Cosmetic Outcomes of the First Bodybuilder Using a Low-Cost Modified Culture Technique for Burn Wound Coverage: A Case Report and Long-Term Follow-Up
Abstract
1. Introduction
2. Detailed Case Description
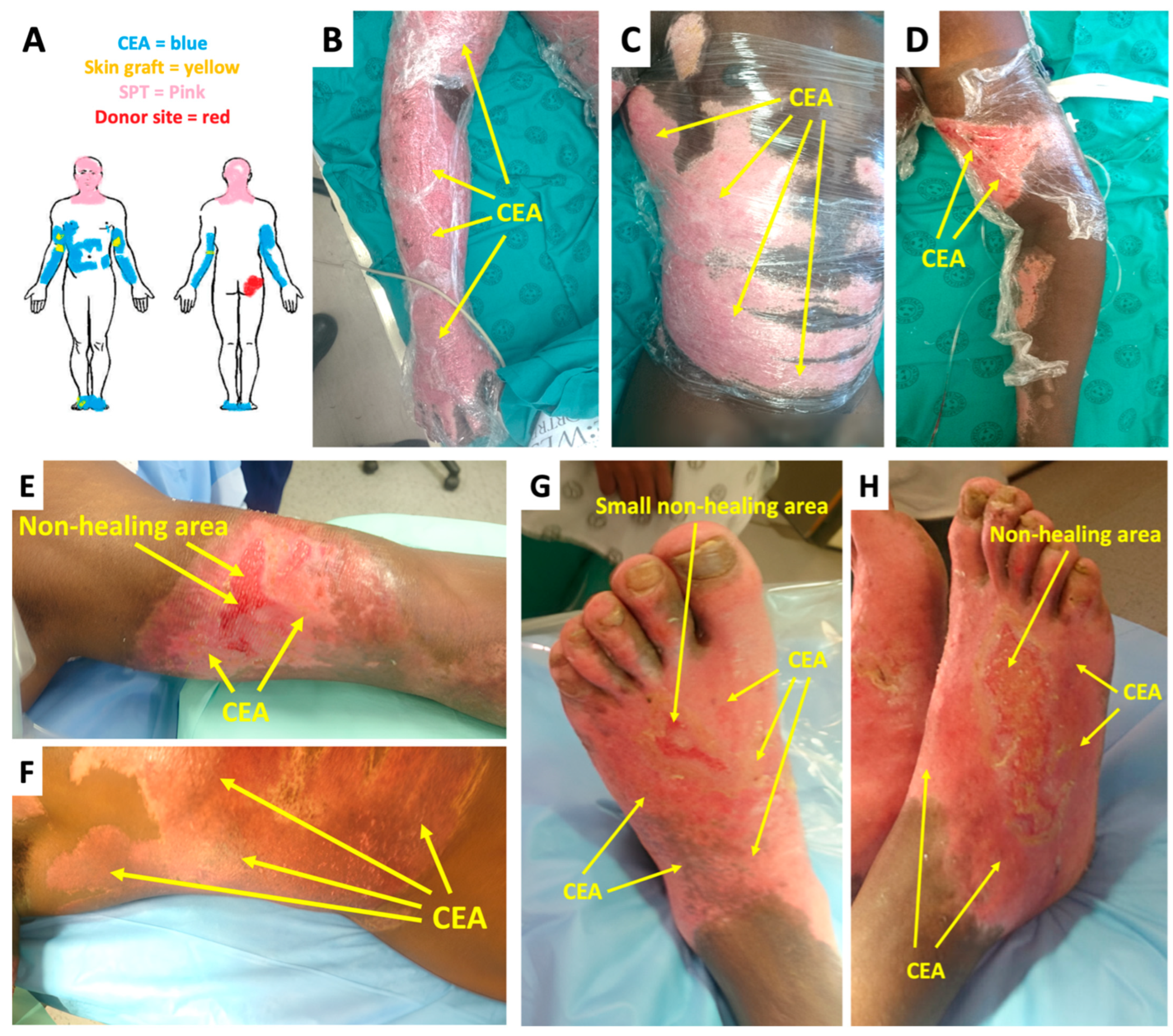
2.1. Case Presentation
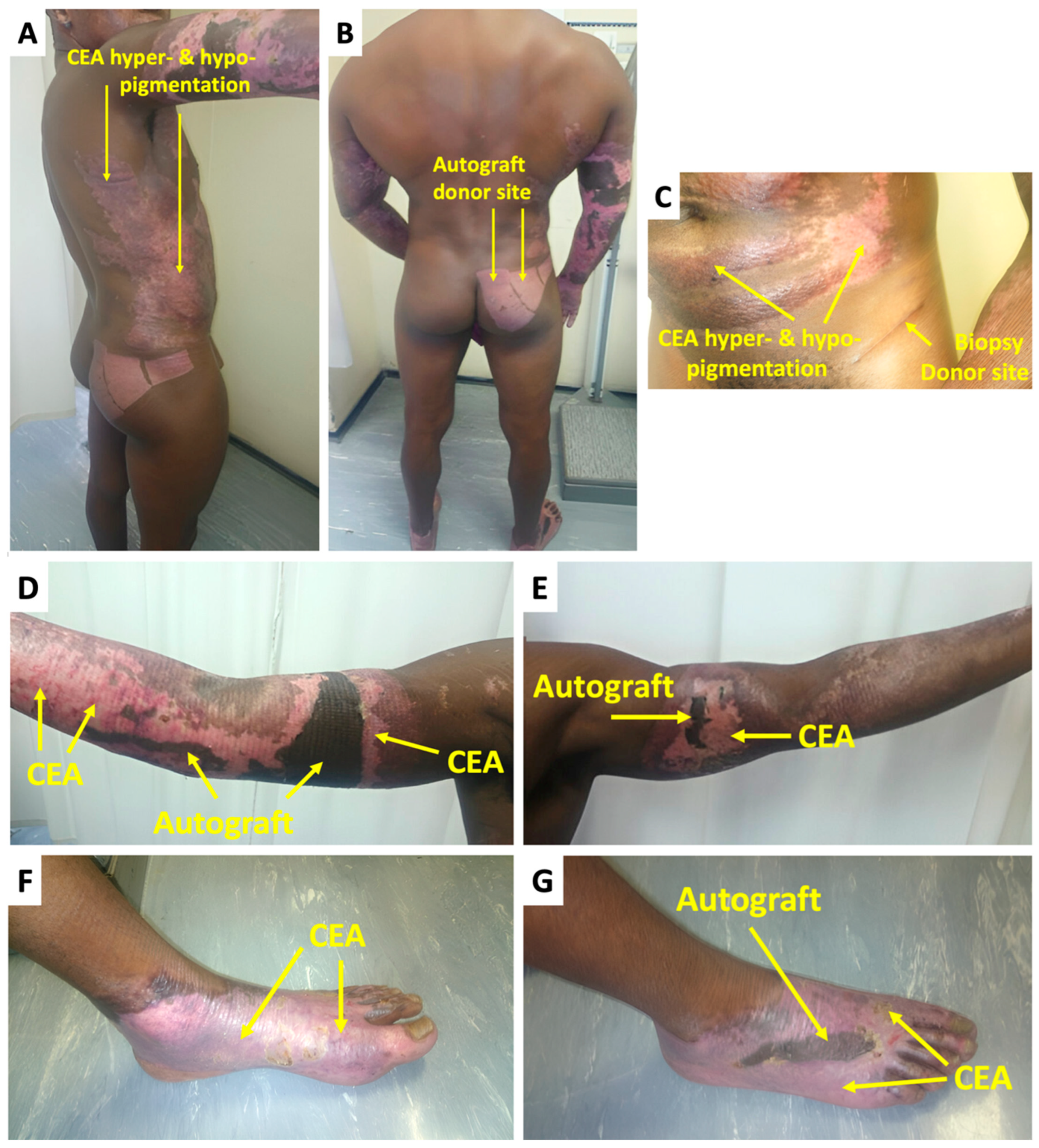
2.2. Treatment and Outcomes
3. Discussion
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CEA | Cultured epidermal autografts |
| STSG | Split-thickness grafts |
| Western Cape Provincial Adult Tertiary Burn Centre | WCPATBC |
| Total body surface area | TBSA |
| Platelet-rich plasma | PRP |
| Vancouver Scar Scale | VSS |
References
- Schlottmann, F.; Bucan, V.; Vogt, P.M.; Krezdorn, N. A Short History of Skin Grafting in Burns: From the Gold Standard of Autologous Skin Grafting to the Possibilities of Allogeneic Skin Grafting with Immunomodulatory Approaches. Medicina 2021, 57, 225. [Google Scholar] [CrossRef] [PubMed]
- Atiyeh, B.S.; Hayek, S.N.; Gunn, S.W. New Technologies for Burn Wound Closure and Healing—Review of the Literature. Burns 2005, 31, 944–956. [Google Scholar] [CrossRef]
- Ronfard, V.; Rives, J.-M.; Neveux, Y.; Carsin, H.; Barrandon, Y. Long-Term Regeneration of Human Epidermis on Third Degree Burns Transplanted with Autologous Cultured Epithelium Grown on a Fibrin Matrix. Transplantation 2000, 70, 1588–1598. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Costagliola, M. Cultured Epithelial Autograft (CEA) in Burn Treatment: Three Decades Later. Burns 2007, 33, 405–413. [Google Scholar] [CrossRef]
- Williamson, J.S.; Snelling, C.F.T.; Clugston, P.; Macdonald, I.B.; Germann, E. Cultured Epithelial Autograft. J. Trauma: Inj. Infect. Crit. Care 1995, 39, 309–319. [Google Scholar] [CrossRef]
- Otene, C.I.; Olaitan, P.B.; Ogbonnaya, I.S.; Nnabuko, R.E. Donor Site Morbidity Following Harvest of Split-Thickness Skin Grafts in South Eastern Nigeria. J. West Afr. Coll. Surg. 2011, 1, 86–96. [Google Scholar]
