Investigation of Five Organic Dyes in Ethanol and Butanol for Two-Color Laser-Induced Fluorescence Ratio Thermometry
Abstract
1. Introduction
2. Experimental Procedure
2.1. Properties of Organic Dyes
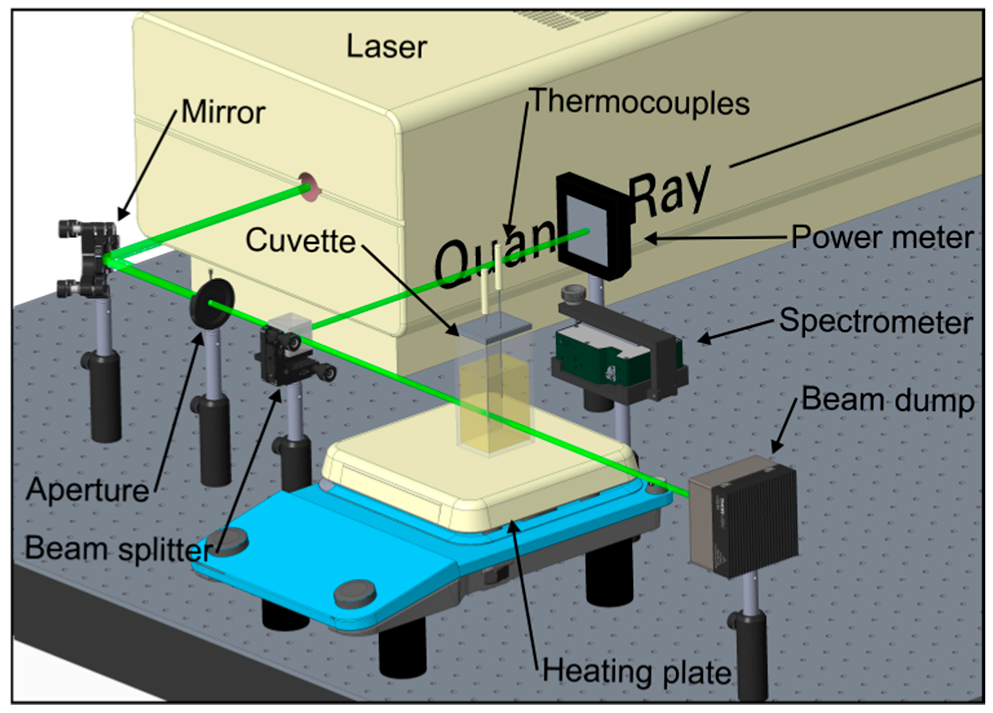
2.2. Measurement Setup
2.2.1. Absorbance Measurements
2.2.2. Temperature-Dependent Fluorescence Measurements
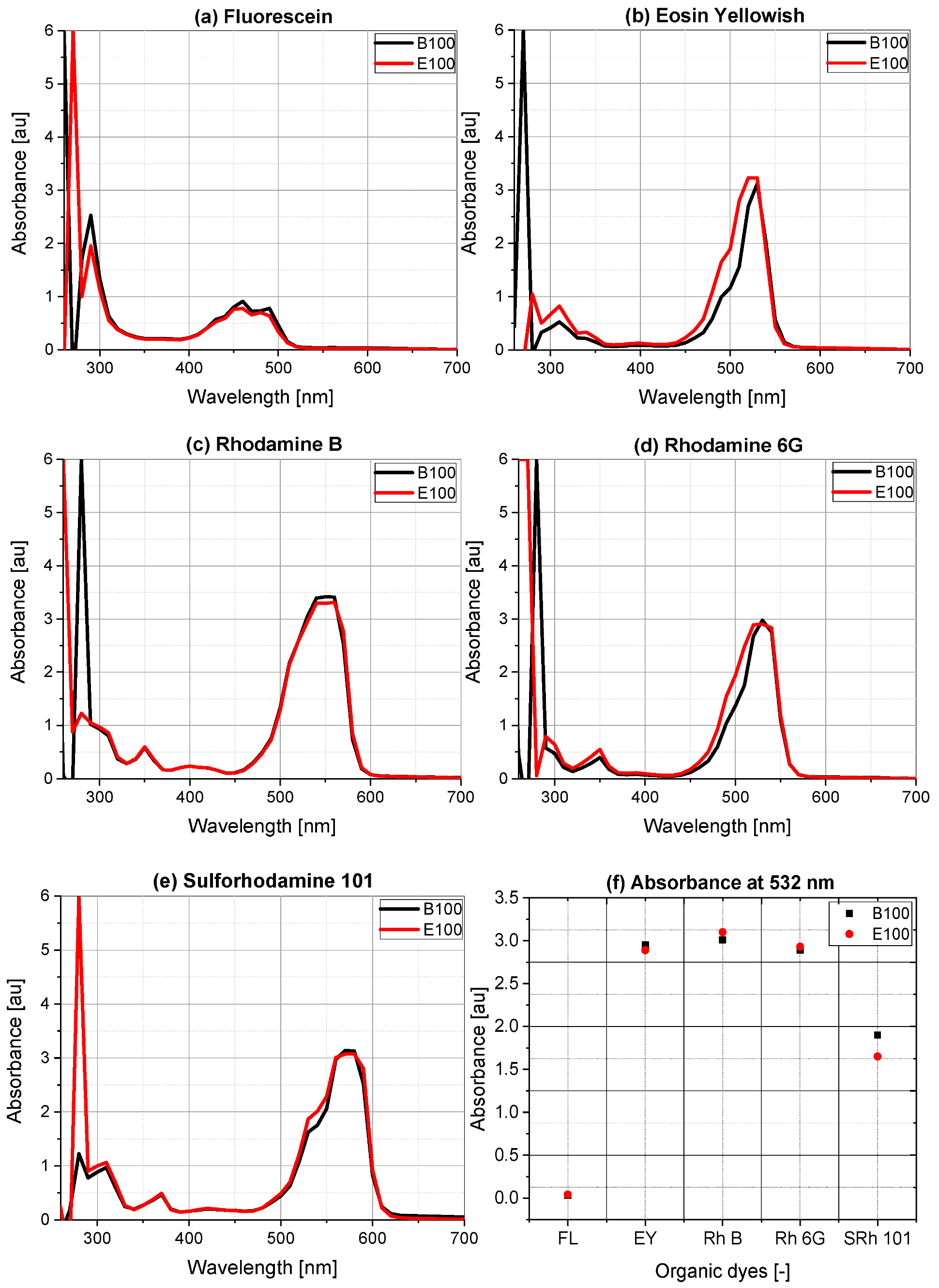
3. Results of Absorption Measurements
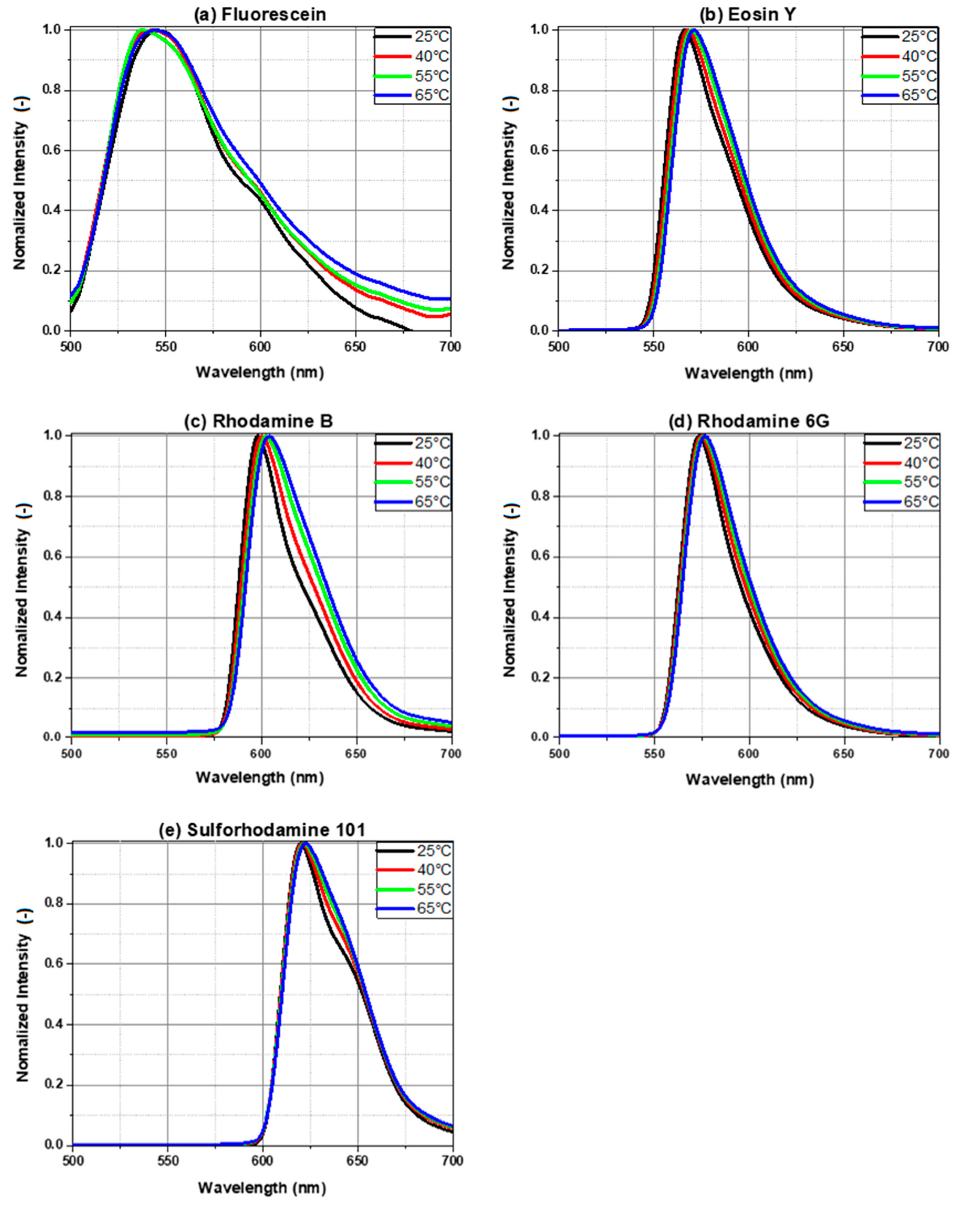
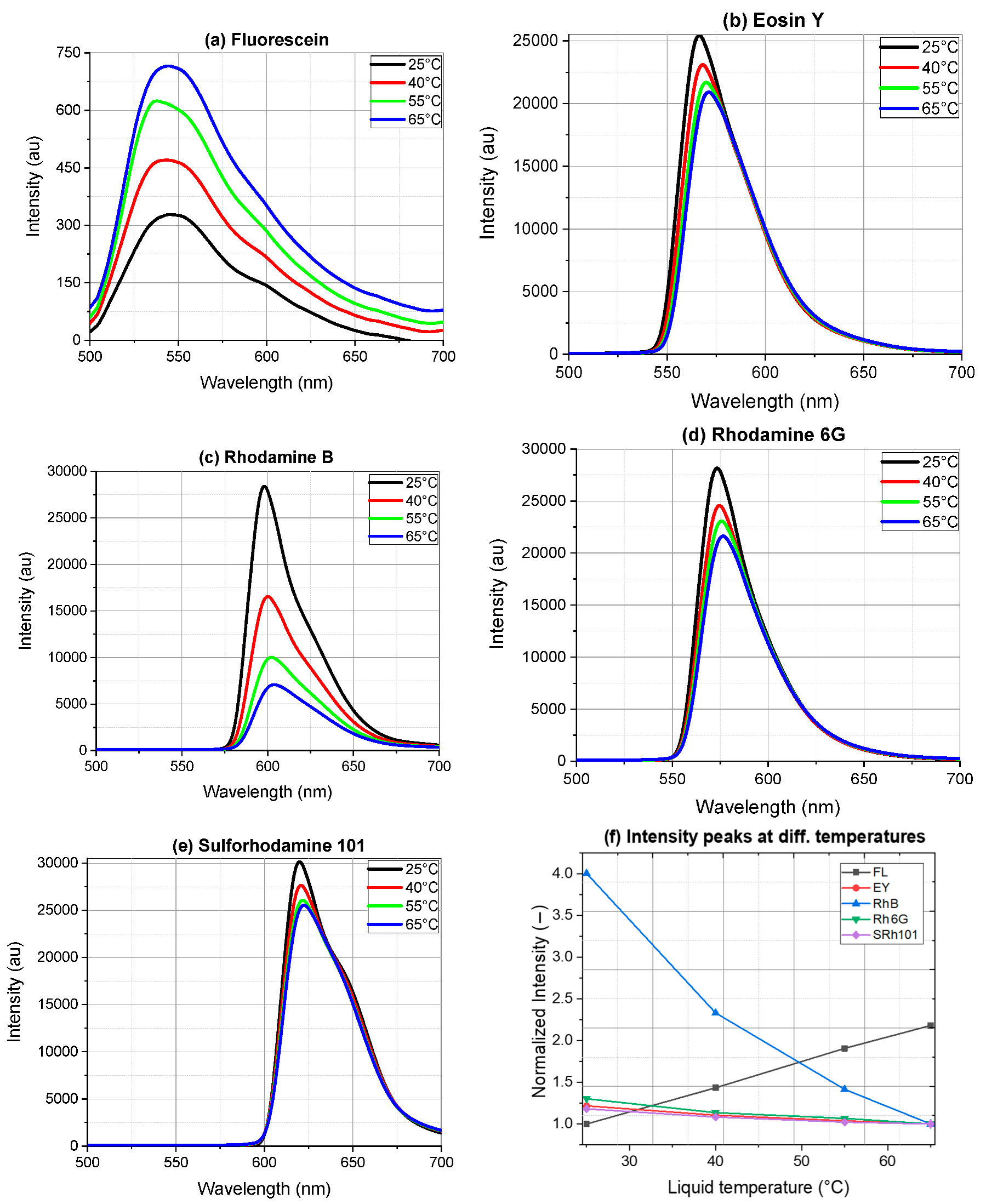
4. Results of Temperature-Dependent Fluorescence
4.1. Emission Spectra of Dyes in Ethanol
4.2. Emission Spectra of Dyes in Butanol
5. Results of Self-Absorption and Two-Color Ratio
5.1. Self-Absorption
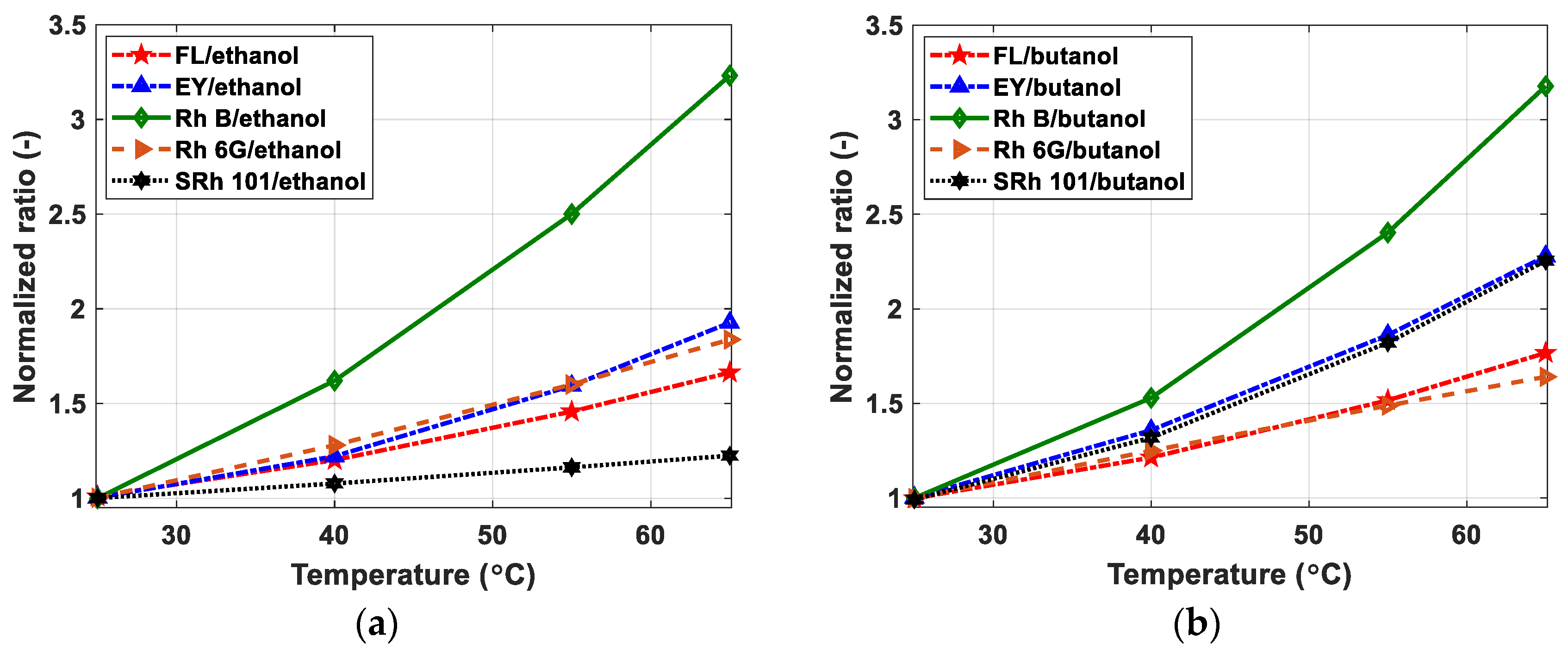
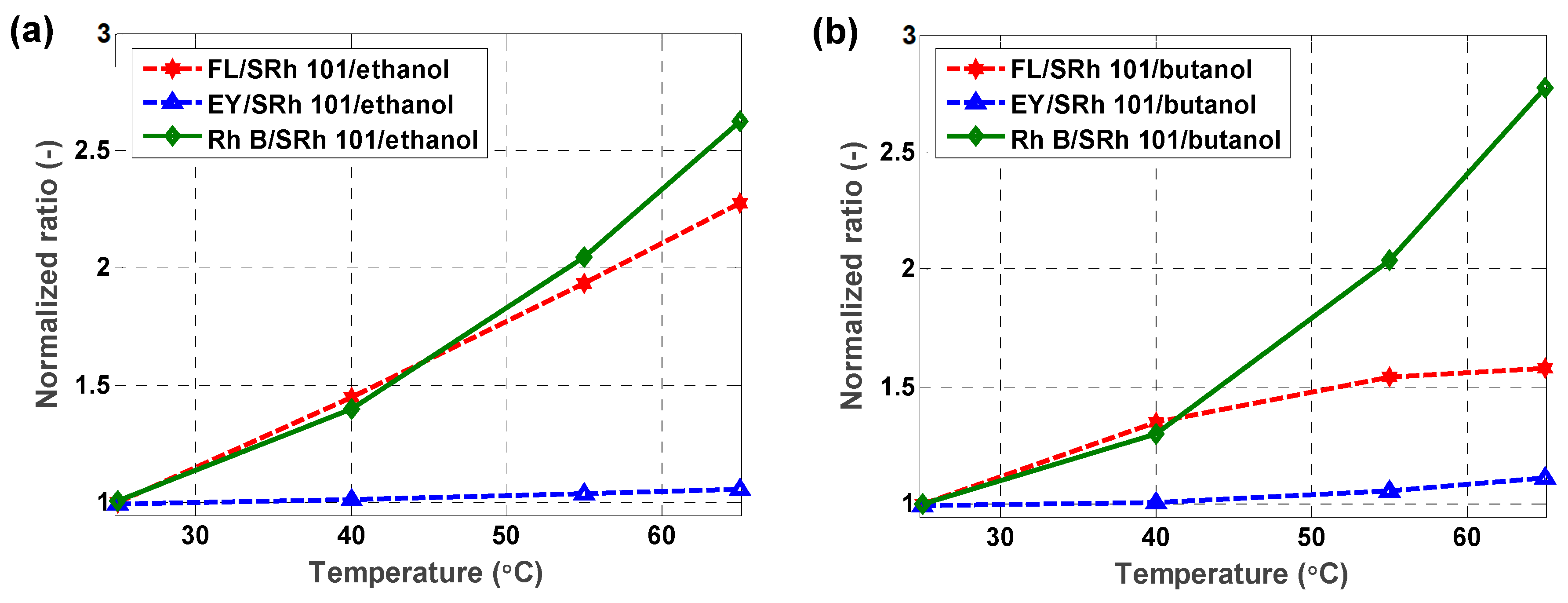
5.2. Two-Color Ratio and Temperature Sensitivity
5.2.1. One Dye Approach
5.2.2. Two-Dyes Approach
6. Outlook and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B

References
- Lemoine, F.; Castanet, G. Temperature and chemical composition of droplets by optical measurement techniques: A state-of-the-art review. Exp. Fluids 2013, 54, 1572. [Google Scholar] [CrossRef]
- Kubin, R.F.; Fletcher, A.N. Fluorescence quantum yields of some rhodamine dyes. J. Lumin. 1982, 27, 455–462. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Zhang, J.; Liu, L. Fluorescence Properties of Twenty Fluorescein Derivatives: Lifetime, Quantum Yield, Absorption and Emission Spectra. J. Fluoresc. 2014, 24, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.M.; Lindqvist, L. The pH dependence of fluorescein fluorescence. J. Lumin. 1975, 10, 381–390. [Google Scholar] [CrossRef]
