Yusof, N.A.; Kusnin, N.; Abdullah, J.; Suraiya, S.; Ong, P.S.; Raston, N.H.A.; Rahman, S.F.A.; Fathil, M.F.M. Sandwich Electrochemical Immunosensor for Early Detection of Tuberculosis Based on Graphene/Polyaniline-Modified Screen-Printed Gold Electrode. Sensors 2018, 18, 3926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valera, E.; Hernández-Albors, A.; Marco, M.-P. Electrochemical coding strategies using metallic nanoprobes for biosensing applications. TrAC Trends Anal. Chem. 2016, 79, 9–22. [Google Scholar] [CrossRef]
- Maiti, D.; Tong, X.; Mou, X.; Yang, K. Carbon-Based Nanomaterials for Biomedical Applications: A Recent Study. Front. Pharmacol. 2019, 9, 1401. [Google Scholar] [CrossRef] [PubMed]
- Heydari-Bafrooei, E.; Ensafi, A.A. Typically Used Carbon-Based Nanomaterials in the Fabrication of Biosensors; Elsevier: Amsterdam, The Netherlands, 2019; pp. 77–98. [Google Scholar] [CrossRef]
- Ramnani, P.; Saucedo, N.M.; Mulchandani, A. Carbon nanomaterial-based electrochemical biosensors for label-free sensing of environmental pollutants. Chemosphere 2016, 143, 85–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene Based Electrochemical Sensors and Biosensors: A Review. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Crevillen, A.G.; Escarpa, A.; Garcia, C.D. Chapter 1 Carbon-Based Nanomaterials in Analytical Chemistry; Royal Society of Chemistry: London, UK, 2018; pp. 1–36. [Google Scholar] [CrossRef]
- Amani, J.; Khoshroo, A.; Rahimi-Nasrabadi, M. Electrochemical immunosensor for the breast cancer marker CA 15–3 based on the catalytic activity of a CuS/reduced graphene oxide nanocomposite towards the electrooxidation of catechol. Microchim. Acta 2017, 185, 79. [Google Scholar] [CrossRef]
- Barman, S.C.; Hossain, M.F.; Park, J.Y. Gold Nanoparticles Assembled Chemically Functionalized Reduced Graphene Oxide Supported Electrochemical Immunosensor for Ultra-Sensitive Prostate Cancer Detection. J. Electrochem. Soc. 2017, 164, B234–B239. [Google Scholar] [CrossRef]
- Gao, Y.-S.; Zhu, X.-F.; Xu, J.; Lu, L.; Wang, W.-M.; Yang, T.-T.; Xing, H.-K.; Yu, Y.-F. Label-free electrochemical immunosensor based on Nile blue A-reduced graphene oxide nanocomposites for carcinoembryonic antigen detection. Anal. Biochem. 2016, 500, 80–87. [Google Scholar] [CrossRef]
- Singh, R.; Hong, S.; Jang, J. Label-free Detection of Influenza Viruses using a Reduced Graphene Oxide-based Electrochemical Immunosensor Integrated with a Microfluidic Platform. Sci. Rep. 2017, 7, 42771. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Tuteja, S.K.; Sillu, D.; Deep, A.; Suri, C.R. Gold nanoparticles-reduced graphene oxide based electrochemical immunosensor for the cardiac biomarker myoglobin. Microchim. Acta 2016, 183, 1729–1738. [Google Scholar] [CrossRef]
- Wang, R.; Feng, J.-J.; Xue, Y.; Wu, L.; Wang, A.-J. A label-free electrochemical immunosensor based on AgPt nanorings supported on reduced graphene oxide for ultrasensitive analysis of tumor marker. Sens. Actuators B Chem. 2018, 254, 1174–1181. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.; Yu, J.; Lian, W.; Cui, M.; Xu, W.; Huang, J. Electrochemical immunosensor based on graphene–polyaniline composites and carboxylated graphene oxide for estradiol detection. Sens. Actuators B Chem. 2013, 188, 99–105. [Google Scholar] [CrossRef]
