Abstract
The purpose of this study was to explain the mechanism of formation and to examine the composition of the anodic film formed on the surface of titanium in an anhydrous neutral methanol solution of electrolytes. In an environment deprived of water molecules, the growth of a 3D-phase titanium oxide layer is not possible. Electrochemical investigations demonstrated that the Ti surface in CH3OH-LiClO4 solutions experienced a pseudo-passivation with the formation of a methoxy layer, which resulted from the reaction of the metal surface with alcohol molecules. The presence of this methoxy surface film was confirmed through XPS and in situ FTIR measurements. The layer blocked the Ti anodic dissolution at the potential range corresponding to the stability of methanol and methoxy ions (i.e., <0.55 V). At potentials over 0.55 V, the methoxy layer was oxidised, which caused the “depassivation” of the metal surface and the etching of titanium. The addition of water changed the properties of Ti in CH3OH-LiClO4 solutions, but only with a water content above 0.2 mole fraction. Below this concentration of water, titanium behaved like it would in an anhydrous solution of methanol. In the range of water concentration of 0.2 to 0.7 mole fraction, the structure of the solution is strengthened because both components of the solvent formed separate percolating networks. The strengthening of the solution structure resulted in a strengthening of the surface layer of Ti(OH)m(OCH3)n. Such a layer had strong barrier properties similar to the properties of an organic polymer film. The formation and growth of a stable layer of TiO2 were possible only in a solvent when the water concentration was higher than ≈0.7 mole fraction.
1. Introduction
Titanium displays a high corrosion immunity in chloride-containing solutions [1,2,3,4,5,6,7], which makes Ti and its alloys very attractive as corrosion-resistant constructional materials that are suitable for applications in aerospace, marine, medical and other industries. The low density and good biocompatibility render titanium alloys especially favourable for the production of biomaterials for implants, prostheses, artificial heart components, etc. [6,7]. Due to the expansion of existing applications and the development of new ones, the knowledge of the properties of titanium in various organic solvents has been systematically expanded [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24].
Particularly interesting is the corrosive behaviour of titanium in alcoholic environments. The reason for this interest is found in the search for efficient anodes for the oxidation of alcohols [25,26], new ways of producing TiO2 nanoparticles [27] and the explanation of titanium stress corrosion in anhydrous alcohol electrolytes [11,12,15]. Most of the studies are devoted to the corrosion behaviour of Ti in neutral and acidic methanol solutions of chlorides [8,13,17,18,19,20,23,28,29]. The literature reports that passive titanium oxide is found to be very sensitive to the presence of aggressive ions in methyl alcohol and undergoes cracking along the intergranular paths due to applied stress [18,19,20,21,22]. The stability of the oxide layer on the Ti surface in methanolic solutions strongly depends on the water concentration [8].
An interesting issue is the phenomenon of the “pseudo-passivity” of metals in anhydrous organic solutions of electrolytes [30]. In these media, the anodic polarisation curve shows a wide range of anodic potentials in which the metal dissolution is inhibited by the formation of an anodic surface product, similar to the passive range in aqueous environments [14,30,31,32]. Such a phenomenon is also observed in the case of titanium [14,30,31,32].
This paper contributes to understanding the mechanism, composition and stability of a pseudo-passive layer formed at the Ti surface in anhydrous methanol containing a LiClO4 solution, which is an electrolyte with weak complexing abilities, as well as what allowed for the evaluation of the role of methanol molecules in anodic passivation processes.
In our work, we also aimed to explain the role of intermolecular water–methanol interactions on the anodic properties of titanium in mixed methanol–aqueous LiClO4 solutions. Considering the behaviour of the metal in water–methanol solutions, the specific nature of this system must be taken into account. Both water and methanol are liquids of high ordering. Water molecules in bulk are organized in tetrahedral ice-like clusters [33]. Molecules in pure methanol form open, non-linear chains with approximately tetrahedral coordination that are either hexa- and/or octamer molecular clusters due to the intermolecular hydrogen bonding [34,35]. The thermodynamic values of water–methanol solutions vary from what would be expected after the mixing of these two liquids [35,36,37,38,39]. Even a small amount of methanol causes a change in the water molecules’ arrangement through the introduction of hydrophobic methyl groups [39]. Due to hydrogen bond interactions, molecules of alcohol and water form clathrates of dodecahedral and tetrahedral structures [39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55]. In water solutions of diluted methanol, the water molecules catch the alcohol in the “cages” [53]; similarly, in diluted solutions of water in methanol, the water molecules are enclosed in CH3OH clathrates [39,43,44,54]. Of particular interest is the concentration region of the water mole fraction in methanol between 0.4 and 0.7, where both components appear to form separate, percolating networks. This is the concentration range where many transport properties and thermodynamic excess functions reach extreme values [38,45,46,50,56,57].
2. Materials and Methods
The electrochemical measurements (i.e., cyclic voltammetry (CV) and impedance measurements (EIS)) were performed with the use of the electrochemical workstations VoltaLab PGZ 301, Radiometer Analytical, Lyon, France and Autolab PGStat 30, Metrohm Autolab Utrecht, Netherlands, in a three-electrode system. Pure titanium (99.99%) was used as a working electrode, a platinum foil was used as a counter electrode and Ag/AgCl was used as a reference electrode. Every potential value was recalculated versus a standard hydrogen electrode (SHE). Before each test, the sample was mechanically ground with sandpapers up to 1000, washed in dry methanol and then electrochemically polished in the tested solution. The study was performed in a CH3OH-0.1 M LiClO4 solution prepared with anhydrous methanol and dried salt, as well as in CH3OH-0.1 M LiClO4 xH2O solutions, with the water content ranging between 0.01 and 0.87 mole fraction (0.5, 1, 5, 10, 20, 50, 75 vol.%). Before each experiment, the tested solutions were deaerated with argon for 2 h at room temperature (25 °C). LiClO4 was used as a supporting electrolyte.
Cyclic polarisation curves were carried out in the potential range −1.75 to 1.5 V. Three cycles were recorded in each experiment. The first cycle was used to remove the spontaneous passive film formed on the titanium surface in the air; therefore, the results presented in this study were obtained from the second cycle of the polarisation experiment. The EIS measurements were recorded over the frequency range of 10 kHz to 10 mHz with an amplitude of 10 mV. The data were then analyzed using Echem Analyst 5.21 software, Gamry Instruments, Warminster, PA, USA, with the help of the opportune equivalent circuit.
The morphology and the chemical composition of the surface layer were examined using a scanning electron microscope (SEM), JEOL JSM 5500 LV, Hitachi, Fukuoka 812-0026, Japan, with the help of electron diffraction (EDX). The composition of the anodic layers was evaluated using an X-ray photoelectron spectroscope ESCA-VSW with a depth and area of approximately 5 nm and 3 mm, respectively. Depending on the elements evaluated, the sensitivity of the method was approximately 0.05–0.5%. The magnesium anode was used for the Kα = 1253.6 eV line (without a monochromator). The voltage was 13 kV and the heater current was 16 mA (power of 208 W). The beam angle was 15°.
The infrared spectra of the investigated solutions were obtained using an FTIR Spectrometer Thermo Scientific Nicolet 6700, Thermo Fisher Scientific, Waltham, MA, USA. The titanium layer was examined with the use of a Seagull FTIR-ATR attachment adapted for an electrochemical cell. In this cell, the working electrode was a Ti layer with a thickness of ≈500 Å, which was obtained by sputtering pure titanium on a germanium hemisphere substrate (e.g., transparent to IR radiation). A platinum tin was used as a counter electrode and an Ag wire covered with AgCl played the role of a reference electrode. This method has been described in detail elsewhere [58,59].