- Lacassagne, T.; Simoëns, S.; El Hajem, M.; Champagne, J.-Y. Ratiometric, single-dye, pH-sensitive inhibited laser-induced fluorescence for the characterization of mixing and mass transfer. Exp. Fluids 2017, 59, 21. [Google Scholar] [CrossRef]
- Coppeta, J.; Rogers, C. Dual emission laser induced fluorescence for direct planar scalar behavior measurements. Exp. Fluids 1998, 25, 1–15. [Google Scholar] [CrossRef]
- Lemoine, F.; Wolff, M.; Lebouche, M. Simultaneous concentration and velocity measurements using combined laser-induced fluorescence and laser Doppler velocimetry: Application to turbulent transport. Exp. Fluids 1996, 20, 319–327. [Google Scholar] [CrossRef]
- Matsumoto, R.; Zadeh, H.F.; Ehrhard, P. Quantitative measurement of depth–averaged concentration fields in microchannels by means of a fluorescence intensity method. Exp. Fluids 2005, 39, 722–729. [Google Scholar] [CrossRef]
- Lavieille, P.; Lemoine, F.; Lavergne, G.; Lebouché, M. Evaporating and combusting droplet temperature measurements using two-color laser-induced fluorescence. Exp. Fluids 2001, 31, 45–55. [Google Scholar] [CrossRef]
- Lavieille, P.; Lemoine, F.; Lavergne, G.; Virepinte, J.F.; Lebouché, M. Temperature measurements on droplets in monodisperse stream using laser-induced fluorescence. Exp. Fluids 2000, 29, 429–437. [Google Scholar] [CrossRef]
- Sakakibara, J.; Adrian, R.J. Whole field measurement of temperature in water using two-color laser induced fluorescence. Exp. Fluids 1999, 26, 7–15. [Google Scholar] [CrossRef]
- Natrajan, V.K.; Christensen, K.T. Two-color laser-induced fluorescent thermometry for microfluidic systems. Meas. Sci. Technol 2009, 20, 015401. [Google Scholar] [CrossRef]