- Halim, A.S.; Khoo, T.L.; Mohd Yussof, S.J. Biologic and Synthetic Skin Substitutes: An Overview. Indian J. Plast. Surg. 2010, 43 (Suppl. S1), S23–S28. [Google Scholar] [CrossRef]
- Kleintjes, W.G.; Prinsloo, T.K. Graft Take Outcomes of Burn Patients Transplanted with CEA Derived From a Modified Composite Culture Technique: A Case Series. J. Burn. Care Res. 2024, irae153. [Google Scholar] [CrossRef]
- Sood, R.; Roggy, D.; Zieger, M.; Balledux, J.; Chaudhari, S.; Koumanis, D.J.; Mir, H.S.; Cohen, A.; Knipe, C.; Gabehart, K.; et al. Cultured Epithelial Autografts for Coverage of Large Burn Wounds in Eighty-Eight Patients: The Indiana University Experience. J. Burn. Care Res. 2010, 31, 559–568. [Google Scholar] [CrossRef]
- Barret, J.P.; Wolf, S.E.; Desai, M.H.; Herndon, D.N. Cost-Efficacy of Cultured Epidermal Autografts in Massive Pediatric Burns. Ann. Surg. 2000, 231, 869–876. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Gilpin, D.A.; Meyer, N.A.; Herndon, D.N. Current Treatment of Severely Burned Patients. Ann. Surg. 1996, 223, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Guilbaud, J. Problems Created by the Use of Cultured Epithelia BT. In The Management of Burns and Fire Disasters: Perspectives 2000; Masellis, M., Gunn, S.W.A., Eds.; Springer: Dordrecht, The Netherlands, 1995; pp. 352–356. [Google Scholar] [CrossRef]
- Kleintjes, W.; Thomas, G.; Thaele, B.S.S.; Stevenson, N.; Warren, B. A Novel Technique for Composite Cultured Epithelial Autograft in a Patient with Extensive Burn Wounds: A Case Report. Clin. Surg. 2017, 2, 1579. [Google Scholar]
- Navarro, F.A.; Stoner, M.L.; Park, C.S.; Huertas, J.C.; Lee, H.B.; Wood, F.M.; Orgill, D.P. Sprayed Keratinocyte Suspensions Accelerate Epidermal Coverage in a Porcine Microwound Model. J. Burn. Care Rehabil. 2000, 21, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Haas, A.F.; Reilly, D.A. Cultured Epithelial Autografts in the Treatment of Extensive Recalcitrant Keloids. Arch. Dermatol. 1998, 134, 549–552. [Google Scholar] [CrossRef][Green Version]
