Laboratory-Generated Autologous Skin Substitutes for Burn Treatment in Europe: Narrative Review, Experts’ Opinion, and Legal Considerations
Abstract
1. Introduction
2. Different Types of Laboratory-Generated Autologous Skin Substitutes
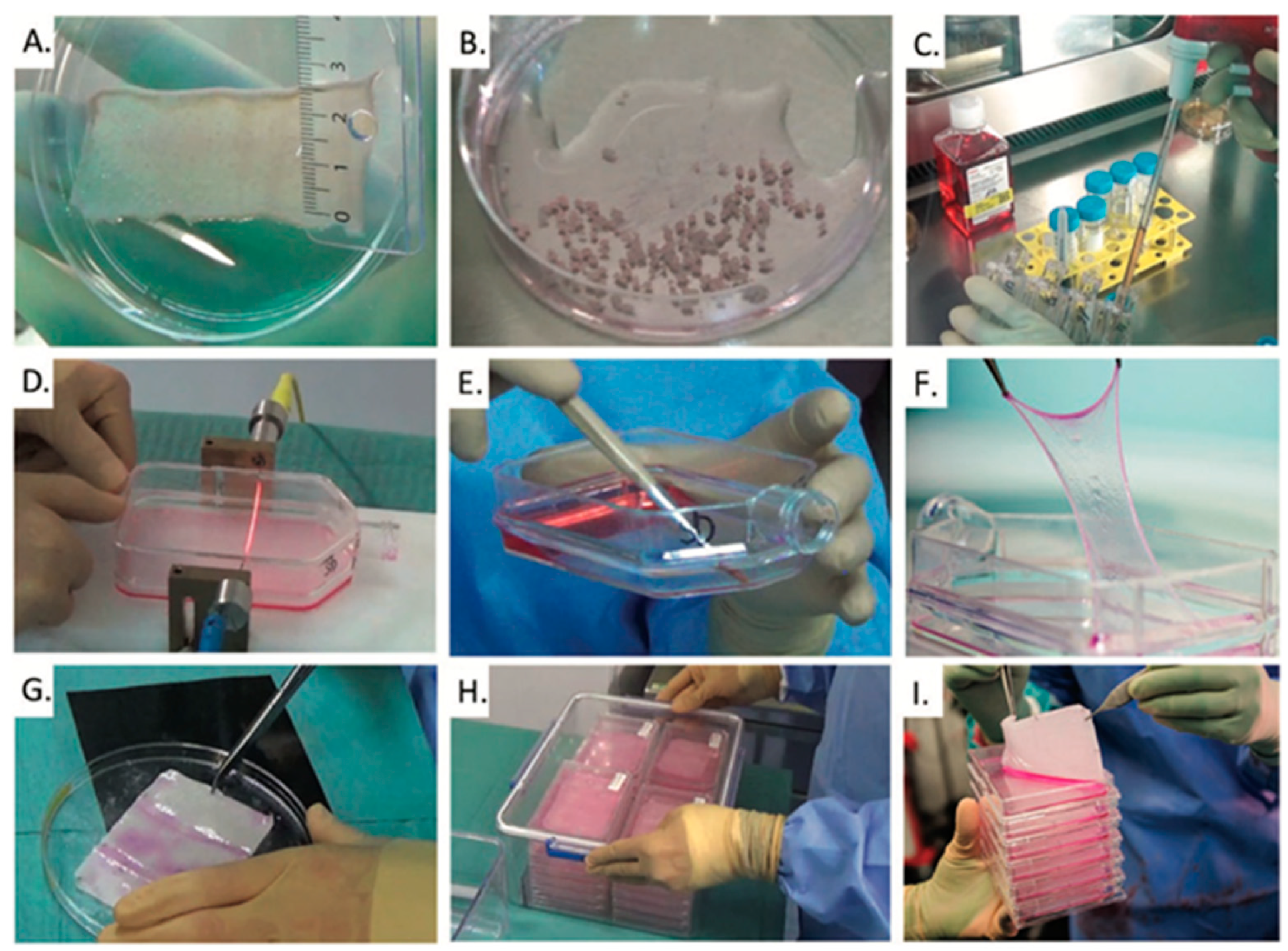
2.1. Cultured Epithelial Autografts (CEAs)
- Skin biopsy collection from the patient, typically a split-thickness specimen;
- Enzymatic digestion to isolate keratinocytes from epidermal tissue;
- Cell expansion in culture, commonly using a feeder layer of fibroblasts;
- Stratified sheet formation over a culture period of approximately 10 to 21 days;
- Harvesting and transfer of the epithelial sheet onto a Vaseline gauze for clinical application or storage.
