Progress and Opportunities for Exsolution in Electrochemistry
Abstract
1. Introduction
2. Discussion
2.1. Recent Advancements in Exsolution Electrodes
2.2. Future Research Directions
2.2.1. Exsolution from Heteroanionic Ceramics
2.2.2. Exsolution in Photocatalysis
2.2.3. Predetermined Location of Exsolution
2.2.4. Exsolved Core-Shell and Core-Skin Particles
2.2.5. Exsolution from Thin Films Cast on High Surface Area Supports
2.2.6. Exsolution Electrodes for Ambient Temperature Applications
3. Conclusions
Funding
Conflicts of Interest
References
- Meille, V. Review on methods to deposit catalysts on structured surfaces. Appl. Catal. A Gen. 2006, 315, 1–17. [Google Scholar] [CrossRef]
- Prieto, G.; Zečević, J.; Friedrich, H.; de Jong, K.P.; de Jongh, P.E. Towards stable catalysts by controlling collective properties of supported metal nanoparticles. Nat. Mater. 2013, 12, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Munnik, P.; de Jongh, P.E.; de Jong, K.P. Recent developments in the synthesis of supported catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, J.; Aikawa, K.; Sato, K.; Uchijima, T. Role of support in reforming of CH4 with CO2 over Rh catalysts. Catal. Lett. 1994, 25, 265–270. [Google Scholar] [CrossRef]
- Schubert, M.M.; Hackenberg, S.; van Veen, A.C.; Muhler, M.; Plzak, V.; Behm, R.J. CO oxidation over supported gold catalysts—“Inert” and “active” support materials and their role for the oxygen supply during reaction. J. Catal. 2001, 197, 113–122. [Google Scholar] [CrossRef]
- Micoud, F.; Maillard, F.; Bonnefont, A.; Job, N.; Chatenet, M. The role of the support in CO ads monolayer electrooxidation on Pt nanoparticles: Pt/WO x vs. Pt/C. Phys. Chem. Chem. Phys. 2010, 12, 1182–1193. [Google Scholar] [CrossRef]
- Neagu, D.; Oh, T.-S.; Miller, D.N.; Ménard, H.; Bukhari, S.M.; Gamble, S.R.; Gorte, R.J.; Vohs, J.M.; Irvine, J.T. Nano-socketed nickel particles with enhanced coking resistance grown in situ by redox exsolution. Nat. Commun. 2015, 6, 8120. [Google Scholar] [CrossRef]
- Ratkovic, S.; Vujicic, D.; Kiss, E.; Boskovic, G.; Geszti, O. Different degrees of weak metal–support interaction in Fe–(Ni)/Al2O3 catalyst governing activity and selectivity in carbon nanotubes’ production using ethylene. Mater. Chem. Phys. 2011, 129, 398–405. [Google Scholar] [CrossRef]
- Park, C.; Keane, M.A. Catalyst support effects in the growth of structured carbon from the decomposition of ethylene over nickel. J. Catal. 2004, 221, 386–399. [Google Scholar] [CrossRef]
- Rosen, B.A.; Singh, S. Fossil Fuels: Coke-Resistant Nanomaterials for Gas-to-Liquid (GTL) Fuels. Nanotechnol. Energy Sustain. 2017, 59–82. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef] [PubMed]
- Sutthiumporn, K.; Maneerung, T.; Kathiraser, Y.; Kawi, S. CO2 dry-reforming of methane over La0.8Sr0.2Ni0.8M0.2O3 perovskite (M = Bi, Co, Cr, Cu, Fe): Roles of lattice oxygen on C–H activation and carbon suppression. Int. J. Hydrogen Energy 2012, 37, 11195–11207. [Google Scholar] [CrossRef]
