Abstract
Developing sustainable and renewable energy sources is critical as higher and higher global energy and environmental challenges arise. Hydrogen has the highest mass/energy density of any fuel and is considered one of the best sources of clean energy. Water splitting is regarded as one of the most promising solutions for hydrogen production on a large scale. Highly efficient, durable, and cost-effective catalysts for hydrogen evolution reaction (HER) are critical in the realization of this goal. Among the many materials proposed, graphene-based materials offer some unique properties for HER catalysis. In this review, we present recent progress on development of graphene-based electrocatalysts toward HER throughout the past few years.
1. Introduction
Most of the energy produced worldwide comes from fossil fuels, which presents several problems. First, the combustion of fossil fuels produces pollutants (CO2, CO, sulfur oxides, nitrogen oxides, unburnt hydrocarbons, etc.) that drive climate change [1]. Second, fossil fuels are not a renewable source and once the reserves are exhausted, another form of storing and producing energy will be needed.
In this scenario, the hydrogen economy appears as the best and most suitable way to produce low-carbon energy source in the near future. The hydrogen evolution reaction (HER) has attracted significant attention during the last decades due to the growing current energy demand and the great potential it presents in terms of energy storage and energy conversion in the future of renewable energies. Water splitting is the best method of converting electrical energy to hydrogen as it requires only water and produces nothing but pure hydrogen (H2) and oxygen (O2). In a typical water electrolysis system, O2 is produced at the anode through the oxygen evolution reaction (OER, Equation (2)) and H2 is produced at the cathode through the hydrogen evolution reaction (HER, Equation (1)) [2]:
4H+ + 4e− ⇌ 2H2
E0 = 0.00 V vs. Reversible Hydrogen Electrode (RHE)
2H2O ⇌ O2 + 4H+ + 4e−
E0 = 1.23 V vs. RHE
Aside from its importance in energy and storage technology, HER is a model reaction for the understanding of electrode kinetics and electrocatalysis and has been extensively studied [3]. The mechanism of HER at noble metal electrodes (e.g., Pt) in electrolyte solutions generally includes two of the following three steps:
(1) Volmer reaction: The first step includes an electrochemical proton transfer step forming an adsorbed H atom (Had) on a suitable free surface site (*):
H+ + e− + * ⇌ Had
Then, two different reaction pathways may occur:
(2) Tafel reaction: A surface chemical recombination of two Had:
2Had ⇌ H2 + 2*
(3) Heyrovsky reaction: An electrochemical water-mediated desorption reaction:
Had + H+ + e− ⇌ H2 + *
Tafel plots and principally Tafel slopes are usually employed to determine the rate determining step (RDS) of the HER. Assuming a charge transfer coefficient (α) = 0.5, Tafel slope values of 120, 30, and 40 mV dec−1 are associated with Volmer, Tafel, and Heyrovsky as RDS, respectively [4]. HER at noble metal electrodes (e.g., Pt) has been widely studied and reported in the literature [3]. By-side reactions such as surface oxide reductions are known to happen very fast, so their contribution to the reduction currents are insignificant. Accordingly, Tafel slopes agree with the theoretical values stated above. Non-noble materials (e.g., graphene-based catalysts) usually reveal slow by-side cathodic reactions (e.g., surface oxide reduction reaction) during the HER, and therefore uncommon Tafel slopes are frequently reported. In order to avoid this problem, application of previous reduction steps (chemical [5] or electrochemical [6]) or follow the HER by differential electrochemical mass spectrometry (DEMS) [7] has been proposed to suppress current contributions from by-side reactions. For the expressed reasons, researchers should pay special attention when interpreting Tafel plots.
Currently, the efficiency of hydrogen production by water electrolysis is too low to be economically competitive for real energy requirements [8]. The best catalysts contain elements from the Pt group metals (PGM), being ideal in terms of thermodynamics and kinetics, but their shortage and high cost impede their large-scale application, requiring cheaper and viable catalysts [9,10]. The principal aim of research in this field is (i) to replace PGM by low-cost materials; (ii) to increase the electrical conductivity; (iii) minimize the required overpotential for HER; (iv) increase the kinetics of HER; and (v) increase the catalytic stability. To overcome these problems, catalysts based on carbonaceous materials have been investigated due to their excellent conductivity, large surface area, and possibility to tune their electronic properties [11].