- Loo, A.H.; Ambrosi, A.; Bonanni, A.; Pumera, M. CVD graphene based immunosensor. RSC Adv. 2014, 4, 23952–23956. [Google Scholar] [CrossRef]
- Hong, C.; Qiao, X.; Wang, H.; Sun, Z.; Qi, Y.; Hong, C. An electrochemical immunosensor for simultaneous point-of-care cancer markers based on the host–guest inclusion of β-cyclodextrin–graphene oxide. J. Mater. Chem. B 2016, 4, 990–996. [Google Scholar] [CrossRef]
- Lai, G.; Cheng, H.; Xin, D.; Zhang, H.; Yu, A. Amplified inhibition of the electrochemical signal of ferrocene by enzyme-functionalized graphene oxide nanoprobe for ultrasensitive immunoassay. Anal. Chim. Acta 2016, 902, 189–195. [Google Scholar] [CrossRef]
- Yáñez-Sedeño, P.; González-Cortés, A.; Agüí, L.; Pingarrón, J.M. Uncommon Carbon Nanostructures for the Preparation of Electrochemical Immunosensors. Electroanalysis 2016, 28, 1679–1691. [Google Scholar] [CrossRef]
- Serafín, V.; Valverde, A.; Martínez-García, G.; Martínez-Periñán, E.; Comba, F.; Garranzo-Asensio, M.; Barderas, R.; Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J. Graphene quantum dots-functionalized multi-walled carbon nanotubes as nanocarriers in electrochemical immunosensing. Determination of IL-13 receptor α2 in colorectal cells and tumor tissues with different metastatic potential. Sens. Actuators B Chem. 2019, 284, 711–722. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Y.; Yan, H.; Wu, Y.; Zhu, C.; Du, D.; Lin, Y. SWCNTs@GQDs composites as nanocarriers for enzyme-free dual-signal amplification electrochemical immunoassay of cancer biomarker. Anal. Chim. Acta 2018, 1042, 44–51. [Google Scholar] [CrossRef]
- Asadian, E.; Ghalkhani, M.; Shahrokhianab, S. Electrochemical sensing based on carbon nanoparticles: A review. Sens. Actuators B Chem. 2019, 293, 183–209. [Google Scholar] [CrossRef]
- Cheng, Z.X.; Ang, W.L.; Bonanni, A. Electroactive Nanocarbon Can Simultaneously Work as Platform and Signal Generator for Label-Free Immunosensing. ChemElectroChem 2019, 6, 3615–3620. [Google Scholar] [CrossRef] [Green Version]
- Allen, B.L.; Kichambare, P.D.; Star, A. Carbon Nanotube Field-Effect-Transistor-Based Biosensors. Adv. Mater. 2007, 19, 1439–1451. [Google Scholar] [CrossRef]
- Kim, S.N.; Rusling, J.F.; Papadimitrakopoulos, F. Carbon Nanotubes for Electronic and Electrochemical Detection of Biomolecules. Adv. Mater. 2007, 19, 3214–3228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhardwaj, J.; Devarakonda, S.; Kumar, S.; Jang, J. Development of a paper-based electrochemical immunosensor using an antibody-single walled carbon nanotubes bio-conjugate modified electrode for label-free detection of foodborne pathogens. Sens. Actuators B Chem. 2017, 253, 115–123. [Google Scholar] [CrossRef]
- Cabral, D.G.; Lima, E.C.; Moura, P.; Dutra, R.A.F. A label-free electrochemical immunosensor for hepatitis B based on hyaluronic acid–carbon nanotube hybrid film. Talanta 2016, 148, 209–215. [Google Scholar] [CrossRef]