- Kleintjes, W.G.; Prinsloo, T.K. Structural Characterization and Safety Validation of Cultured Skin Cells Grown on Routinely Used Dressing in Pediatric Incubators: A Preliminary Report. World J. Surg. Res. 2024, 7, 1569. [Google Scholar]
- Sullivan, T.; Smith, J.; Kermode, J.; Mclver, E.; Courtemanche, D.J. Rating the Burn Scar. J. Burn. Care Rehabil. 1990, 11, 256–260. [Google Scholar] [CrossRef]
- Klein, A.M. Little Big Men: Bodybuilding Subculture and Gender Construction; State University of New York Press: New York, NY, USA, 1993. [Google Scholar]
- Şentürk, G.; Gobel, P. The Relationship Between Body Composition and Self-Esteem and Body Image in Male Bodybuilding Athletes. Int. J. Exerc. Psychol. 2023, 5, 9–14. [Google Scholar] [CrossRef]
- Aimé, A.; Fuller-Tyszkiewicz, M.; Dion, J.; Markey, C.H.; Strodl, E.; McCabe, M.; Mellor, D.; Granero Gallegos, A.; Pietrabissa, G.; Alcaraz-Ibánez, M.; et al. Assessing Positive Body Image, Body Satisfaction, Weight Bias, and Appearance Comparison in Emerging Adults: A Cross-Validation Study across Eight Countries. Body Image 2020, 35, 320–332. [Google Scholar] [CrossRef]
- Macho, J.; Mudrak, J.; Slepicka, P. Enhancing the Self: Amateur Bodybuilders Making Sense of Experiences with Appearance and Performance-Enhancing Drugs. Front. Psychol. 2021, 12, 648467. [Google Scholar] [CrossRef]
- Andreasson, J.; Johansson, T. Bodybuilding and Fitness Doping in Transition. Historical Transformations and Contemporary Challenges. Soc. Sci. 2019, 8, 80. [Google Scholar] [CrossRef]
- Horch, R.E.; Kopp, J.; Kneser, U.; Beier, J.; Bach, A.D. Tissue Engineering of Cultured Skin Substitutes. J. Cell. Mol. Med. 2005, 9, 592–608. [Google Scholar] [CrossRef] [PubMed]
- Kleintjes, W.G.; Prinsloo, T.K. Case Report of the First Caucasian Burn Patient Transplanted with Cutimed Sorbact®-Based Cultured Epithelial Autografts Technique at Tygerberg Hospital, Cape Town, South Africa: An 8-Year Follow-Up. SAGE Open Med. Case Rep. 2024, 16, 2050313X231223462. [Google Scholar] [CrossRef] [PubMed]