3. Results
3.1. Anhydrous CH3OH-LiClO4 Solutions
3.1.1. Polarisation Measurements
Figure 1a shows the cycle polarisation curves carried out at different scan rates on pure titanium in a CH3OH-0.1 M LiClO4 solution. The figure shows the presence of two anodic (i.e., a1 and a2) and two poorly defined cathodic peaks (i.e., c1 and c2). The anodic peaks were attributed to the formation of surface compounds. The linear dependence of the current density of anodic peaks with the potential sweep, presented in Figure 1b, revealed the surface control of the oxidation/reduction processes.
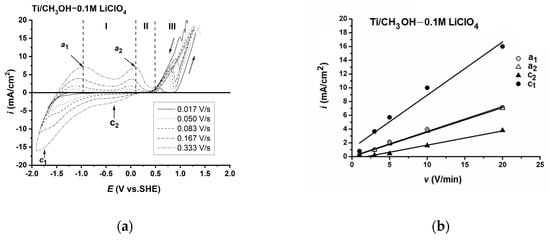
Figure 1.
Polarisation curves of Ti in anhydrous CH3OH-0.1 M LiClO4: I—first pseudopassive range, II—second pseudopassive range and III—transpassive range (a). Linear dependence of anodic and cathodic peaks current on the potential sweep (b).
The first anodic peak (i.e., in the range of −1.25 to −1.0V) could be attributed to the formation of the Ti3+ surface product as a result of the Ti→Ti2+→Ti3+ pathway. According to the literature, the metal/metal ion standard potentials only slightly differ in aqueous and methanol solutions [60]. In aqueous solutions, the equilibrium potential of the Ti/Ti2+ oxidation process is −1.75 V. The zero current potential on the anodic polarisation curve is very close to this value. According to the E–pH diagram, for titanium in neutral aqueous solutions, oxide/hydroxide passivation with the participation of Ti3+ ions and water molecules occurs in the potential range −1.53 to −1.31 V [61]. The equilibrium potentials of the reactions of methoxides formation, similar to the formation of hydroxides in water, are not known. We assumed that, as in aqueous solutions, the oxidation reaction of Ti2+→Ti3+ already occurs within the first anodic peak. In methanol solutions, metal ions at a lower oxidation state are relatively stable. This also applies to Ti3+ ions [62].
The potential of the second peak (i.e., about 0 V) may have been related to the reaction of Ti3+→Ti4+ in the anodic film. The equilibrium potential of the redox reaction Ti3+/Ti4+ in aqueous solutions is −0.04 V [61]. In CH3OH-0.01 M HCl-0.2 M LiCl solution, the half-wave potential of Ti4+ → Ti3+ is E0.5 = −0.103 V vs. SHE [63].
The surface compounds formed at the anodic peaks caused the blocking of the titanium surface at potentials up to 0.55 V. At the more positive potential, the polarisation curve describes the activation of the titanium electrode due to the removal of the methoxy film blocking the metal surface in the pseudo-passive range. This process was the result of the oxidation of methoxy anions and methanol molecules.
The mechanism of oxidation of absolute methanol was proposed by Bélanger [64] and Vassiliev et al. [65] based on measurements of platinum/anhydrous methanol solutions in an electrolytes system. It can be described by the following reactions [64]:
CH3O-layer − e → CH3O*Solution
CH3OH → CH3O* + H+ + e
CH3O* + CH3O* ↔ CH2O + CH3OH
The course of voltammetric curves at a high anodic overvoltage (>0.55 V) is the result of the overlapping of three processes: the oxidation of the methoxy film, the oxidation of methanol on the bare surface and the corrosion of titanium in the exposed areas. The high rate of the anodic reactions in this transpassive range (the increase of the current density by almost three decades) leads to surface oversaturation with the oxidation products of both titanium and methanol. An additional peak appears indicating the adsorption or precipitation of the anodic product inhibiting the dissolution. This anodic product can be adsorbed organic compounds (mainly CH2O [64,65] and/or precipitated Ti(OCH3)3). The presence of the latter was confirmed by the purple color of the solution after longer etching at potentials higher than 1 V.
From the Koutecky–Levich plots (Figure 2a) obtained from the anodic and cathodic branches of the polarisation curves presented in Figure S1 (supplement materials) and its extrapolation to ω−0.5→0, we could calculate the values of the kinetic current ik. Over the entire investigated potential range, the current ik was anodic (Figure 2b). The small dependence of ik on the potential up to 0.2 V showed that the anodic dissolution of titanium was effectively blocked. The imposition of the diffusion-controlled cathodic current (likely corresponding to the reduction of methanol) and the anodic current of the oxidation reaction of titanium (passivation process) was also observed. The reduction of methanol proceeded with the formation of CH3O− anions that were readily adsorbed on the electrode surface, especially in the potential range of the parallel anodic reaction of the formation of titanium compounds at lower oxidation states (Ti2+ and Ti3+). The formation of a Ti-OCH3 surface compound led to surface blocking and inhibition of the methanol reduction.
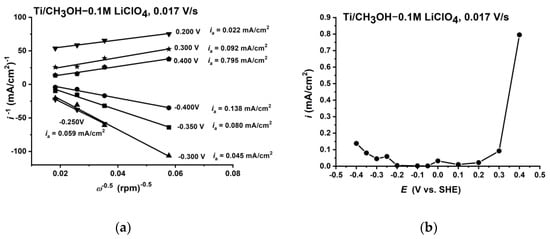
Figure 2.
Koutecky–Levich plots of current values obtained from the polarisation curve in the anodic direction (a) and kinetic current ik obtained via extrapolation of ω−0.5→0 (b).
3.1.2. Impedance Measurements
The Bode diagrams for Ti polarised potentiostatically at specified potentials (−0.510, −0.010, 0.340 and 0.540 V vs. SHE) in the tested solution (Figure 3) revealed blocking properties related to the formation of a barrier surface layer. The analysis of the dlog|Z|/dlog(f) slope (Figure 3a) allowed for distinguishing a low-frequency range with a slope value close to −0.5 and a medium-frequency range with a slope close to −1.0. The relationship between the negative phase angle and frequency exhibited two minima (Figure 3b). The first high-frequency minimum (f > 105 Hz) was probably associated with the electronic system. We did not interpret this range because it was not related to Faraday processes or the pseudocapacity of the layer on the Ti surface.
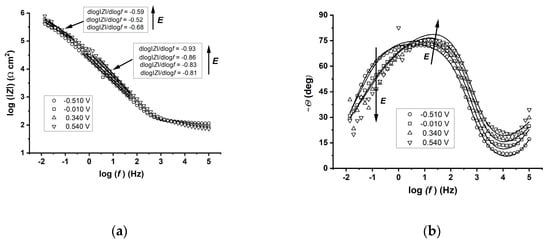
Figure 3.
Effect of the potential on the impedance spectrum of titanium in anhydrous CH3OH-0.1 M LiClO4 and the fitted equivalent circuit. Bode (a) and Phase (b).
The second flat minimum in a low-frequency range (f < 102 Hz) resulted from the overlapping of two relaxation processes associated with the capacitive and diffusive properties of the metal/film/electrolyte system. The impedance in the low-frequency range was attributed to the diffusion processes, whereas the medium-frequency range was associated with the capacitive behaviour of the surface film.
The local etching of the titanium surface at a high anodic overvoltage (1.020 V) was reflected in a significant decrease in the impedance. The EIS spectrum was highly disturbed in the low-frequency range due to the instability of the investigated system. This instability was caused by the changes in the active surface area during the local etching of titanium.