In the last decades, graphene has attracted extensive attention due to its excellent physical and chemical properties such as large surface area, extraordinary electrical conductivity, and high mechanical strength [12]. Extensive research has been devoted to elucidating the catalytic properties of graphene-based materials toward several chemical reactions. Hence, the current review is dedicated to summarizing the most important findings of the HER in diverse catalysts involving graphene.
2. Non-Precious Metal Graphene-Based Materials
Pure graphene is catalytically inactive for HER due to its flat and inert surface [13]. Therefore, graphene undergoes different doping, functionalization, and strain processes with the objective of tuning its chemical properties. Fortunately, graphene derivatives such as reduced graphene oxide (rGO) can be synthesized by the simple Hummers method to produce graphene oxide (GO), followed by a reduction step and used as precursor for a wide range of graphene-based materials [14]. The reduction step can be done in a number of ways, some of them leading to an already doped product, as is the case for heteroatom doping [10,11,12]. Due to residual oxygenated groups and defects, the conductivity of rGO is lower than that of pristine graphene. However, reactive surfaces of GO and rGO enable the optimization of specific properties and ease the incorporation of the dopant or composite. rGO has proven unique chemical, electronic, mechanical and optical properties. The fabrication of hybrids combining various semiconductor materials and rGO has showed excellent performances compared with single transition metal catalysts due to the interface-induced effect [15].
Doping has been widely investigated in order to specifically tailor the catalytic properties [16]. The creation of charged groups and defects in the graphene network by modifying the carbon atom surface gives place to defective graphene materials that present an alteration of the geometry and the electronic structure. These materials are used as electrocatalysts since they can induce lower activation barriers or improved adsorption energy to reactants, products, or intermediate species, being able to achieve higher catalytic activities or selectivity toward certain reactions [17]. Notably, the strong coupling between metal centers and doped graphene facilitates the electron transference improving the electroactivity. Moreover, the durability of the catalyst increases due to the existence of covalent bonds between the dopants and the metal [11]. Furthermore, it is well known that topological defects or edges are related to the electrocatalytic performance, so a large number of defects can lead to enhancement of the graphene catalytic activity [18]. However, the number of defects should not be so high as to destroy the overall graphene structure, as that would lead to the loss of important properties such as conductivity and stability.
3. Heteroatom-Doped Metal-Free Graphene Catalysts
Heteroatom-doped metal-free graphene catalysts have been synthesized (i.e., nitrogen, sulfur, fluorine, boron, phosphorus, etc.), tuning its electronic properties to enhance HER. In this regard, nitrogen doping has been an effective way to tune the properties of graphene, contributing to its development for various applications. The normally obtained nitrogen bonding configuration are pyridinic N, pyrrolic N, and graphitic N [13]. The pyridine N (sp2) is bonded to two carbon atoms at the graphene edges, contributing one electron to the π system; pyrrolic N (sp3) contributes two electrons in a five-member ring; graphite N (sp2) substitutes C-atoms in the hexagonal rings [18].
Sulfur bond length (C-S, 1.78 Å) is longer than the C–C bond distance, therefore, sulfur doping offers a stable structure in which each S atom is above the graphene plane. Unlike the N-doping effect, there is an insubstantial polarization (or charge transfer) on the C–S bond due to the similar electronegativity of the S (2.58) and C (2.55) [19]. It has been reported that S-doping can improve the graphene conductivity due to a more effective reduction and its greater electron donor capacity [16]. In this regard, sulfur-doped graphene has recently attracted attention as a promising material beyond N-doped graphene, and shown to be competitive or even better compared to N-doped materials for ORR activity. Recently, it has been investigated as a noble metal-free catalyst for HER in acidic media, obtaining a significant enhancement to the HER activity of graphene, attributed to the presence of high S-doping level with thiophene-S rich species [20]. Tian et al. [20] have reported the high activity of the S-doped graphene catalyst prepared through solvothermal synthesis followed by plasma-etching in an Ar atmosphere. Plasma-etching post-treatment could improve the HER activity of the S-doped graphene materials by introduction of defect sites. Figure 1 shows the role of the S-doping level, species types/contents, and defect level toward HER in acid media.