- Huang, K.; Niu, D.-J.; Xie, W.-Z.; Wang, W. A disposable electrochemical immunosensor for carcinoembryonic antigen based on nano-Au/multi-walled carbon nanotubes–chitosans nanocomposite film modified glassy carbon electrode. Anal. Chim. Acta 2010, 659, 102–108. [Google Scholar] [CrossRef]
- Malhotra, R.; Patel, V.; Vaqué, J.P.; Gutkind, J.S.; Rusling, J.F. Ultrasensitive Electrochemical Immunosensor for Oral Cancer Biomarker IL-6 Using Carbon Nanotube Forest Electrodes and Multilabel Amplification. Anal. Chem. 2010, 82, 3118–3123. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Tirado, E.; Salvo, C.; González-Cortés, A.; Yáñez-Sedeño, P.; Langa, F.; Pingarrón, J. Electrochemical immunosensor for simultaneous determination of interleukin-1 beta and tumor necrosis factor alpha in serum and saliva using dual screen printed electrodes modified with functionalized double–walled carbon nanotubes. Anal. Chim. Acta 2017, 959, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Valverde, A.; Serafín, V.; Montero-Calle, A.; González-Cortés, A.; Barderas, R.; Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Carbon/Inorganic Hybrid Nanoarchitectures as Carriers for Signaling Elements in Electrochemical Immunosensors: First Biosensor for the Determination of the Inflammatory and Metastatic Processes Biomarker RANK-ligand. ChemElectroChem 2020, 7, 810–820. [Google Scholar] [CrossRef]
- Serafín, V.; Valverde, A.; Garranzo-Asensio, M.; Barderas, R.; Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Simultaneous amperometric immunosensing of the metastasis-related biomarkers IL-13Rα2 and CDH-17 by using grafted screen-printed electrodes and a composite prepared from quantum dots and carbon nanotubes for signal amplification. Microchim. Acta 2019, 186, 411. [Google Scholar] [CrossRef]
- Pumera, M. Electrochemistry of graphene: New horizons for sensing and energy storage. Chem. Rec. 2009, 9, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Xiang, G.; Jiang, D.; Liu, L.; Liu, C.; Liu, F.; Pu, X.; And, F.L. Electrochemical Immunoassay forCytomegalovirusAntigen Detection with Multiple Signal Amplification Using HRP and Pt-Pd Nanoparticles Functionalized Single-walled Carbon Nanohorns. Electroanalysis 2016, 28, 1126–1133. [Google Scholar] [CrossRef]
- Tirado, E.S.; Cortés, A.G.; Yudasaka, M.; Iijima, S.; Langa, F.; Sedeño, P.Y.; Pingarrón, J. Electrochemical immunosensor for the determination of 8-isoprostane aging biomarker using carbon nanohorns-modified disposable electrodes. J. Electroanal. Chem. 2017, 793, 197–202. [Google Scholar] [CrossRef]
- Gao, Z. Electrochemical Immunosensor for Monocyte Chemoattractant Protein-1 Detection Based on Pt Nanoparticles Functionalized Single-walled Carbon Nanohorns. Int. J. Electrochem. Sci. 2018, 13, 3923–3934. [Google Scholar] [CrossRef]
- Yang, M.; Wu, X.; Hu, X.-L.; Wang, K.; Zhang, C.; Gyimah, E.; Yakubu, S.; Zhang, Z. Electrochemical immunosensor based on Ag+-dependent CTAB-AuNPs for ultrasensitive detection of sulfamethazine. Biosens. Bioelectron. 2019, 144, 111643. [Google Scholar] [CrossRef]