2.2. Co-Cultures of Autologous Keratinocytes and Fibroblasts (CDEAs)
2.3. Cell Suspension/Spray
- A spray containing only cultured keratinocytes from the patient’s skin biopsy. Expansion of the cells is achieved basically according to the abovementioned techniques. However, the cells are passed before they reach confluence. Once enough cell number is reached, the keratinocytes are detached as above, mixed and washed, and centrifuged to a cell pellet. In the operating theater, the cell pellet is re-suspended in the thrombin part of a commercially available fibrinogen–thrombin tissue glue and sprayed onto the wound surface.
- A spray containing non-cultured cells, mainly keratinocytes, but also other cells such as melanocytes, which are isolated from a small biopsy and immediately processed into a spray directly in the operating theater and then returned to the patient. In this case, the spray functions as a tool to evenly distribute the cells on the wound surface, where they then start multiplying.
2.4. Autologous Two-Layer Skin Substitutes
3. Indications
3.1. Indications for CEAs and CDEAs
3.2. Indications for Cell Spray
3.3. Indications for Autologous Two-Layer Skin Substitutes
3.4. General Remarks on the Indication of All Types of Laboratory-Generated Autologous Skin Substitutes
4. Regulatory Issues
4.1. European Regulatory Issues Until 2009
4.2. European Regulatory Issues After 2009
5. Research and Future Perspectives
5.1. Basic Research
5.2. Translational Research
5.3. Clinical Research
6. Opportunities for Further Development in Europe
- A constructive dialogue is needed with those officially responsible for the regulatory framework in Europe (EMA) and all the stakeholders including the users; producers, in particular the European burn centers; and most importantly, burn survivors. The aim of this constructive dialogue should be to define with users the minimum necessary regulatory requirements for laboratory-produced autologous skin substitutes as AMTPs, on the premise that these products have already been in use for over 40 years.
- Despite the regulatory requirements for patient safety, it would be important to allow European burn centers to set up a network of laboratories with affordable financial resources to ensure the provision of care to severely burned patients for all the European centers.
- For their part, European centers should work collaboratively towards establishing general guidelines and uniform outcome research for this innovative therapy.
- Regulatory requirements;
- Production conditions;
- Clinical application;
- Database.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| ESEN | European Skin Engineering Network |
| ESENBURN | COST Action project of ESEN |
| EBA | European Burns Association |
| GMP | Good Manufacturing Practice |
| CRC | Childrens Research Center |
| CPC | Cell Production Center |
| CHUV | Lausanne University Hopsital |
| AND&TAT | Andalusian Network for the Design and Translation of Advanced Therapies |