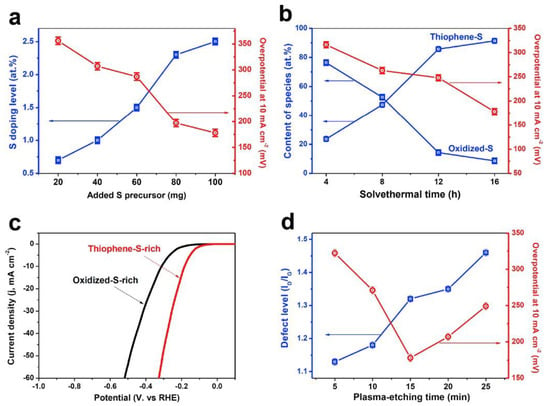
Figure 1.
(a) Hydrogen evolution reaction (HER) activities (overpotential at 10 mA cm−2) of sulfur-doped graphene (SG) samples with different S-doping levels obtained by varying the amount of added S precursor. (b) HER activities of SG samples with different compositions of thiophene-S/oxidized-S species obtained by varying the solvothermal time during the synthesis of SG. (c) Polarization curves of thiophene-S-rich and oxidized-S-rich SG samples in 0.5 M H2SO4 solution at a scan rate of 5 mV s−1. (d) HER activities of SG samples with different topological defect levels (ID/IG ratio) obtained by varying the plasma-etching time. Reproduced with permission [20].
On the other hand, it has also been reported that nitrogen and sulfur co-doping leads to higher activity of graphene in HER at low operating overpotential (Figure 2), obtaining comparable results to platinum [12]. Chemically doped nanoporous graphene was developed by chemical vapor deposition at different deposition temperature (500 and 800 °C) with nanoporous Ni (np-Ni) as both template and substrate; and thiophene and pyridine were used as sulfur and nitrogen sources, respectively [12].
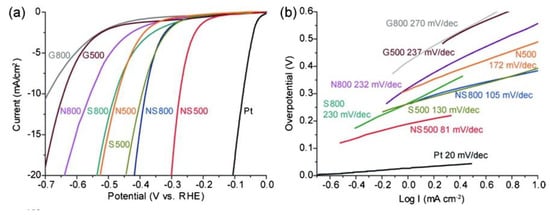
Figure 2.
HER activity of chemically doped nanoporous graphene: G (graphene), N (N-doped graphene), S (S-doped graphene), and NS (NS-doped graphene). (a) Linear sweep voltammetry (LSV) curves of the samples produced at different chemical vapor deposition temperatures and with different dopants in comparison to Pt in H2SO4 0.5 M at a scan rate of 10 mV s-1; (b) Tafel plots for the different samples. Reproduced with permission [12].
P and N have the same number of valence electrons, but P has a different doping effect because of the additional orbital electron donation capacity compared to N. In addition, the electronegativity of P (2.19) is less than N (4.39), thus the C–P bond polarity is opposite to the C–N bond. In P-doping materials, a strong hybridization between P 3p and C 2p converts the sp2 C into sp3 hybridization giving place to a pyramidal bonding configuration with three C atoms. In this structure, P overhangs from the graphene plane by 1.33 Å, increasing the P–C bond length (1.77 Å) compared to the C–C bond distance, producing a structural distortion and inducing defect sites in the graphene sheet [21]. In addition, nitrogen and phosphorus dual-doped graphene has been studied, exhibiting comparable onset overpotential, Tafel slope, and exchange current density to some of the traditional metallic catalysts due to the synergistic effect of the dopants [22,23].
Boron has also emerged as a doping heteroatom in several technological fields [17,24]. It has been demonstrated that B-substituted graphene, synthetized by controlled substitution of the C atoms, is an efficient metal-free electrocatalyst for HER [12] and B-doped graphene can lower the conversion barriers for the transformation of H+ ions to H2, showing a better HER activity than undoped graphene [11]. In graphene B-doping materials, the B atoms are sp2-hybridized in the carbon network due to the resemblance between C and B atoms. However, the lattice parameters are slightly changed because of the B–C bond length that causes some distortion of the planar structure of the graphene [20]. Boron has one less valence electron than C, which induces a charge polarization in the graphene network, becoming negatively charged. This circumstance occurs because B-doping provides more holes to the valence band of graphene, increasing the carrier concentration and hence improving the electrocatalyst activity [25].