The analysed impedance spectrum obtained in the second pseudo-passive range (<0.55 V) was well-described by an equivalent circuit R(Q1R1(Q2R2)) consisting of two parallel components (Q1R1) in the medium and (Q2R2) in the low-frequency range. The first element (Q1R1) corresponded to the capacitive behaviour of the surface film, while the second (Q2R2) was related to the diffusion constituent at low frequencies (Table S1 in the Supplementary Materials).
The Mott–Schottky relationship (Figure S2) for potentials ranging from −0.5 to 0.5 V (the pseudo-passive range), constructed using the parameter C, calculated for the Q1R1 element using the formula: C = (Q1Rel(1–n))1/n [66], demonstrated that the anodic film generated at potentials corresponding to the stability of Ti(IV) compounds was an n-type semiconductor layer, probably doped with Ti3+ ions. For the lower potentials, there was no such relationship, which indicates the adsorption nature of the surface layer.
3.1.3. SEM Measurements
The titanium sample etched in the first pseudo-passive range (E = −0.3 V vs. SHE) was covered with a layer consisting mainly of O- and C-containing compounds (Figure 4a). The dark spots indicated a layer with high carbon content and with a small contribution of titanium. The SEM picture of the Ti-etched surface in the second pseudo-passive range (E = 0.123 V vs. SHE) (Figure 4b) showed a surface covered with a thin compact film with white spots. These spots consisted mostly of oxygen and carbon. The analysis of the Ti etched surface at E = 0.6 V vs. SHE (Figure 4c) revealed the strong corrosion of metal. The bright areas consisted of pure Ti, whereas the darker regions were enriched with O and C. The presence of such a large amount of carbon in the surface layer suggests that the layer was not an oxide layer, but rather a methoxy film.
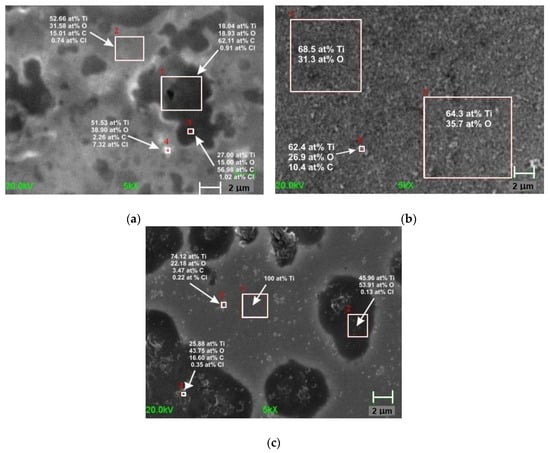
Figure 4.
SEM images (with EDX analysis) of the Ti surface polarized in CH3OH-0.1 M LiClO4 at the following potentials: −0.3 V vs. SHE for 40 min (a), 0.12 V vs. SHE for 20 min (b), and 0.6 V vs. SHE for 2min (c) [58].
3.1.4. XPS Analysis
The XPS spectra are presented in Figure 5. The high-resolution peak core levels were analysed through a deconvolution fitting of the complex spectra. The binding energies and the corresponding quantification (%) of each peak component are presented in Table S2. The analysis of the C1s level spectra for the Ti etched in CH3OH-0.1 M LiClO4 at the potential of the first pseudo-passive range (E = −0.3 V vs. SHE) indicated the presence of carbon bonded with oxygen and carbon in the hydrocarbon group. The dominant peak, with the energy of approximately 285 eV, corresponded to the presence of the C–C and C–H bonds (adventitious carbon) [67,68,69]. The peak of energy of about 286 eV was assigned to C–O bonding and the signal at 289 eV was related to C=O bonding [70]. The same binding energies were recorded for the species of the surface layer formed on Ti after etching at the potential in the second pseudo-passive range (E = 0.123 V vs. SHE) and at the potential of the transpassive region (E = 0.6 V vs. SHE). The only change was the intensity of the bands.
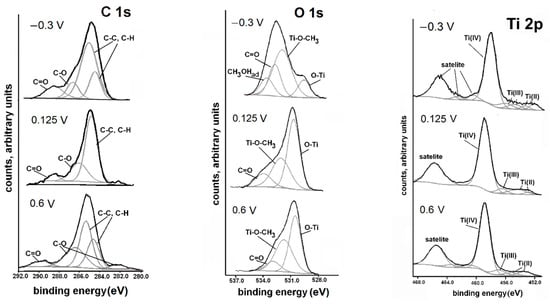
Figure 5.
XPS spectra for the Ti surface that was potentiostatically etched in anhydrous CH3OH-0.1 M LiClO4 at the prepassive range (−0.3 V vs. SHE for 90 min), passive range (0.125 V vs. SHE for 60 min) and transpassive range (0.6 V vs. SHE for 2 min).
The spectra recorded in the O1s band for all potentials contained signals from oxygen bound with titanium –O–Ti (530.5 eV) [70,71]. The peaks at the binding energy of 531.8–532.6 eV, according to Attard and co-authors as well as Atuchin et al. [70,71], were attributed to the oxygen in the OCH3 group bound with the metal surface. The band of energy at 533 eV originated from C=O bonding [72].
The spectrum in the Ti2p band at the first pseudo-passive range was dominated by a signal originating from Ti(IV) compounds (459 eV), the signal was about one-fifth the size of the peak recorded at a higher potential (0.125 V). The spectrum also contained signals from the metallic Ti and titanium of the lower valance states (456–457 eV for trivalent Ti and 455 eV for divalent Ti) [68,70].
The XPS analysis indicated that in the first pseudo-passive range, the surface film was dominated by carbon compounds. At higher potentials, the surface film contained mainly titanium (IV) but the participation of methoxy groups continued to be significant.
3.1.5. FTIR-ATR Measurements
To clarify the chemical nature of the surface layer formed on the Ti surface in the CH3OH-0.1 M LiClO4 solution, in situ FTIR-ATR experiments were performed. The spectra for specified polarisation potentials (E = −0.800 to +0.800 V vs. SHE) were obtained by subtracting the background, which represented the spectrum recorded at an open circuit potential (Figure 6). The most important was the lack of the signal from the OH group (1600 cm−1 and 3750 cm−1) and the presence of the peak originating from the Ti-OCH3 bond [73] appearing near the signals from C–O stretching for alcohols at a wavelength of ≈1029 cm−1.
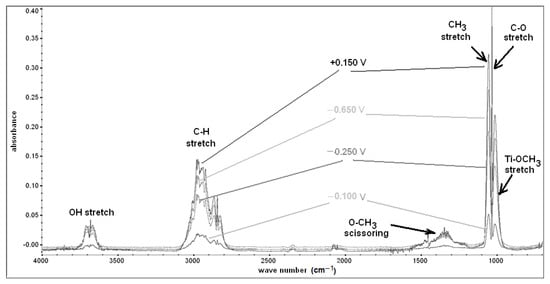
Figure 6.
FTIR-ATR spectra of the Ti layer obtained at various polarisation potentials (E = −0.650, −0.250, −0.100, +0.150 V vs. SHE) in an anhydrous deaerated CH3OH-0.1 M LiClO4 solution [58].
The analysis of the intensity of bands that came from the Ti-OCH3 group compared with the polarisation curve of Ti on Ge demonstrated the increase of the methoxy group’s intensity towards both cathodic and anodic potentials (Figure 7).
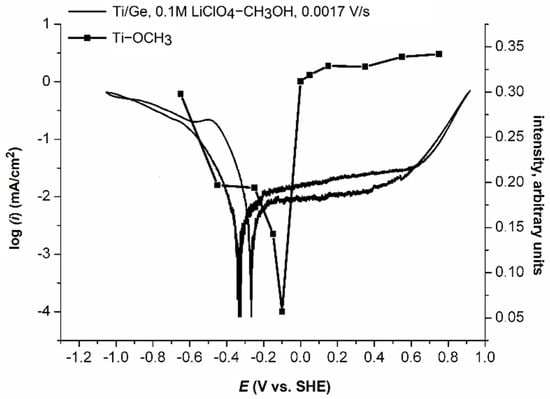
Figure 7.
