Co-Electrodeposition of Au–TiO2 Nanocomposite and the Micro-Mechanical Properties
Abstract
1. Introduction
2. Materials and Methods
Co-Electrodeposition of Au–TiO2 Composite Films
3. Results
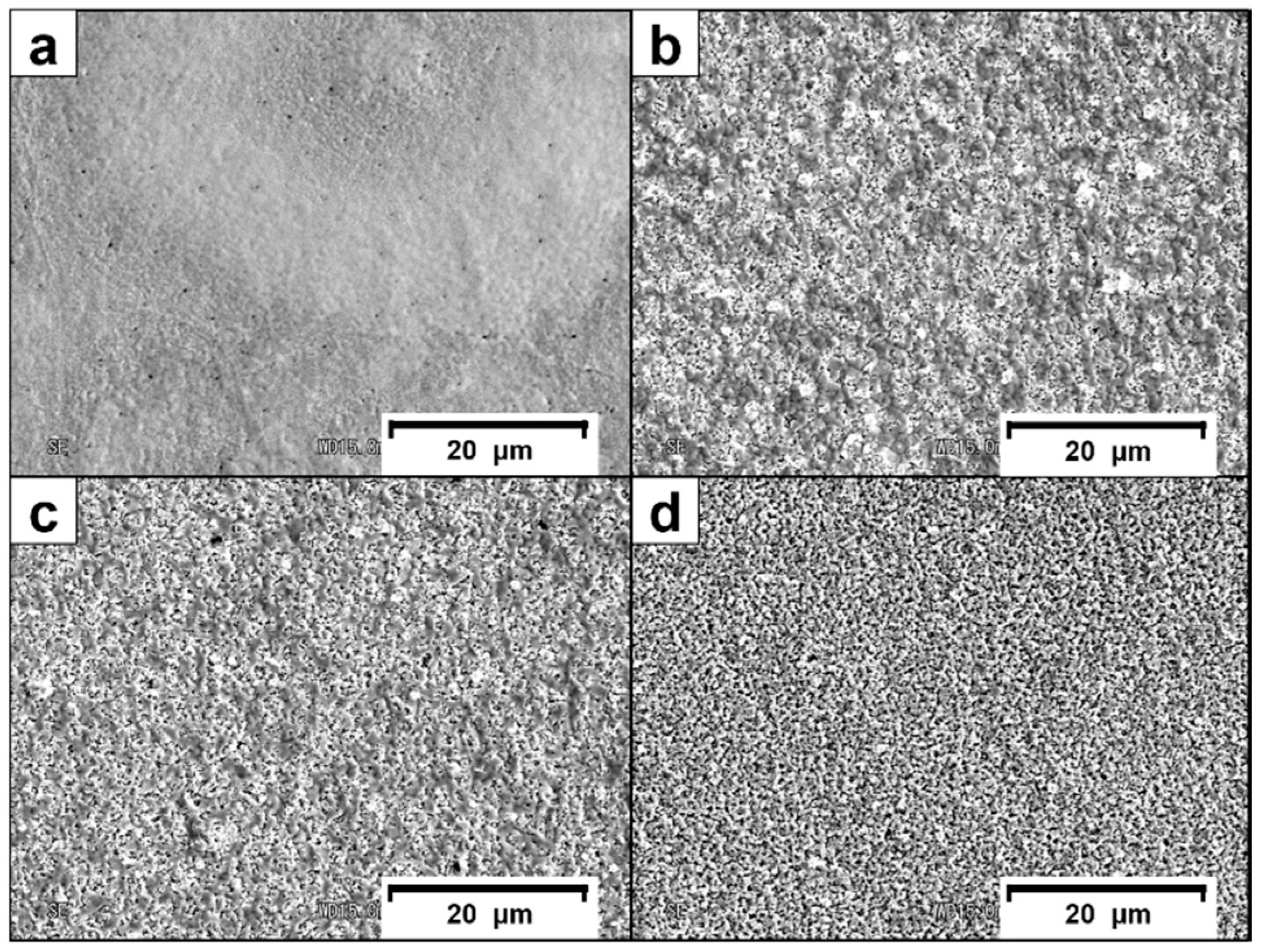
3.1. Surface Morphology and Crystalline Structure
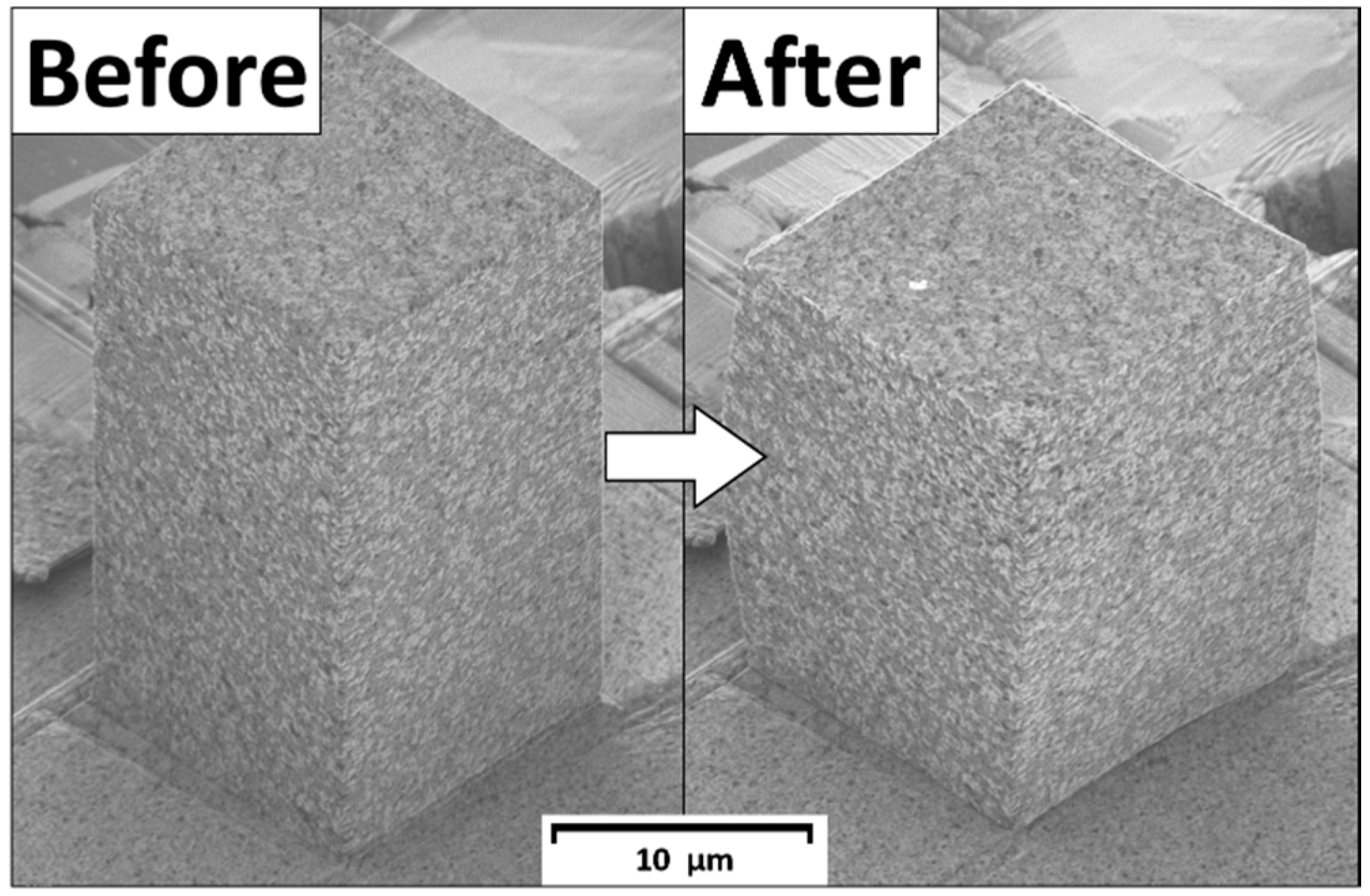
3.2. Mechanical Properties of Au–TiO2 Composite
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yamane, D.; Konishi, T.; Matsushima, T.; Machida, K.; Toshiyoshi, H.; Masu, K. Design of sub-1g microelectromechanical systems accelerometers. Appl. Phys. 2014, 104, 074102. [Google Scholar] [CrossRef]
- Masu, K.; Machida, K.; Yamane, D.; Ito, H.; Ishihara, N.; Chang, T.F.M.; Sone, M.; Shigeyama, R.; Ogata, T.; Miyake, Y. CMOS-MEMS based microgravity sensor and its application. ECS Trans. 2020, 97, 91–108. [Google Scholar] [CrossRef]
- Chiu, S.Y.; Chung, S.T.; Lin, C.Y.; Tsai, W.T. Electrodeposition of Ni-Al2O3 composite coatings employing supercritical CO2 baths. Surf. Coat. Technol. 2014, 247, 68–73. [Google Scholar] [CrossRef]
- Imbaby, M.F.; Jiang, K. Stainless steel–titania composite micro gear fabricated by soft moulding and dispersing technique. Microelectron. Eng. 2010, 87, 1650–1654. [Google Scholar] [CrossRef]
