An Insight into the Reactivity of the Electrogenerated Radical Cation of Caffeine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cyclic Voltammetry
2.3. Controlled Potential Electrolysis
2.4. HPLC-PDA-ESI-MS/MS Analysis
3. Results
3.1. Anodic oxidation of CAF
3.2. HPLC-PDA-ESI-MS/MS Analysis of Oxidation Products
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shechter, M.; Shalmon, G.; Scheinowitz, M.; Koren-Morag, N.; Feinberg, M.S.; Harats, D.; Sela, B.A.; Sharabi, Y.; Chouraqui, P. Impact of acute caffeine ingestion on endothelial function in subjects with and without coronary artery disease. Am. J. Cardiol. 2011, 107, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Gaytan, S.P.; Pasaro, R. Neonatal caffeine treatment up-regulates adenosine receptors in brainstem and hypothalamic cardio-respiratory related nuclei of rat pups. Exp. Neurol. 2012, 237, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Barrès, R.; Yan, J.; Egan, B.; Treebak, J.T.; Rasmussen, M.; Fritz Caidahl, K.; Krook, A.; O’Gorman, D.J.; Zierath, J.R. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 2012, 15, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Wardle, M.C.; Treadway, M.T.; de Wit, H. Caffeine increases psychomotor performance on the effort expenditure for rewards task. Pharmacol. Biochem. Behav. 2012, 102, 526–531. [Google Scholar] [CrossRef]
- Ward, N.; Whitney, C.; Avery, D.; Dunner, D. The analgesic effects of caffeine in headache. Pain 1991, 44, 151–155. [Google Scholar] [CrossRef]
- Renner, B.; Clarke, G.; Grattan, T.; Beisel, A.; Muller, C.; Werner, U.; Kobal, G.; Brune, K. Caffeine accelerates absorption and enhances the analgesic effect of acetaminophen. J. Clin. Pharmacol. 2007, 47, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.; Alves, M.G.; Oliveira, P.F.; Silva, B.M. Pharmacological potential of methylxanthines: Retrospective analysis and future expectations. Crit. Rev. Food Sci. Nutr. 2019, 59, 2597–2625. [Google Scholar] [CrossRef]
- Prasanthi, J.R.P.; Dasari, B.; Marwarha, G.; Larson, T.; Chen, X.; Geiger, J.D.; Ghribi, O. Caffeine protects against oxidative stress and Alzheimer’s disease-like pathology in rabbit hippocampus induced by cholesterol-enriched diet. Free Radic. Biol. Med. 2010, 49, 1212–1220. [Google Scholar] [CrossRef]
- Devasagayam, T.P.A.; Kamat, J.P.; Mohan, H.; Kesavan, P.C. Caffeine as an antioxidant: Inhibition of lipid peroxidation induced by reactive oxygen species. Biochim. Biophys. Acta 1996, 1282, 63–70. [Google Scholar] [CrossRef]
- Varma, S.D.; Hegde, K.R. Kynurenine-induced photo oxidative damage to lens in vitro: Protective effect of caffeine. Mol. Cell. Biochem. 2010, 340, 49–54. [Google Scholar] [CrossRef]
- León-Carmona, J.R.; Galano, A. Is Caffeine a Good Scavenger of Oxygenated Free Radicals? J. Phys. Chem. 2011, 115, 4538–4546. [Google Scholar] [CrossRef] [PubMed]
- Brezová, V.; Šlebodová, A.; Staško, A. Coffee as a source of antioxidants: An EPR study. Food Chem. 2009, 114, 859–868. [Google Scholar] [CrossRef]
- Załuski, M.; Schabikowski, J.; Schlenk, M.; Olejarz-Maciej, A.; Kubas, B.; Karcz, T.; Kuder, K.; Latacz, G.; Zygmunt, M.; Synak, D.; et al. Novel multi-target directed ligands based on annelated xanthine scaffold with aromatic substituents acting on adenosine receptor and monoamine oxidase B. Synthesis, in vitro and in silico studies. Bioorg. Med. Chem. 2019, 27, 1195–1210. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Kumar Shreshtha, A.; Thakur, M.S.; Patra, S. Xanthine scaffold: Scope and potential in drug development. Heliyon 2018, 4, e00829. [Google Scholar] [CrossRef]