- Arandiyan, H.; Li, J.; Ma, L.; Hashemnejad, S.M.; Mirzaei, M.Z.; Chen, J.; Chang, H.; Liu, C.; Wang, C.; Chen, L. Methane reforming to syngas over LaNixFe1−xO3 (0 ≤ x ≤ 1) mixed-oxide perovskites in the presence of CO2 and O2. J. Ind. Eng. Chem. 2012, 18, 2103–2114. [Google Scholar] [CrossRef]
- Gallego, G.S.; Batiot-Dupeyrat, C.; Barrault, J.; Florez, E.; Mondragon, F. Dry reforming of methane over LaNi1−yByO3 ± δ (B = Mg, Co) perovskites used as catalyst precursor. Appl. Catal. A Gen. 2008, 334, 251–258. [Google Scholar] [CrossRef]
- Tsai, C.; Abild-Pedersen, F.; Nørskov, J.K. Tuning the MoS2 edge-site activity for hydrogen evolution via support interactions. Nano Lett. 2014, 14, 1381–1387. [Google Scholar] [CrossRef]
- Neagu, D.; Tsekouras, G.; Miller, D.N.; Ménard, H.; Irvine, J.T.S. In situ growth of nanoparticles through control of non-stoichiometry. Nat. Chem. 2013, 5, 916–923. [Google Scholar] [CrossRef]
- Huang, K. An emerging platform for electrocatalysis: Perovskite exsolution. Sci. Bull. 2016, 61, 1783–1784. [Google Scholar] [CrossRef]
- Kan, W.H.; Samson, A.J.; Thangadurai, V. Trends in electrode development for next generation solid oxide fuel cells. J. Mater. Chem. A 2016, 4, 17913–17932. [Google Scholar] [CrossRef]
- Irvine, J.T.S.; Neagu, D.; Verbraeken, M.C.; Chatzichristodoulou, C.; Graves, C.; Mogensen, M.B. Evolution of the electrochemical interface in high-temperature fuel cells and electrolysers. Nat. Energy 2016, 1, 15014. [Google Scholar] [CrossRef]
- Nishihata, Y.; Mizuki, J.; Akao, T.; Tanaka, H.; Uenishi, M.; Kimura, M.; Okamoto, T.; Hamada, N. Self-regeneration of a Pd-perovskite catalyst for automotive emissions control. Nature 2002, 418, 164–167. [Google Scholar] [CrossRef]
- Myung, J.-H.; Neagu, D.; Miller, D.N.; Irvine, J.T.S. Switching on electrocatalytic activity in solid oxide cells. Nature 2016, 537, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Neagu, D.; Kyriakou, V.; Roiban, I.-L.; Aouine, M.; Tang, C.; Caravaca, A.; Kousi, K.; Schreur-Piet, I.; Metcalfe, I.S.; Vernoux, P.; et al. In Situ Observation of Nanoparticle Exsolution from Perovskite Oxides: From Atomic Scale Mechanistic Insight to Nanostructure Tailoring. ACS Nano 2019, 13, 12996–13005. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.-S.; Rahani, E.K.; Neagu, D.; Irvine, J.T.S.; Shenoy, V.B.; Gorte, R.J.; Vohs, J.M. Evidence and Model for Strain-Driven Release of Metal Nanocatalysts from Perovskites during Exsolution. J. Phys. Chem. Lett. 2015, 6, 5106–5110. [Google Scholar] [CrossRef] [PubMed]
- Haag, J.M.; Barnett, S.A.; Richardson, J.W.; Poeppelmeier, K.R. Structural and Chemical Evolution of the SOFC Anode La0.30Sr0.70Fe0.70Cr0.30O3-δ upon Reduction and Oxidation: An in Situ Neutron Diffraction Study. Chem. Mater. 2010, 22, 3283–3289. [Google Scholar] [CrossRef]
