A Miniature pH Probe Using Functional Microfiber Bragg Grating †
Abstract
1. Introduction
2. Methods and Principle
2.1. Materials
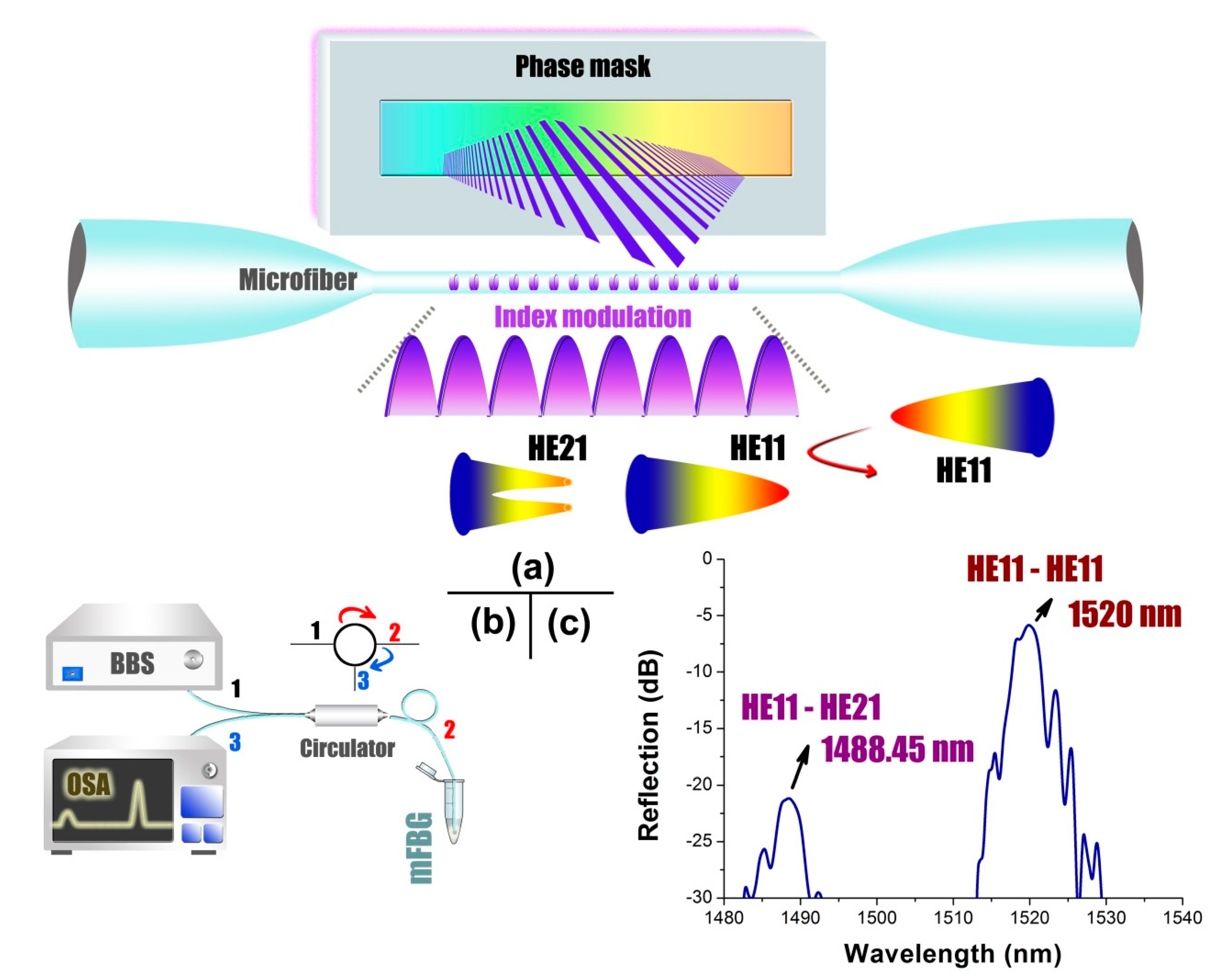
2.2. Fabrication of Microfiber Bragg Grating
2.3. RI-Response-Characterization of Microfiber Bragg Grating
