Mind the Gap! Core-Peripheral Temperature Gradient and Its Relationship to Mortality in Major Burns
Abstract
1. Introduction
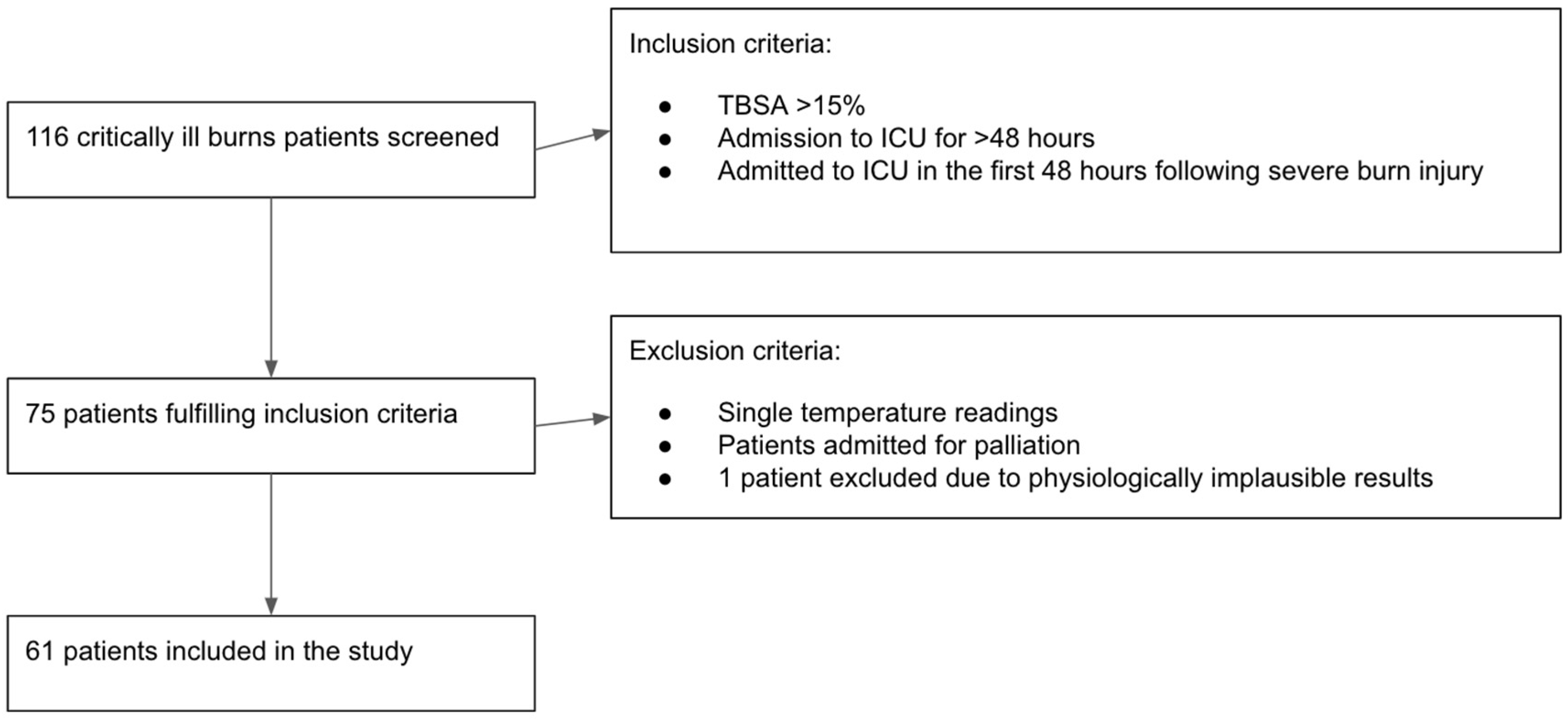
2. Materials and Methods
2.1. Aim of the Study
2.2. Methodology
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Cohort
3.2. Temperature Values in the Initial 48 h