- Buharon, M.; Singh, S.; Komarala, E.P.; Rosen, B.A. Expanding possibilities for solid-phase crystallization by exsolving tunable Pd–NiO core–shell nanostructures. CrystEngComm 2018, 20, 6372–6376. [Google Scholar] [CrossRef]
- Bergamaschini, R.; Rosen, B.A.; Montalenti, F.; Colin, J. Motion of crystalline inclusions by interface diffusion in the proximity of free surfaces. J. Nanopart. Res. 2019, 21, 271. [Google Scholar] [CrossRef]
- Gao, Y.; Lu, Z.; You, T.L.; Wang, J.; Xie, L.; He, J.; Ciucci, F. Energetics of Nanoparticle Exsolution from Perovskite Oxides. J. Phys. Chem. Lett. 2018, 9, 3772–3778. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction to the High Temperature Oxidation of Metals; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Sun, Y.; Li, J.; Zeng, Y.; Amirkhiz, B.S.; Wang, M.; Behnamian, Y.; Luo, J. A-site deficient perovskite: The parent for in situ exsolution of highly active, regenerable nano-particles as SOFC anodes. J. Mater. Chem. A 2015, 3, 11048–11056. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Li, J.-H.; Wang, M.-N.; Hua, B.; Li, J.; Luo, J.-L. A-site deficient chromite perovskite with in situ exsolution of nano-Fe: A promising bi-functional catalyst bridging the growth of CNTs and SOFCs. J. Mater. Chem. A 2015, 3, 14625–14630. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Li, J.-H.; Cui, L.; Hua, B.; Cui, S.-H.; Li, J.; Luo, J.-L. A-site-deficiency facilitated in situ growth of bimetallic Ni–Fe nano-alloys: A novel coking-tolerant fuel cell anode catalyst. Nanoscale 2015, 7, 11173–11181. [Google Scholar] [CrossRef]
- Kousi, K.; Neagu, D.; Bekris, L.; Papaioannou, E.I.; Metcalfe, I.S. Endogenous Nanoparticles Strain Perovskite Host Lattice Providing Oxygen Capacity and Driving Oxygen Exchange and CH4 Conversion to Syngas. Angew. Chem. Int. Ed. 2019. [Google Scholar] [CrossRef]
- Zhu, T.; Troiani, H.E.; Mogni, L.V.; Han, M.; Barnett, S.A. Ni-Substituted Sr(Ti,Fe)O3 SOFC Anodes: Achieving High Performance via Metal Alloy Nanoparticle Exsolution. Joule 2018, 2, 478–496. [Google Scholar] [CrossRef]
- Zhu, T.; Troiani, H.; Mogni, L.V.; Santaya, M.; Han, M.; Barnett, S.A. Exsolution and electrochemistry in perovskite solid oxide fuel cell anodes: Role of stoichiometry in Sr(Ti,Fe,Ni)O3. J. Power Sources 2019, 439, 227077. [Google Scholar] [CrossRef]
- Kwon, O.; Kim, K.; Joo, S.; Jeong, H.Y.; Shin, J.; Han, J.W.; Sengodan, S.; Kim, G. Self-assembled alloy nanoparticles in a layered double perovskite as a fuel oxidation catalyst for solid oxide fuel cells. J. Mater. Chem. A 2018, 6, 15947–15953. [Google Scholar] [CrossRef]
- Chen, X.; Ni, W.; Wang, J.; Zhong, Q.; Han, M.; Zhu, T. Exploration of Co-Fe alloy precipitation and electrochemical behavior hysteresis using Lanthanum and Cobalt co-substituted SrFeO3-δ SOFC anode. Electrochim. Acta 2018, 277, 226–234. [Google Scholar] [CrossRef]
- Zubenko, D.; Singh, S.; Rosen, B.A. Exsolution of Re-alloy catalysts with enhanced stability for methane dry reforming. Appl. Catal. B Environ. 2017, 209, 711–719. [Google Scholar] [CrossRef]