- Kleintjes, W.G.; Prinsloo, T.K. A case report of the first CEA transplant in an HIV-positive burn patient in South Africa using a novel composite culture technique. Indian J. Plast. Surg. 2024, 57, 401–403. [Google Scholar] [CrossRef]
- Coleman III, J.J.; Siwy, B.K. Cultured Epidermal Autografts: A Life-Saving and Skin-Saving Technique in Children. J. Pediatr. Surg. 1992, 27, 1029–1032. [Google Scholar] [CrossRef]
| VSS Criteria and Score | 2 Weeks | 2 Months | 6 Months |
|---|---|---|---|
| Vascularity (Normal = 0, Pink = 1, Red = 2, Purple = 3) | |||
| 2 | 1 | 1 | |
| Pigmentation (Normal = 0, Hypopigmentation = 1, Hyperpigmentation = 2) | |||
| 2 | 1 | 1 | |
| Pliability (Normal = 0, Supple = 1, Yielding = 2, Firm = 3, Ropes = 4, Contractures = 5) | |||
| 3 | 2 | 1 | |
| Height (Flat = 0, <2 mm = 1, 2–5 mm = 2, <5 mm = 3) | |||
| 1 | 0 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the European Burns Association. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleintjes, W.G.; Prinsloo, T.K. Cosmetic Outcomes of the First Bodybuilder Using a Low-Cost Modified Culture Technique for Burn Wound Coverage: A Case Report and Long-Term Follow-Up. Eur. Burn J. 2025, 6, 29. https://doi.org/10.3390/ebj6020029
Kleintjes WG, Prinsloo TK. Cosmetic Outcomes of the First Bodybuilder Using a Low-Cost Modified Culture Technique for Burn Wound Coverage: A Case Report and Long-Term Follow-Up. European Burn Journal. 2025; 6(2):29. https://doi.org/10.3390/ebj6020029
Chicago/Turabian StyleKleintjes, Wayne George, and Tarryn Kay Prinsloo. 2025. "Cosmetic Outcomes of the First Bodybuilder Using a Low-Cost Modified Culture Technique for Burn Wound Coverage: A Case Report and Long-Term Follow-Up" European Burn Journal 6, no. 2: 29. https://doi.org/10.3390/ebj6020029
APA StyleKleintjes, W. G., & Prinsloo, T. K. (2025). Cosmetic Outcomes of the First Bodybuilder Using a Low-Cost Modified Culture Technique for Burn Wound Coverage: A Case Report and Long-Term Follow-Up. European Burn Journal, 6(2), 29. https://doi.org/10.3390/ebj6020029