- Parida, G.; Chaira, D.; Chopkar, M.; Basu, A. Synthesis and characterization of Ni-TiO2 composite coatings by electro-co-deposition. Surf. Coat. Technol. 2011, 205, 4871–4879. [Google Scholar] [CrossRef]
- Srivastava, M.; Balaraju, J.N.; Ravishankar, B.; Rajam, K.S. Improvement in the properties of nickel by nano-Cr2O3 incorporation. Surf. Coat. Technol. 2010, 205, 66–75. [Google Scholar] [CrossRef]
- Maurin, G.; Lavanant, A. Electrodeposition of nickel/silicon carbide composite coatings on a rotating disc electrode. J. Appl. Electrochem. 1995, 25, 1113–1121. [Google Scholar] [CrossRef]
- Arai, S.; Endo, M. Carbon nanofiber–copper composite powder prepared by electrodeposition. Electrochem. Commun. 2003, 5, 797–799. [Google Scholar] [CrossRef]
- Arai, S.; Saito, T.; Endo, M. Cu–MWCNT composite films fabricated by electrodeposition. J. Electrochem. Soc. 2010, 157, D147–D153. [Google Scholar] [CrossRef]
- Graydon, J.W.; Kirk, D.W. Suspension Electrodeposition of Phosphorus and Copper. J. Electrochem. Soc. 1990, 137, 2061–2066. [Google Scholar] [CrossRef]
- Hu, C.C.; Chen, E.; Lin, J.Y. Capacitive and textural characteristics of polyaniline-platinum composite films. Electrochim. Acta 2002, 47, 2741–2749. [Google Scholar] [CrossRef]