- Li, M.; Hua, B.; Zeng, Y.; Amirkhiz, B.S.; Luo, J.-L. Thermally stable and coking resistant CoMo alloy-based catalysts as fuel electrodes for solid oxide electrochemical cells. J. Mater. Chem. A 2018, 6, 15377–15385. [Google Scholar] [CrossRef]
- Fowler, D.E.; Messner, A.C.; Miller, E.C.; Slone, B.W.; Barnett, S.A.; Poeppelmeier, K.R. Decreasing the Polarization Resistance of (La,Sr)CrO3-δ Solid Oxide Fuel Cell Anodes by Combined Fe and Ru Substitution. Chem. Mater. 2015, 27, 3683–3693. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Guan, K.; Meng, J.; Wei, Z.; Liu, X.; Meng, J. Enhanced Anode Performance and Coking Resistance by In Situ Exsolved Multiple-Twinned Co–Fe Nanoparticles for Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2019. [Google Scholar] [CrossRef]
- Wang, L.; Hu, R.; Liu, H.; Wei, Q.; Gong, D.; Mo, L.; Tao, H.; Zhang, Z. Encapsulated Ni@La2O3/SiO2 Catalyst with a One-Pot Method for the Dry Reforming of Methane. Catalysts 2019, 10, 38. [Google Scholar] [CrossRef]
- Papargyriou, D.; Miller, D.N.; Irvine, J.T.S. Exsolution of Fe–Ni alloy nanoparticles from (La,Sr)(Cr,Fe,Ni)O3 perovskites as potential oxygen transport membrane catalysts for methane reforming. J. Mater. Chem. A 2019, 7, 15812–15822. [Google Scholar] [CrossRef]
- Singh, S.; Prestat, E.; Huang, L.-F.; Rondinelli, J.M.; Haigh, S.J.; Rosen, B.A. Role of 2D and 3D defects on the reduction of LaNiO3 nanoparticles for catalysis. Sci. Rep. 2017, 7, 10080. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Jia, L.; Luo, J.-L.; Chi, B.; Pu, J.; Li, J. CO2 dry reforming of CH4 with Sr and Ni co-doped LaCrO3 perovskite catalysts. Appl. Surf. Sci. 2020, 506, 144699. [Google Scholar] [CrossRef]
- Oh, J.H.; Kwon, B.W.; Cho, J.; Lee, C.H.; Kim, M.K.; Choi, S.H.; Yoon, S.P.; Han, J.; Nam, S.W.; Kim, J.Y.; et al. Importance of Exsolution in Transition-Metal (Co, Rh, and Ir)-Doped LaCrO3 Perovskite Catalysts for Boosting Dry Reforming of CH4 Using CO2 for Hydrogen Production. Ind. Eng. Chem. Res. 2019, 58, 6385–6393. [Google Scholar] [CrossRef]
- Vecino-Mantilla, S.; Gauthier-Maradei, P.; Huvé, M.; Serra, J.M.; Roussel, P.; Gauthier, G.H. Nickel Exsolution-Driven Phase Transformation from an n = 2 to an n = 1 Ruddlesden-Popper Manganite for Methane Steam Reforming Reaction in SOFC Conditions. ChemCatChem 2019, 11, 4631–4641. [Google Scholar] [CrossRef]
- Atkinson, A.; Barnett, S.; Gorte, R.J.; Irvine, J.T.S.; McEvoy, A.J.; Mogensen, M.; Singhal, S.C.; Vohs, J. Advanced anodes for high-temperature fuel cells. In Materials for Sustainable Energy; Co-Published with Macmillan Publishers Ltd.: London, UK, 2010; pp. 213–223. [Google Scholar]
- Zhu, J.; Zhang, W.; Li, Y.; Yue, W.; Geng, G.; Yu, B. Enhancing CO2 catalytic activation and direct electroreduction on in-situ exsolved Fe/MnOx nanoparticles from (Pr,Ba)2Mn2-yFeyO5 + δ layered perovskites for SOEC cathodes. Appl. Catal. B Environ. 2019, 268, 118389. [Google Scholar] [CrossRef]