| CEA | Cultured Epithelial Auografts |
| SOP | Standard Operating Procedure |
| STSG | Split-Thickenss Skin Graft |
| AMM | Autorisation de mise sur le marché |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| CDEA | cultured dermal–epidermal autografts |
| DIZG | German Institute for Cells and Tissue |
| PHIT | Piel humana obtenida por ingeniería de tejidos |
| SSTaRC | Skin and Soft Tissue Research Center |
| TBSA | total body surface area |
| ATMPs | Advanced Therapy Medical Products |
| NMAs | National Medicine Agencies |
| EMA | European Medicine Agency |
| PV-Skin | Pigmented–Vascularized Skin |
References
- Billingham, R.E.; Reynolds, J. Transplantation studies on sheets of pure epidermal epithelium and on epidermal cell suspensions. Br. J. Plast. Surg. 1952, 5, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Rheinwald, J.G.; Green, H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature 1977, 265, 421–424. [Google Scholar] [CrossRef]
- Rheinwald, J.G.; Green, H. Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells. Cell 1975, 6, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Boyce, S.T.; Kagan, R.J.; Meyer, N.A.; Yakuboff, K.P.; Warden, G.D. The 1999 clinical research award. Cultured skin substitutes combined with Integra Artificial Skin to replace native skin autograft and allograft for the closure of excised full-thickness burns. J. Burn. Care Rehabil. 1999, 20, 453–461. [Google Scholar] [CrossRef]
- Green, J.R.; James, R. Atkinson (January 6, 1920–February 12, 1978). ARIZ Med. 1978, 35, 419–420. [Google Scholar] [PubMed]
- Green, H.; Kehinde, O.; Thomas, J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc. Natl. Acad. Sci. USA 1979, 76, 5665–5668. [Google Scholar] [CrossRef]
- Rheinwald, J.G.; Green, H. Formation of a keratinizing epithelium in culture by a cloned cell line derived from a teratoma. Cell 1975, 6, 317–330. [Google Scholar] [CrossRef]
- Chemali, M.; Laurent, A.; Scaletta, C.; Waselle, L.; Simon, J.P.; Michetti, M.; Brunet, J.F.; Flahaut, M.; Hirt-Burri, N.; Raffoul, W.; et al. Burn Center Organization and Cellular Therapy Integration: Managing Risks and Costs. J. Burn. Care Res. 2021, 42, 911–924. [Google Scholar] [CrossRef]
- De Luca, M.; Albanese, E.; Bondanza, S.; Megna, M.; Ugozzoli, L.; Molina, F.; Cancedda, R.; Santi, P.L.; Bormioli, M.; Stella, M.; et al. Multicentre experience in the treatment of burns with autologous and allogenic cultured epithelium, fresh or preserved in a frozen state. Burns 1989, 15, 303–309. [Google Scholar] [CrossRef]
- Deglise, B.; Benathan, M.; Frenk, E.; Krupp, S. Preliminary results of burn treatment using an autograft of cultured epidermis. Schweiz. Med. Wochenschr. 1987, 117, 1380–1383. [Google Scholar]
- Braye, F.; Hautier, A.; Bouez, C.; Damour, O. Skin substitutes reconstructed in the laboratory: Application in burn treatment. Pathol. Biol. 2005, 53, 613–617. [Google Scholar] [CrossRef]