For instance, B-substituted graphene (B-SuG) has been reported as an efficient metal-free electrocatalyst for HER. Figure 3 shows a significantly higher electrical conductivity (Figure 3b) and catalytic activity (Figure 3a) displayed by B-SuG than defective graphene (DeG). The last is explained by the B-doping that creates surface defect sites and a large number of surface-active reduction centers (electron rich) [17].
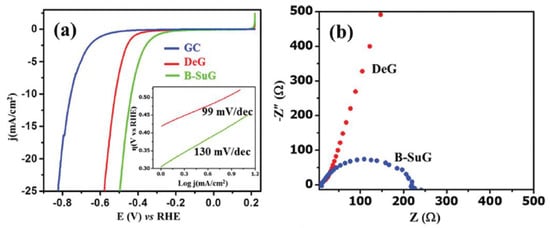
Figure 3.
(a) Superimposed linear sweep voltammetry (LSV) curves of B-substituted graphene (B-SuG), defective graphene (DeG), and glassy carbon (GC) electrode in 0.5 M H2SO4 at a scan rate of 10 mV s-1, with their corresponding Tafel plots shown in the inset. (b) Electrochemical impedance spectra (EIS) of DeG and B-SuG at an overpotential of 0.201 V vs. Reversible Hydrogen Electrode (RHE). Reproduced with permission [17].
4. Non-Precious Transition Metal Graphene Catalysts
The nonprecious transition metal insertion (Mn, Co, Cu, Ni, etc.) has also been investigated, obtaining satisfactory results and displaying high HER performances due to an active metal–H bond interaction and similar electronic structure to Pt [11,26]. In this field, Deng et al. found that increasing the amount of nitrogen doping and reducing the number of graphene layers that encapsulate a CoNi nanoparticle can significantly increase the electron density, enhancing the HER activity in acidic media [27].
In addition, co-doping materials with metal and non-metal pairs with high catalytic performance have also been reported, showing that dual-doped multilayer graphene exhibits higher HER activity than mono-doped materials, as a result of a synergetic dual-doped effect [21]. Regarding this, nitrogen-doped graphene materials are the ones with the highest HER performance [28], in particular, Co and Cu embedded N-enriched mesoporous carbon showed high catalytic performance toward HER [29]. A catalyst made up of holey reduced graphene oxide, coupled with a small-sized Mo2N–Mo2C heterojunction (Mo2N–Mo2C/HGr) that exhibits an outstanding stability and superior activity toward HER compared to Pt/C in alkaline media at large current densities (>88 mA·cm−2), has also been reported [30]. In addition, Pt-analogous catalytic activity is expected for Ni–N and V–N co-doped graphenes [31].
Recent study of nickel ferrite (NFO) embedded into rGO catalyst (NFO/rGO) shows superior performance toward HER in acidic medium due to the electronic interaction between metal cations with the rGO sheet, which offers high nanoparticle dispersibility and a large surface area. This gives rise to a large ionic conductivity and a low agglomeration, providing small charge transfer resistance and many accessible catalytic sites as well as an excellent stability of the electrode [32]. The high catalytic performance of NFO/rGO is attributed to the strong electrical and chemical coupling between rGO and NFO nanoparticles. The chemical coupling supports the creation of uniformly dispersed NFO nanoparticles (10.5 nm) on the rGO (Figure 4), meanwhile the electrical coupling offers a unified conductive network displaying fast electron transport, a large cathodic current density, small overpotential of 5 mV, and relatively small Tafel slope of 59 mV·dec-1 (Figure 5) [32].
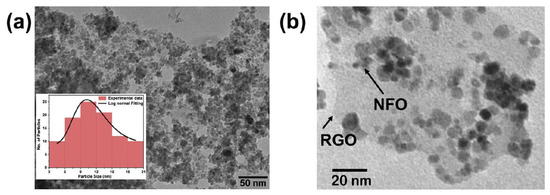
Figure 4.
(a) Transmission Electron Microscopy (TEM) image of nickel ferrite (NFO), the histogram displays the particle size with log-normal fitting, (b) TEM image of nickel ferrite embedded into reduced graphene oxide (NFO/rGO). Reproduced with permission [32].
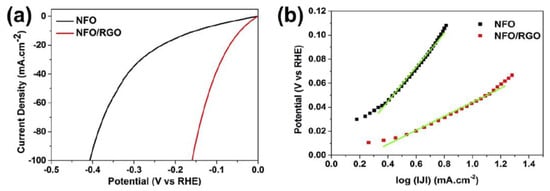
Figure 5.