- Yang, F.; Han, J.; Zhuo, Y.; Yang, Z.; Chai, Y.-Q.; Yuan, R. Highly sensitive impedimetric immunosensor based on single-walled carbon nanohorns as labels and bienzyme biocatalyzed precipitation as enhancer for cancer biomarker detection. Biosens. Bioelectron. 2014, 55, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xiang, G.; Yuan, R.; Chen, X.; Luo, F.; Jiang, D.; Huang, S.; Li, Y.; Pu, X. Procalcitonin sensitive detection based on graphene–gold nanocomposite film sensor platform and single-walled carbon nanohorns/hollow Pt chains complex as signal tags. Biosens. Bioelectron. 2014, 60, 210–217. [Google Scholar] [CrossRef]
- Pilehvar, S.; De Wael, K. Recent Advances in Electrochemical Biosensors Based on Fullerene-C60 Nano-Structured Platforms. Biosensors 2015, 5, 712–735. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, A. Functionalization of fullerenes and carbon nanotubes. Phys. Status Solidi 2006, 243, 3209–3212. [Google Scholar] [CrossRef]
- Demirbakan, B.; Sezgintürk, M.K. A novel immunosensor based on fullerene C60 for electrochemical analysis of heat shock protein 70. J. Electroanal. Chem. 2016, 783, 201–207. [Google Scholar] [CrossRef]
- Li, Y.; Fang, L.; Cheng, P.; Deng, J.; Jiang, L.; Huang, H.; Zheng, J. An electrochemical immunosensor for sensitive detection of Escherichia coli O157:H7 using C60 based biocompatible platform and enzyme functionalized Pt nanochains tracing tag. Biosens. Bioelectron. 2013, 49, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, Z.; Cai, Y.; Wei, Z.; Fang, Y.; Ren, G.; Huang, Y. Electrochemical impedance spectroscopy for analytical determination of paraquat in meconium samples using an immunosensor modified with fullerene, ferrocene and ionic liquid. Electrochim. Acta 2011, 56, 1117–1122. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Hosseinzadeh, L.; Khoshroo, A. Label-free electrochemical immunosensor for detection of tumor necrosis factor α based on fullerene-functionalized carbon nanotubes/ionic liquid. J. Electroanal. Chem. 2015, 757, 58–64. [Google Scholar] [CrossRef]
- Zhang, W.; Patel, K.; Schexnider, A.; Banu, S.; Radadia, A.D. Nanostructuring of Biosensing Electrodes with Nanodiamonds for Antibody Immobilization. ACS Nano 2014, 8, 1419–1428. [Google Scholar] [CrossRef]
- Pashchenko, O.; Shelby, T.; Banerjee, T.; Santra, S. A Comparison of Optical, Electrochemical, Magnetic, and Colorimetric Point-of-Care Biosensors for Infectious Disease Diagnosis. ACS Infect. Dis. 2018, 4, 1162–1178. [Google Scholar] [CrossRef] [PubMed]
- Beitollahi, H.; Khalilzadeh, M.A.; Tajik, S.; Safaei, M.; Zhang, K.; Jang, H.W.; Shokouhimehr, M. Recent Advances in Applications of Voltammetric Sensors Modified with Ferrocene and Its Derivatives. ACS Omega 2020, 5, 2049–2059. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Nagpal, M.; Singh, P.; Chauhan, P.; Zaidi, M.A. Tumor markers: A diagnostic tool. Natl. J. Maxillofac. Surg. 2016, 7, 17–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Mohan, A.; Guleria, R. Biomarkers in cancer screening, research and detection: Present and future: A review. Biomarkers 2006, 11, 385–405. [Google Scholar] [CrossRef]
- Wu, J.; Fu, Z.; Yan, F.; Ju, H. Biomedical and clinical applications of immunoassays and immunosensors for tumor markers. TrAC Trends Anal. Chem. 2007, 26, 679–688. [Google Scholar] [CrossRef]