- Coolen, M.C.J.; Kieft, R.N.; Rindt, C.C.M.; van Steenhoven, A.A. Application of 2-D LIF temperature measurements in water using a Nd: YAG laser. Exp. Fluids 1999, 27, 420–426. [Google Scholar] [CrossRef]
- Mishra, Y.N.; Abou Nada, F.; Polster, S.; Kristensson, E.; Berrocal, E. Thermometry in aqueous solutions and sprays using two-color LIF and structured illumination. Opt. Express 2016, 24, 4949–4963. [Google Scholar] [CrossRef]
- Kan, T.; Aoki, H.; Binh-Khiem, N.; Matsumoto, K.; Shimoyama, I. Ratiometric Optical Temperature Sensor Using Two Fluorescent Dyes Dissolved in an Ionic Liquid Encapsulated by Parylene Film. Sensors 2013, 13, 4138–4145. [Google Scholar] [CrossRef]
- Kim, H.J.; Kihm, K.D.; Allen, J.S. Examination of ratiometric laser induced fluorescence thermometry for microscale spatial measurement resolution. Int. J. Heat Mass Transf. 2003, 46, 3967–3974. [Google Scholar] [CrossRef]
- Estrada-Pérez, C.E.; Hassan, Y.A.; Tan, S. Experimental characterization of temperature sensitive dyes for laser induced fluorescence thermometry. Rev. Sci. Instrum. 2011, 82, 074901. [Google Scholar] [CrossRef]
- Shafii, M.B.; Lum, C.L.; Koochesfahani, M.M. In situ LIF temperature measurements in aqueous ammonium chloride solution during uni-directional solidification. Exp. Fluids 2010, 48, 651–662. [Google Scholar] [CrossRef]
- Chaze, W.; Caballina, O.; Castanet, G.; Lemoine, F. Spatially and temporally resolved measurements of the temperature inside droplets impinging on a hot solid surface. Exp. Fluids 2017, 58, 96. [Google Scholar] [CrossRef]
- Castanet, G.; Chaze, W.; Caballina, O.; Collignon, R.; Lemoine, F. Transient evolution of the heat transfer and the vapor film thickness at the drop impact in the regime of film boiling. Phys. Fluids 2018, 30, 122109. [Google Scholar] [CrossRef]