- Park, S.; Kim, Y.; Han, H.; Chung, Y.S.; Yoon, W.; Choi, J.; Kim, W.B. In situ exsolved Co nanoparticles on Ruddlesden-Popper material as highly active catalyst for CO2 electrolysis to CO. Appl. Catal. B Environ. 2019, 248, 147–156. [Google Scholar] [CrossRef]
- Lv, H.; Lin, L.; Zhang, X.; Gao, D.; Song, Y.; Zhou, Y.; Liu, Q.; Wang, G.; Bao, X. In situ exsolved FeNi3 nanoparticles on nickel doped Sr2Fe1.5Mo0.5O6−δ perovskite for efficient electrochemical CO2 reduction reaction. J. Mater. Chem. A 2019, 7, 11967–11975. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Q.; Luo, J.-L. Highly Stable and Efficient Catalyst with In Situ Exsolved Fe–Ni Alloy Nanospheres Socketed on an Oxygen Deficient Perovskite for Direct CO2 Electrolysis. ACS Catal. 2016, 6, 6219–6228. [Google Scholar] [CrossRef]
- Kyriakou, V.; Neagu, D.; Zafeiropoulos, G.; Sharma, R.K.; Tang, C.; Kousi, K.; Metcalfe, I.S.; van de Sanden, M.C.M.; Tsampas, M.N. Symmetrical Exsolution of Rh Nanoparticles in Solid Oxide Cells for Efficient Syngas Production from Greenhouse Gases. ACS Catal. 2019, 1278–1288. [Google Scholar] [CrossRef]
- Bahout, M.; Managutti, P.; Dorcet, V.; la Salle, A.L.G.; Paofai, S.; Hansen, T.C. In situ exsolution of Ni particles on the PrBaMn2O5 SOFC electrode material monitored by high temperature neutron powder diffraction under hydrogen. J. Mater. Chem. A 2020, 8, 3590–3597. [Google Scholar] [CrossRef]
- Lee, J.G.; Myung, J.-H.; Naden, A.B.; Jeon, O.S.; Shul, Y.G.; Irvine, J.T.S. Replacement of Ca by Ni in a Perovskite Titanate to Yield a Novel Perovskite Exsolution Architecture for Oxygen-Evolution Reactions. Adv. Energy Mater. 2020, 1903693. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.; Zhang, X.; Zhang, H.; Yu, N.; Liu, T.; Wang, Y. Thermal Stability of an in Situ Exsolved Metallic Nanoparticle Structured Perovskite Type Hydrogen Electrode for Solid Oxide Cells. ACS Sustain. Chem. Eng. 2019, 7, 17834–17844. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Yang, Z.; Lei, Z.; Jin, C.; Liu, Y.; Wang, Y.; Peng, S. Co-substituted Sr2Fe1.5Mo0.5O6-δ as anode materials for solid oxide fuel cells: Achieving high performance via nanoparticle exsolution. J. Power Sources 2019, 438, 226989. [Google Scholar] [CrossRef]
- Wan, Y.; Xing, Y.; Xie, Y.; Shi, N.; Xu, J.; Xia, C. Vanadium-Doped Strontium Molybdate with Exsolved Ni Nanoparticles as Anode Material for Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2019, 11, 42271–42279. [Google Scholar] [CrossRef]
- Jo, Y.-R.; Koo, B.; Seo, M.-J.; Kim, J.K.; Lee, S.; Kim, K.; Han, J.W.; Jung, W.; Kim, B.-J. Growth Kinetics of Individual Co Particles Ex-solved on SrTi0.75Co0.25O3-δ Polycrystalline Perovskite Thin Films. J. Am. Chem. Soc. 2019, 141, 6690–6697. [Google Scholar] [CrossRef]