- Filik, H.; Avan, A.A. Nanostructures for nonlabeled and labeled electrochemical immunosensors: Simultaneous electrochemical detection of cancer markers: A review. Talanta 2019, 205, 120153. [Google Scholar] [CrossRef]
- Kufe, D. Mucins in cancer: Function, prognosis and therapy. Nat. Rev. Cancer 2009, 9, 874–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rittenhouse, H.G.; Manderino, G.L.; Hass, G.M. Mucin-Type Glycoproteins as Tumor Markers. Lab. Med. 1985, 16, 556–560. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, S.C.; Kumar, D.; Jaggi, M. Mucins in ovarian cancer diagnosis and therapy. J. Ovarian Res. 2009, 2, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chikkaveeraiah, B.V.; Bhirde, A.A.; Morgan, N.Y.; Eden, H.S.; Chen, X. Electrochemical Immunosensors for Detection of Cancer Protein Biomarkers. ACS Nano 2012, 6, 6546–6561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, A.; Tang, Y.-H.; Lai, C.; Chang, C.-J.; Chang, S.-C.; Wu, T.-I.; Hsueh, S.; Wang, C.-J.; Chou, H.-H.; Chang, T.-C. Potential of an age-stratified CA125 cut-off value to improve the prognostic classification of patients with endometrial cancer. Gynecol. Oncol. 2013, 129, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Sharma, S.; Nara, S. Dual gold nanostructure-based electrochemical immunosensor for CA125 detection. Appl. Nanosci. 2018, 8, 1843–1853. [Google Scholar] [CrossRef]
- Pakchinab, P.; Fathib, M.; Ghanbaria, H.; Saber, R.; Omidi, Y. A novel electrochemical immunosensor for ultrasensitive detection of CA125 in ovarian cancer. Biosens. Bioelectron. 2020, 153, 112029. [Google Scholar] [CrossRef]
- Reijnen, C.; Visser, N.C.M.; Kasius, J.C.; Boll, D.; Geomini, P.M.; Ngo, H.; Van Hamont, D.; Pijlman, B.M.; Vos, M.C.; Bulten, J.; et al. Improved preoperative risk stratification with CA-125 in low-grade endometrial cancer: A multicenter prospective cohort study. J. Gynecol. Oncol. 2019, 30, e70. [Google Scholar] [CrossRef]
- Huang, Z.; Jiang, Z.; Zhao, C.; Han, W.; Lin, L.; Liu, A.; Weng, S.; Lin, X. Simple and effective label-free electrochemical immunoassay for carbohydrate antigen 19-9 based on polythionine-Au composites as enhanced sensing signals for detecting different clinical samples. Int. J. Nanomed. 2017, 12, 3049–3058. [Google Scholar] [CrossRef] [Green Version]
- Imaoka, H.; Shimizu, Y.; Senda, Y.; Natsume, S.; Mizuno, N.; Hara, K.; Hijioka, S.; Hieda, N.; Tajika, M.; Tanaka, T.; et al. Post-adjuvant chemotherapy CA19-9 levels predict prognosis in patients with pancreatic ductal adenocarcinoma: A retrospective cohort study. Pancreatology 2016, 16, 658–664. [Google Scholar] [CrossRef]