- Fenner, A.; Stephan, P. Two dye combinations suitable for two-color/two-dye laser-induced fluorescence thermography for ethanol. Exp. Fluids 2017, 58, 65. [Google Scholar] [CrossRef]
- Castanet, G.; Lavieille, P.; Lebouché, M.; Lemoine, F. Measurement of the temperature distribution within monodisperse combusting droplets in linear streams using two-color laser-induced fluorescence. Exp. Fluids 2003, 35, 563–571. [Google Scholar] [CrossRef]
- Castanet, G.; Liénart, T.; Lemoine, F. Dynamics and temperature of droplets impacting onto a heated wall. Int. J. Heat Mass Transf. 2009, 52, 670–679. [Google Scholar] [CrossRef]
- Labergue, A.; Deprédurand, V.; Delconte, A.; Castanet, G.; Lemoine, F. New insight into two-color LIF thermometry applied to temperature measurements of droplets. Exp. Fluids 2010, 49, 547–556. [Google Scholar] [CrossRef][Green Version]
- Vetrano, M.R.; Simonini, A.; Steelant, J.; Rambaud, P. Thermal characterization of a flashing jet by planar laser-induced fluorescence. Exp. Fluids 2013, 54, 1573. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, W.; Yin, Y.; Chen, S.; Yan, B.; Mu, J.; Qi, X. Nonlinear temperature calibration equation for Rhodamine B in different solutions for wide-temperature-range applications. Appl. Opt. 2019, 58, 1514–1518. [Google Scholar] [CrossRef]
- Perrin, L.; Castanet, G.; Lemoine, F. Characterization of the evaporation of interacting droplets using combined optical techniques. Exp. Fluids 2015, 56, 29. [Google Scholar] [CrossRef]
- Palmer, J.; Reddemann, M.A.; Kirsch, V.; Kneer, R. Temperature measurements of micro-droplets using pulsed 2-color laser-induced fluorescence with MDR-enhanced energy transfer. Exp. Fluids 2016, 57, 177. [Google Scholar] [CrossRef]