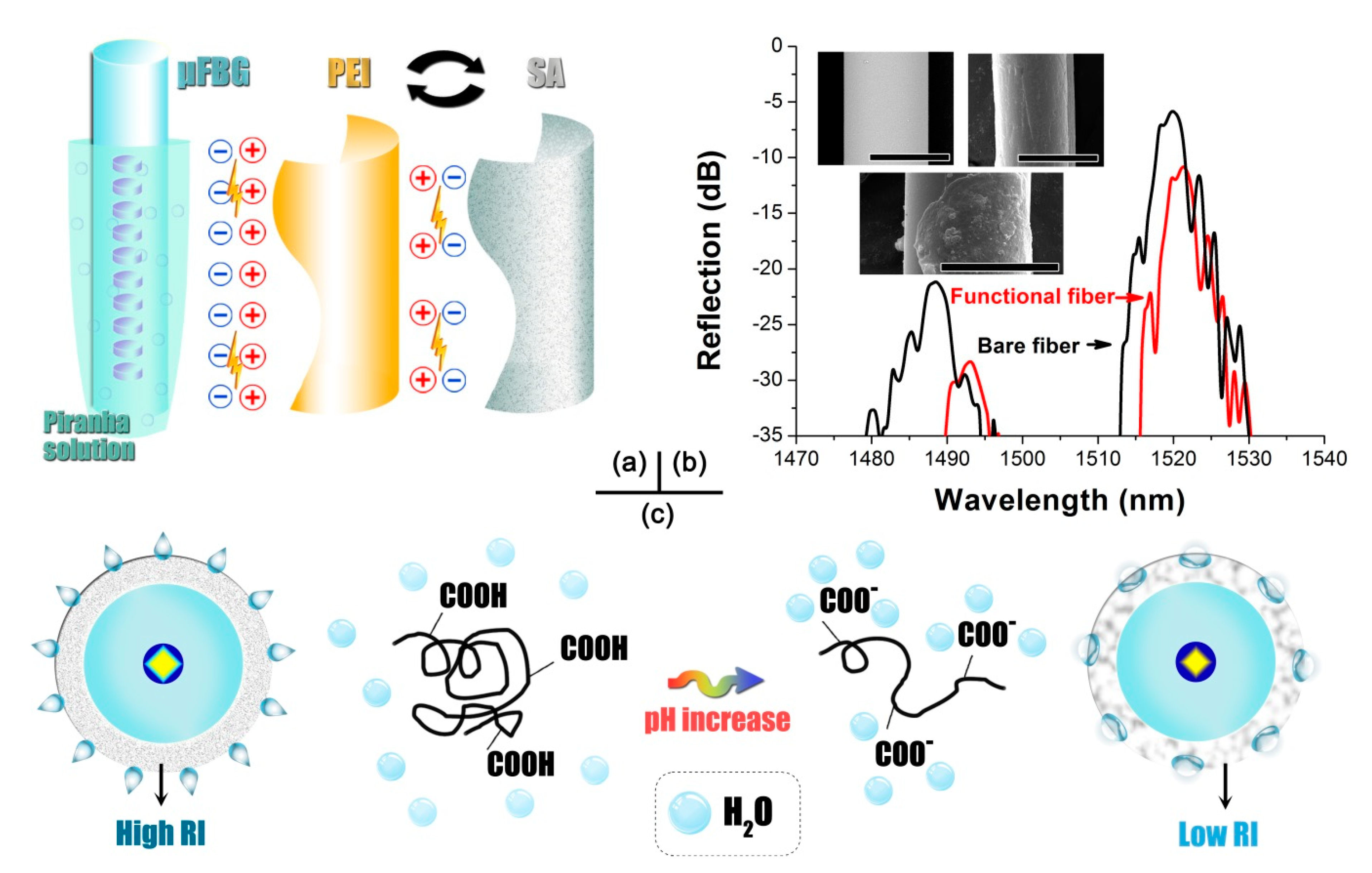
2.4. Functionalization of Microfiber Bragg Grating for pH Sensing
- Prepare the piranha solution by mixing the 98% sulfuric acid and 30% hydrogen peroxide with a volume ratio of 4:1. Dissolve the SA and PEI with DI water to an identical density of 0.01 mol/L.