- Stieber, P.; Nagel, R.; Blankenburg, I.; Heinemann, V.; Untch, M.; Bauerfeind, I.; Di Gioia, D. Diagnostic efficacy of CA 15-3 and CEA in the early detection of metastatic breast cancer—A retrospective analysis of kinetics on 743 breast cancer patients. Clin. Chim. Acta 2015, 448, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Jiao, X.; Chen, D. Ultrasensitive electrochemical immunosensor for CA 15-3 using thionine-nanoporous gold-graphene as a platform and horseradish peroxidase-encapsulated liposomes as signal amplification. Analyst 2012, 137, 4440–4447. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.-C.; Yan, W.-L.; Liu, X. CA19-9 and CA242 as tumor markers for the diagnosis of pancreatic cancer: A meta-analysis. Clin. Exp. Med. 2013, 14, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zheng, X.; Zhang, Z.; Wu, X.; Sun, L.; Zhou, J.; Liu, M. A Label-Free Electrochemical Immunosensor for Detection of the Tumor Marker CA242 Based on Reduced Graphene Oxide-Gold-Palladium Nanocomposite. Nanomaterials 2019, 9, 1335. [Google Scholar] [CrossRef] [Green Version]
- George, S.M.; Tandon, S.; Kandasubramanian, B. Advancements in Hydrogel-Functionalized Immunosensing Platforms. ACS Omega 2020, 5, 2060–2068. [Google Scholar] [CrossRef]
- Tang, Z.; Fu, Y.; Ma, Z. Multiple signal amplification strategies for ultrasensitive label-free electrochemical immunoassay for carbohydrate antigen 24-2 based on redox hydrogel. Biosens. Bioelectron. 2017, 91, 299–305. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, G.; Zhang, Y.; Pang, X.; Cao, W.; Du, B.; Wei, Q. Sandwich-type electrochemical immunosensor for CEA detection based on Ag/MoS2@Fe3O4 and an analogous ELISA method with total internal reflection microscopy. Sens. Actuators B Chem. 2018, 266, 561–569. [Google Scholar] [CrossRef]
- Zhao, C.; Ma, C.; Wu, M.; Li, W.; Song, Y.; Hong, C.; Qiao, X. A novel electrochemical immunosensor based on CoS2 for early screening of tumor marker carcinoembryonic antigen. New J. Chem. 2020, 44, 3524–3532. [Google Scholar] [CrossRef]
- Idris, A.O.; Mabuba, N.; Arotiba, O.A. An Exfoliated Graphite-Based Electrochemical Immunosensor on a Dendrimer/Carbon Nanodot Platform for the Detection of Carcinoembryonic Antigen Cancer Biomarker. Biosensors 2019, 9, 39. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Wang, Y.; Cao, W.; Zhang, Y.; Yan, T.; Du, B.; Wei, Q. An ultrasensitive electrochemical immunosensor for CEA using MWCNT-NH2 supported PdPt nanocages as labels for signal amplification. J. Mater. Chem. B 2015, 3, 2006–2011. [Google Scholar] [CrossRef]
- Galle, P.; Foerster, F.; Kudo, M.; Chan, S.L.; Llovet, J.M.; Qin, S.; Schelman, W.R.; Chintharlapalli, S.; Abada, P.B.; Sherman, M.; et al. Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int. 2019, 39, 2214–2229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, L.; Mu, Z.; Zhu, C.; Wei, Q.; Li, H.; Du, D.; Lin, Y. Graphene loaded bimetallic Au@Pt nanodendrites enhancing ultrasensitive electrochemical immunoassay of AFP. Sens. Actuators B Chem. 2016, 231, 513–519. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, T.; Ye, Y.; Chen, P.; Ren, X.-N.; Rao, A.; Wan, Y.; Wang, B.; Luo, Z. A highly sensitive label-free electrochemical immunosensor based on an aligned GaN nanowires array/polydopamine heterointerface modified with Au nanoparticles. J. Mater. Chem. B 2019, 7, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Blumenfeld, N.R.; Laksanasopin, T.; Sia, S.K. Point-of-Care Diagnostics: Recent Developments in a Connected Age. Anal. Chem. 2017, 89, 102–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaFleur, J.P.; Jönsson, A.; Senkbeil, S.; Kutter, J.P. Recent advances in lab-on-a-chip for biosensing applications. Biosens. Bioelectron. 2016, 76, 213–233. [Google Scholar] [CrossRef] [PubMed]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.-H.; Choi, M.; Ku, K.B.; Lee, C.-S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef] [Green Version]