(a) Polarization curves of NFO and NFO/rGO (b) Tafel plot of NFO and NFO/rGO at a scan rate of 5 mV s−1 in 0.5 M H2SO4. Reproduced with permission [32].
Furthermore, phosphorus-modified tungsten nitride supported on reduced graphene oxide (P-WN/rGO) was designed as an efficient and low-cost electrocatalyst for HER. Figure 6 shows the polarization curves of commercial Pt/C (20 wt %), P-WN/rGO, WN-rGO, P-rGO, and GO, revealing that despite the lower P-doping, its modification can intensely improve the catalytic performance by an increment of the negative charges on the surface catalyst. Additionally, the small size of the tungsten nitride (WN) nanoparticles increases the number of active sites for H+ adsorption, while rGO contributes an excellent electrical conductivity to the catalyst [33].
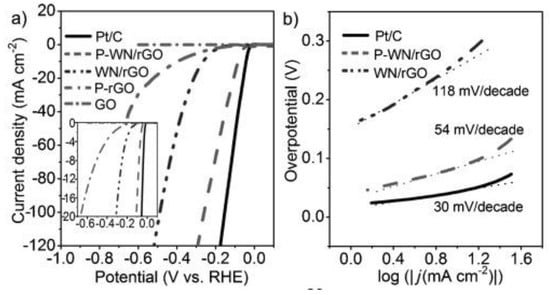
Figure 6.
(a) Polarization curves for phosphorus-modified tungsten nitride supported on reduced graphene oxide (P-WN/rGO), tungsten nitride supported on reduced graphene oxide (WN/rGO), reduced graphene oxide doped with phosphorus (P-rGO), platinum supported on carbon (Pt/C), and graphene oxide (GO) in 0.5 M H2SO4 with a scan rate of 5 mV s−1. (b) Tafel plots for P-WN/rGO, WN/rGO, and Pt/C. Reproduced with permission [33].
Moreover, graphene-encapsulated CoNi nanoalloys have been reported to have a high HER activity due to the thin nitrogen-doped graphene layer protecting the alloy from corrosion, while simultaneously promoting the electron transfer from the transition metals to the carbon surface [34]. A similar synergistic effect has been shown for iron nanoparticles [35].
5. Non-Precious Metal Graphene Composite Catalysts
Graphenic materials have been used to synthesize different 1D, 2D, and 3D nanocomposites with enhanced catalytic activity toward the HER. For instance, 2D nanomaterials composed of MoCoFeS supported on reduced graphene oxide (rGO) showed good electrochemical performance for HER [36]. Additionally, Figure 7 reveals that iron-doped tungsten oxide nanoplate supported on rGO nanocomposite (Fe-WOxP/rGO) exhibited excellent electrocatalytic activity toward HER due to the coupled synergic effect between the formation of many oxygen vacancies on tungsten oxide in the nanoplate structure of Fe-WOxP and rGO nanosheet [37].
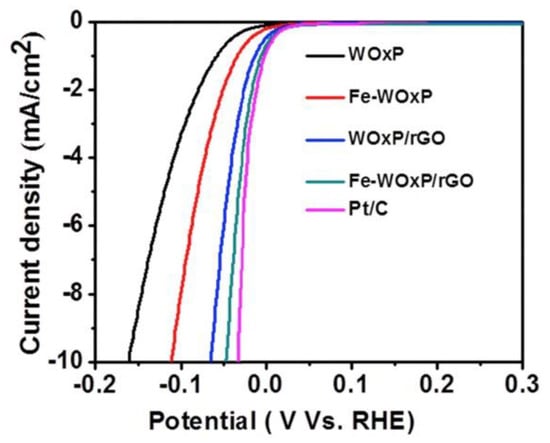
Figure 7.
Hydrogen evolution on WOxP, Fe-WOxP, WOx/PrGO, Fe-WOxP/rGO, and Pt/C recorded in 0.5 M H2SO4 at 2 mV s−1. Reproduced with permission [37].
Furthermore, a novel hybrid material of [Mo3S13]2- clusters supported on sulfur doped rGO (S-rGO) with outstanding catalytic performance toward the HER has been reported [38]. Figure 8 depicts significant improvements in HER activity compared to four hybrid catalysts to simple [Mo3S13]2− clusters. The last was attributed to the S-rGO support that provides properties such as high electrical conductivity, elevated porosity, an increase in the electrochemical active surface area, facilitates the mass transport, and decreases the value of the Tafel slope [38].
