A First Step towards Determining the Ionic Content in Water with an Integrated Optofluidic Chip Based on Near-Infrared Absorption Spectroscopy
Abstract
1. Introduction
1.1. The Relevance of Detecting Ionic Content in Water
1.1.1. Government Sector
1.1.2. Industry
1.2. Choice of Spectral Region
1.3. Method of Detection
1.4. Integrated Optofluidic Chips
2. Simulated Device Performance
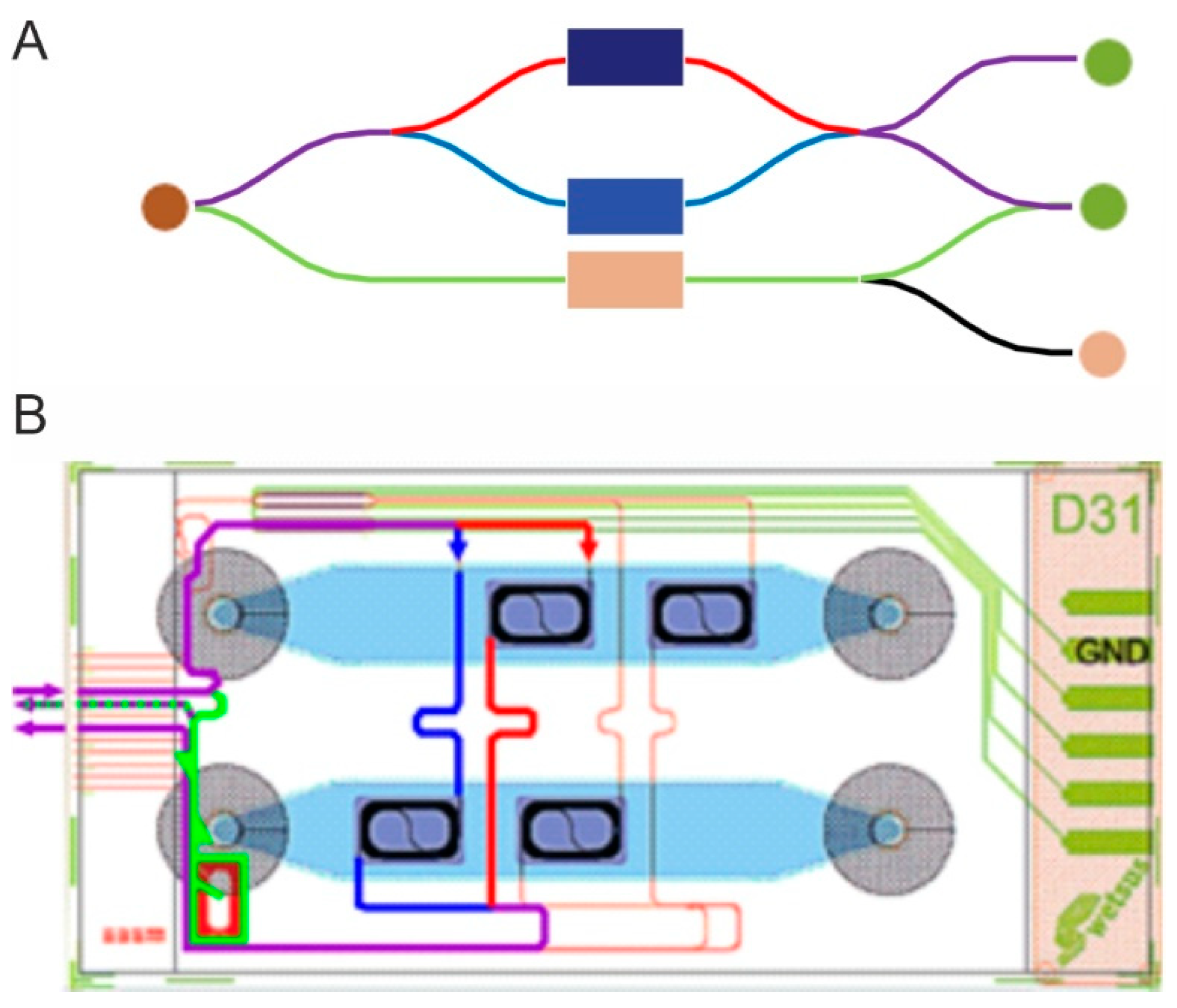
2.1. Mach–Zehnder Interferometer (MZI)
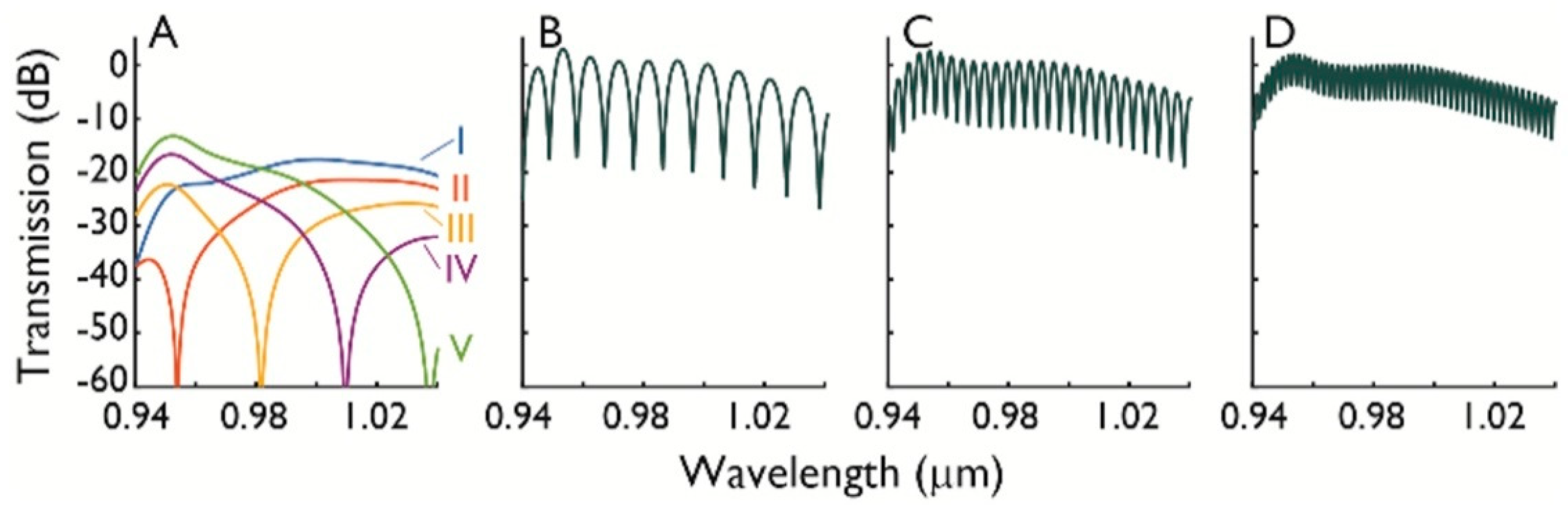
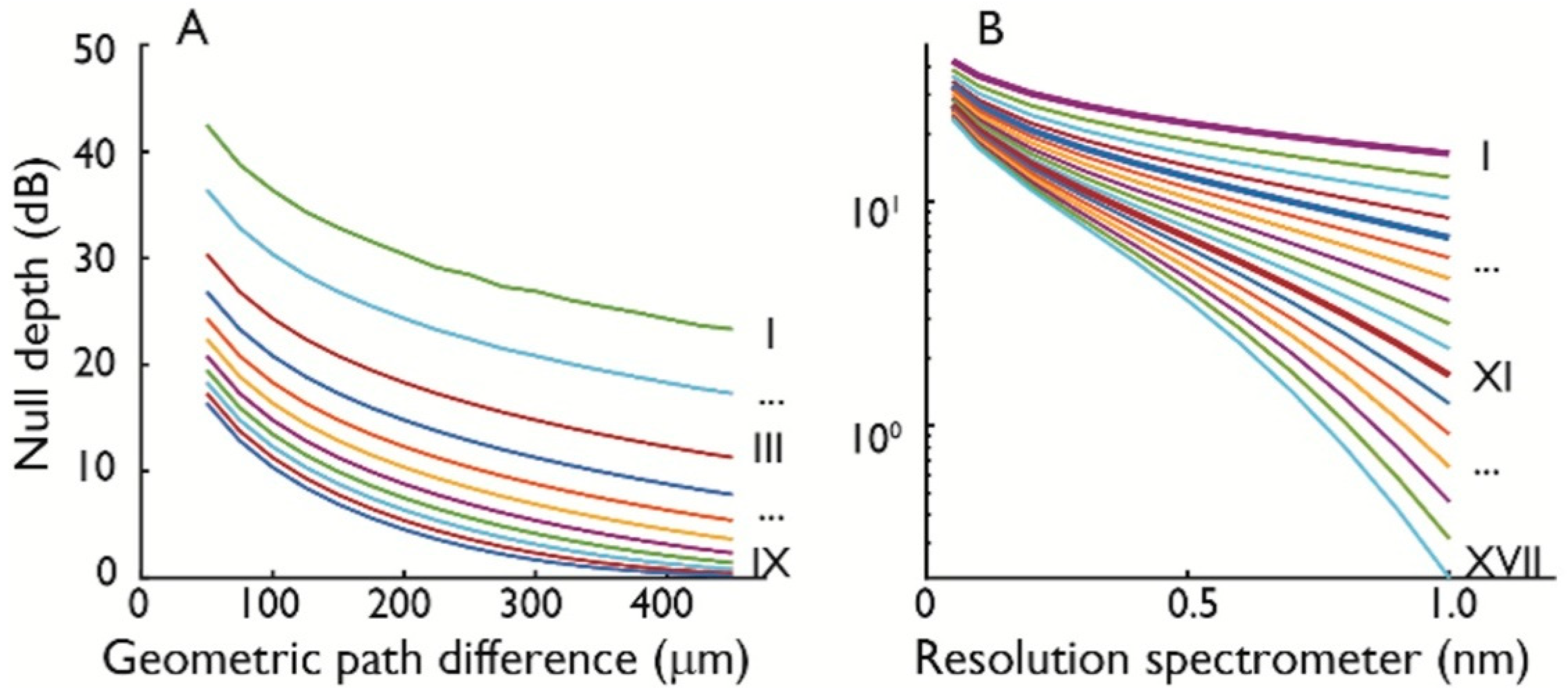
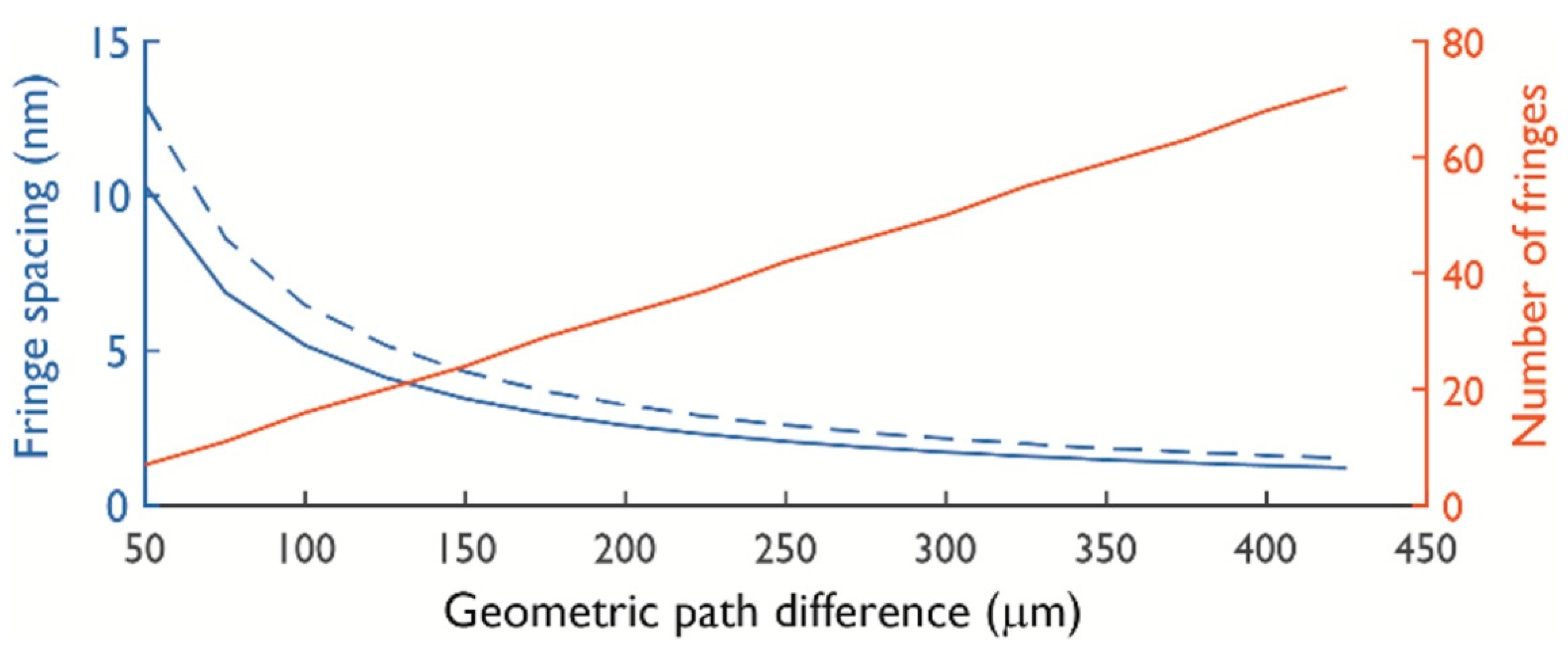
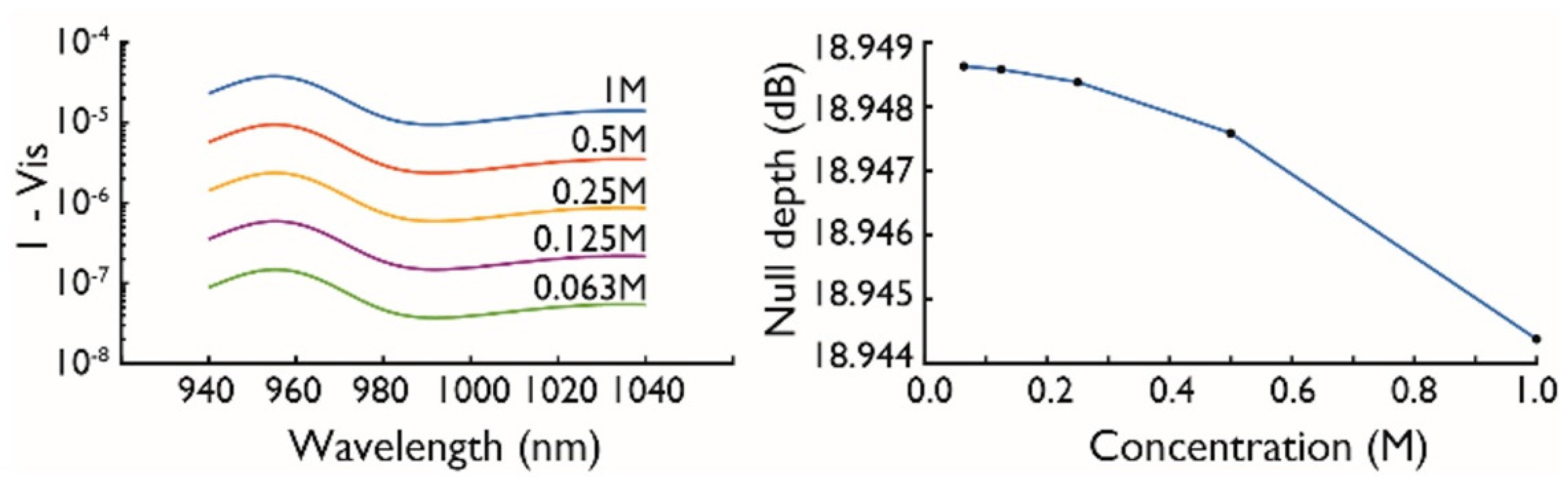
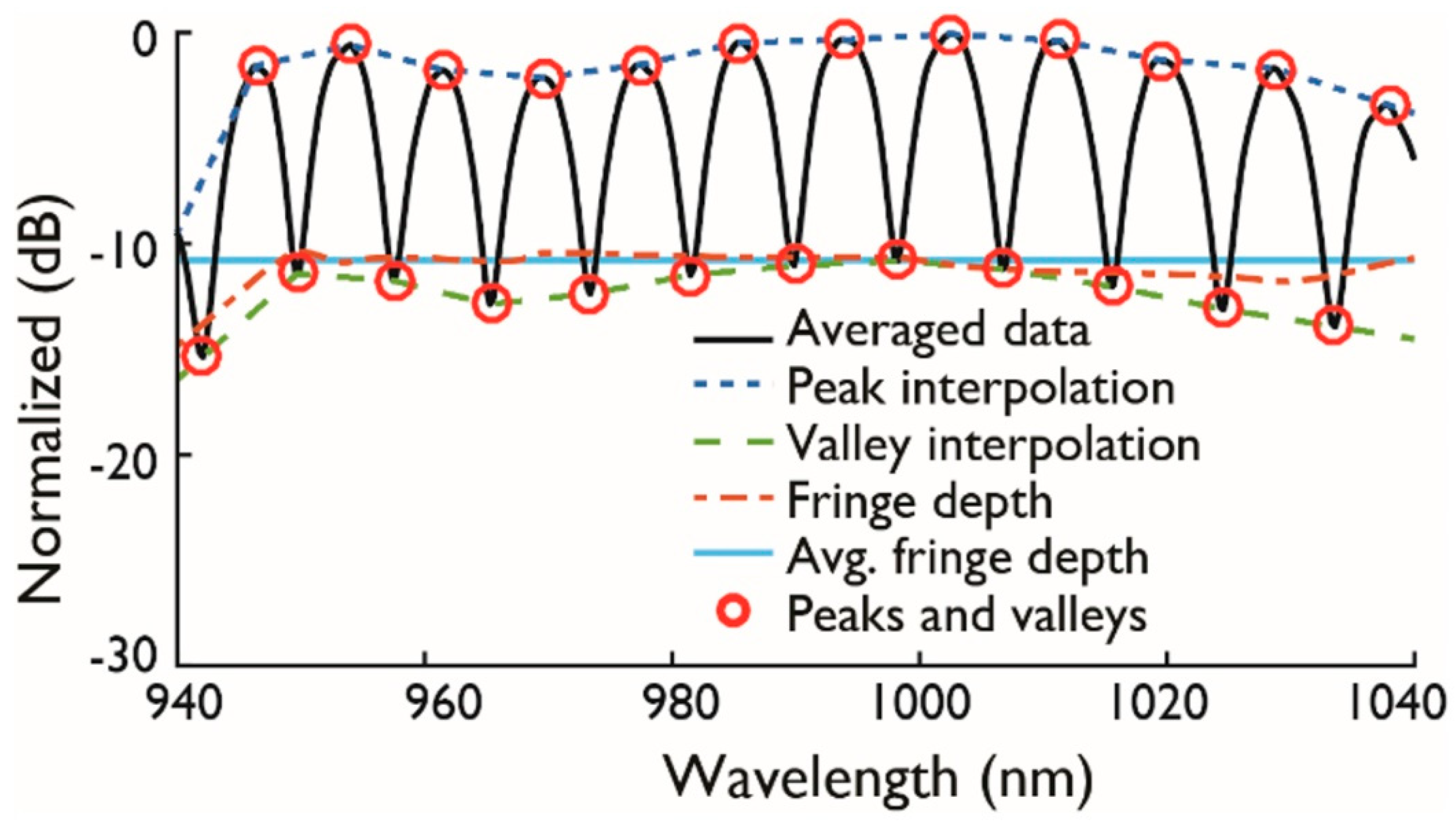