- Immerse the μFBG into the piranha solution for 1 h to endow the silica surface with abundant negative charges. Rinse the μFBG with DI water several times to eliminate the residual piranha solution.
- Move the μFBG into the PEI solution. After a standing of 15 min, the PEI layer would be anchored on the fiber through ironic adsorption and provides a positive charge. Rinse the positively charged μFBG with DI water several times to remove the residual PEI solution.
- Dip the μFBG into the SA solution, lasting for 15 min. The functional layer of SA could be immobilized and enable a negative charge. Rinse the functional μFBG with DI water several times to get rid of the unbind SA.
- Repeat Step 3 and Step 4 alternatively to obtain the required coating thickness. In this study, three double-layers were decorated on the μFBG surface.
- Dehumidify the functional device using an incubator that keeps a constant temperature of 70 °C and humidity of 30% for 12 h, and a pH-sensitive μFBG was ready for service.
3. Results and Discussion
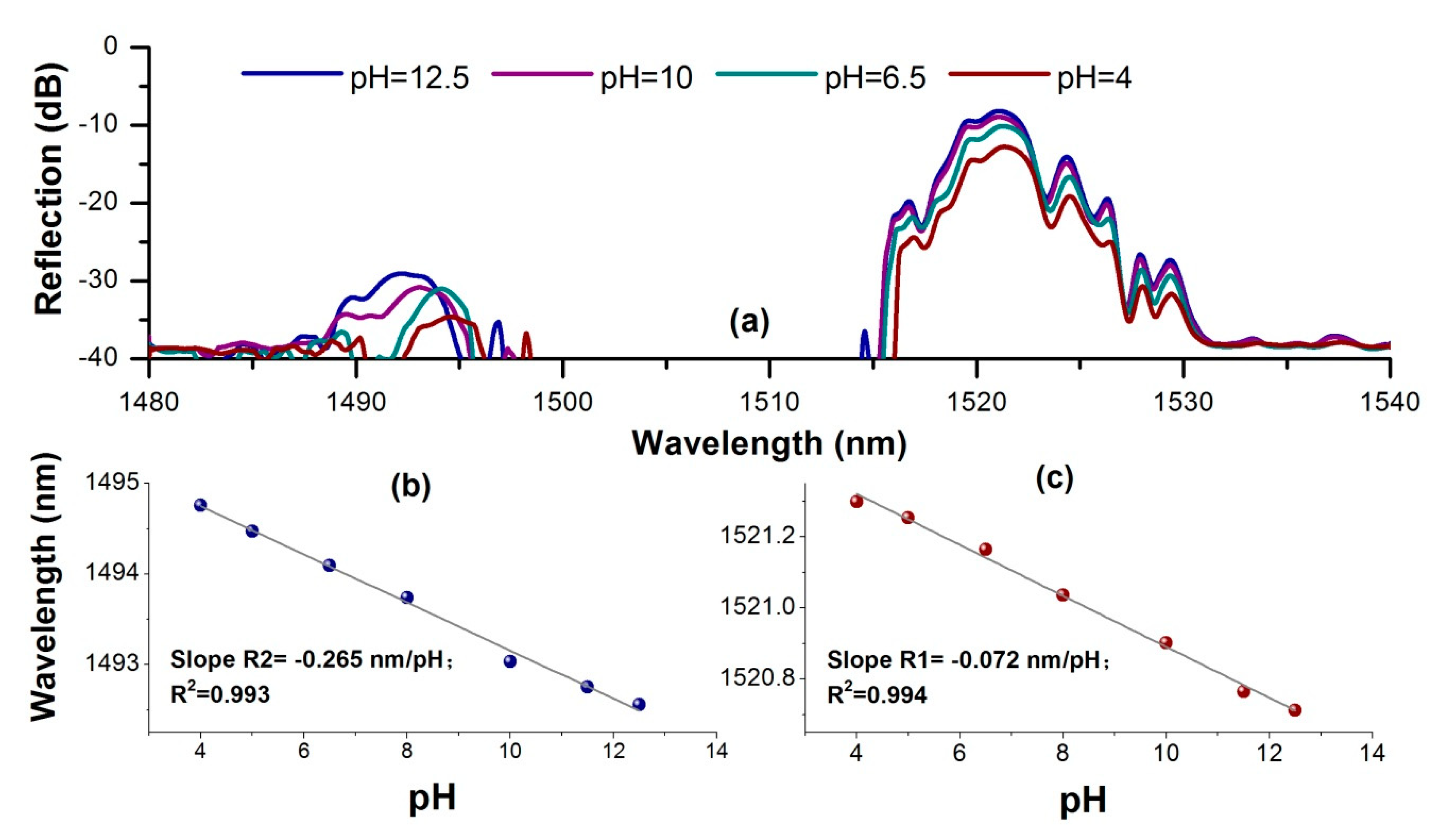
3.1. pH Sensing Characterization
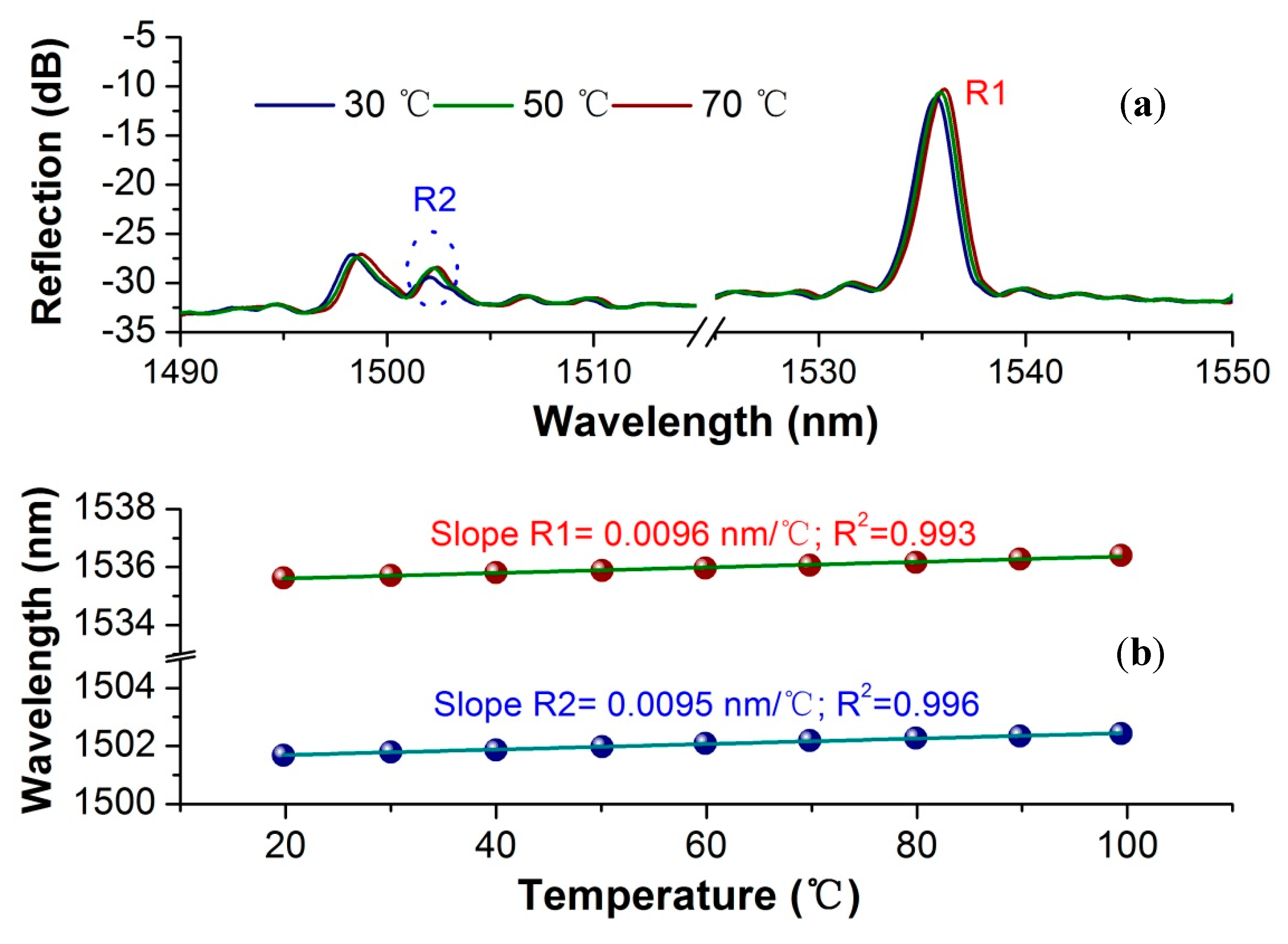
3.2. Temperature Cross-Sensitivity Characterization
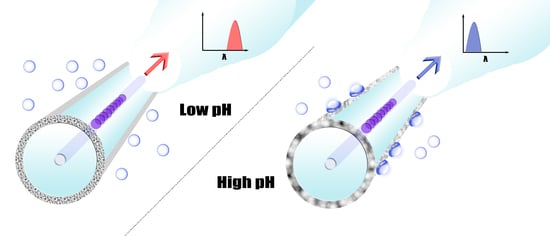
3.3. Model Analysis for Improving pH Sensitivity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chiavaioli, F.; Gouveia, A.J.C.; Jorge, A.S.P.; Baldini, F. Towards a Uniform Metrological Assessment of Grating-Based Optical Fiber Sensors: From Refractometers to Biosensors. Biosensors 2017, 7, 23. [Google Scholar] [CrossRef]
- Socorro-Leránoz, A.B.; Santano, D.; Del Villar, I.; Matias, I.R. Trends in the design of wavelength-based optical fibre biosensors (2008–2018). Biosens. Bioelectron. X 2019, 1, 100015. [Google Scholar] [CrossRef]
- Wang, X.-D.; Wolfbeis, O.S. Fiber-Optic Chemical Sensors and Biosensors (2015–2019). Anal. Chem. 2020, 92, 397–430. [Google Scholar] [CrossRef] [PubMed]
- Scaffidi, J.P.; Gregas, M.K.; Seewaldt, V.; Vo-Dinh, T. SERS-based plasmonic nanobiosensing in single living cells. Anal. Bioanal. Chem. 2009, 393, 1135–1141. [Google Scholar] [CrossRef]