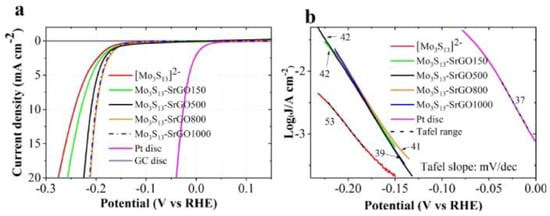
Figure 8.
(a) LSV curves of [Mo3S13]2− clusters, [Mo3S13]2-SrGO150, [Mo3S13]2−SrGO500, [Mo3S13]2−SrGO800, [Mo3S13]2−SrGO1000 HER catalyst, Pt disc, and glassy carbon disc in Ar-saturated 0.5 M H2SO4 at scan rate of 15 mVs−1. (b) Tafel plots derived from LSV curves in (a). Reproduced with permission [38].
Furthermore, a reduced graphene oxide/metallic MoSe2:Cu nanosheet has been synthesized and shown to be effective toward the HER as the Cu doping and the interface effect between MoSe2 and rGO increased the electrical conductivity of the material, resulting in an active and stable catalyst [39].
Finally, Table 1 reports and compares the main physicochemical properties and catalytic performances toward HER in acidic and alkaline media of non-precious metal graphene-based catalysts discussed in the current manuscript.

Table 1.
Physicochemical properties of non-precious metal graphene-based catalysts.
6. Summary
Herein, the catalytic performance of the latest non-precious metal graphene-based catalysts for HER has been presented. HER importance is paramount in fields as diverse as energy production and storage, fuel cells, and production of bulk chemicals. In this context, HER is a crucial electrochemical reaction for the desired hydrogen economy since it has the potential to provide pure hydrogen for fuel cells. With the aim to solve the principal catalytic problems at the cathode electrode in which HER takes place, the new advances in non-precious metal graphene materials are reported and a summary of the main catalytic properties is provided in Table 1. With the aim of tailoring the properties for future design and synthesis of innovative and sustainable catalysts for HER, diverse methods to modify graphene are mentioned along the manuscript as well as the electrochemical performance toward HER of the resulting materials. Thus, the current work may help improve the fabrication of novel electrodes in order to decrease the cost and enhance the performance of HER catalysts.
Author Contributions
M.L.-S., L.R., E.P., and G.G. contributed to the design and writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The Spanish Ministry of Economy and Competitiveness (MINECO) supported this work under project ENE2017-83976-C2-2-R (co-funded by FEDER). Financial support from E3TECH (CTQ2017-90659-REDT) and REPICOMES (ENE2017- 90932-REDT) Excellence Networks is acknowledged. G.G acknowledges the Viera y Clavijo program (ACIISI and ULL) for financial support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Solomon, S.; Plattner, G.; Knutti, R.; Friedlingstein, P. Irreversible climate change due to carbon dioxide emissions. PNAS 2009, 106, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; JOHN WILEY & SONS, INC.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Duca, M.; Koper, M.T.M. Fundamental Aspects of Electrocatalysis Surface and Interface Science; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2020; Volume 8, pp. 773–890. [Google Scholar]
- Fletcher, S. Tafel slopes from first principles. J. Solid State Electr. 2009, 13, 537–539. [Google Scholar] [CrossRef]
- Kimmel, Y.C.; Xu, X.; Yu, W.; Yang, X.; Chen, J.G. Trends in electrochemical stability of transition metal carbides and their potential use as supports for low-cost electrocatalysts. ACS Catal. 2014, 4, 1558–1562. [Google Scholar] [CrossRef]
- Schalenbach, M.; Speck, F.D.; Ledendecker, M.; Kasian, O.; Goehl, D.; Mingers, A.M.; Breitbach, B.; Springer, H.; Cherevko, S.; Mayrhofer, K.J.J. Nickel- molybdenum alloy catalysts for the hydrogen evolution reaction: Activity and stability revised. Electrochim. Acta 2018, 259, 1154–1161. [Google Scholar] [CrossRef]