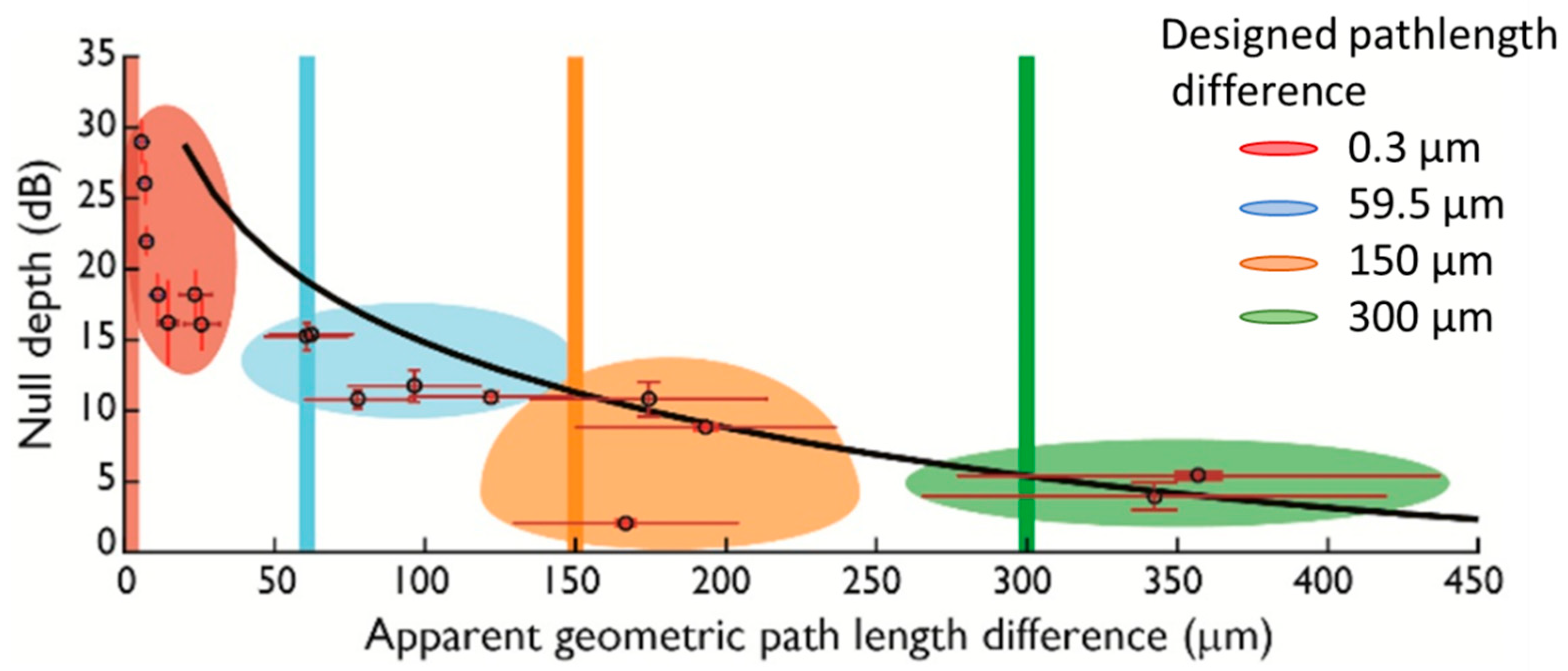
2.2. Null Depth and Fringe Spacing
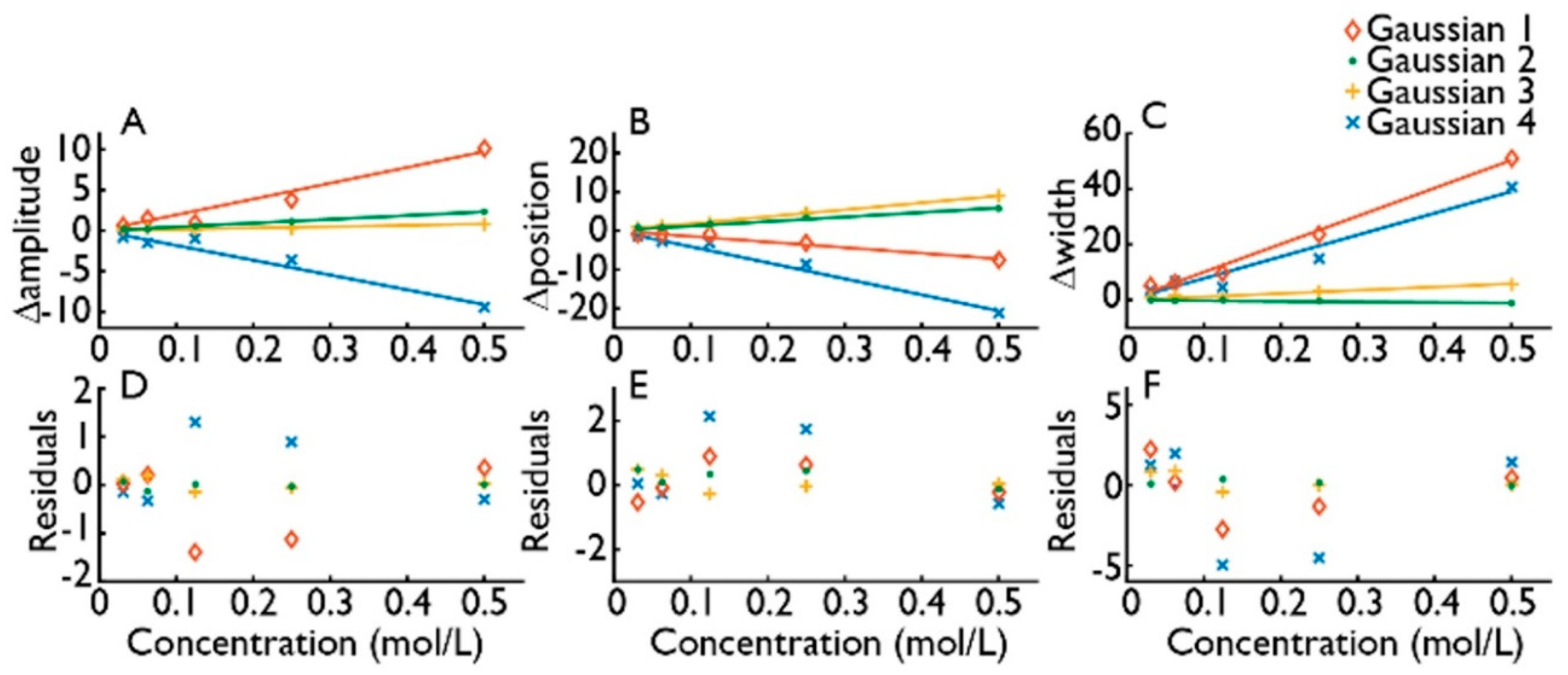
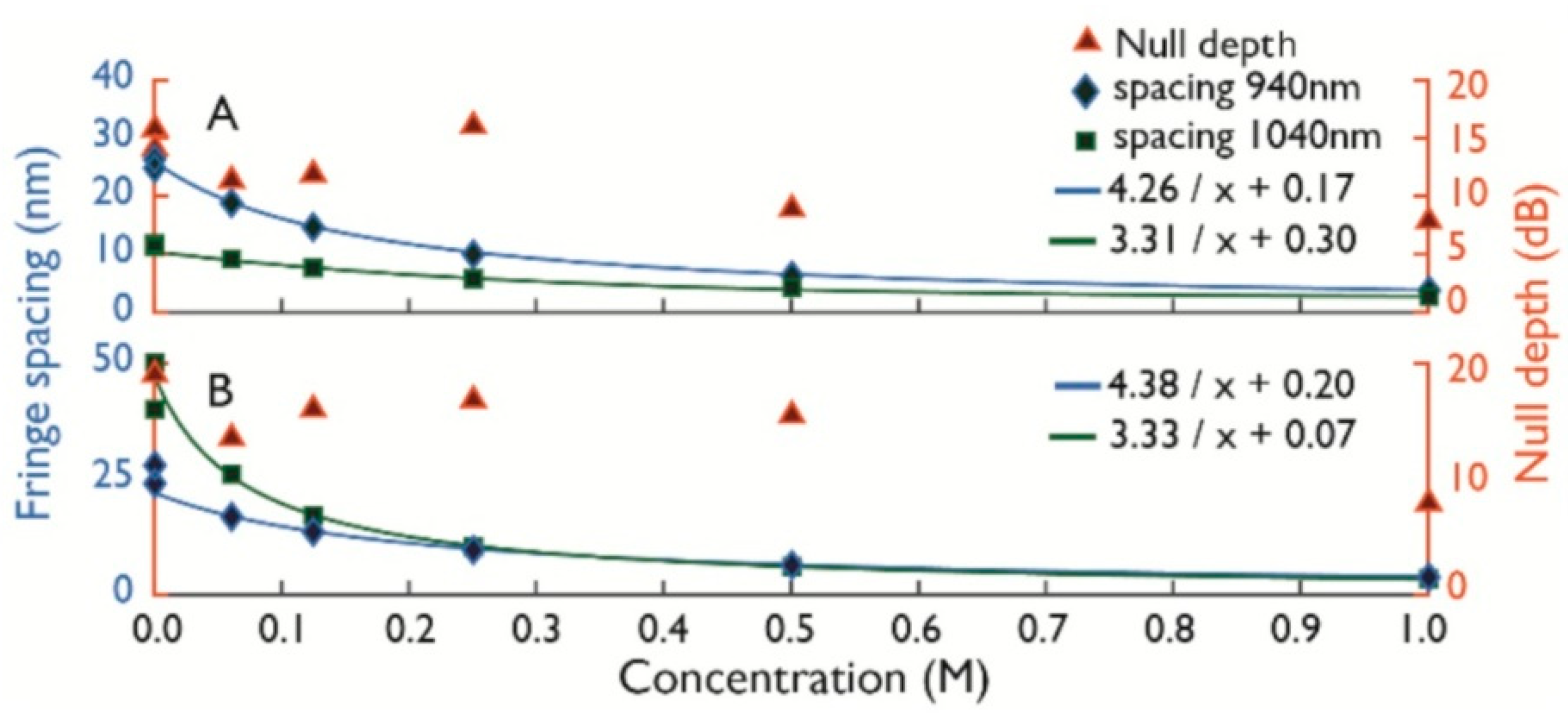
2.3. Differential Absorbance
3. Experimental Testing and Validation
3.1. Methodology
3.1.1. Chip Testing Hardware
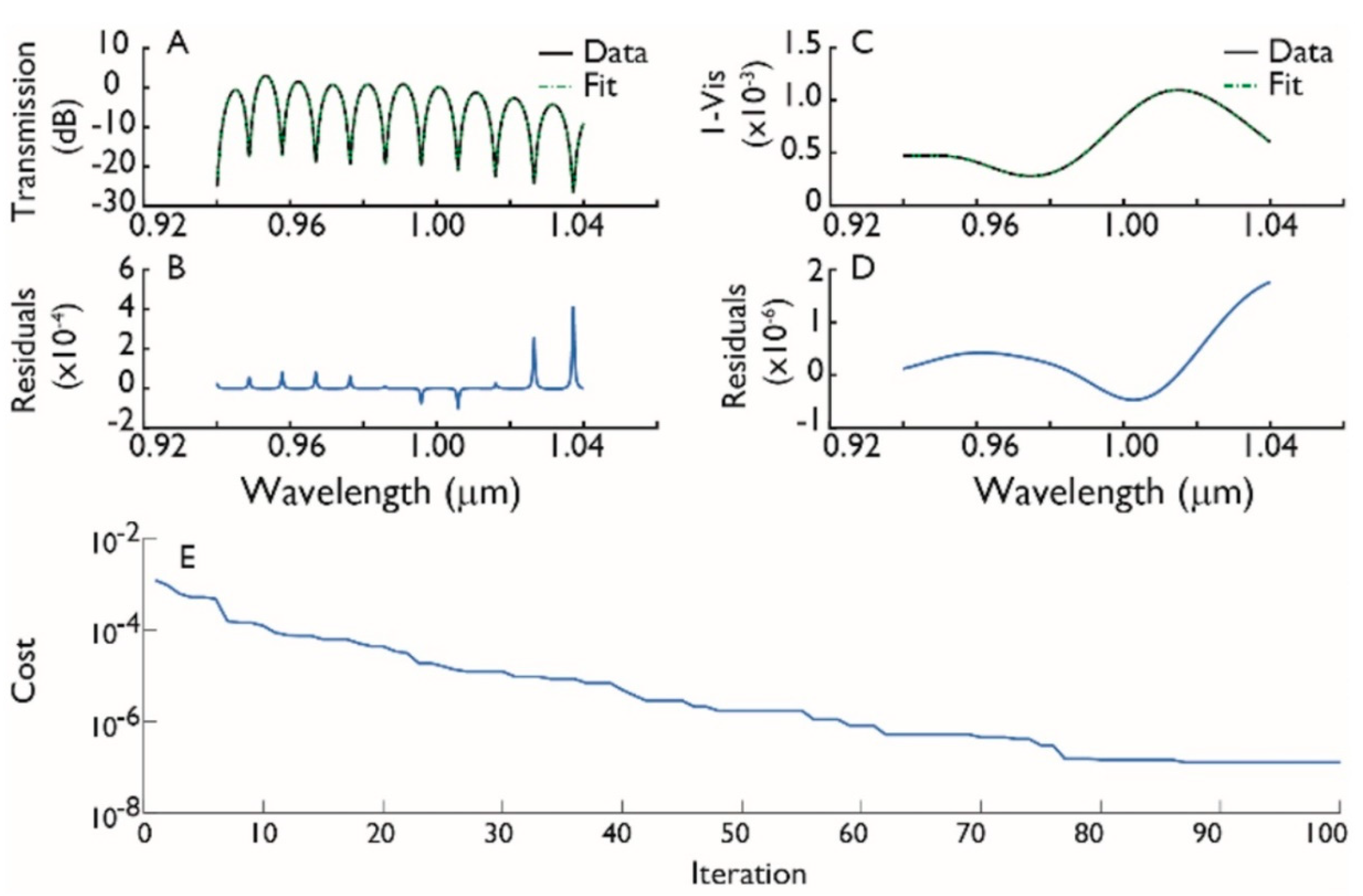
3.1.2. Chip Validation Procedure
4. Experimental Results and Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Plant, J.A.; Voulvoulis, N.; Ragnarsdottir, K.V. (Eds.) Pollutants, Human Health and the Environment: A Risk Based Approach; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Argos, M.; Kalra, T.; Rathouz, P.J.; Chen, Y.; Pierce, B.; Parvez, F.; Sarwar, G. Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): A prospective cohort study. Lancet 2010, 376, 252–258. [Google Scholar] [CrossRef]
- Knobeloch, L.; Salna, B.; Hogan, A.; Postle, J.; Anderson, H. Blue babies and nitrate-contaminated well water. Environ. Health Perspect. 2000, 108, 675. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Puri, A. A review of permissible limits of drinking water. Indian J. Occup. Environ. Med. 2012, 16, 40. [Google Scholar] [PubMed]
- World Health Organization, WHO/UNICEF Joint Water Supply, & Sanitation Monitoring Programme. Progress on Sanitation and Drinking Water: 2015 Update and MDG Assessment; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Alblasserdamsnieuws (Online-Newspaper), Ook Genx Aangetroffen in Alblasserdams Leidingwater. Available online: http://www.alblasserdamsnieuws.nl/wordpress/2017/04/21/ook-genx-aangetroffen-in-alblasserdams-leidingwater-d66-wil-landelijke-aandacht (accessed on 12 July 2017).
- AD (Online-Newspaper), Bron van Drinkwater in Deel Randstad Vervuild Met Gif. Available online: http://www.ad.nl/nieuws/bron-van-drinkwater-in-deel-randstad-vervuild-met-gif~a3350191/ (accessed on 12 July 2017).
- Huisman, J. Ziektegevallen na Verontreiniging van Drinkwater Met Fenol; een Engelse Ervaring. H twee O Tijdschr. Watervoorzien. Afvalw. 1985, 18, 433. [Google Scholar]
- Coussens, C. (Ed.) Global Environmental Health: Research Gaps and Barriers for Providing Sustainable Water, Sanitation, and Hygiene Services: Workshop Summary; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Reynolds, K.A.; Mena, K.D.; Gerba, C.P. Risk of waterborne illness via drinking water in the United States. In Reviews of Environmental Contamination and Toxicology; Springer New York: New York, NY, USA, 2008; pp. 117–158. [Google Scholar]
- Parnell, G.S.; Smith, C.M.; Moxley, F.I. Intelligent adversary risk analysis: A bioterrorism risk management