- Wang, J.; Geng, Y.; Shen, Y.; Shi, W.; Xu, W.; Xu, S. SERS-active fiber tip for intracellular and extracellular pH sensing in living single cells. Sens. Actuators B Chem. 2019, 290, 527–534. [Google Scholar] [CrossRef]
- Wencel, D.; Kaworek, A.; Abel, T.; Efremov, V.; Bradford, A.; Carthy, D.; Coady, G.; McMorrow, R.C.N.; McDonagh, C. Optical Sensor for Real-Time pH Monitoring in Human Tissue. Small 2018, 14, 1803627. [Google Scholar] [CrossRef] [PubMed]
- Elsherif, M.; Moreddu, R.; Hassan, M.U.; Yetisen, A.K.; Butt, H. Real-time optical fiber sensors based on light diffusing microlens arrays. Lab Chip 2019, 19, 2060–2070. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-H.; Hsieh, J.-C.; Chen, C.-C.; Zan, H.-W.; Meng, H.-F.; Kuo, S.-Y.; Nguyễn, M.T.N. A low-cost, portable and easy-operated salivary urea sensor for point-of-care application. Biosens. Bioelectron. 2019, 132, 352–359. [Google Scholar] [CrossRef]
- Khan, R.M.; Kang, S.-W. Highly Sensitive and Wide-Dynamic-Range Multichannel Optical-Fiber pH Sensor Based on PWM Technique. Sensors 2016, 16. [Google Scholar] [CrossRef]
- Gu, B.; Yin, M.-J.; Zhang, A.P.; Qian, J.-W.; He, S. Low-cost high-performance fiber-optic pH sensor based on thin-core fiber modal interferometer. Opt. Express 2009, 17, 22296–22302. [Google Scholar] [CrossRef]
- Gu, B.; Yin, M.; Zhang, A.P.; Qian, J.; He, S. Biocompatible Fiber-Optic pH Sensor Based on Optical Fiber Modal Interferometer Self-Assembled With Sodium Alginate/Polyethylenimine Coating. IEEE Sens. J. 2012, 12, 1477–1482. [Google Scholar] [CrossRef]
- Zhao, Q.; Yin, M.; Zhang, A.P.; Prescher, S.; Antonietti, M.; Yuan, J. Hierarchically Structured Nanoporous Poly(Ionic Liquid) Membranes: Facile Preparation and Application in Fiber-Optic pH Sensing. J. Am. Chem. Soc. 2013, 135, 5549–5552. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Albri, F.; Maier, R.R.J.; Shu, W.; Sun, J.; Hand, D.P.; MacPherson, W.N. A Micro-Machined Optical Fiber Cantilever as a Miniaturized pH Sensor. IEEE Sens. J. 2015, 15, 7221–7228. [Google Scholar] [CrossRef]