- Alexaline, M.M.; Trouillas, M.; Nivet, M.; Bourreau, E.; Leclerc, T.; Duhamel, P.; Martin, M.T.; Doucet, C.; Fortunel, N.O.; Lataillade, J.J. Bioengineering a human plasma-based epidermal substitute with efficient grafting capacity and high content in clonogenic cells. Stem Cells Transl. Med. 2015, 4, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Alexaline, M.M.; Magne, B.; Zuleta Rodriguez, A.; Nivet, M.; Bacqueville, D.; Lataillade, J.J.; Trouillas, M. Influence of fibrin matrices and their released factors on epidermal substitute phenotype and engraftment. J. Tissue Eng. Regen. Med. 2019, 13, 1362–1374. [Google Scholar] [CrossRef]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte-fibroblast interactions in wound healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef]
- Zaulyanov, L.; Kirsner, R.S. A review of a bi-layered living cell treatment (Apligraf) in the treatment of venous leg ulcers and diabetic foot ulcers. Clin. Interv. Aging 2007, 2, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Koch, N.; Erba, P.; Benathan, M.; Raffoul, W. New developments in skin reconstruction—Cell cultures and skin substitutes plus review of the literature. Ann. Burns Fire Disasters 2010, 23, 131–136. [Google Scholar] [PubMed]
- Stark, G.B.; Kaiser, H.W. Cologne Burn Centre experience with glycerol-preserved allogeneic skin: Part II: Combination with autologous cultured keratinocytes. Burns 1994, 20 (Suppl. 1), S34–S38. [Google Scholar] [CrossRef]
- Silla, R.C.; Fong, J.; Wright, J.; Wood, F. Infection in acute burn wounds following the Bali bombings: A comparative prospective audit. Burns 2006, 32, 139–144. [Google Scholar] [CrossRef]
- Hartmann, B.; Ekkernkamp, A.; Johnen, C.; Gerlach, J.C.; Belfekroun, C.; Kuntscher, M.V. Sprayed cultured epithelial autografts for deep dermal burns of the face and neck. Ann. Plast. Surg. 2007, 58, 70–73. [Google Scholar] [CrossRef]
- Gerlach, J.C.; Johnen, C.; Ottomann, C.; Brautigam, K.; Plettig, J.; Belfekroun, C.; Munch, S.; Hartmann, B. Method for autologous single skin cell isolation for regenerative cell spray transplantation with non-cultured cells. Int. J. Artif. Organs 2011, 34, 271–279. [Google Scholar] [CrossRef]
- Nuutila, K.; Katayama, S.; Laitinen, A.; Siltanen, A.; Patrikoski, M.; Valtonen, J.; Kankainen, M.; Kerkela, E.; Kaartinen, T.; Juteau, S.; et al. ATMP-classified, scalable, autologous cell spray for the treatment of skin wounds and assessment of its effects on wound healing clinically and on a molecular level. Burns 2023, 49, 1144–1156. [Google Scholar] [CrossRef] [PubMed]
- Karström, A.; Huss, F. Guide to the Quality and Safety of Tissues and Cells for Human Application; Keitel, S., Ed.; European Directorate for the Quality of Medicines & HealthCare: Strasbourg, France, 2015; pp. 471–477. [Google Scholar]