The intensity of the signal from the Ti-OCH3 group compared to the polarisation curve (scan rate v = 0.0017 V/s) of Ti sputtered on a germanium hemisphere in CH3OH-0.1 M LiClO4 solution.
3.2. The Effect of the Methanol–Water Interaction on the Passivation Process of Titanium
3.2.1. The Structure of the CH3OH–H2O–0.1 M LiClO4 Solutions
Our study of the physicochemical properties, as well as the FTIR spectra of the methanol–water–LiClO4 solutions, was convergent with the structure of the methanol–water system described in the introduction. The properties of solutions containing less than 0.2 mole fraction of water (i.e., viscosity and conductivity) were practically the same as those observed in the case of the anhydrous methanolic solutions (Figure S3). The slight minimum observed of the viscosity curve, and the maximum observed in the conductivity curve after the addition of a small amount of water (x = 0.01), could be associated with the relaxation of the methanol structure [56]. The maximum strength of the mixed solvent that was observed in viscosity measurements occurred at a water content of x = 0.7, which is consistent with the literature [45,46,74,75]. The minimum conductivity for the methanol–water mixture was visible for a ≈0.2 mole fraction of water (20% mol), while the minimum for a solution containing 0.1 M LiClO4 was observed at a mole fraction of water equal to 0.7, (corresponding to a water content of approx. 50%). Shifting of the conductivity minimum in the presence of dissolved salts may be related to the preferential hydration of the ions by water molecules, and thus its lower content in the solution.
The FTIR study of the structure of CH3OH 0.1 M LiClO4 xH2O solutions was carried out in three frequency regions (Figure S4). In the first frequency region of 1635 cm−1, the signal was only caused by the OH groups of water molecules. The small addition of methanol caused the shift of the δOH band to a higher wavelength/lower frequency, i.e., a redshift. In solutions with a water content above 0.7 mol fraction, the frequency of the band was practically constant, which means that methanol had little influence on the solution’s properties. The second frequency range (≈1447 cm−1) corresponded to the bending vibrations of the C–H bond in the OCH3 group. The spectrum shifted towards lower wavelengths (higher frequencies). In the third range, the frequency range of the stretching vibration of the C–O bond in the CH3O group was the result of the imposition of the five bands. Every sub-component corresponded to a specific type of hydrogen bond [76,77,78]. The first band (1032 cm−1) could be attributed to the C–O vibrations in the methanol molecule hydrogen-bonded with two other CH3OH molecules, forming a polymeric chain [76,78]. A narrow and intensive band at 1026 cm−1, which was noticeable only for the solutions containing less than 0.2 mole fraction of water, corresponded to non-hydrogen-bonded methanol molecules [76,78]. In the concentration range of water up to 0.4 mole fraction, at the frequencies of 1019 cm−1, 1016 cm−1, and 1014 cm−1, three additional bands could be observed. According to the literature [76,78], these bands may be assigned to the methanol molecules associated with two water molecules via hydrogen bridges. Above the concentration of 0.6 mol fraction, a wideband at 1012 cm−1 could be observed. This value is similar to the frequency attributable to the hydrogen bridges between oxygen and the hydrogen atom of the water molecule [76,78]. The interaction is stronger than in the bridges, where the hydrogen origin forms the alcohol molecule [76].
The structure of the methanol–water interactions in the tested solution can be described using the scheme proposed by Dixit et al. [57]. The figure reveals three ranges of solution structure associated with the low, medium and high concentrations of water (Figure 8).
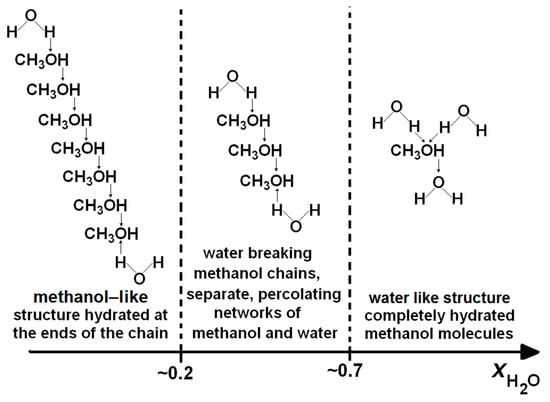
Figure 8.
The effect of the methanol–water system structure on the anodic properties of the potentiodynamic polarisation curves of titanium in CH3OH-LiClO4-H2O solutions (scan rate: 0.17 V/s) [57].
At lower concentrations (x ≤ 0.2), the structure of the liquid corresponds to the structure of pure methanol. The addition of small amounts of water does not cause a substantial change in the pattern of the liquid. Methanol molecules, linked by hydrogen bridges, form chains containing up to 10 molecules. The chains are mostly linearly dominated by clusters containing six and eight molecules of methanol [35]. Hydration takes place at the ends of methanol chains via hydrogen bonds with water molecules as hydrogen donors [57].
At medium concentrations (−0.2 < x < −0.7), the water molecules cause cracking of alcohol chains and create a percolating network of methanol and water clusters, i.e., incomplete mixing occurs at the microscopic level [79]. In this range, the strengthening of the solution structure occurs.
At higher concentrations (x > −0.7), the complete hydration of methanol molecules takes place and hetero-clathrates are formed and dispersed in the water structure.
This model illustrates the methanol–water interactions. For solutions of lithium perchlorate, it is expected that additional Li+ cations and ClO4− anions have an additional but not determinant impact on the structure of the solution.
3.2.2. Polarisation Measurements
Figure 9 illustrates the effect of water on the course of the anodic polarization in a 0.1 M LiClO4-CH3OH xH2O solution. Regardless of the presence of water, the titanium surface remained blocked at the potential range corresponding to Ti(III) stability (the area between the first and second anodic peak). This pseudo-passivation formed in the anhydrous methanol-LiClO4 solution was the effect of the methoxylation of the metal electrode surface and formation of the Ti-OCH3 surface film, whose formation was described in the previous section.
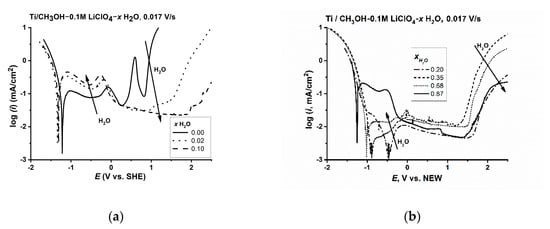
Figure 9.
Effect of water on the polarisation of titanium in CH3OH-LiClO4-H2O solutions: (a) low water content (x ≤ 0.1) for 0.1 M LiClO4, and (b) medium and high water content (x > 0.2) for 0.1 M LiClO4.
The effect of water on the composition of the surface layer in this potential range was complicated. The current value in this range (ia1) reached a minimum at the concentration of water of 0.2 to 0.69 mole fraction, which reflected the impact of the strengthening of the solution structure through the methanol–water interaction. The forming surface layer had strong barrier properties but was not stable at the anodic potentials corresponding to the growth of TiO2. At high anodic potentials (>1.5 V), the layer dissolved and the local corrosion of the titanium surface was observed. The formation of a stable oxide layer took place over the potential corresponding to the formation of Ti(IV) species (above the second peak) only after the water concentration exceeded about 0.7 mole fraction. This was the concentration range in which the water molecules were no longer fully bonded by the methanol particles and may contribute to the growth of the surface layer.
