Abstract
Atomic-level gold clusters are decorated on a polyaniline (PANI) support by a cyclic atomic electrodeposition process, and the catalytic activity in the oxidation of glucose is studied. The evaluation is conducted by cyclic voltammetry using atomic-level gold clusters-decorated PANI (PANI/AuN, where N indicates the atomic size of the Au cluster and N = 1~3 in this study) as the working electrode and a solution containing 0 to 50.0 mM of glucose in phosphate-buffered saline. The catalytic activity is determined from the oxidation current observed at around +0.6 V vs. Ag/AgCl. The catalytic activity is found to be affected by the size of gold clusters decorated on the PANI/AuN, whereby the catalytic activity is low when N is 1 or 3. On the other hand, an obvious enhancement in the catalytic activity is observed for the PANI/Au2 electrode.
1. Introduction
Gold is often used as the catalyst in the oxidization of organic materials [1,2], and the catalytic activity is improved following a reduction in size of the gold catalyst [3], or an increase in the specific surface area. On the other hand, from simulation results, properties of metal clusters are reported to fluctuate when the size is at the atomic level [4,5,6]. For gold clusters composed of 2 to 12 atoms, the second total energy differences fluctuate between a high value at roughly 1.0 eV when the number of gold atoms in the gold cluster is an even number and a low value at roughly −1.0 eV when the number is an odd number [4]. The even–odd pattern is also observed in the HOMO–LUMO gap of atomic-level gold clusters, where the gold cluster switches between semiconductor and conductor when the number of atoms in the cluster is an even or odd number [4].
In order to evaluate the properties of atomic-level clusters experimentally, Janata et al. reported a cyclic atomic electrodeposition process to allow electrodeposition of gold clusters one atom by one atom [7,8,9,10]. In the preparation of atomic-level gold clusters, a solution containing the tetrachloroaurate anion (AuCl4−) is used. AuCl4− has a high affinity for the imine sites of polyaniline (PANI). Upon immersing PANI into a solution containing AuCl4−, AuCl4− is spontaneously reduced to metallic gold as shown in the following equations:
By controlling the potential applied to the PANI electrode, deposition of one atom by one atom of gold per imine site of PANI is realized. Formation of metallic gold at the imine sites would free up the imine sites of PANI again and makes them accessible for the next deposition cycle.
The even–odd pattern reported in the properties of atomic-level gold clusters from simulation results [4,5,6] is also observed in experiments of normal propanol and isopropanol oxidation using atomic-level gold clusters-decorated PANI (PANI/AuN, where N indicates the atomic size of the Au cluster) electrodes prepared by the cyclic atomic electrodeposition process [8,11]. PANI/AuN electrodes with N = 1 and 3 are both not effective in promoting oxidation of both normal propanol and isopropanol, which is similar to the result when using a bare PANI electrode as the working electrode, and then an obvious enhancement in the oxidization current is obtained when using PANI/AuN electrodes with N = 2 and 4 [11]. In addition, discrimination of normal propanol and isopropanol is difficult because of the identical molecular mass and close chemical structure, but the current responses in oxidizations of the two propanols are different when using PANI/AuN (N = 2 and 4) electrodes.
The PANI/Au2 electrode prepared by cyclic atomic electrodeposition is used in the sensing of ethanol, normal propanol, isopropanol and ethyl formate vapors [10]. PANI/Au2 shows decent catalytic activity in sensing these short-chain aliphatic esters. Patterns of current responses from the oxidation of ethanol, normal propanol and ethyl formate are similar, and the isopropanol vapor generates a different pattern of the current responses.
In this study, PANI/AuN (N = 1 to 3) electrodes prepared by cyclic atomic electrodeposition are used as the working electrode in the oxidation of glucose in phosphate buffer solution (PBS), and effects of the gold cluster size on glucose oxidation are evaluated for application in glucose electrochemical sensors.
2. Materials and Methods
2.1. Chemicals
Tetrafluoroboric acid (HBF4, 48%), perchloric acid (HClO4, 70%), aniline (C6H5NH2, 99.5+%), potassium tetrachloroaurate (KAuCl4, 98%), glucose oxidase enzyme (GOD, ≥65%) and PBS (P5493) were all purchased from Sigma-Aldrich. Potassium chloride (KCl, 99.5+%) was provided by Wako Pure Chemical Industries, Ltd. Hydrochloric acid (HCl, 35.0~37.0%), potassium hydroxide (KOH, 86.0+%) and glutaraldehyde solution (CH2(CH2CHO)2, 25% in water) were obtained from Kanto Chemical Co., Inc. β-D-Glucose (C6H12O6, >85.0%) was purchased from Tokyo Chemical Industry Co., Ltd.