- Palmer, J.; Reddemann, M.A.; Kirsch, V.; Kneer, R. Comparison of 2c- and 3cLIF droplet temperature imaging. Exp. Fluids 2018, 59, 102. [Google Scholar] [CrossRef]
- Palmer, J.; Reddemann, M.A.; Kirsch, V.; Kneer, R. Applying 2D-2cLIF-EET thermometry for micro-droplet internal temperature imaging. Exp. Fluids 2018, 59, 51. [Google Scholar] [CrossRef]
- Wolff, M.; Delconte, A.; Schmidt, F.; Gucher, P.; Lemoine, F. High-pressure Diesel spray temperature measurements using two-colour laser-induced fluorescence. Meas. Sci. Technol. 2007, 18, 697. [Google Scholar] [CrossRef]
- Deprédurand, V.; Delconte, A.; Lemoine, F. Combined PDA and LIF applied to size–temperature correlations measurements in a heated spray. Exp. Fluids 2011, 50, 561–571. [Google Scholar] [CrossRef]
- Deprédurand, V.; Miron, P.; Labergue, A.; Wolff, M.; Castanet, G.; Lemoine, F. A temperature-sensitive tracer suitable for two-colour laser-induced fluorescence thermometry applied to evaporating fuel droplets. Meas. Sci. Technol. 2008, 19, 105403. [Google Scholar] [CrossRef]
- Sanned, D. Study of Fluorescence Dyes for Tracing Lubrication Oil Inside Marine Engines. Bachelor’s Thesis, Lund University, Lund, Sweden, 2017. [Google Scholar]
- Ippen, E.; Shank, C.; Dienes, A. Rapid photobleaching of organic laser dyes in continuously operated devices. IEEE J. Quantum Electron. 1971, 7, 178–179. [Google Scholar] [CrossRef]
- Chaze, W.; Caballina, O.; Castanet, G.; Lemoine, F. The saturation of the fluorescence and its consequences for laser-induced fluorescence thermometry in liquid flows. Exp. Fluids 2016, 57, 58. [Google Scholar] [CrossRef]