- Pathak, A.K.; Chaudhary, D.K.; Singh, V.K. Broad range and highly sensitive optical pH sensor based on Hierarchical ZnO microflowers over tapered silica fiber. Sens. Actuators A Phys. 2018, 280, 399–405. [Google Scholar] [CrossRef]
- Zamarreño, C.R.; Hernáez, M.; Del Villar, I.; Matías, I.R.; Arregui, F.J. Optical fiber pH sensor based on lossy-mode resonances by means of thin polymeric coatings. Sens. Actuators B Chem. 2011, 155, 290–297. [Google Scholar] [CrossRef]
- Rivero, P.J.; Goicoechea, J.; Hernaez, M.; Socorro, A.B.; Matias, I.R.; Arregui, F.J. Optical fiber resonance-based pH sensors using gold nanoparticles into polymeric layer-by-layer coatings. Microsyst. Technol. 2016, 22, 1821–1829. [Google Scholar] [CrossRef]
- Yin, M.-J.; Yao, M.; Gao, S.; Zhang, A.P.; Tam, H.-Y.; Wai, P.-K.A. Rapid 3D Patterning of Poly(acrylic acid) Ionic Hydrogel for Miniature pH Sensors. Adv. Mater. 2016, 28, 1394–1399. [Google Scholar] [CrossRef]
- Mishra, S.K.; Zou, B.; Chiang, K.S. Wide-Range pH Sensor Based on a Smart- Hydrogel-Coated Long-Period Fiber Grating. IEEE J. Quantum Elect. 2017, 23, 284–288. [Google Scholar] [CrossRef]
- Ni, Y.-Q.; Ding, S.; Han, B.; Wang, H. Layer-by-layer assembly of polyelectrolytes-wrapped multi-walled carbon nanotubes on long period fiber grating sensors. Sens. Actuators B Chem. 2019, 301, 127120. [Google Scholar] [CrossRef]
- Hartings, M.R.; Castro, N.J.; Gill, K.; Ahmed, Z. A photonic pH sensor based on photothermal spectroscopy. Sens. Actuators B Chem. 2019, 301, 127076. [Google Scholar] [CrossRef]
- Lopez Aldaba, A.; González-Vila, Á.; Debliquy, M.; Lopez-Amo, M.; Caucheteur, C.; Lahem, D. Polyaniline-coated tilted fiber Bragg gratings for pH sensing. Sens. Actuators B Chem. 2018, 254, 1087–1093. [Google Scholar] [CrossRef]
- Cheng, X.; Bonefacino, J.; Guan, B.O.; Tam, H.Y. All-polymer fiber-optic pH sensor. Opt. Express 2018, 26, 14610–14616. [Google Scholar] [CrossRef] [PubMed]