model. Risk Anal. 2010, 30, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.C. Bacteria as potential tools in bioterrorism, with an emphasis on bacterial toxins. Br. J. Biomed. Sci. 2005, 62, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Gleick, P.H. Water and terrorism. Water Policy 2006, 8, 481–503. [Google Scholar] [CrossRef]
- Pujol, L.; Evrard, D.; Groenen-Serrano, K.; Freyssinier, M.; Ruffien-Cizsak, A.; Gros, P. Electrochemical sensors and devices for heavy metals assay in water: The French groups’ contribution. Front. Chem. 2014, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Bodini, M.E.; Sawyer, D.T. Voltammetric determination of nitrate ion at parts-per-billion levels. Anal. Chem. 1977, 49, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Murrihy, J.P.; Breadmore, M.C.; Tan, A.; McEnery, M.; Alderman, J.; O’Mathuna, C.; Glennon, J.D. Ion chromatography on-chip. J. Chromatogr. A 2001, 924, 233–238. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, C.C. A simple, cost-effective sensor for detecting lead ions in water using under-potential deposited bismuth sub-layer with differential pulse voltammetry (DPV). Sensors 2017, 17, 950. [Google Scholar]
- Chaplin. Available online: http://www1.lsbu.ac.uk/water/water_vibrational_spectrum.html (accessed on 21 June 2018).