- Fabiani, L.; Saroglia, M.; Galatà, G.; De Santis, R.; Fillo, S.; Luca, V.; Faggioni, G.; D’Amore, N.; Regalbuto, E.; Salvatori, P.; et al. Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: A reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva. Biosens. Bioelectron. 2021, 171, 112686. [Google Scholar] [CrossRef]
- Gan, N.; Du, X.; Cao, Y.; Hu, F.; Lia, T.; Jiang, Q.-L. An Ultrasensitive Electrochemical Immunosensor for HIV p24 Based on Fe3O4@SiO2 Nanomagnetic Probes and Nanogold Colloid-Labeled Enzyme–Antibody Copolymer as Signal Tag. Materials 2013, 6, 1255–1269. [Google Scholar] [CrossRef] [Green Version]
- Ding, C.; Li, H.; Hu, K.; Lin, J.-M. Electrochemical immunoassay of hepatitis B surface antigen by the amplification of gold nanoparticles based on the nanoporous gold electrode. Talanta 2010, 80, 1385–1391. [Google Scholar] [CrossRef]
- Shen, G.; Zhang, Y. Highly sensitive electrochemical stripping detection of hepatitis B surface antigen based on copper-enhanced gold nanoparticle tags and magnetic nanoparticles. Anal. Chim. Acta 2010, 674, 27–31. [Google Scholar] [CrossRef]
- Wei, S.; Xiao, H.; Cao, L.; Chen, Z. A Label-Free Immunosensor Based on Graphene Oxide/Fe3O4/Prussian Blue Nanocomposites for the Electrochemical Determination of HBsAg. Biosensors 2020, 10, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Layqah, L.A.; Eissa, S. An electrochemical immunosensor for the corona virus associated with the Middle East respiratory syndrome using an array of gold nanoparticle-modified carbon electrodes. Microchim. Acta 2019, 186, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zong, C.; Wu, J.; Xu, J.; Ju, H.; Yan, F. Multilayer hemin/G-quadruplex wrapped gold nanoparticles as tag for ultrasensitive multiplex immunoassay by chemiluminescence imaging. Biosens. Bioelectron. 2013, 43, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Jiang, J.-H.; Luo, Y.; Shen, G.; Yu, R. Copper-enhanced gold nanoparticle tags for electrochemical stripping detection of human IgG. Talanta 2007, 73, 420–424. [Google Scholar] [CrossRef]
- Yin, Y.; Wunderink, R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology 2017, 23, 130–137. [Google Scholar] [CrossRef] [Green Version]

| Target Antigen | Electrode Configuration | Label | Detection | LOD | Linear Range | Ref. |
|---|---|---|---|---|---|---|
| CA125 | Ab/rGO/Thi/AuNPs/GC | Label-free | DPV | 0.01 U/mL | 0.1–200 U/mL | [20] |
| Ab/AuNPs-PB-PtNP-PANI/GC | Label-free | SWV | 4.4 mU/mL | 0.01–5000 U/mL | [24] | |
| ITO-AuNRs-Ab | AuNPs-Ab-Ca2+ | DPV | 3.4 U/mL | 20–100 U/mL | [100] | |
| Ab-3DrGO-MWCNTs- PAMAM/AuNPs-GC | Ab-Suc-CS@MNPs-TB | SWV | 6 μU/mL | 0.0005–75 U/mL | [101] | |
| CA19-9 | Ab-AuNPs/ AuNPs@PThi/GC | Label-free | DPV | 0.26 U/mL | 6.5–520 U/mL | [103] |
| CA15-3 | CuS-rGO/Ab | Label-free | DPV | 0.3 U/mL | 1.0–150 U/mL | [51] |