- Załuski, M.; Stanuch, K.; Karcz, T.; Hinz, S.; Latacz, G.; Szymańska, E.; Schabikowski, J.; Doroż-Płonka, A.; Handzlik, J.; Drabczyńska, A.; et al. Tricyclic xanthine derivatives containing a basic substituent: Adenosine receptor affinity and drug-related properties. Med. Chem. Commun. 2018, 9, 951–962. [Google Scholar] [CrossRef]
- Kascatan-Nebioglu, A.; Melaiye, A.; Hindi, K.; Durmus, S.; Panzner, M.J.; Hogue, L.A.; Mallett, R.J.; Hovis, C.E.; Coughenour, M.; Crosby, S.D.; et al. Synthesis from Caffeine of a Mixed N-Heterocyclic Carbene-Silver Acetate Complex Active against Resistant Respiratory Pathogens. J. Med. Chem. 2006, 49, 6811–6818. [Google Scholar] [CrossRef]
- Pandolfi, F.; Chiarotto, I.; Mattiello, L.; Rocco, D.; Feroci, M. Cathodic reduction of caffeine: Synthesis of an amino-finctionalized imidazole from a biobased reagent. Synlett 2019, 30, 1215–1218. [Google Scholar] [CrossRef]
- Pandolfi, F.; Mattiello, L.; Zane, D.; Feroci, M. Electrochemical behaviour of 9-methylcaffeinium iodide and in situ electrochemical synthesis of hymeniacidin. Electrochim. Acta 2018, 280, 71–76. [Google Scholar] [CrossRef]
- Pandolfi, F.; Chiarotto, I.; Mattiello, L.; Petrucci, R.; Feroci, M. Two Different Selective Ways in the Deprotonation of β-Bromopropionanilides: β-Lactams or Acrylanilides Formation. ChemistrySelect 2019, 4, 12871–12874. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M. Sustainable management of coffee industry by-products and value addition. A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Švorc, L. Determination of caffeine: A comprehensive review on electrochemical method. Int. J. Electrochem. Sci. 2013, 8, 5755–5773. [Google Scholar]
- Trani, A.; Petrucci, R.; Marrosu, G.; Zane, D.; Curulli, A. Selective electrochemical determination of caffeine at a gold-chitosan nanocomposite sensor: May little change on nanocomposites synthesis affect selectivity? J. Electroanal. Chem. 2017, 788, 99–106. [Google Scholar] [CrossRef]
- Trani, A.; Petrucci, R.; Marrosu, G.; Curulli, A. Determination of Caffeine @ Gold Nanoparticles Modified Gold (Au) Electrode: A Preliminary Study. Sensors 2015, 319, 147–151. [Google Scholar] [CrossRef]
- Lovecchio, N.; Costantini, F.; Nascetti, A.; Petrucci, R.; de Cesare, G.; Caputo, D. Development of an Electrochemiluminescence-based Lab-on-Chip Using Thin/Thick Film Technologies. In Proceedings of the 8th International Workshop on Advances in Sensors and Interfaces, IWASI 2019, Otranto, Italy, 13–14 June 2019; pp. 79–83. [Google Scholar]
- Petrucci, R.; Chiarotto, I.; Mattiello, L.; Passeri, D.; Rossi, M.; Zollo, G.; Feroci, M. Graphene Oxide: A Smart (Starting) Material for Natural Methylxanthines Adsorption and Detection. Molecules 2019, 24, 4247. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.H.; Dryhurst, G. Electrochemical oxidation of theobromine and caffeine at the pyrolitic graphite electrode. Electroanal. Chem. Interfacial Electrochem. 1971, 30, 407–416. [Google Scholar] [CrossRef]
- Hansen, B.H.; Dryhurst, G. Voltammetric oxidation of some biologically important xanthines at the pyrolitic graphite electrode. Electroanal. Chem. Interfacial Electrochem. 1971, 30, 417–426. [Google Scholar] [CrossRef]
- Kumar, M.R.; Adinarayana, M. Oxidation of caffeine by phosphate radical anion in aqueous solution under anoxic conditions. Proc. Indian Acad. Sci. Chem. Sci. 2000, 112, 551–557. [Google Scholar] [CrossRef]
- Dalmázio, I.; Santos, L.S.; Lopes, R.P.; Eberlin, M.N.; Augusti, R. Advanced Oxidation of Caffeine in Water: On-line and Real-Time Monitoring by Electrospray Ionization Mass Spectrometry. Environ. Sci. Technol. 2005, 39, 5982–5988. [Google Scholar] [CrossRef]