- Qi, H.; Yang, T.; Li, W.; Ma, L.; Hu, S.; Shi, W.; Sabolsky, E.M.; Zondlo, J.W.; Hart, R.; Hackett, G.A.; et al. Reversible In-Situ Exsolution of Fe Catalyst in La0.5Sr1.5Fe1.5Mo0.5O6-δ Anode for SOFCs. ECS Trans. 2019, 91, 1701–1710. [Google Scholar] [CrossRef]
- Li, X.; Dai, L.; He, Z.; Meng, W.; Li, Y.; Wang, L. In situ exsolution of PdO nanoparticles from non-stoichiometric LaFePd0.05O3 + δ electrode for impedancemetric NO2 sensor. Sens. Actuators B Chem. 2019, 298, 126827. [Google Scholar] [CrossRef]
- Li, X.; Dai, L.; He, Z.; Meng, W.; Li, Y.; Wang, L. Enhancing NH3 sensing performance of mixed potential type sensors by chemical exsolution of Ag nanoparticle on AgNbO3 sensing electrode. Sens. Actuators B Chem. 2019, 298, 126854. [Google Scholar] [CrossRef]
- Kim, K.J.; Rath, M.K.; Kwak, H.H.; Kim, H.J.; Han, J.W.; Hong, S.-T.; Lee, K.T. A Highly Active and Redox-Stable SrGdNi0.2Mn0.8O4 ± δ Anode with in Situ Exsolution of Nanocatalysts. ACS Catal. 2019, 9, 1172–1182. [Google Scholar] [CrossRef]
- Han, H.; Park, J.; Nam, S.Y.; Kim, K.J.; Choi, G.M.; Parkin, S.S.P.; Jang, H.M.; Irvine, J.T.S. Lattice strain-enhanced exsolution of nanoparticles in thin films. Nat. Commun. 2019, 10, 1471. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hong, T.; Cheng, J.; Chen, Z.; Xu, C. Enhanced proton conductivity of La0.95Ca0.05NixNb1-xO4 (0.01 ≤ x ≤ 0.3) through in-situ exsolution of metallic nanocatalysts. Mater. Chem. Phys. 2018, 208, 226–236. [Google Scholar] [CrossRef]
- Tan, J.; Lee, D.; Ahn, J.; Kim, B.; Kim, J.; Moon, J. Thermally driven in situ exsolution of Ni nanoparticles from (Ni, Gd)CeO2 for high-performance solid oxide fuel cells. J. Mater. Chem. A 2018, 6, 18133–18142. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Yang, Y.-L.; Chen, J.; Li, M.; Zhang, Y.-Q.; Li, J.-H.; Hua, B.; Luo, J.-L. Toward a rational photocatalyst design: A new formation strategy of co-catalyst/semiconductor heterostructures via in situ exsolution. Chem. Commun. 2018, 54, 1505–1508. [Google Scholar] [CrossRef]
- Arandiyan, H.; Wang, Y.; Scott, J.; Mesgari, S.; Dai, H.; Amal, R. In Situ Exsolution of Bimetallic Rh–Ni Nanoalloys: A Highly Efficient Catalyst for CO2 Methanation. ACS Appl. Mater. Interfaces 2018, 10, 16352–16357. [Google Scholar] [CrossRef]
- Kwak, N.W.; Jeong, S.J.; Seo, H.G.; Lee, S.; Kim, Y.; Kim, J.K.; Byeon, P.; Chung, S.-Y.; Jung, W. In situ synthesis of supported metal nanocatalysts through heterogeneous doping. Nat. Commun. 2018, 9, 4829. [Google Scholar] [CrossRef]
- Kwon, O.; Sengodan, S.; Kim, K.; Kim, G.; Jeong, H.Y.; Shin, J.; Ju, Y.-W.; Han, J.W.; Kim, G. Exsolution trends and co-segregation aspects of self-grown catalyst nanoparticles in perovskites. Nat. Commun. 2017, 8, 15967. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Ran, R.; Chen, Y.; Shao, Z.; Liu, M. Promotion of Oxygen Reduction by Exsolved Silver Nanoparticles on a Perovskite Scaffold for Low-Temperature Solid Oxide Fuel Cells. Nano Lett. 2016, 16, 512–518. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Zhang, Y.-Q.; Chen, J.; Li, J.-H.; Zhu, Y.-T.; Zeng, Y.-M.; Amirkhiz, B.S.; Li, J.; Hua, B.; Luo, J.-L. New Opportunity for in Situ Exsolution of Metallic Nanoparticles on Perovskite Parent. Nano Lett. 2016, 16, 5303–5309. [Google Scholar] [CrossRef]