- Le Gal, P.; Farrugia, N.; Greenhalgh, D.A. Laser Sheet Dropsizing of dense sprays. Opt. Laser Technol. 1999, 31, 75–83. [Google Scholar] [CrossRef]
- Domann, R.; Hardalupas, Y. Quantitative Measurement of Planar Droplet Sauter Mean Diameter in Sprays using Planar Droplet Sizing. Part. Part. Syst. Charact. 2003, 20, 209–218. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry, 4th ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010. [Google Scholar]
- Taniguchi, M.; Lindsey, J.S. Database of Absorption and Fluorescence Spectra of >300 Common Compounds for use in PhotochemCAD. Photochem. Photobiol. 2018, 94, 290–327. [Google Scholar] [CrossRef]
- Koegl, M.; Hofbeck, B.; Baderschneider, K.; Mishra, Y.N.; Huber, F.J.T.; Berrocal, E.; Will, S.; Zigan, L. Analysis of LIF and Mie signals from single micrometric droplets for instantaneous droplet sizing in sprays. Opt. Express 2018, 26, 31750–31766. [Google Scholar] [CrossRef]
- Koegl, M.; Mishra, Y.N.; Storch, M.; Conrad, C.; Berrocal, E.; Will, S.; Zigan, L. Analysis of ethanol and butanol direct-injection spark-ignition sprays using two-phase structured laser illumination planar imaging droplet sizing. Int. J. Spray Combust. Dyn. 2019, 11, 1–16. [Google Scholar] [CrossRef]
- Durst, A.; Wensing, M.; Berrocal, E. Light sheet fluorescence microscopic imaging for the primary breakup of diesel and gasoline sprays with real-world fuels. Appl. Opt. 2018, 57, 2704–2714. [Google Scholar] [CrossRef] [PubMed]
- Koegl, M.; Mull, C.; Baderschneider, K.; Wislicenus, J.; Will, S.; Zigan, L. Characterization of Nile Red as a Tracer for Laser-Induced Fluorescence Spectroscopy of Gasoline and Kerosene and Their Mixture with Biofuels. Sensors 2019, 19, 2822. [Google Scholar] [CrossRef] [PubMed]