- Chan, K.K.; Ganguly, R.; Li, Y.; Webster, R.D. Electrochemically Controlled One-Electrone Oxidation Coupled to Consecutive Hydrogen Atom Transfer of Caffeine. ChemElectroChem 2014, 1, 1557–1562. [Google Scholar] [CrossRef]
- Petrucci, R.; Zollo, G.; Curulli, A.; Marrosu, G. A new insight into the oxidative mechanism of caffeine and related methylxanythines in aprotic medium: May caffeine be really considered as an antioxidant? BBA Gen. Subj. 2018, 1862, 1781–1789. [Google Scholar] [CrossRef]
- Chiarotto, I.; Mattiello, L.; Pandolfi, F.; Rocco, D.; Feroci, M.; Petrucci, R. Electrochemical oxidation of theophylline in organic solvents: HPLC-PDA-ESI-MS/MS analysis of the oxidation products. ChemElectroChem 2019, 6, 4511–4521. [Google Scholar] [CrossRef]
- Feroci, M.; Civitarese, T.; Pandolfi, F.; Petrucci, R.; Rocco, D.; Zane, D.; Zollo, G.; Mattiello, L. Electrochemical Studies of New Donor-Acceptor Oligothiophenes. ChemElectroChem 2019, 6, 4016–4021. [Google Scholar] [CrossRef]
- Struck, W.A.; Elving, P.J. Electrolytic oxidation of uric acid: Products and mechanism. Biochemistry 1965, 4, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Al-Qaim, F.F.; Mussa, Z.H.; Othman, M.R.; Abdullah, M.P. Removal of caffeine from aqueous solution by indirect electrochemical oxidation using a graphite-PVC composite electrode: A role of hypochlorite ion as an oxidizing agent. J. Hazard. Mater. 2015, 300, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Panusa, A.; Petrucci, R.; Lavecchia, R.; Zuorro, A. UHPLC-PDA-ESI-TOF/MS metabolic profiling and antioxidant capacity of arabica and robusta coffee silverskin: Antioxidants vs phytotoxins. Food Res. Inter. 2017, 99, 155–165. [Google Scholar] [CrossRef]


| ACN | ACN/1eq H2O | |
|---|---|---|
| Eap (V) a | +1.87 | +1.92 |
| I (A cm−2) b | 6.64 × 10−4 | 1.4 × 10−3 |
| I2F/I0c | 42% | 14% |
| Compound | RT (min) | M (Da) caltd | [M+H]+ caltd/expt | [M-H]− cald/expt | λ (nm) | Structure | Data in SM | 2 F/1 F |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.75 | 250.09 | 251.10/251.22 | - | 294 a | 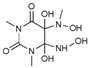 | Figure S3 | +57% |
| 2 | 3.35 | 188.09 | [M+Na]+ 211.08/211.06 | 187.08/187.09 | 203; 234 |  | Figure S4 | +71% |
| 3 | 5.39 | 194.08 | 195.09/195.04 | - | 205; 272 | 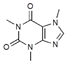 | −70% | |
| 4 | 6.94 | 213.07 | - | 212.07/212.03 | 220–240 b | 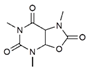 | Figure S5 | +97% |
| 5 | 8.37 | 228.09 | 229.09/229.10 | - | 209; 276 | 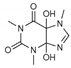 | Figure S6 | −28% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feroci, M.; Bortolami, M.; Chiarotto, I.; Di Matteo, P.; Mattiello, L.; Pandolfi, F.; Rocco, D.; Petrucci, R. An Insight into the Reactivity of the Electrogenerated Radical Cation of Caffeine. Electrochem 2020, 1, 44-55. https://doi.org/10.3390/electrochem1010005
Feroci M, Bortolami M, Chiarotto I, Di Matteo P, Mattiello L, Pandolfi F, Rocco D, Petrucci R. An Insight into the Reactivity of the Electrogenerated Radical Cation of Caffeine. Electrochem. 2020; 1(1):44-55. https://doi.org/10.3390/electrochem1010005
Chicago/Turabian StyleFeroci, Marta, Martina Bortolami, Isabella Chiarotto, Paola Di Matteo, Leonardo Mattiello, Fabiana Pandolfi, Daniele Rocco, and Rita Petrucci. 2020. "An Insight into the Reactivity of the Electrogenerated Radical Cation of Caffeine" Electrochem 1, no. 1: 44-55. https://doi.org/10.3390/electrochem1010005
APA StyleFeroci, M., Bortolami, M., Chiarotto, I., Di Matteo, P., Mattiello, L., Pandolfi, F., Rocco, D., & Petrucci, R. (2020). An Insight into the Reactivity of the Electrogenerated Radical Cation of Caffeine. Electrochem, 1(1), 44-55. https://doi.org/10.3390/electrochem1010005