2.2. Deposition of PANI
Pretreatment was conducted to clean the surface of the working electrode (WE) before the deposition of PANI. The WE was a ⌀3 mm platinum disk electrode (PTE platinum electrode, #002422) provided by ALS Co., Ltd. A piece of platinum plate with 2 cm2 of surface area was used as the counter electrode (CE), and the reference electrode (RE) was a double-junction silver chloride (Ag/AgCl/3 M KCl) electrode (PINE research). The electrolyte was 1 M HCl solution. A constant potential of +1.5 V was applied to the WE for 180 s while stirring the electrolyte at 200 RPM. The WE electrode was rinsed with ultrapure water after the pretreatment to remove the residual electrolyte on its surface.
PANI was deposited by the electropolymerization of the anline monomer on the pretreated platinum disk electrode. The electrolyte was composed of 0.1 M aniline monomer in 2 M HBF4. The electropolymerization was conducted in two steps. In step 1, linear sweep voltammetry from 0 to 1.5 V at 50 mV/s was conducted. In step 2, a constant current of 0.05 mA was applied for 260 s. The color of the surface of the WE changed from silver (color of Pt) to blue (color of PANI emeraldine base) after the electropolymerization process.
2.3. Cyclic Atomic Electrodepostion of Atomic-Level Gold Clusters on PANI
Cyclic atomic electrodeposition was conducted using a reaction flow cell as shown in Figure 1a. One cycle of the cyclic atomic electrodeposition process could be summarized in four steps as shown in Figure 1b. In step i, 0.1 M HClO4 solution was introduced into the cell, and an applied potential at +0.8 V was applied to the WE. By doing this, the PANI on the WE would transform to the pernigraniline phase, which is a highly oxidized state with positively charged nitrogen sites in its main chain. In step ii, the solution inside the flow cell was exchanged to 0.1 M HClO4 solution containing 0.2 mM potassium tetrachloroaurate. In step iii, the surface of the WE was rinsed with 0.1 M HClO4 solution to ensure that one and only one tetrachloroaurate anion was attracted to each imine group in PANI. In step iv, the single tetrachloroaurate anion attached to the imine group was reduced to form one gold atom by sweeping the applied potential from +0.8 to −0.2 V.
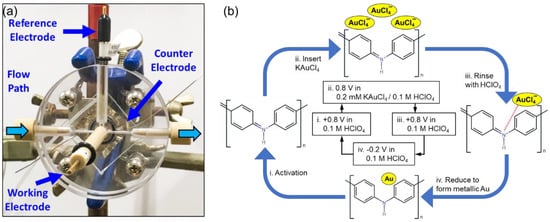
Figure 1.
(a) Reaction flow cell and (b) schematic of the cyclic atomic electrodeposition process. N atoms containing atomic gold clusters are formed by repeating the cycle N times.
2.4. Glucose Oxidation
Immobilization of the GOD on the PANI/AuN electrode was conducted by first dipping 10 μL of 0.1% glutaraldehyde solution onto the PANI/AuN electrode and drying under ambient conditions to remove unbounded glutaraldehyde molecules. Then, the electrode was rinsed with ultrapure water. Next, 3 mg of the GOD was dissolved in 1 mL of 0.1 M PBS, and 30 μL of this solution was dipped onto the PANI/AuN electrode and kept for 48 h at room temperature. After that, the electrode was rinsed with ultrapure water to remove unbounded GOD and stored at 4 °C before the next step [2].
Activation of the GOD-bounded PANI/AuN electrode was conducted before evaluation of the catalytic activity in the oxidation of glucose. The activation was conducted by conducting cyclic voltammetry (CV) in 0.1 M PBS in a range of −0.2 to +1.0 V at a scan rate of 100 mV/s for 20 cycles. The performance in glucose oxidation was evaluated by conducting CV in a range of −0.2 to +1.0 V at a scan rate of 50 mV/s using 0.1 M PBS containing 0 to 50.0 mM of glucose. Five cycles of CV were performed to ensure a steady state was achieved.
3. Results and Discussion
The catalytic activity of the PANI/Au1 electrode in the oxidation of glucose could be observed from the CVs shown in Figure 2. The oxidation current responses at roughly +0.6 V were suggested to be contributed by the oxidation of glucose. In CVs of the electrolyte without the introduction of glucose (0.0 mM case), no obvious oxidation current was observed at around +0.6 V. For electrolytes containing 0.5 and 5.0 mM of glucose, the current responses from the oxidation of glucose were still not observable. An increase in the oxidation current was observed when the concentration of glucose increased to 10.0 mM, in which the oxidation current density was about 33.0 μA/cm2. The oxidation current density further increased to roughly 37.0 μA/cm2 as the glucose concentration increased to 20.0 mM.