- Carriel, V.; Garzon, I.; Jimenez, J.M.; Oliveira, A.C.; Arias-Santiago, S.; Campos, A.; Sanchez-Quevedo, M.C.; Alaminos, M. Epithelial and stromal developmental patterns in a novel substitute of the human skin generated with fibrin-agarose biomaterials. Cells Tissues Organs 2012, 196, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Garzon, I.; Miyake, J.; Gonzalez-Andrades, M.; Carmona, R.; Carda, C.; Sanchez-Quevedo Mdel, C.; Campos, A.; Alaminos, M. Wharton’s jelly stem cells: A novel cell source for oral mucosa and skin epithelia regeneration. Stem Cells Transl. Med. 2013, 2, 625–632. [Google Scholar] [CrossRef]
- Martin-Piedra, M.A.; Alfonso-Rodriguez, C.A.; Zapater, A.; Durand-Herrera, D.; Chato-Astrain, J.; Campos, F.; Sanchez-Quevedo, M.C.; Alaminos, M.; Garzon, I. Effective use of mesenchymal stem cells in human skin substitutes generated by tissue engineering. Eur. Cell Mater. 2019, 37, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.M.; Chato-Astrain, J.; Cardona Perez, J.C.; Campos, F.; Perez Gomez, M.; Alaminos, M.; Garzon Bello, I. Evaluation of the optical and biomechanical properties of bioengineered human skin generated with fibrin-agarose biomaterials. J. Biomed. Opt. 2020, 25, 055002. [Google Scholar] [CrossRef]
- Ruiz-Lopez, J.; Cardona, J.C.; Garzon, I.; Perez, M.M.; Alaminos, M.; Chato-Astrain, J.; Ionescu, A.M. Optical Behavior of Human Skin Substitutes: Absorbance in the 200-400 nm UV Range. Biomedicines 2022, 10, 1640. [Google Scholar] [CrossRef]
- Egea-Guerrero, J.J.; Carmona, G.; Correa, E.; Mata, R.; Arias-Santiago, S.; Alaminos, M.; Gacto, P.; Cuende, N. Transplant of Tissue-Engineered Artificial Autologous Human Skin in Andalusia: An Example of Coordination and Institutional Collaboration. Transplant. Proc. 2019, 51, 3047–3050. [Google Scholar] [CrossRef]
- Martin-Piedra, M.A.; Carmona, G.; Campos, F.; Carriel, V.; Fernandez-Gonzalez, A.; Campos, A.; Cuende, N.; Garzon, I.; Gacto, P.; Alaminos, M. Histological assessment of nanostructured fibrin-agarose skin substitutes grafted in burnt patients. A time-course study. Bioeng. Transl. Med. 2023, 8, e10572. [Google Scholar] [CrossRef]
- Braziulis, E.; Biedermann, T.; Hartmann-Fritsch, F.; Schiestl, C.; Pontiggia, L.; Bottcher-Haberzeth, S.; Reichmann, E.; Meuli, M. Skingineering I: Engineering porcine dermo-epidermal skin analogues for autologous transplantation in a large animal model. Pediatr. Surg. Int. 2011, 27, 241–247. [Google Scholar] [CrossRef]
- Schiestl, C.; Biedermann, T.; Braziulis, E.; Hartmann-Fritsch, F.; Bottcher-Haberzeth, S.; Arras, M.; Cesarovic, N.; Nicolls, F.; Linti, C.; Reichmann, E.; et al. Skingineering II: Transplantation of large-scale laboratory-grown skin analogues in a new pig model. Pediatr. Surg. Int. 2011, 27, 249–254. [Google Scholar] [CrossRef]