3.2.3. EIS Investigations
The results of impedance measurements, performed in the potential range of −0.510 V to −0.01 V (range of pseudo-passive layer stability) confirmed the important role of the water–methanol interactions on the anodic behavior of titanium (Figure 10). Impedance diagrams for the methanolic solutions at low (≤0.02 mol fraction) and high (≥0.69 mol fraction) water content had a shape corresponding to the titanium passivation. In the low water-content range, there was methanolate pseudo-passivation, while in the high water-content solutions, oxide passivation took place. In the range of medium water content (0.1 to 0.36 mole fraction), the impedance spectrum corresponded to the metallic surface being blocked with the membrane of very high resistance and very low capacitance, which is presented in Figure S5.
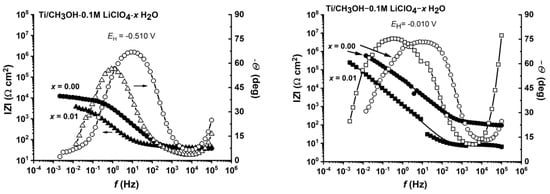
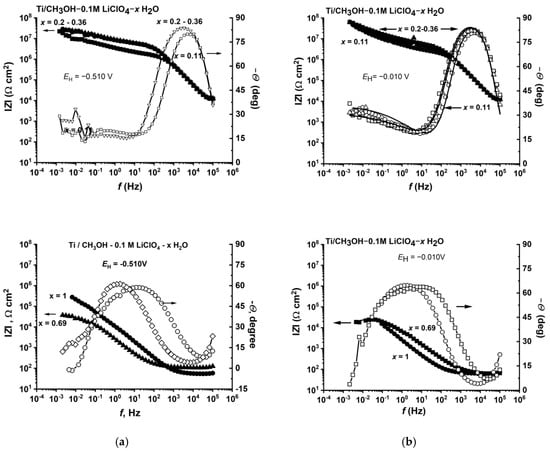
Figure 10.
Effect of the molar fraction of water on the impedance spectra of titanium in CH3OH-H2O-0.1 M LiClO4 solutions at the potential of −0.510 V vs. SHE (a) and −0.010 V vs. SHE (b).
3.2.4. FTIR-ATR Measurements
The in situ FTIR-ATR measurements of Ti in 0.1 M LiClO4 CH3OH x H2O solutions at potentials of the pseudo-passive range of polarisation (−0.1 V) confirmed the results of the polarisation studies. The intensity of the Ti-OCH3 band (Figure S6) decreased with an increase in water content (Figure 11 and Table S3). The band intensity due to the water hydroxyl group started to increase only when the water concentration exceeded the value of 0.2 mole fraction, which means that the OH groups took part in the formation of the Ti(OH)m(OCH3)n surface film, where m + n = 3.
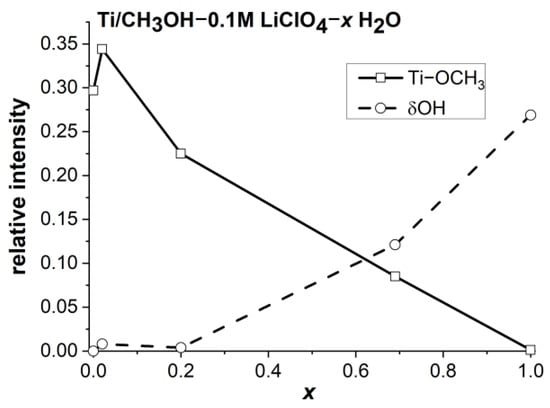
Figure 11.
Effect of water concentration on the intensity of FTIR-ATR bands due to Ti-OCH3 and OH groups in CH3OH-0.1 MLiClO4-H2O solutions.
4. Discussion
The study of the anodic properties of titanium in an anhydrous CH3OH-LiClO4 solution showed that the metal underwent pseudo-passivation, which was associated with the formation of a methoxy layer. The formation of this layer was similar to the formation of the passivation process observed in aqueous solutions. In water solutions, the passivation process is the direct reaction of titanium with water molecules [80,81] in the Ti→Ti2+→Ti3+→Ti4+ sequence, resulting in the amorphous Ti2O3/TiOOH layer transforming into TiO2 film as a result of further oxidation. In the case of greater anodic overvoltage (>1 V), titanium behaves in aqueous solutions like typical valve metal. The increase in anodic overpotential results in increasing the film thickness and the layer growth is determined by a high field mechanism [82]. In methanol solutions, in the range of potential up to about 0.5 V, the anodic properties of Ti are similar to those in aqueous solutions, where the water molecules behave similar to methanol and hydroxyl ions behave similarly to the methoxy anions. The formation of the surface methoxy film is a result of the direct reaction of titanium with methanol molecules. The presence of this film was confirmed by in situ spectroelectrochemical measurements (FTIR-ATR).
In contrast to the oxide film in aqueous solutions, the methoxy layer is stable in a relatively narrow potential range up to about 0.55 V. This range is limited by the stability of methanol and methoxy anions. Above this potential, both methoxy anions and methanol are oxidised (reactions (1)–(3)) and the formation of methoxy film stops. There is also no possibility of the formation of a Ti oxide layer due to the absence of water molecules. Under such conditions, the titanium loses its corrosion resistance and undergoes etching. We have named this range of potential “transpassive” since the methoxy anions, as a component of the barrier film, undergo oxidation in this potential range. The activated titanium (devoid of the layer) dissolves in the form of trivalent ions, as evidenced by the violet colour of the solution.
The effect of the methanol–water interaction is summarised in Figure 12. Changing the properties of the solution affected the anodic behaviour of Ti and the nature of the surface film that was formed on the metal surface.
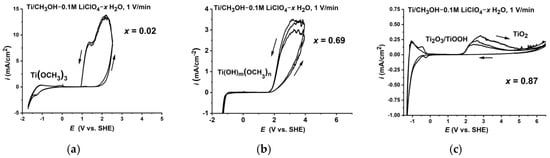
Figure 12.
The effect of the methanol–water system structure on the anodic properties of the potentiodynamic polarization curves of titanium in CH3OH-LiClO4-H2O solutions (scan rate: 0.017 V/s). (a) low water content; (b) medium water content; (c) high water content.
In solutions with a low water content, where the molecules of water are enclosed in methanol clathrates and thus separated from the titanium surface, Ti reacts with methanol, forming a pseudo-passive methanolate layer (Figure 12a).
An increase in the H2O concentration above 0.2 mole fraction up to 0.7 mole fraction changes the nature of the methanol–water interaction, followed by the strengthening of the solution. It seems that in this range of water concentrations, the film properties changed and the impact of the hydroxyl groups began to dominate the interactions of methoxy groups, which was followed by the formation of the surface film consisting of Ti(OH)m(OCH3)n, where m + n = 3 (Figure 12b).
When the water content in the solution exceeded 0.7 mole fraction, the situation reversed and the water molecules formed clathrates in which methanol molecules were enclosed such that their access to the metal surface was cut off. Ti in such a solution behaved like it would in water and its surface was covered with a stable TiO2 layer (Figure 12c).
5. Conclusions
Titanium underwent pseudo-passivation in anhydrous methanol as a result of the anodic reaction of Ti with methanol molecules, leading to the formation of methoxide layer.
The presence of the titanium methoxide film was confirmed using SEM, XPS, and FTIR-ATR in situ techniques. The barrier layer on the metal surface was stable only at relative low anodic overpotentials (<0.55 V). At higher anodic voltages, the layer underwent dissolution and intensive metal etching occurred. The instability of the methoxide layer at high anodic overvoltages could be associated with the lack of a source of methoxy ions/radicals, which are necessary for film formation, because of methanol oxidation.