- Janting, J.; Pedersen, J.K.M.; Woyessa, G.; Nielsen, K.; Bang, O. Small and Robust All-Polymer Fiber Bragg Grating Based pH Sensor. J. Lightwave Technol. 2019, 37, 4480–4486. [Google Scholar] [CrossRef]
- Tong, L.; Gattass, R.R.; Ashcom, J.B.; He, S.; Lou, J.; Shen, M.; Maxwell, I.; Mazur, E. Subwavelength-diameter silica wires for low-loss optical wave guiding. Nature 2003, 426, 816. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, G. Optical fibre nanowires and microwires: A review. J. Opt. 2010, 12, 043001. [Google Scholar] [CrossRef]
- Ismaeel, R.; Lee, T.; Ding, M.; Belal, M.; Brambilla, G. Optical microfiber passive components. Laser Photonics Rev. 2013, 7, 350–384. [Google Scholar] [CrossRef]
- Lou, J.; Wang, Y.; Tong, L. Microfiber Optical Sensors: A Review. Sensors 2014, 14. [Google Scholar] [CrossRef]
- Chen, J.; Li, D.; Xu, F. Optical Microfiber Sensors: Sensing Mechanisms, and Recent Advances. J. Lightwave Technol. 2019, 37, 2577–2589. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, Y.; Tong, L. Micro-/Nanofiber Optics: Merging Photonics and Material Science on Nanoscale for Advanced Sensing Technology. iScience 2020, 23, 100810. [Google Scholar] [CrossRef]
- Kou, J.-L.; Ding, M.; Feng, J.; Lu, Y.-Q.; Xu, F.; Brambilla, G. Microfiber-Based Bragg Gratings for Sensing Applications: A Review. Sensors 2012, 12. [Google Scholar] [CrossRef]
- Guan, B.-O.; Li, J.; Jin, L.; Ran, Y. Fiber Bragg gratings in optical microfibers. Opt. Fiber Technol. 2013, 19, 793–801. [Google Scholar] [CrossRef]
- Fang, X.; Liao, C.R.; Wang, D.N. Femtosecond laser fabricated fiber Bragg grating in microfiber for refractive index sensing. Opt. Lett. 2010, 35, 1007–1009. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; Tan, Y.-N.; Sun, L.-P.; Gao, S.; Li, J.; Jin, L.; Guan, B.-O. 193nm excimer laser inscribed Bragg gratings in microfibers for refractive index sensing. Opt. Express 2011, 19, 18577–18583. [Google Scholar] [CrossRef]
- Ran, Y.; Jin, L.; Tan, Y.N.; Sun, L.P.; Li, J.; Guan, B.O. High-efficiency ultraviolet inscription of Bragg gratings in microfibers. IEEE Photon. J. 2012, 4, 181–186. [Google Scholar] [CrossRef]