- Meuli, M.; Hartmann-Fritsch, F.; Huging, M.; Marino, D.; Saglini, M.; Hynes, S.; Neuhaus, K.; Manuel, E.; Middelkoop, E.; Reichmann, E.; et al. A Cultured Autologous Dermo-epidermal Skin Substitute for Full-Thickness Skin Defects: A Phase I, Open, Prospective Clinical Trial in Children. Plast. Reconstr. Surg. 2019, 144, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Schiestl, C.; Neuhaus, K.; Meuli, M.; Farkas, M.; Hartmann-Fritsch, F.; Elrod, J.; Bressan, J.; Reichmann, E.; Bottcher-Haberzeth, S. Long-term outcomes of a cultured autologous dermo-epidermal skin substitute in children: 5 year results of a phase I clinical trial. J. Burn. Care Res. 2024, 46, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Gilpin, D.A.; Meyer, N.A.; Herndon, D.N. Current treatment of severely burned patients. Ann. Surg. 1996, 223, 14–25. [Google Scholar] [CrossRef]
- Auxenfans, C.; Menet, V.; Catherine, Z.; Shipkov, H.; Lacroix, P.; Bertin-Maghit, M.; Damour, O.; Braye, F. Cultured autologous keratinocytes in the treatment of large and deep burns: A retrospective study over 15 years. Burns 2015, 41, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Auxenfans, C.; Shipkov, H.; Bach, C.; Catherine, Z.; Lacroix, P.; Bertin-Maghit, M.; Damour, O.; Braye, F. Cultured allogenic keratinocytes for extensive burns: A retrospective study over 15 years. Burns 2014, 40, 82–88. [Google Scholar] [CrossRef]
- Glat, P.; Quirk, L.; Hultman, S.; Kesey, J.; Jain, A.; Griswald, J.; Natalie, F.; Wibbenmeyer, L.; Amani, H.; Cramer, C.; et al. Establishing Consensus of Best Practice for CEA Use in Treatment of Severe Burns: A US Burn Provider Delphi Study. J. Burn. Care Res. 2024, 45, 1287–1293. [Google Scholar] [CrossRef]
- Gobet, R.; Raghunath, M.; Altermatt, S.; Meuli-Simmen, C.; Benathan, M.; Dietl, A.; Meuli, M. Efficacy of cultured epithelial autografts in pediatric burns and reconstructive surgery. Surgery 1997, 121, 654–661. [Google Scholar] [CrossRef]
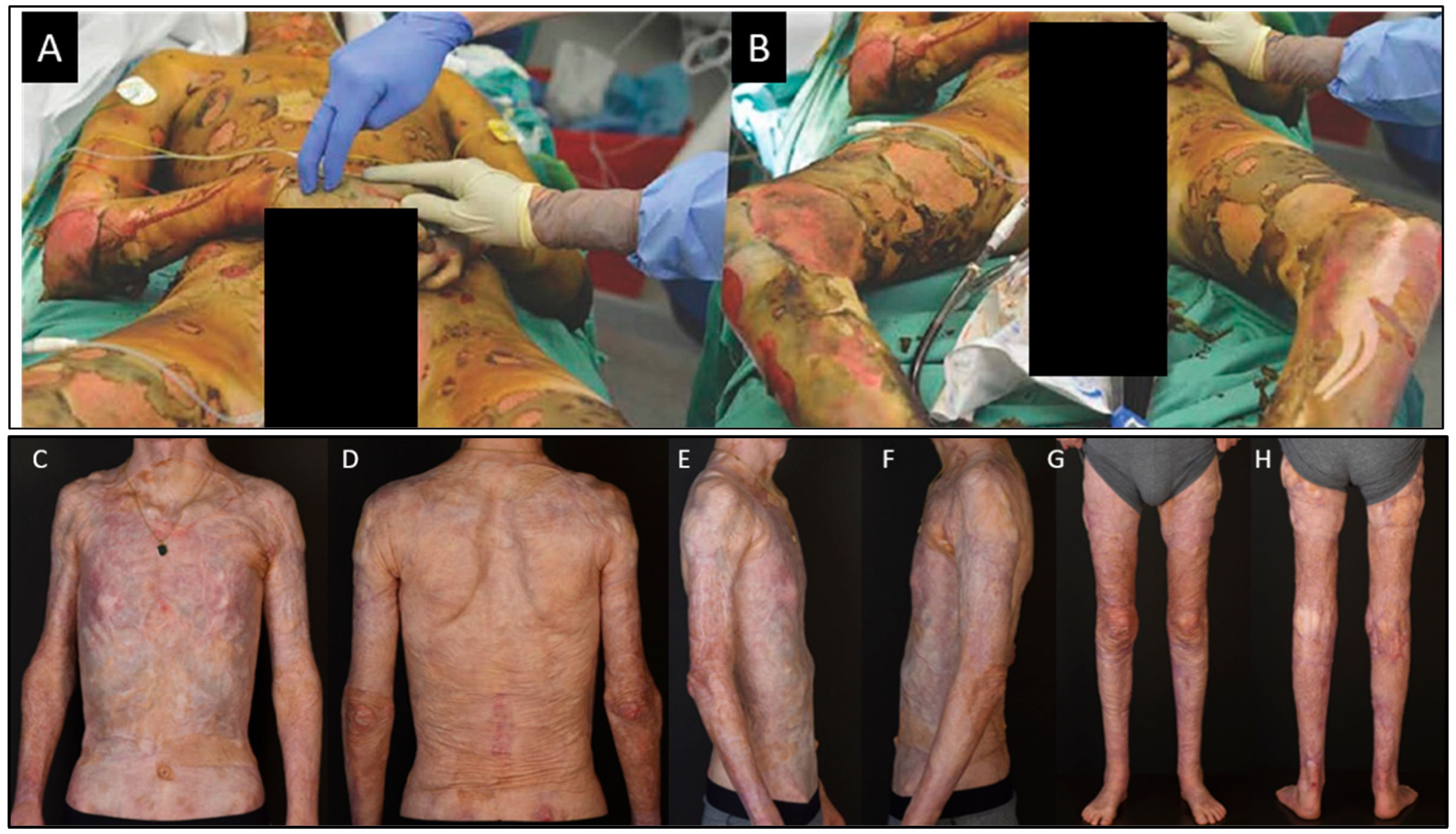
- Abdel-Sayed, P.; Michetti, M.; Scaletta, C.; Flahaut, M.; Hirt-Burri, N.; de Buys Roessingh, A.; Raffoul, W.; Applegate, L.A. Cell therapies for skin regeneration: An overview of 40 years of experience in burn units. Swiss Med. Wkly. 2019, 149, w20079. [Google Scholar] [CrossRef]
- McHeik, J.N.; Barrault, C.; Levard, G.; Morel, F.; Bernard, F.X.; Lecron, J.C. Epidermal healing in burns: Autologous keratinocyte transplantation as a standard procedure: Update and perspective. Plast. Reconstr. Surg. Glob. Open 2014, 2, e218. [Google Scholar] [CrossRef]
- James, S.E.; Booth, S.; Dheansa, B.; Mann, D.J.; Reid, M.J.; Shevchenko, R.V.; Gilbert, P.M. Sprayed cultured autologous keratinocytes used alone or in combination with meshed autografts to accelerate wound closure in difficult-to-heal burns patients. Burns 2010, 36, e10–e20. [Google Scholar] [CrossRef]
- Menon, S.; Li, Z.; Harvey, J.G.; Holland, A.J. The use of the Meek technique in conjunction with cultured epithelial autograft in the management of major paediatric burns. Burns 2013, 39, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Schiestl, C.; Meuli, M.; Vojvodic, M.; Pontiggia, L.; Neuhaus, D.; Brotschi, B.; Reichmann, E.; Böttcher-Haberzeth, S.; Neuhaus, K. Expanding into the future: Combining a novel dermal template with distinct variants of autologous cultured skin substitutes in massive burns. Burns Open 2021, 5, 145–153. [Google Scholar] [CrossRef]