| Ab/TH-NPG-GN/GC | HRP@liposomes/Ab2 | DPV | 5 μU/mL | 2 × 10−5–40 U/mL | [106] | |
| CA242 | rGO-Au-Pd-Ab/GC | Label-free | DPV | 0.00154 mU/mL | 0.001–10,000 U/mL | [108] |
| Ab/Chit-Pb2+-/ SA-Pb2+-GO/GC | Label-free | SWV | 0.067 mU/mL | 0.005–500 U/mL | [110] | |
| CEA | Ab-biotin-streptavidin-PtNPs@rGO@PS NSs/GC | Label-free | DPV | 0.01 ng/mL | 0.05–70 ng/mL | [25] |
| Ab/AuNPs/NB-ERGO | Label-free | DPV | 0.00045 ng/mL | 0.001–40 ng/mL | [53] | |
| Ab/AgPt NRs-rGO | Label-free | EIS | 1.43 fg/mL | 5 fg/mL–50 ng/mL | [56] | |
| Ab/rGO-AuNPs/GC | SWCNTs@GQDs/Ab2 | EIS, CV | 5.3 pg/mL | 50–650 pg/mL | [63] | |
| Ab/AuNPs-MWCNTs-Chits/ GC | Label-free | DPV | 0.01 ng/mL | 0.3–20 ng/mL | [70] | |
| EG/CNDTs@PPI/Ab | Label-free | DPV | 0.00145 ng/mL | 0.005–300 ng/mL | [113] | |
| Ab/NH2-GS/GC | PdPt nanocages/ MWCNTs-NH2-Ab2 | CA | 0.2 pg/mL | 0.001–20 ng/mL | [114] | |
| AFP | ADA-Ab/CD-GS | PdNi/N-GNRs-Ab2 | CV | 0.03 pg/mL | 0.0001–16 ng/mL | [35] |
| PDA-N-MWCNTs/GC | NH2-GS-Au@Pt | EIS | 0.05 pg/mL | 0.1–10 ng/mL | [116] | |
| Ab/AuNPs/PDA/GaN nanowires | Label-free | DPV | 0.003 ng/mL | 0.01–100 ng/mL | [117] | |
| PSA | Ab/PdNP@PANI-C60/GC | HRP-Ab2 | DPV | 1.95 × 10−5 ng/mL | 1.6 × 10−4–38 ng/mL | [23] |
| Ab/HQ@CuNPs-reduced- fullerene-C60/GC | HRP-Ab2 | DPV | 0.002 ng/mL | 0.005–20 ng/mL | [27] | |
| Ab/AuNPS/rGO/Au | Label-free | DPV | 3 pg/mL | 6 pg/mL–30 ng/mL | [52] | |
| HBV | HBsAb-MNPs | Ab2AuNPs/hemin/ G-Quadruplex/MB | SWV | 0.19 pg/mL | 0.3–1000 pg/mL | [42] |
| HRP-Ab–AuNPs/DTSP/NPG | HRP | DPV | 2.3 pg/mL | 0.01–0.1ng/mL | [123] | |
| HBsAb-MNPs | HBsAb-AuNPs/Cu | ASW | 87 pg/mL | 0.1–1500 ng/mL | [124] | |
| GO/Fe3O4/ PB@AuNPs/SPE | Label-free | CV | 0.16 pg/mL | 0.5–200 ng/mL | [125] | |
| CoV | Au/MNPs | Label-free | SWV | 0.1 pg/mL | 0.001–100 ng/mL | [126] |
| Ab-PBASE/GN | Label free | FET | 2.42 × 102 cp/mL | - | [120] | |
| antimIgG-Ab-MNPs | antirIgG-Ab2-AP | DPV | 19 ng/mL (S) 8 ng/mL (N) | - | [121] | |
| H1N1 | Ab/RGO/Au | Label-free | CA | 0.5 pfu/mL | 1–104 pfu/mL | [54] |
| HIV | MNPs-Ab | AuNPs/Ac-HRP-Ab2 | DPV | 0.5 pg/mL | 0.001–10.00 ng/mL | [122] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zumpano, R.; Polli, F.; D’Agostino, C.; Antiochia, R.; Favero, G.; Mazzei, F. Nanostructure-Based Electrochemical Immunosensors as Diagnostic Tools. Electrochem 2021, 2, 10-28. https://doi.org/10.3390/electrochem2010002
Zumpano R, Polli F, D’Agostino C, Antiochia R, Favero G, Mazzei F. Nanostructure-Based Electrochemical Immunosensors as Diagnostic Tools. Electrochem. 2021; 2(1):10-28. https://doi.org/10.3390/electrochem2010002
Chicago/Turabian StyleZumpano, Rosaceleste, Francesca Polli, Cristine D’Agostino, Riccarda Antiochia, Gabriele Favero, and Franco Mazzei. 2021. "Nanostructure-Based Electrochemical Immunosensors as Diagnostic Tools" Electrochem 2, no. 1: 10-28. https://doi.org/10.3390/electrochem2010002
APA StyleZumpano, R., Polli, F., D’Agostino, C., Antiochia, R., Favero, G., & Mazzei, F. (2021). Nanostructure-Based Electrochemical Immunosensors as Diagnostic Tools. Electrochem, 2(1), 10-28. https://doi.org/10.3390/electrochem2010002