- Ran, Y.; Jin, L.; Sun, L.P.; Li, J.; Guan, B.O. Temperature-Compensated Refractive-Index Sensing Using a Single Bragg Grating in an Abrupt Fiber Taper. IEEE Photon. J. 2013, 5, 7100208. [Google Scholar] [CrossRef]
- Ran, Y.; Jin, L.; Sun, L.-P.; Li, J.; Guan, B.-O. Bragg gratings in rectangular microfiber for temperature independent refractive index sensing. Opt. Lett. 2012, 37, 2649–2651. [Google Scholar] [CrossRef]
- Sun, D.; Guo, T.; Ran, Y.; Huang, Y.; Guan, B.-O. In-situ DNA hybridization detection with a reflective microfiber grating biosensor. Biosens. Bioelectron. 2014, 61, 541–546. [Google Scholar] [CrossRef]
- Ran, Y.; Jin, L.; Gao, S.; Sun, L.-P.; Huang, Y.-Y.; Li, J.; Guan, B.-O. Type IIa Bragg gratings formed in microfibers. Opt. Lett. 2015, 40, 3802–3805. [Google Scholar] [CrossRef]
- Yu, Z.; Jin, L.; Chen, L.; Li, J.; Ran, Y.; Guan, B. Microfiber Bragg Grating Hydrogen Sensors. IEEE Photon. Technol. Lett. 2015, 27, 2575–2578. [Google Scholar] [CrossRef]
- Xiao, P.; Liu, T.; Feng, F.-R.; Sun, L.-P.; Liang, H.; Ran, Y.; Jin, L.; Guan, B.-O. Spectral tuning of the diameter-dependent-chirped Bragg gratings written in microfibers. Opt. Express 2016, 24, 29749–29759. [Google Scholar] [CrossRef]
- Liu, T.; Liang, L.-L.; Xiao, P.; Sun, L.-P.; Huang, Y.-Y.; Ran, Y.; Jin, L.; Guan, B.-O. A label-free cardiac biomarker immunosensor based on phase-shifted microfiber Bragg grating. Biosens. Bioelectron. 2018, 100, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; Long, J.; Xu, Z.; Hu, D.; Guan, B.-O. Temperature monitorable refractometer of microfiber Bragg grating using a duet of harmonic resonances. Opt. Lett. 2019, 44, 3186–3189. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Yang, K.; Wang, J.; Bai, Z.; Gan, Z.; Wang, Y. Helical Microfiber Bragg Grating Printed by Femtosecond Laser for Refractive Index Sensing. IEEE Photon. Technol. Lett. 2019, 31, 971–974. [Google Scholar] [CrossRef]
- Ran, Y.; Huang, Y.; Shen, X.; Sun, D.; Wang, X.; Jin, L.; Li, J.; Guan, B. Biofuncationalized microfiber Bragg grating for acid-based sensing. SPIE 2014, 9157, 915742. [Google Scholar]