- Verbeken, G.; Draye, J.; Fauconnier, A.; Vanlaere, I.; Huys, I.; De Corte, P.; Verween, G.; Pascual, B.; Delmotte, N.; Pierlot, A. The magistral preparation of advanced therapy medicinal products (ATMPs). J. Surg. Pract. 2020, 2, 1–7. [Google Scholar]
- Good manufacturing practice (GMP) Guidelines. In EudraLex; European Commission: Brussels, Belgium, 2003; Volume 4.
- Kamolz, L.P.; Luegmair, M.; Wick, N.; Eisenbock, B.; Burjak, S.; Koller, R.; Meissl, G.; Frey, M. The Viennese culture method: Cultured human epithelium obtained on a dermal matrix based on fibroblast containing fibrin glue gels. Burns 2005, 31, 25–29. [Google Scholar] [CrossRef]
- Braye, F.; Oddou, L.; Bertin-Maghit, M.; Belgacem, S.; Damour, O.; Spitalier, P.; Guillot, M.; Bouchard, C.; Gueugniaud, P.Y.; Goudeau, M.; et al. Widely meshed autograft associated with cultured autologous epithelium for the treatment of major burns in children: Report of 12 cases. Eur. J. Pediatr. Surg. 2000, 10, 35–40. [Google Scholar] [CrossRef]
- Braye, F.; Pascal, P.; Bertin-Maghit, M.; Colpart, J.J.; Tissot, E.; Damour, O. Advantages of using a bank of allogenic keratinocytes for the rapid coverage of extensive and deep second-degree burns. Med. Biol. Eng. Comput. 2000, 38, 248–252. [Google Scholar] [CrossRef]
- Gomez, C.; Galan, J.M.; Torrero, V.; Ferreiro, I.; Perez, D.; Palao, R.; Martinez, E.; Llames, S.; Meana, A.; Holguin, P. Use of an autologous bioengineered composite skin in extensive burns: Clinical and functional outcomes. A multicentric study. Burns 2011, 37, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Bian, D.; Wu, Y.; Song, G.; Azizi, R.; Zamani, A. The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: A comprehensive review. Stem Cell Res. Ther. 2022, 13, 24. [Google Scholar] [CrossRef]
- Biedermann, T.; Klar, A.S.; Bottcher-Haberzeth, S.; Michalczyk, T.; Schiestl, C.; Reichmann, E.; Meuli, M. Long-term expression pattern of melanocyte markers in light- and dark-pigmented dermo-epidermal cultured human skin substitutes. Pediatr. Surg. Int. 2015, 31, 69–76. [Google Scholar] [CrossRef]
- Bottcher-Haberzeth, S.; Klar, A.S.; Biedermann, T.; Schiestl, C.; Meuli-Simmen, C.; Reichmann, E.; Meuli, M. “Trooping the color”: Restoring the original donor skin color by addition of melanocytes to bioengineered skin analogs. Pediatr. Surg. Int. 2013, 29, 239–247. [Google Scholar] [CrossRef]
- Klar, A.S.; Guven, S.; Biedermann, T.; Luginbuhl, J.; Bottcher-Haberzeth, S.; Meuli-Simmen, C.; Meuli, M.; Martin, I.; Scherberich, A.; Reichmann, E. Tissue-engineered dermo-epidermal skin grafts prevascularized with adipose-derived cells. Biomaterials 2014, 35, 5065–5078. [Google Scholar] [CrossRef] [PubMed]