- Diaz-Coello, S.; García, G.; Arévalo, M.C.; Pastor, E. Precise determination of Tafel slopes by DEMS. Hydrogen evolution on tungsten-based catalysts in alkaline solution. Int. J. Hydrogen Energ. 2019, 44, 12576–12582. [Google Scholar] [CrossRef]
- Rashid, M.; Al Mesfer, M.K.; Naseem, H.; Danish, M. Hydrogen production by water electrolysis: A review of alkaline water electrolysis, PEM water electrolysis and high temperature water electrolysis. Int. J. Adv. 2015, 4, 80–93. [Google Scholar]
- Sammes, N. Fuel Cell Technology: Reaching Towards Commercialization in Engineering Materials and Processes Series; Springer: London, UK, 2006. [Google Scholar]
- Eftekhari, A. Electrocatalysts for hydrogen evolution reaction. Int. J. Hydrogen Energ. 2017, 42, 11053–11077. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Jaroniec, M.; Qiao, S.Z. Heteroatom-doped graphene-based materials for energy-relevant electrocatalytic processes. ACS Catal. 2015, 5, 5207–5234. [Google Scholar] [CrossRef]
- Ito, Y.; Cong, W.; Fujita, T.; Tang, Z.; Chen, M. High catalytic activity of nitrogen and sulfur co-doped nanoporous graphene in the hydrogen evolution reaction. Angew 2014, 127, 2159–2164. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on recent progress in nitrogen-doped graphene: Synthesis, characterization, and its potential applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef] [PubMed]
- He, H.Y.; He, Z.; Shen, Q. Efficient hydrogen evolution catalytic activity of graphene/metallic MoS2 nanosheet heterostructures synthesized by a one-step hydrothermal process. Int. J. Hydrog. Energ. 2018, 43, 21835–21843. [Google Scholar] [CrossRef]
- Rivera, L.M.; Fajardo, S.; Arévalo, M.C.; García, G.; Pastor, E. S- and N-doped graphene nanomaterials for the oxygen reduction reaction. Catalysts 2017, 7, 278. [Google Scholar] [CrossRef]
- Sathe, B.R.; Zou, X.; Asefa, T. Metal-free B-doped graphene with efficient electrocatalytic activity for hydrogen evolution reaction. Catal. Sci. Technol. 2014, 4, 2023–2030. [Google Scholar] [CrossRef]
- Carr, L.D.; Lusk, M.T. Graphene gets designer defects. Nat. Publ. Gr. 2010, 5, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Denis, P.A.; Faccio, R.; Mombru, A.W. Is it possible to dope single-walled carbon nanotubes and graphene with sulfur? ChemPhysChem 2009, 10, 715–722. [Google Scholar] [CrossRef]
- Tian, Y.; Wei, Z.; Wang, X.; Peng, S.; Zhang, X. Plasma-etched, S-doped graphene for effective hydrogen evolution reaction. Int. J. Hydrog. Energ. 2016, 42, 4184–4192. [Google Scholar] [CrossRef]
- Cruz-Silva, E.; López-Urías, F.; Muñoz-Sandoval, E.; Sumpter, B.G.; Terrones, H.; Charlier, J.-C.; Terrones, M. Electronic transport and mechanical properties of phosphorus- and phosphorus−nitrogen-doped carbon nanotubes. ACS Nano 2009, 3, 1913–1921. [Google Scholar] [CrossRef]
- Jiang, H.; Zhu, Y.; Su, Y.; Yao, Y.; Liu, Y.; Yang, X.; Li, C. Highly dual-doped multilayer nanoporous graphene: Efficient metal-free electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A. 2015, 3, 12642–12645. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Xue, Y.; Xu, Z.; Pei, J.; Zhuang, Z. Self-assembly precursor-derived MoP supported on N, P-Codoped reduced graphene oxides as efficient catalysts for hydrogen evolution reaction. Inorg. Chem. 2018, 57, 13859–13865. [Google Scholar] [CrossRef]
- Agnoli, S.; Favaro, M. Doping graphene with boron: A review of synthesis. J. Mater. Chem. A 2016, 4, 5002–5025. [Google Scholar] [CrossRef]