- Ibrahim, I.A.; Mohamed, F.A.; Lavernia, E.J. Particulate reinforced metal matrix composites—A review. J. Mater. Sci. 1991, 26, 1137–1156. [Google Scholar] [CrossRef]
- Greer, J.R.; Oliver, W.C.; Nix, W.D. Size dependence of mechanical properties of gold at the micron scale in the absence of strain gradients. Acta Mater. 2005, 53, 1821–1830. [Google Scholar] [CrossRef]
- Takashima, K.; Higo, Y.; Sugiura, S.; Shimojo, M. Fatigue crack growth behavior of micro-sized specimens prepared from an electroless plated Ni-P amorphous alloy thin film. Mater. Trans. 2001, 42, 68–73. [Google Scholar] [CrossRef]
- Chen, C.Y.; Yoshiba, M.; Nagoshi, T.; Chang, T.F.M.; Yamane, D.; Machida, K.; Masu, K.; Sone, M. Pulse electroplating of ultra-fine grained Au films with high compressive strength. Electrochem. Commun. 2016, 67, 51–54. [Google Scholar] [CrossRef]
- Zhang, P.; Li, S.X.; Zhang, Z.F. General relationship between strength and hardness. Mater. Sci. Eng. A 2011, 529, 62–73. [Google Scholar] [CrossRef]


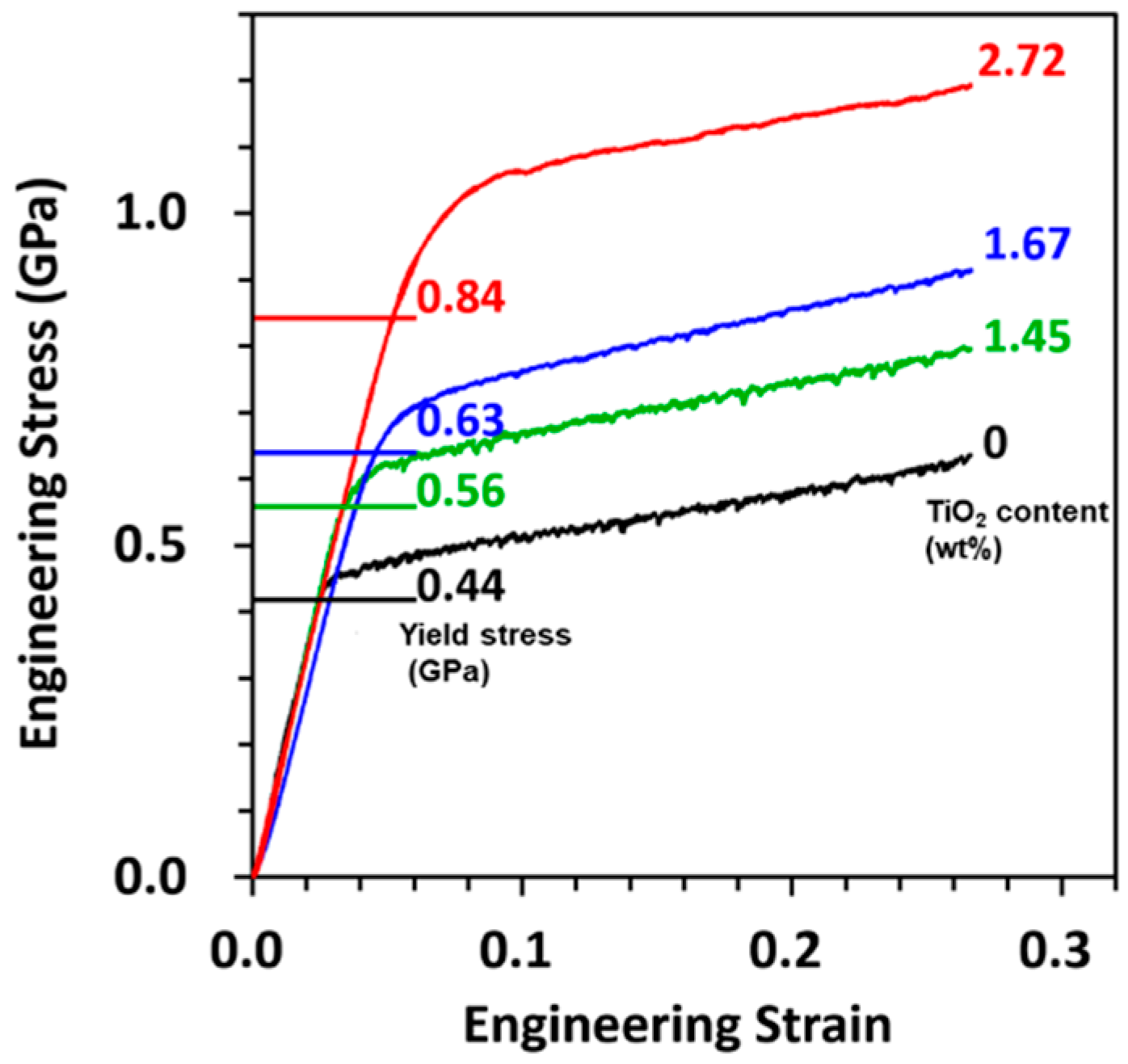
| Electrolyte | TiO2 Content (wt%) | HV (Hv) | σy (GPa) | HV/σy | Mass Density (g/cm3) |
|---|---|---|---|---|---|
| 0 g/L TiO2 | - | 135 | 0.44 | 3.01 | 19.32 |
| 10 g/L TiO2 | 1.45 | 162 | 0.56 | 2.84 | 19.08 |