- Seybold, P.G.; Gouterman, M.; Callis, J. Calorimetric, Photometric and Lifetime Determinations of Fluorescence Yields of Fluorescein Dyes. Photochem. Photobiol. 1969, 9, 229–242. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Zhang, Y.; Liu, L. Fluorescence lifetimes and quantum yields of ten rhodamine derivatives: Structural effect on emission mechanism in different solvents. J. Lumin. 2014, 145, 448–453. [Google Scholar] [CrossRef]
- Terdale, S.; Tantray, A. Spectroscopic study of the dimerization of rhodamine 6G in water and different organic solvents. J. Mol. Liq. 2017, 225, 662–671. [Google Scholar] [CrossRef]
- Penzkofer, A.; Leupacher, W. Fluorescence behaviour of highly concentrated rhodamine 6G solutions. J. Lumin. 1987, 37, 61–72. [Google Scholar] [CrossRef]


| Organic Dyes (Xanthene Family) | CAS [40] | Melting Point (°C) | FQY in Ethanol [40] | Conc. In Ethanol/Butanol (10−4 M/L) |
|---|---|---|---|---|
| FL | 2321-07-5 | 314 | 0.97 | 34.9/34.6 |
| EY | 17372-87-1 | 295 | 0.67 | 1.2/0.9 |
| RhB | 81-88-9 | 210 | 0.70 | 1.3/1.31 |
| Rh6G | 989-38-8 | 290 | 0.95 | 1.5/1.5 |
| SRh101 | 60311-02-6 | >300 | 0.90 | 1.2/1.0 |
| Dyes | Self-Absorption Band |
|---|---|
| FL | 500–525 |
| EY | 540–575 |
| RhB | 575–610 |
| Rh6G | 550–575 |
| SRh101 | 590–625 |
| Dyes | LIF1 Band (Semrock Order #) | LIF2 Band (Semrock Order #) |
|---|---|---|
| FL | 505–515 (FF02-510/10-25) | 604–679 (FF02-641/75-25) |
| EY | 553–567 (FF01-560/14-25) | 620–680 (FF01-650/60-25) |
| RhB | 580–591 (FF01-585/11-25) | 620–680 (FF01-650/60-25) |
| Rh6G | 553–567 (FF01-560/14-25) | 604–679 (FF02-641/75-25) |
| SRh101 | 598–613 (FF01-605/15-25) | 620–680 (FF01-650/60-25) |