- Li, X.; Fan, L.; Li, Z.; Wang, K.; Zhong, M.; Wei, J.; Zhu, H. Boron doping of graphene for graphene-silicon p-n junction solar cells. Adv. Energy Mater. 2012, 2, 425–429. [Google Scholar] [CrossRef]
- Qiu, H.; Ito, Y.; Cong, W.; Tan, Y.; Liu, P.; Hirata, A.; Fujita, T.; Tang, Z.; Chen, M. Nanoporous graphene with single-atom nickel dopants: An efficient and stable catalyst for electrochemical hydrogen production. Angew 2015, 54, 14031–14035. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Ren, P.; Deng, D.; Bao, X. Enhanced electron penetration through an ultrathin graphene layer for highly efficient catalysis of the hydrogen evolution reaction. Angew 2015, 54, 2100–2104. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lv, Y.; Cao, D. Co, N-codoped nanotube/graphene 1D/2D heterostructure for efficient oxygen reduction and hydrogen evolution reactions. J. Mater. Chem. A 2018, 6, 3926–3932. [Google Scholar] [CrossRef]
- Kuang, M.; Wang, Q.; Han, P.; Zheng, G. Cu, Co-Embedded N-Enriched Mesoporous Carbon for Efficient Oxygen Reduction and Hydrogen Evolution Reactions. Adv. Energy. Mater. 2017, 7, 1700193. [Google Scholar] [CrossRef]
- Yan, H.; Xie, Y.; Jiao, Y.; Wu, A.; Tian, C.; Zhang, X.; Wang, L.; Fu, H. Holey reduced graphene oxide coupled with an Mo2N-Mo2C heterojunction for efficient hydrogen evolution. Adv. Mater. 2018, 30, 1704156. [Google Scholar] [CrossRef]
- Qu, Y.; Ke, Y.; Shao, Y.; Chen, W.; Kwok, C.T.; Shi, X.; Pan, H. Effect of curvature on the hydrogen evolution reaction of grapheme. K. Phys. Chem. C 2018, 122, 25331–25338. [Google Scholar] [CrossRef]
- Mukherjee, A.; Chakrabarty, S.; Su, W.-N.; Basu, S. Nanostructured nickel ferrite embedded in reduced graphene oxide for electrocatalytic hydrogen evolution reaction. Mater. Today Energy 2018, 8, 118–124. [Google Scholar] [CrossRef]
- Yan, H.; Tian, C.; Wang, L.; Wu, A.; Meng, M.; Zhao, L.; Fu, H. Phosphorus-modified tungsten nitride/reduced graphene oxide as a high-performance, non-noble-metal electrocatalyst for the hydrogen evolution reaction. Angew 2015, 54, 6325–6329. [Google Scholar] [CrossRef]
- Su, J.; Yang, Y.; Xia, G.; Chen, J.; Jiang, P.; Chen, Q. Ruthenium-cobalt nanoalloys encapsulated in nitrogen-doped graphene as active electrocatalysts for producing hydrogen in alkaline media. Nat. Commun. 2017, 8, 14969. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, G.; Miao, S.; Li, J.; Bao, X. Graphene-supported iron-based nanoparticles encapsulated in nitrogen-doped carbon as a synergistic catalyst for hydrogen evolution and oxygen reduction reactions. Faraday Discuss 2014, 176, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Bagher, M.; Salarizadeh, P.; Mohammad, S. MoCoFeS hybridized with reduced graphene oxide as a new electrocatalyst for hydrogen evolution reaction. Chem. Phys. Lett. 2018, 711, 32–36. [Google Scholar] [CrossRef]
- Wondimu, T.H.; Chen, G.; Kabtamu, D.M.; Chen, H.; Bayeh, A.W.; Huang, H.; Wang, H.C. Highly efficient and durable phosphine reduced iron-doped tungsten oxide / reduced graphene oxide nanocomposites for the hydrogen evolution reaction. Int. J. Hydrog. Energ. 2018, 43, 6481–6490. [Google Scholar] [CrossRef]
- Pham, C.V.; Zana, A.; Arenz, M.; Thiele, S. [Mo3S13]2− Cluster decorated sulfur-doped reduced graphene oxide as noble metal-free catalyst for hydrogen evolution reaction in polymer electrolyte membrane electrolyzers. ChemElectroChem 2018, 5, 2672–2680. [Google Scholar] [CrossRef]
- He, H.-Y.; He, Z.; Shen, Q. Reduced graphene oxide/metallic MoSe2: Cu nanosheet nanostructures grown by a chemical process for highly efficient water splitting. Mater. Res. Bull. 2019, 111, 183–190. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).