The growth of a TiO2 oxide film in anhydrous methanol is impossible due to the lack of a source of oxygen; therefore, in the potential range over 0.55 V, titanium underwent rapid dissolution because of the decomposition of methoxide layer.
The oxide passivation of titanium was not possible in the solutions with a water content of less than 0.2 mole fraction. However, there was a possible formation of a methanolate or hydroxy-methanolate surface layer involving titanium at a lower oxidation state (Ti (III)). This pseudo-passive film was stable only in a limited range of potential (up to about 0.55 V) corresponding to the stability of the compound Ti(OH)m(OCH3)n, where m + n = 3.
In the range of 0.2–0.7 mole fraction, strengthening of the solution structure occurred, resulting in a strengthening of the surface layer of Ti(OH)m(OCH3)n. Such a layer had a strong barrier property similar to the properties of organic polymer film blocking the growth of the oxide layer. This film was corrosion-resistant only within the range of potentials corresponding to the stability of the methoxy anions and methanol (<−0.5 V). Above this potential, the film was destroyed by oxidation of the methoxy anions and local titanium corrosion was observed.
The formation and the growth of a stable oxide layer of TiO2 were possible only at water concentrations above 0.7 mole fraction (50 vol.%). Methanol molecules were completely “enclosed” inside the water clathrates in this case and did not block the admission of water molecules to the titanium surface.
Supplementary Materials
The following are available online at https://www.mdpi.com/2673-3293/1/2/9/s1, Figure S1 Effect of RDE rotation rate on polarization of Ti in CH3OH – 0.1M LiClO4 (v = 0.17 V/s), (a) scan in anodic direction, (b) reverse scan, Figure S2 Mott-Schottky diagram for Ti polarized in anhydrous CH3OH-0.1 M LiClO4, Figure S3. Effect of water content on viscosity (a) and conductivity of CH3OH-electrolyte-H2O solutions, Figure S4. Effect of water content on the FTIR spectrum of CH3OH-x H2O-0.1M LiClO4 solutions, Figure S5. Effect of water concentration on parameters of Rel(Q1R1) element of equivalent circuit of impedance spectrum of titanium in CH3OH-H2O-0.1M LiClO4, Figure S6. FTIR-ATR spectra of layer formed on Ti in the pseudo-passive range in CH3OH-0.1 M LiClO4-H2O solutions, Table S1 Parameters of equivalent circuit Rel(Q1R1(Q2R2)) calculated for impedance spectra presented in Figure 3, Table S2. Interpretation of XPS spectra for Ti surface etched potentiostatically in anhydrous CH3OH-0.1M LiClO4 at the prepassive range (−0.3 V vs. NHE, 90 min), passive range (0.125 V vs. NHE, 60 min) and transpassive range (0.6 V vs. NHE, 2 min), Table S3. Parameters of equivalent circuits Rel(Q1R1) and Rel(Q1R1(Q2R2)) calculated for impedance spectra of Ti in CH3OH-H2O-0.1M LiClO4 solutions at potentials of −0.0510 V and −0.010 V, Table S4. Interpretation of band positions in FTIR-ATR spectrum of Ti surface in 0.1M LiClO4-CH3OH-xH2O solution.
Author Contributions
Conceptualization, U.L.-B. and J.B.; Formal analysis, U.L.-B. and J.B.; Investigation, U.L.-B.; Writing—original draft preparation, U.L.-B. and J.B.; Writing—review and editing, G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nishimura, R.; Kudo, K. Anodic oxidation and kinetics of titanium in 1 M chloride solutions. Corros. Sci. 1982, 22, 637–645. [Google Scholar] [CrossRef]
- Basame, S.B.; White, H.S. Pitting corrosion of titanium the relationship between pitting potential and competitive anion adsorption at the oxide film/electrolyte interface. J. Electrochem. Soc. 2000, 147, 1376–1381. [Google Scholar] [CrossRef]
- Burstein, G.T.; Souto, R.M. Observations of localised instability of passive titanium in chloride solution. Electrochim. Acta 1995, 40, 1881–1888. [Google Scholar] [CrossRef]
- Liuz, J.; Alfantazi, A.; Asselin, E. The anodic passivity of titanium in mixed sulfate-chloride solutions. J. Electrochem. Soc. 2015, 162, E289–E295. [Google Scholar]
- Trompette, J.L.; Massot, L.; Arurault, L.; Fontorbes, S. Influence of the anion specificity on the anodic polarization of titanium. Corros. Sci. 2011, 53, 1262–1268. [Google Scholar] [CrossRef]
- Welsch, G.; Boyer, R.; Collings, E. Materials Properties Handbook: Titanium Alloys; ASM International: Metals Park, OH, USA, 1993. [Google Scholar]
- Schenk, R. The corrosion properties of titanium and titanium alloys. In Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications; Springer: Berlin/Heidelberg, Germany, 2001; pp. 145–170. [Google Scholar]
- Cerquetti, A.; Mazza, F. Electrochemical behaviour and stress-corrosion cracking of titanium in alcoholic solutions. Corros. Sci. 1973, 13, 337–349. [Google Scholar] [CrossRef]
- Fushimi, K.; Habazaki, H. Anodic dissolution of titanium in NaCl-containing ethylene glycol. Electrochim. Acta 2008, 53, 3371–3376. [Google Scholar] [CrossRef]
- Fushimi, K.; Kondo, H.; Konno, H. Anodic dissolution of titanium in chloride-containing ethylene glycol solution. Electrochim. Acta 2009, 55, 258–264. [Google Scholar] [CrossRef]
- Trasatti, S.P.; Sivieri, E. Electrochemical and stress corrosion cracking behaviour of titanium in n-propanol and iso-propanol solutions. Mater. Chem. Phys. 2004, 83, 367–372. [Google Scholar] [CrossRef]
- Trasatti, S.P.; Sivieri, E. Corrosion behaviour of titanium in non-aqueous solvents. Mater. Chem. Phys. 2005, 92, 475–479. [Google Scholar] [CrossRef]
- Sanderson, G.; Scully, J.C. The stress corrosion of ti alloys in methanolic solutions. Corros. Sci. 1968, 8, 541–548. [Google Scholar] [CrossRef]
- Banaś, K.; Banaś, J. Anodic behaviour of titanium in methanol solutions of chlorides. Metall. Foundry Eng. 2003, 29, 123–133. [Google Scholar]
- Burstein, G.T.; Whillock, G.O.H. The dissolution and repassivation of new titanium surfaces in alkaline methanolic solution:The phenomena. J. Electrochem. Soc. 1989, 136, 1313–1319. [Google Scholar] [CrossRef]
- Whillock, G.O.H.; Burstein, G.T. The dissolution and repassivation of new tiitanium surfaces in alkaline methanolic solution: The kinetics. J. Electrochem. Soc. 1989, 136, 1320–1327. [Google Scholar] [CrossRef]
- Mazza, F.; Puschmann, H. Anodisches verhalten und korrosion von titan in methanolischen lösungen. Mater. Corros. 1969, 20, 199–205. [Google Scholar] [CrossRef]