| Dyes | Sensitivity (%/°C) in Ethanol | Sensitivity (%/°C) in Butanol |
|---|---|---|
| FL | 1.27 | 1.42 |
| EY | 1.64 | 2.06 |
| RhB | 2.93 | 2.89 |
| Rh6G | 1.52 | 1.24 |
| SRh101 | 0.51 | 2.03 |
| Dyes | LIF1 Band (Semrock Order #) | LIF2 Band (Semrock Order #) |
|---|---|---|
| FL/SRh101 | 543–555 (FF01-549/12-25) | 620–680 (FF01-650/60-25) |
| EY/SRh101 | 580–591 (FF01-585/11-25) | 620–680 (FF01-650/60-25) |
| RhB/SRh101 | 613–627 (FF01-620/14-25) | 634–686 (FF01-660/52-25) |
| Dyes | Sensitivity (%/°C) in Ethanol | Sensitivity (%/°C) in Butanol |
|---|---|---|
| FL/SRh101 | 2.05 | 1.14 |
| EY/SRh101 | 0.15 | 0.27 |
| RhB/SRh101 | 2.39 | 2.54 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, Y.N.; Yoganantham, A.; Koegl, M.; Zigan, L. Investigation of Five Organic Dyes in Ethanol and Butanol for Two-Color Laser-Induced Fluorescence Ratio Thermometry. Optics 2020, 1, 1-17. https://doi.org/10.3390/opt1010001
Mishra YN, Yoganantham A, Koegl M, Zigan L. Investigation of Five Organic Dyes in Ethanol and Butanol for Two-Color Laser-Induced Fluorescence Ratio Thermometry. Optics. 2020; 1(1):1-17. https://doi.org/10.3390/opt1010001
Chicago/Turabian StyleMishra, Yogeshwar Nath, Ajeth Yoganantham, Matthias Koegl, and Lars Zigan. 2020. "Investigation of Five Organic Dyes in Ethanol and Butanol for Two-Color Laser-Induced Fluorescence Ratio Thermometry" Optics 1, no. 1: 1-17. https://doi.org/10.3390/opt1010001
APA StyleMishra, Y. N., Yoganantham, A., Koegl, M., & Zigan, L. (2020). Investigation of Five Organic Dyes in Ethanol and Butanol for Two-Color Laser-Induced Fluorescence Ratio Thermometry. Optics, 1(1), 1-17. https://doi.org/10.3390/opt1010001