| 30 g/L TiO2 | 1.67 | 176 | 0.63 | 2.74 | 19.05 |
| 50 g/L TiO2 | 2.72 | 207 | 0.84 | 2.42 | 18.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chien, Y.-A.; Chang, T.-F.M.; Chen, C.-Y.; Yamane, D.; Ito, H.; Machida, K.; Masu, K.; Sone, M. Co-Electrodeposition of Au–TiO2 Nanocomposite and the Micro-Mechanical Properties. Electrochem 2020, 1, 388-393. https://doi.org/10.3390/electrochem1040025
Chien Y-A, Chang T-FM, Chen C-Y, Yamane D, Ito H, Machida K, Masu K, Sone M. Co-Electrodeposition of Au–TiO2 Nanocomposite and the Micro-Mechanical Properties. Electrochem. 2020; 1(4):388-393. https://doi.org/10.3390/electrochem1040025
Chicago/Turabian StyleChien, Yu-An, Tso-Fu Mark Chang, Chun-Yi Chen, Daisuke Yamane, Hiroyuki Ito, Katsuyuki Machida, Kazuya Masu, and Masato Sone. 2020. "Co-Electrodeposition of Au–TiO2 Nanocomposite and the Micro-Mechanical Properties" Electrochem 1, no. 4: 388-393. https://doi.org/10.3390/electrochem1040025
APA StyleChien, Y.-A., Chang, T.-F. M., Chen, C.-Y., Yamane, D., Ito, H., Machida, K., Masu, K., & Sone, M. (2020). Co-Electrodeposition of Au–TiO2 Nanocomposite and the Micro-Mechanical Properties. Electrochem, 1(4), 388-393. https://doi.org/10.3390/electrochem1040025