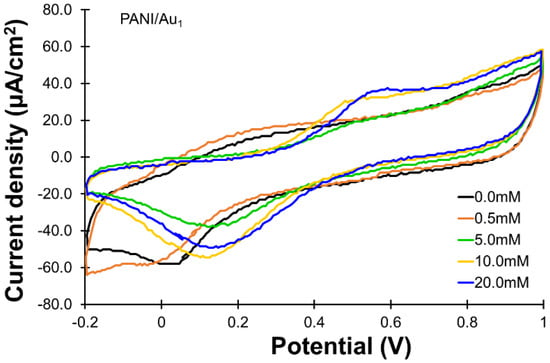
Figure 2.
Cyclic voltammograms (CVs) for the oxidation of glucose (0~20 mM) using the PANI/Au1 electrode. All the CVs were recorded with the same scan rate of 50 mV/s and the same potential range of −0.2 to 1.0 V.
The catalytic activity of the PANI working electrode experienced two cycles of the cyclic atomic deposition process (PANI/Au2) and was significantly better than that of the PANI/Au1 electrode, as shown in Figure 3. The current responses did not change much when the glucose concentration increased from 0 to 0.5 mM. The oxidation current responses noticeably increased as the glucose concentration increased to 5.0 mM, and the current density reached 51.0 μA/cm2, which is higher than the oxidation current density observed for PANI/Au1 in the electrolyte containing 20.0 mM of glucose. The current response reached roughly 69.0 μA/cm2 when 10.0 mM of glucose was used and 104 μA/cm2 when the glucose concentration was increased to 20.0 mM.
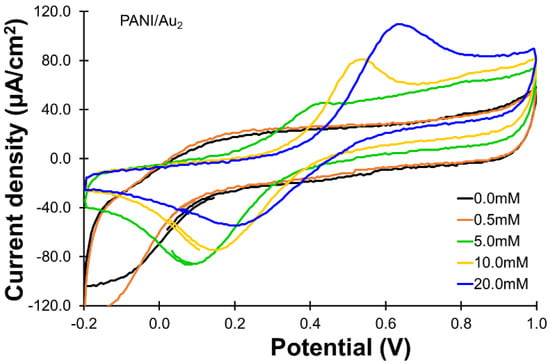
Figure 3.
CVs for the oxidation of glucose (0~20 mM) using the PANI/Au2 electrode. All the CVs were recorded with the same scan rate of 50 mV/s and the same potential range of −0.2 to 1.0 V.
For the PANI/Au3 electrode, the oxidation of glucose was still not observable using the PBS solution containing 0.5 mM of glucose, as shown in Figure 4. The oxidation current response increased to 45.0 μA/cm2 when 5.0 mM of glucose was used. The oxidation reached 55.0 and 78.0 μA/cm2 as the glucose concentration increased to 10.0 and 20.0 mM, respectively.
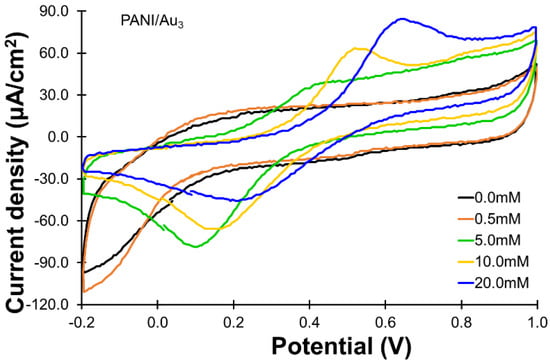
Figure 4.
CVs for the oxidation of glucose (0~20.0 mM) using the PANI/Au3 electrode. All the CVs were recorded with the same scan rate of 50 mV/s and the same potential range of −0.2 to 1.0 V.
The even–odd pattern observed in the oxidation of short-chain aliphatic esters [10,11] was also observed from comparisons of the current responses generated by the three PANI/AuN electordes (N = 1–3) in this study, as shown in Figure 5. The PANI/Au2 electrode showed the best catalytic activity in the oxidation of glucose among the three electrodes. A similar trend is also reported in the oxidation of short-chain aliphatic esters, in which the even-numbered electrode (PANI/Au2 in this case) shows better catalytic activity when compared to the odd-numbered electrode, such as PANI/Au1 and PANI/Au3. Fernandez et al. reported that even-numbered atomic-level gold clusters would behave like semiconductors and the odd-numbered atomic-level gold clusters would be like conductors from simulation results [4]. Gold clusters with better electrical conductivity are expected to have better catalytic activity in the oxidation of organics [1,2], but results obtained in this study show a different trend. Results obtained in this study suggest that the electrical conductivity is not the sole factor affecting the catalytic activity. Other factors, such as the shape of the atomic-level gold cluster, could also be involved. Further study is still required to clarify this point.