- Menzies, I.A.; Averill, A.F. The anodic behaviour of titanium in HCl-methanol solutions. Electrochim. Acta 1968, 13, 807–824. [Google Scholar] [CrossRef]
- Parry, E.P.; Hern, D.H. Effect of chloride on the anodic dissolution of titanium in methanolic solutions. J. Electrochem. Soc. 1972, 119, 1141–1147. [Google Scholar] [CrossRef]
- Powell, D.T.; Scully, J.C. Fractographic observations of the stress corrosion cracking of titanium alloys in methanolic environments. Corrosion 1969, 25, 483–492. [Google Scholar] [CrossRef]
- Qin, Z.; Pang, X.; Qiao, L.J.; Khodayari, M.; Volinsky, A. Water molecules effect on pure Ti passive film structure in methanol solution. Appl. Surf. Sci. 2014, 303, 282–289. [Google Scholar] [CrossRef]
- Qin, Z.; Pang, X.; Yan, Y.; Qiao, L.; Tran, H.T.; Volinsky, A.A. Passive film-induced stress and mechanical properties of α-Ti in methanol solution. Corros. Sci. 2014, 78, 287–292. [Google Scholar] [CrossRef]
- Ebtehaj, K.; Hardie, D.; Parkins, R.N. The stress corrosion and pre-exposure embrittlement of titanium in methanolic solutions of hydrochloric acid. Corros. Sci. 1985, 25, 415–429. [Google Scholar] [CrossRef]
- Xingfu, Z.; Daobao, C.; Jiashan, G.; Changjian, L.; Huashui, L.; Zhongqun, T. Direct electrochemical preparation of titanium alkoxides. Acta Chim. Sin. 2000, 58, 1327–1331. [Google Scholar]
- Chen, J.; Ollis, D.F.; Rulkens, W.H.; Bruning, H. Photocatalyzed oxidation of alcohols and organochlorides in the presence of native TiO2 and metallized TiO2 suspensions: Photocatalytic activity and pH influence. Water Res. 1999, 33, 661–668. [Google Scholar] [CrossRef]
- Gupta, S.S.; Datta, J. An investigation into the electro-oxidation of ethanol and 2-propanol for application in direct alcohol fuel cells (DAFCs). J. Chem. Sci. 2005, 117, 337–344. [Google Scholar] [CrossRef]
- Zhou, X.F.; Chu, D.B.; Lin, C.J. Anodic dissolution of spongy titanium in ethanol solution for preparation of nano-sized TiO2 powder. Electrochim. Acta 2002, 47, 2769–2773. [Google Scholar] [CrossRef]
- Al-Abdallah, M.M. Chemical and electrochemical behaviour of titanium in methanol-water-HCl mixtures. Br. Corros. J. 1991, 26, 133–134. [Google Scholar] [CrossRef]
- Mansfeld, F. The effect of water on passivity and pitting of titanium in solutions of methanol and hydrogen chloride. J. Electrochem. Soc. 1971, 118, 1412–1415. [Google Scholar] [CrossRef]
- Banaś, J.; Stypuła, B.; Banaś, K.; Światowska-Mrowiecka, J.; Starowicz, M.; Lelek-Borkowska, U. Corrosion and passivity of metals in methanol solutions of electrolytes. J. Solid State Electrochem. 2009, 13, 1669. [Google Scholar] [CrossRef]
- Grigoriew, W.P.; Nieczajewa, O.N.; Popowa, A.A. Formirovanie anodnyh plienok na titanie v vodnyh i organichieskih pierhloratnych sriedah. Russ. J. Electrochem. 1992, 28, 2–4. [Google Scholar]
- Nieczajewa, O.N.; Grigoriew, W.P.; Popowa, A.A. Izuchienie kinietiki formirovania anodnyh plienok na titanie v pierhloratnyh spirtovyh spriedah. Zashchita Met. 1992, 28, 4–6. [Google Scholar]
- Sastry, S. Water structure: Order and oddities. Nature 2001, 409, 300–301. [Google Scholar] [CrossRef]
- Sarkar, S.; Joarder, R.N. Molecular clusters and correlations in liquid methanol at room temperature. J. Chem. Phys. 1993, 99, 2032–2039. [Google Scholar] [CrossRef]
- Guo, J.H.; Luo, Y.; Augustsson, A.; Kashtanov, S.; Rubensson, J.E.; Shuh, D.K.; Ågren, H.; Nordgren, J. Molecular structure of alcohol-water mixtures. Phys. Rev. Lett. 2003, 91, 157401. [Google Scholar] [CrossRef]
- Bosch, E.; Bou, P.; Allemann, H.; Rosés, M. Retention of ionizable compounds on HPLC. pH scale in methanol-water and the pK and pH values of buffers. Anal. Chem. 1996, 68, 3651–3657. [Google Scholar] [CrossRef]
- Galicia-Andrés, E.; Dominguez, H.; Pusztai, L.; Pizio, O. Composition dependence of thermodynamic, dynamic and dielectric properties of water–methanol model mixtures. Molecular dynamics simulation results with the OPLS-AA model for methanol. J. Mol. Liq. 2015, 212, 70–78. [Google Scholar]
- Dougan, L.; Bates, S.P.; Hargreaves, R.; Fox, J.P.; Crain, J.; Finney, J.L.; Réat, V.; Soper, A.K. Methanol-water solutions: A bi-percolating liquid mixture. J. Chem. Phys. 2004, 121, 6456–6462. [Google Scholar] [CrossRef]
- Noskov, S.Y.; Kiselev, M.G.; Kolker, A.M.; Rode, B.M. Structure of methanol-methanol associates in dilute methanol-water mixtures from molecular dynamics simulation. J. Mol. Liq. 2001, 91, 157–165. [Google Scholar] [CrossRef]
- Tanaka, H.; Gubbins, K.E. Structure and thermodynamic properties of water–methanol mixtures: Role of the water–water interaction. J. Chem. Phys. 1992, 97, 2626–2634. [Google Scholar] [CrossRef]
- Mandal, A.; Prakash, M.; Kumar, M.R.; Parthasarathi, R.; Subramanian, V. Ab initio and DFT studies on methanol-water clusters. J. Phys. Chem. A 2010, 114, 2250–2258. [Google Scholar] [CrossRef]
- Laaksonen, A.; Kusalik, P.G.; Svishchev, I.M. Three-dimensional structure in water-methanol mixtures. J. Phys. Chem. A 1997, 101, 5910–5918. [Google Scholar] [CrossRef]
- Dixit, S.; Crain, J.; Poon, W.C.K.; Finney, J.L.; Soper, A.K. Molecular segregation observed in a concentrated alcohol-water solution. Nature 2002, 416, 829–832. [Google Scholar] [CrossRef]
- Dixit, S.; Soper, A.K.; Finney, J.L.; Crain, J. Water structure and solute association in dilute aqueous methanol. Europhys. Lett. 2002, 59, 377–383. [Google Scholar] [CrossRef]
- Neidig, H.A.; Yingling, R.T.; Lockwood, K.L.; Teates, T.G. Interaction in chemical systems. The methanol-water system. J. Chem. Educ. 1965, 42, 309. [Google Scholar] [CrossRef]
- Neidig, H.A.; Yingling, R.T.; Lockwood, K.L.; Teates, T.G. Interaction in chemical systems. The KCl-methanol-water system. J. Chem. Educ. 1965, 42, 368. [Google Scholar] [CrossRef]
- Benson, S.P.; Pleiss, J. Incomplete mixing versus clathrate-like structures: A molecular view on hydrophobicity in methanol–water mixtures. J. Mol. Model. 2013, 19, 3427–3436. [Google Scholar] [CrossRef]
- Hernández-Cobos, J.; Ortega-Blake, I. Hydrophobic hydration in methanol aqueous solutions. J. Chem. Phys. 1995, 103, 9261. [Google Scholar] [CrossRef]
- Masella, M.; Flament, J.P. Relation between cooperative effects in cyclic water, methanol/water, and methanol trimers and hydrogen bonds in methanol/water, ethanol/water, and dimethylether/water heterodimers. J. Chem. Phys. 1998, 108, 7141–7151. [Google Scholar] [CrossRef]