- Steen, G.W.; Fuchs, E.C.; Wexler, A.D.; Offerhaus, H.L. Design Considerations to Realize Differential Absorption-Based Optofluidic Sensors for Determination of Ionic Content in Water. IEEE Sens. J. 2018, 18, 6051–6058. [Google Scholar] [CrossRef]
- Zhuang, L.; Marpaung, D.A.I.; Burla, M.; Beeker, W.; Beeker, W.P.; Leinse, A.; Roeloffzen, C.G.H. Low-loss, high-index-contrast Si3N4SiO2 optical waveguides for optical delay lines in microwave photonics signal processing. Opt. Express 2011, 19, 23162–23170. [Google Scholar] [CrossRef] [PubMed]
- Steen, G.W.; Fuchs, E.C.; Wexler, A.D.; Offerhaus, H.L. Identification and quantification of 16 inorganic ions in water by Gaussian curve fitting of near-infrared difference absorbance spectra. Appl. Opt. 2015, 54, 5937–5942. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roeloffzen, C.G.H.; Hoekman, M.; Klein, E.J.; Wevers, L.S.; Timens, R.B.; Marchenko, D.; Geskus, D.; Dekker, R.; Alippi, A.; Grootjans, R.; et al. Low-Loss Si3N4 TriPleX Optical Waveguides: Technology and Applications Overview. IEEE J. Sel. Top. Quant. Electr. 2018, 24, 4400321. [Google Scholar] [CrossRef]
- Steen, G.W.; Wexler, A.D.; Fuchs, E.C.; Bakker, H.A.; Nguyen, P.D.; Offerhaus, H.L. Role of temperature in de-mixing absorbance spectra composed of compound electrolyte solutions. Appl. Opt. 2018, 57, 7871–7877. [Google Scholar] [CrossRef] [PubMed]
- Pissadakis, S.; Selleri, S. (Eds.) Optofluidics, Sensors and Actuators in Microstructured Optical Fibers; Woodhead Publishing: Sawston, UK, 2015. [Google Scholar]
- Fainman, Y.; Lee, L.; Psaltis, D.; Yang, C. Optofluidics: Fundamentals, Devices, and Applications; McGraw-Hill, Inc.: New York, NY, USA, 2009. [Google Scholar]
- Hlubina, P. Dispersive white-light spectral two-beam interference under general measurement conditions. Opt. Int. J. Light Electron Opt. 2003, 114, 185–190. [Google Scholar] [CrossRef]
- Eberhart, R.; Kennedy, J. A new optimizer using particle swarm theory. In Micro Machine and Human Science, 1995. MHS’95, Proceedings of the Sixth International Symposium on Micro Machine and Human Science, Nagoya, Japan, 4–6 October 1995; IEEE: Piscataway Township, NJ, USA, 1995; pp. 39–43. [Google Scholar]
- Clerc, M.; Kennedy, J. The particle swarm-explosion, stability, and convergence in a multidimensional complex space. IEEE Trans. Evol. Comput. 2002, 6, 58–73. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steen, G.W.; Wexler, A.D.; Fuchs, E.C.; Offerhaus, H.L. A First Step towards Determining the Ionic Content in Water with an Integrated Optofluidic Chip Based on Near-Infrared Absorption Spectroscopy. Optics 2020, 1, 175-190. https://doi.org/10.3390/opt1020014
Steen GW, Wexler AD, Fuchs EC, Offerhaus HL. A First Step towards Determining the Ionic Content in Water with an Integrated Optofluidic Chip Based on Near-Infrared Absorption Spectroscopy. Optics. 2020; 1(2):175-190. https://doi.org/10.3390/opt1020014
Chicago/Turabian StyleSteen, Gerwin W., Adam D. Wexler, Elmar C. Fuchs, and Herman L. Offerhaus. 2020. "A First Step towards Determining the Ionic Content in Water with an Integrated Optofluidic Chip Based on Near-Infrared Absorption Spectroscopy" Optics 1, no. 2: 175-190. https://doi.org/10.3390/opt1020014
APA StyleSteen, G. W., Wexler, A. D., Fuchs, E. C., & Offerhaus, H. L. (2020). A First Step towards Determining the Ionic Content in Water with an Integrated Optofluidic Chip Based on Near-Infrared Absorption Spectroscopy. Optics, 1(2), 175-190. https://doi.org/10.3390/opt1020014