- Marino, D.; Luginbuhl, J.; Scola, S.; Meuli, M.; Reichmann, E. Bioengineering dermo-epidermal skin grafts with blood and lymphatic capillaries. Sci. Transl. Med. 2014, 6, 221ra214. [Google Scholar] [CrossRef]
| Type of Skin Substitute | Clinical Use Since | Indication | Production Time | Estimated Cost | Regulatory Requirements |
|---|---|---|---|---|---|
| Cultured Epithelial Autografts (CEAs) | Early 1980s → since 2009 with national exemption authorisation | ≥50% TBSA Deep dermal burns (2° deep) or 3° if needed; often combined with widely meshed STSG | 10–21 days | 7 EUR per cm2 |
|
| Cultured Dermal–Epidermal Autografts (CDEAs) | 2000s → since 2009 with national exemption authorisation | ≥50% TBSA | 28–42 days | 10–12 EUR per cm2 |
|
| Cell Suspension/Spray (cultured) | 2000s → notably after Bali bombings 2002 → since 2009 with national exemption authorisation | 50% TBSA, combined with: widely meshed STSG or MEEK | 28–35 days | 5–7 EUR per cm2 |
|
| Autologous two-layered Skin Substitutes | 2000 → only compassionate use | Experimental therapy for burns ≥ 80% TBSA; currently under compassionate use or hospital exemption | 28–42 days | Not yet available * |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the European Burns Association. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auxenfans, C.; Valencia, R.G.; Abdel-Sayed, P.; Alaminos, M.; Brunet, J.-F.; Campos, F.; Chato-Astrain, J.; Carmona, G.; de Buys Roessingh, A.; Droz-Georget, S.; et al. Laboratory-Generated Autologous Skin Substitutes for Burn Treatment in Europe: Narrative Review, Experts’ Opinion, and Legal Considerations. Eur. Burn J. 2025, 6, 30. https://doi.org/10.3390/ebj6020030
Auxenfans C, Valencia RG, Abdel-Sayed P, Alaminos M, Brunet J-F, Campos F, Chato-Astrain J, Carmona G, de Buys Roessingh A, Droz-Georget S, et al. Laboratory-Generated Autologous Skin Substitutes for Burn Treatment in Europe: Narrative Review, Experts’ Opinion, and Legal Considerations. European Burn Journal. 2025; 6(2):30. https://doi.org/10.3390/ebj6020030
Chicago/Turabian StyleAuxenfans, Celine, Rocio G. Valencia, Philippe Abdel-Sayed, Miguel Alaminos, Jean-François Brunet, Fernando Campos, Jesus Chato-Astrain, Gloria Carmona, Anthony de Buys Roessingh, Stephanie Droz-Georget, and et al. 2025. "Laboratory-Generated Autologous Skin Substitutes for Burn Treatment in Europe: Narrative Review, Experts’ Opinion, and Legal Considerations" European Burn Journal 6, no. 2: 30. https://doi.org/10.3390/ebj6020030
APA StyleAuxenfans, C., Valencia, R. G., Abdel-Sayed, P., Alaminos, M., Brunet, J.-F., Campos, F., Chato-Astrain, J., Carmona, G., de Buys Roessingh, A., Droz-Georget, S., Farkas, M., Fernandez Gonzalez, A., Gönczi, E., Huss, F., Hartmann, B., Heusi, B., Karström, A., Moiemen, N., Sartoris, G., ... Böttcher, S. (2025). Laboratory-Generated Autologous Skin Substitutes for Burn Treatment in Europe: Narrative Review, Experts’ Opinion, and Legal Considerations. European Burn Journal, 6(2), 30. https://doi.org/10.3390/ebj6020030