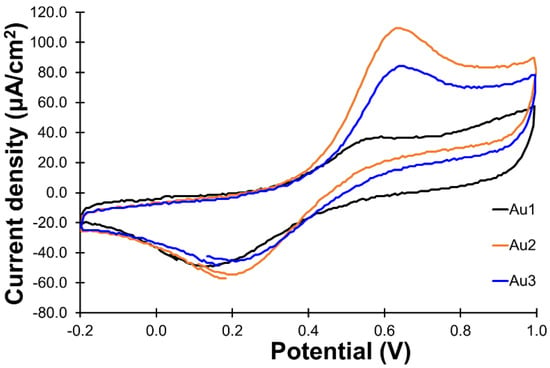
Figure 5.
CVs for the oxidation of glucose for PANI/Au1, PANI/Au2 and PANI/Au3. All the CVs were recorded with 20 mM glucose in the solution, the same scan rate of 50 mV/s and the same potential range of −0.2 to 1.0 V.
4. Conclusions
Atomic-level gold clusters were decorated on a PANI electrode by the cyclic atomic electrodeposition process. The catalytic activity of the atomic-level gold clusters-decorated PANI electrode toward the oxidation of glucose was clarified by cyclic voltammetry, and the catalytic activity was determined by the oxidation current at roughly +0.6 V vs. Ag/AgCl. The even–odd pattern observed in the physical properties of atomic-level gold clusters and in the oxidation of short-chain aliphatic esters was confirmed in the oxidation of glucose, in which the even-numbered PANI/Au2 electrode showed better catalytic activity than that of the odd-numbered PANI/Au1 and PANI/Au3 electrodes.
Author Contributions
Conceptualization, T.-F.M.C., T.N., M.S.; methodology, Y.I., Y.-A.C., P.C.; validation, C.-Y.C., T.-F.M.C.; formal analysis, Y.I., T.-F.M.C.; investigation, Y.I., T.-F.M.C.; resources, T.N., M.S.; data curation, Y.I.; writing—original draft preparation, Y.I.; writing—review and editing, Y.I., T.-F.M.C.; visualization, Y.I.; supervision, T.-F.M.C. and M.S.; project administration, T.N., M.S.; funding acquisition, T.-F.M.C., T.N., M.S. All authors have read and agreed to the published version of the manuscript.
Funding
The New Energy and Industrial Technology Development Organization (NEDO).
Acknowledgments
This study is based on results obtained from a project commissioned by the New Energy and Industrial Technology Development Organization (NEDO).
Conflicts of Interest
On behalf of all of the co-authors, the corresponding author states that there is no conflict of interest.
References
- Hashmi, A.S.K.; Hutchings, G.J. Gold catalysis. Angew. Chem. Int. Edit. 2006, 45, 7896–7936. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.; Lemire, C.; Shaikhutdinov, S.K.; Freund, H. Surface chemistry of catalysis by gold. Gold Bull. 2004, 37, 72–124. [Google Scholar] [CrossRef]
- Fang, X.F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar]
- Fernandez, E.M.; Soler, J.M.; Garzon, I.L.; Balbas, L.C. Trends in the structure and bonding of noble metal clusters. Phys. Rev. B 2004, 70, 165403. [Google Scholar] [CrossRef]
- Hakkinen, H.; Landman, U. Gold clusters () and their anions. Phys. Rev. B 2000, 62, R2287–R2290. [Google Scholar] [CrossRef]
- Majumder, C.; Kulshreshtha, S.K. Structural and electronic properties of () clusters and their interactions with single S atoms: Ab initio molecular dynamics simulations. Phys. Rev. B 2006, 73, 155427. [Google Scholar] [CrossRef]
- Jonke, A.P.; Josowicz, M.; Janata, J.; Engelhard, M.H. Electrochemically controlled atom by atom deposition of gold to polyaniline. J. Electrochem. Soc. 2010, 157, P83–P87. [Google Scholar] [CrossRef]
- Jonke, A.P.; Josowicz, M.; Janata, J. Odd-even pattern observed in polyaniline/(Au0–Au8) composites. J. Electrochem. Soc. 2012, 159, 40–43. [Google Scholar] [CrossRef]
- Jonke, A.P.; Steeb, J.L.; Josowicz, M.; Janata, J. Atomic clusters of Pd and AuNPdM in polyaniline. Catal. Lett. 2013, 143, 531–538. [Google Scholar] [CrossRef]
- Chakraborty, P.; Chien, Y.A.; Chang, T.F.M.; Sone, M.; Nakamoto, T. Indirect sensing of lower aliphatic ester using atomic gold decorated polyaniline electrode. Sensors 2020, 20, 3640. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Chien, Y.A.; Chiu, W.T.; Chang, T.F.M.; Sone, M.; Nakamoto, T. Design and development of amperometric gas sensor with atomic Au–Polyaniline/Pt composite. IEEE Sens. J. 2020, 20, 12479–12487. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).