- Blandino, A.; Macías, M.; Cantero, D. Glucose oxidase release from calcium alginate gel capsules. Enzyme Microb. Tech. 2000, 27, 319–324. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232. [Google Scholar] [CrossRef]
- Ju, H.K.; Kim, S.Y.; Lee, Y.M. pH/temperature-responsive behaviors of semi-IPN and comb-type graft hydrogels composed of alginate and poly(N-isopropylacrylamide). Polymer 2001, 42, 6851–6857. [Google Scholar] [CrossRef]
- Yuan, W.; Dong, H.; Li, C.M.; Cui, X.; Yu, L.; Lu, Z.; Zhou, Q. pH-Controlled Construction of Chitosan/Alginate Multilayer Film: Characterization and Application for Antibody Immobilization. Langmuir 2007, 23, 13046–13052. [Google Scholar] [CrossRef]
- González-Vila, Á.; Debliquy, M.; Lahem, D.; Zhang, C.; Mégret, P.; Caucheteur, C. Molecularly imprinted electropolymerization on a metal-coated optical fiber for gas sensing applications. Sens. Actuators B Chem. 2017, 244, 1145–1151. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, X.; Luo, B.; Pan, W.; Yan, L.; Peng, W. Optically functionalized microfiber Bragg grating for RH sensing. Opt. Lett. 2019, 44, 4646–4649. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ran, Y.; Xiao, P.; Zhang, Y.; Hu, D.; Xu, Z.; Liang, L.; Guan, B.-O. A Miniature pH Probe Using Functional Microfiber Bragg Grating. Optics 2020, 1, 202-212. https://doi.org/10.3390/opt1020016
Ran Y, Xiao P, Zhang Y, Hu D, Xu Z, Liang L, Guan B-O. A Miniature pH Probe Using Functional Microfiber Bragg Grating. Optics. 2020; 1(2):202-212. https://doi.org/10.3390/opt1020016
Chicago/Turabian StyleRan, Yang, Peng Xiao, Yongkang Zhang, Deming Hu, Zhiyuan Xu, Lili Liang, and Bai-Ou Guan. 2020. "A Miniature pH Probe Using Functional Microfiber Bragg Grating" Optics 1, no. 2: 202-212. https://doi.org/10.3390/opt1020016
APA StyleRan, Y., Xiao, P., Zhang, Y., Hu, D., Xu, Z., Liang, L., & Guan, B.-O. (2020). A Miniature pH Probe Using Functional Microfiber Bragg Grating. Optics, 1(2), 202-212. https://doi.org/10.3390/opt1020016