- Fuertes, A. Chemistry and applications of oxynitride perovskites. J. Mater. Chem. 2012, 22, 3293–3299. [Google Scholar] [CrossRef]
- Yang, M.; Oró-Solé, J.; Rodgers, J.A.; Jorge, A.B.; Fuertes, A.; Attfield, J.P. Anion order in perovskite oxynitrides. Nat. Chem. 2011, 3, 47. [Google Scholar] [CrossRef] [PubMed]
- Padi, S.P.; Shelly, L.; Komarala, E.P.; Schweke, D.; Hayun, S.; Rosen, B.A. Coke-free methane dry reforming over nano-sized NiO-CeO2 solid solution after exsolution. Catal. Commun. 2020, 138, 105951. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, D.; Saccoccio, M.; Lu, Z.; Ciucci, F. From material design to mechanism study: Nanoscale Ni exsolution on a highly active A-site deficient anode material for solid oxide fuel cells. Nano Energy 2016, 27, 499–508. [Google Scholar] [CrossRef]
- Singh, S.; Zubenko, D.; Rosen, B.A. Influence of LaNiO3 Shape on Its Solid-Phase Crystallization into Coke-Free Reforming Catalysts. ACS Catal. 2016, 6, 4199–4205. [Google Scholar] [CrossRef]
- Fang, F.; Feng, N.; Zhao, P.; Chen, C.; Li, X.; Meng, J.; Liu, G.; Chen, L.; Wan, H.; Guan, G. In situ exsolution of Co/CoOx core-shell nanoparticles on double perovskite porous nanotubular webs: A synergistically active catalyst for soot efficient oxidation. Chem. Eng. J. 2019, 372, 752–764. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Li, J.-H.; Zhang, Y.-Q.; Hua, B.; Luo, J.-L. Bifunctional Catalyst of Core–Shell Nanoparticles Socketed on Oxygen-Deficient Layered Perovskite for Soot Combustion: In Situ Observation of Synergistic Dual Active Sites. ACS Catal. 2016, 6, 2710–2714. [Google Scholar] [CrossRef]
- Ruban, A.V.; Skriver, H.L.; Nørskov, J.K. Surface segregation energies in transition-metal alloys. Phys. Rev. B 1999, 59, 15990–16000. [Google Scholar] [CrossRef]
- Lin, C.; Foucher, A.C.; Ji, Y.; Curran, C.D.; Stach, E.A.; McIntosh, S.; Gorte, R.J. “Intelligent” Pt Catalysts Studied on High-Surface-Area CaTiO3 Films. ACS Catal. 2019, 9, 7318–7327. [Google Scholar] [CrossRef]
- Lai, K.-Y.; Manthiram, A. Evolution of Exsolved Nanoparticles on a Perovskite Oxide Surface during a Redox Process. Chem. Mater. 2018, 30, 2838–2847. [Google Scholar] [CrossRef]
- Jiang, Y.; Geng, Z.; Sun, Y.; Wang, X.; Huang, K.; Cong, Y.; Shi, F.; Wang, Y.; Zhang, W.; Feng, S. Highly Efficient B-Site Exsolution Assisted by Co Doping in Lanthanum Ferrite toward High-Performance Electrocatalysts for Oxygen Evolution and Oxygen Reduction. ACS Sustain. Chem. Eng. 2019, 8, 302–310. [Google Scholar] [CrossRef]

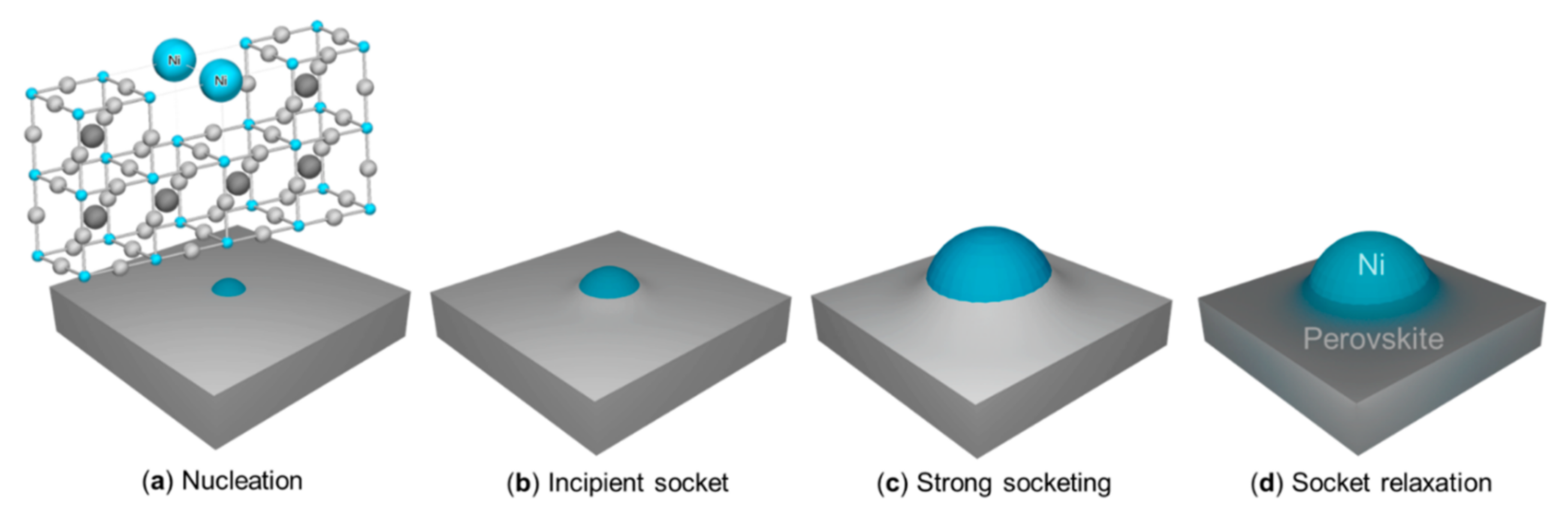
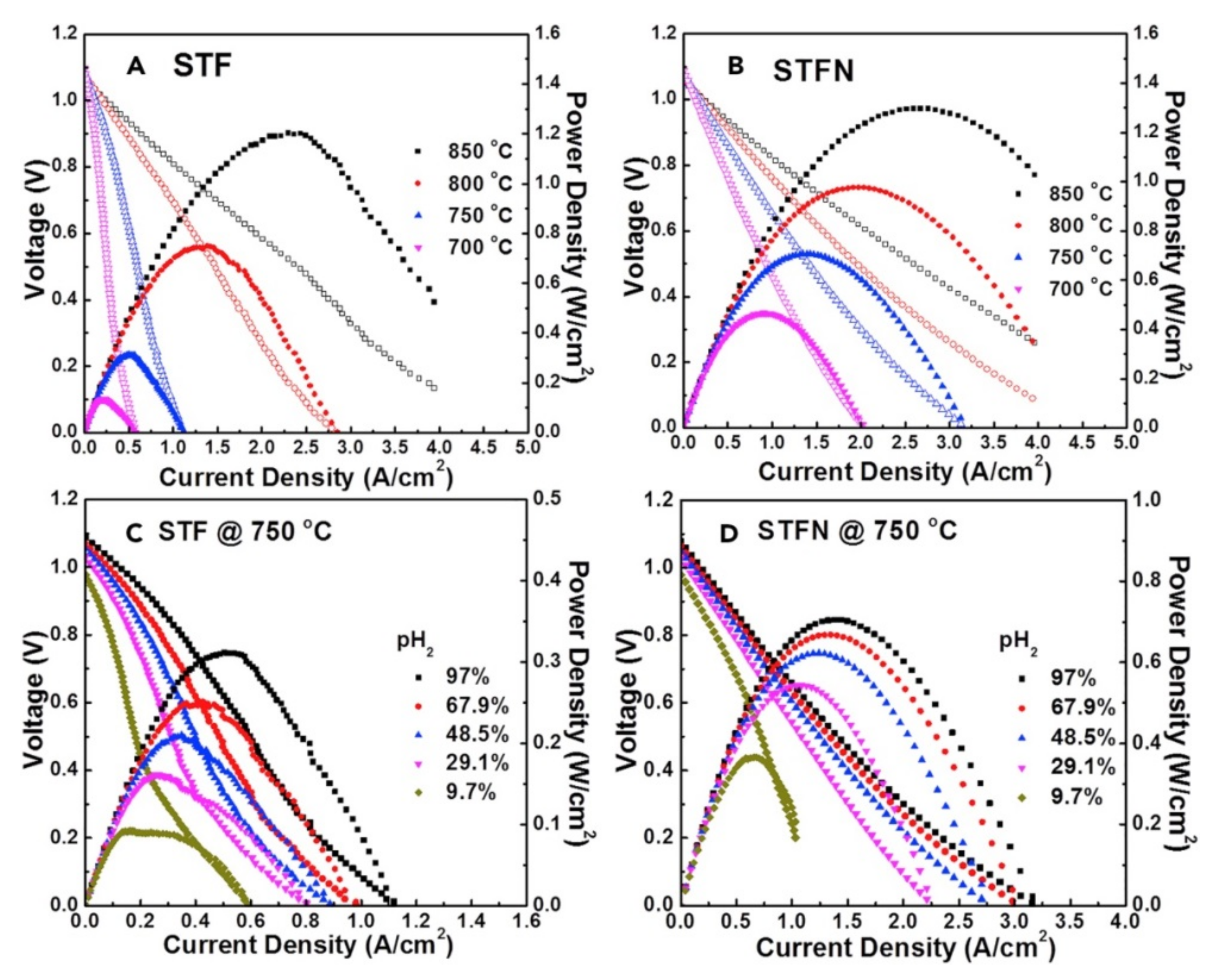
| Material | System | Notes | Year | Ref |
|---|---|---|---|---|