- Bolis, G.; Corongiu, G.; Clementi, E. Methanol in water solution at 300 K. Chem. Phys. Lett. 1982, 86, 299–306. [Google Scholar] [CrossRef]
- Van Erp, T.S.; Meijer, E.J. Hydration of methanol in water. A DFT-based molecular dynamics study. Chem. Phys. Lett. 2001, 333, 290–296. [Google Scholar] [CrossRef]
- Soper, A.K.; Finney, J.L. Hydration of methanol in aqueous solution. Phys. Rev. Lett. 1993, 71, 4346–4349. [Google Scholar] [CrossRef]
- Ebukuro, T.; Takami, A.; Oshima, Y.; Koda, S. Raman spectroscopic studies on hydrogen bonding in methanol and methanol/water mixtures under high temperature and pressure. J. Supercrit. Fluids 1999, 15, 73–78. [Google Scholar] [CrossRef]
- Sameti, M.R.; Bayat, M.; Salehzadeh, S. The DFT study of hydrogen bonding and thermodynamic parameters of (CH3OH)n(H2O)m (n, m = 1–8) clusters at different temperatures. Arab. J. Chem. 2016, 9 (Suppl. 1), S41–S46. [Google Scholar] [CrossRef]
- Moin, S.T.; Hofer, T.S.; Randolf, B.R.; Rode, B.M. Structure and dynamics of methanol in water: A quantum mechanical charge field molecular dynamics study. J. Comput. Chem. 2011, 32, 886–892. [Google Scholar] [CrossRef]
- Batista da Silva, J.A.; Moreira, F.G.B.; Leite dos Santos, V.M.; Longo, R.L. On the hydrogen bond networks in the water-methanol mixtures: Topology, percolation and small-world. Phys. Chem. Chem. Phys. 2011, 13, 6452–6461. [Google Scholar] [CrossRef]
- Dixit, S.; Poon, W.C.K.; Crain, J. Hydration of methanol in aqueous solutions: A Raman spectroscopic study. J. Phys. Condens. Matter 2000, 12, L323. [Google Scholar] [CrossRef]
- Lelek-Borkowska, U.; Talar-Westenholtz, I.; Banaś, J. Badanie produktów procesów elektrochemicznych zachodzących na powierzchni tytanu w roztworze CH3OH-LiClO4 metodą spektroskopii FTIR-ATR. Ochr. Przed Korozją 2010, 53, 599–601. [Google Scholar]
- Bisztyga, M.; Lelek-Borkowska, U.; Proniewicz, E.; Banaś, J. Cathodic behaviour of nickel in alcohol solutions of electrolytes. Electrochim. Acta 2016, 207, 1–8. [Google Scholar] [CrossRef]
- Dobos, D. Electrochemical Data; Akadémiai Kiadó, Budapest: Budapest, Hungary, 1976. [Google Scholar]
- Pourbaix, M. Atlas of Eectrochemical Equilibriain Aqueous Solutions; NACE International: Houston, TX, USA, 1974. [Google Scholar]
- Sibrell, P.L. Electrochemical Reduction of Titanium in Nonaqueous Solvents; Bureau of Mines: Pittsburgh, PA, USA, 1995.
- Hartmann, H.; Schläfer, H.L.; Hansen, K.H. Uber lichtabsorption vom dipolkomplexen des III-wertigen titans vom typ [TiA6]3+ mit A = H2O, CH3OH, C2H5OH und (NH2)2CO. Z. Anorg. Allg. Chem. 1957, 289, 40–65. [Google Scholar] [CrossRef]
- Bélanger, G. Anodic oxidation of anhydrous methanol. J. Electrochem. Soc. 1976, 123, 818–823. [Google Scholar] [CrossRef]
- Vassiliev, Y.B.; Lotvin, B.M. Specific features of electrooxidation of alcohols on platinum in absolute alcohol solutions. Role of water in chemisorption and dehydrogenation processes. Electrochim. Acta 1985, 30, 1345–1354. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Sundberg, P.; Larsson, R.; Folkesson, B. On the core electron binding energy of carbon and the effective charge of the carbon atom. J. Electron. Spectrosc. Relat. Phenom. 1988, 46, 19–29. [Google Scholar] [CrossRef]
- Muilenberg, G.E.; Wagner, C.D. Handbook of X-Ray Photoelectron Spectroscopy: A Reference Book of Standard Data for Use in X-Ray Photoelectron Spectroscopy; Perkin-Elmer: Eden Prairie, MN, USA, 1979. [Google Scholar]
- Barr, T.L.; Seal, S. Nature of the use of adventitious carbon as a binding energy standard. J. Vac. Sci. Technol. A Vac. Surf. Film. 1995, 13, 1239–1246. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Kesler, V.G.; Pervukhina, N.V.; Zhang, Z. Ti 2p and O 1s core levels and chemical bonding in titanium-bearing oxides. J. Electron. Spectrosc. Relat. Phenom. 2006, 152, 18–24. [Google Scholar] [CrossRef]
- Attard, G.A.; Chibane, K.; Ebert, H.D.; Parsons, R. The adsorption and decomposition of methanol on Pt(110). Surf. Sci. 1989, 224, 311–326. [Google Scholar] [CrossRef]
- Beccaria, A.M.; Poggi, G.; Castello, G. Influence of passive film composition and sea water pressure on resistance to localised corrosion of some stainless steels in sea water. Br. Corros. J. 1995, 30, 283–287. [Google Scholar] [CrossRef]
- Kakos, G.A.; Winter, G. C-O and Ti-O vibration frequencies in alkyltitanates. Aust. J. Chem. 1968, 21, 793–795. [Google Scholar] [CrossRef]
- Gupta, A. Thermodynamics of electrolytes in mixed solvents. Application of Pitzer’s thermodynamic equations to activity coefficients of 1:1 electrolytes in methanol-water mixtures. J. Phys. Chem. 1979, 83, 2986–2990. [Google Scholar] [CrossRef]
- Mazzarese, J.; Popovych, O. Standard potentials of Li, Na, and K ellectrodes and transfer free energies of LiCl, NaCl, and KCl in selected ethanol-water and methanol-water solvents. J. Electrochem. Soc. 1983, 130, 2032–2037. [Google Scholar] [CrossRef]
- Loboda, O.; Goncharuk, V. Theoretical study on icosahedral water clusters. Chem. Phys. Lett. 2010, 484, 144–147. [Google Scholar] [CrossRef]
- Buffey, I.P.; brown, W.B.; Gebbie, H.A. Icosahedral water clusters. Chem. Phys. Lett. 1988, 148, 281–284. [Google Scholar] [CrossRef]
- Kabisch, G.; Pollmer, K. Hydrogen bonding in methanol-organic solvent and methanol-water mixtures as studied by the vco and voh. J. Mol. Struct. 1982, 81, 35–50. [Google Scholar]
- Guo, X.Z.; Gao, K.W.; Chu, W.Y.; Qiao, L.J. Correlation between passive film-induced stress and stress corrosion cracking of α-Ti in a methanol solution at various potentials. Mater. Sci. Eng. A 2003, 346, 1–7. [Google Scholar] [CrossRef]
- Kelly, E.J. Anodic dissolution of titanium in acidic sulfate solutions: Effects of Ti(III) and Ti(IV) ions. J. Electrochem. Soc. 1976, 123, 162–170. [Google Scholar] [CrossRef]
- Kelly, E.J. Electrochemical behavior of titanium. In Modern Aspects of Electrochemistry; Bockris, J.O., Conway, B.E., White, R.E., Eds.; Plenum Press: New York, NY, USA, 1982. [Google Scholar]
- Huang, Y.Z.; Blackwood, D.J. Characterisation of titanium oxide film grown in 0.9% NaCl at different sweep rates. Electrochim. Acta 2005, 51, 1099–1107. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).