| Pr0.65Ba0.35Mn0.975Ni0.025O3 | H2 SOFC | In-situ neutron diffraction of Ni exsolution process | 2020 | [53] |
| CaTi0.94Ni0.04O3−δ | Oxygen reduction | A-site exsolution | 2020 | [54] |
| Sr0.95(Ti0.3Fe0.63Ni0.07)O3−δ | H2 SOFC | Effect of non-stoichiometry in Sr(Ti,Fe,Ni)O3 | 2019 | [34] |
| (Pr,Ba)2Mn2-yFeyO5+δ | CO2 SOEC | Exsolution of Fe/MnOx for CO2 reduction | 2019 | [48] |
| Sr2CoMo0.95Fe0.05O6−δ | H2/CH4 SOFC | Twinning in Co-Fe exsolution | 2019 | [40] |
| Sr2Fe1.4Ni0.1Mo0.5O6 | H2 SOFC | Thermal stability of Ni exsolution | 2019 | [55] |
| Sr2Fe1.5Mo0.5O6−δ | H2 SOFC | Co exsolution from double-perovskites | 2019 | [56] |
| SrV0.5Mo0.5Ni0.1O4−δ | H2 SOFC | B-site excess doping | 2019 | [57] |
| La1.5Sr1.5Mn1.5Ni0.5O7±δ | H2 SOFC | Exsolution in Ruddlesden Popper Phases | 2019 | [46] |
| SrTi0.75Co0.25O3−δ | CO oxidation | Particle density and growth kinetics | 2019 | [58] |
| La0.5Sr1.5Fe1.5Mo0.5O6−δ | H2 SOFC | Reversible Fe exsolution | 2019 | [59] |
| La0.6Sr0.4Co0.7Mn0.3O3 | CO2 SOEC | Exsolution in Ruddlesden Popper Phases | 2019 | [49] |
| La0.43Ca0.37Ni0.06Ti0.94O3 | Fundamental | In-situ TEM of Ni exsolution | 2019 | [22] |
| LaFePd0.05O3+δ | CO sensor | Pd exsolution | 2019 | [60] |
| AgNbO3 | NH3 sensor | Ag exsolution | 2019 | [61] |
| SrGdNi0.2Mn0.8O4±δ | H2 SOFC | Improved redox stability | 2019 | [62] |
| La0.2Sr0.7Ni0.1Ti0.9O3−δ | Fundamental | Strain enhanced exsolution | 2019 | [63] |
| Sr0.95(Ti0.3Fe0.63Ni0.07)O3 | H2 SOFC | High current density | 2018 | [33] |
| La0.95Ca0.05NixNb1−xO4 | H2 SOFC | Enhanced proton conductivity | 2018 | [64] |
| (Gd0.2−xNixCe0.8O2−δ | H2 SOFC | Exsolution in Gd doped perovskites | 2018 | [65] |
| SrTiWO3 | H2 Production | Exsolution in photocatalysis | 2018 | [66] |
| Rh/3DOM LaNi0.08Al0.92O3 | CO2 reduction | Rh-Ni exsolution for methanation | 2018 | [67] |
| Many | Co oxidation | Predetermined location | 2018 | [68] |
| LaNiO3 | Fundamental | Role of extended defects | 2017 | [43] |
| Pr0.5Ba0.5Mn0.85T0.15O3-δ | H2 SOFC | Exsolution in layered perovskites | 2017 | [69] |
| Sr0.95Ag0.05Nb0.1Co0.9O3−δ | LT-SOFC | Oxygen reduction at low temperature | 2016 | [70] |
| Co-doped Pr0.5Ba0.5MnOx | SOFC/SOEC | High population, dual use | 2016 | [71] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosen, B.A. Progress and Opportunities for Exsolution in Electrochemistry. Electrochem 2020, 1, 32-43. https://doi.org/10.3390/electrochem1010004
Rosen BA. Progress and Opportunities for Exsolution in Electrochemistry. Electrochem. 2020; 1(1):32-43. https://doi.org/10.3390/electrochem1010004
Chicago/Turabian StyleRosen, Brian A. 2020. "Progress and Opportunities for Exsolution in Electrochemistry" Electrochem 1, no. 1: 32-43. https://doi.org/10.3390/electrochem1010004
APA StyleRosen, B. A. (2020). Progress and Opportunities for Exsolution in Electrochemistry. Electrochem, 1(1), 32-43. https://doi.org/10.3390/electrochem1010004