3.3. Predictors of Core-Peripheral Temperature Gap at the Beginning and the End of the Resuscitation Period
3.3.1. Core-Peripheral Gap at Admission to ICU
3.3.2. Core-Peripheral Gap at 48 h
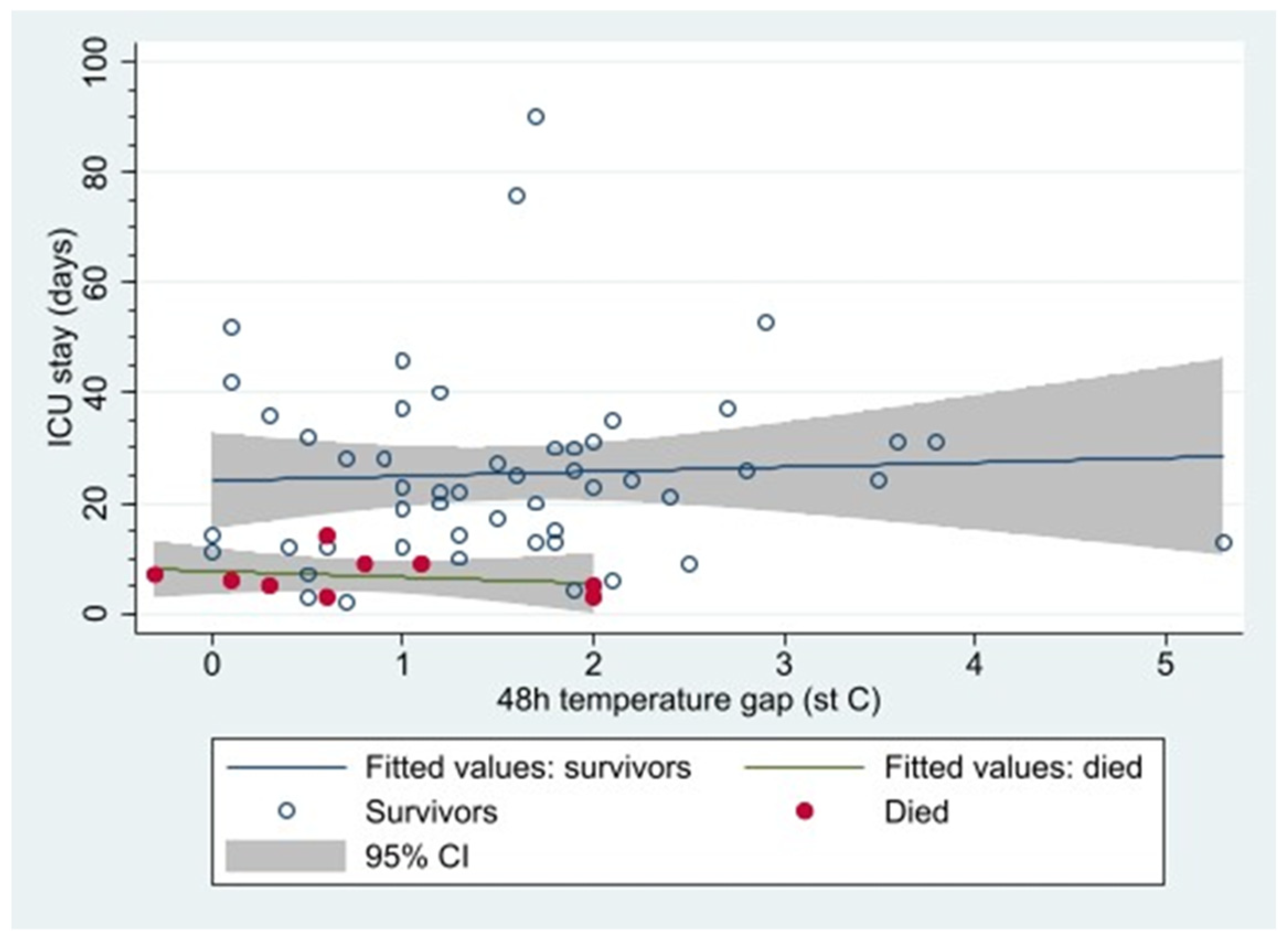
3.3.3. In-ICU Mortality and Core-Peripheral Gap
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahuja, R.B. ISBI practice guidelines for burn care. Editorial. Burn 2016, 42, 951–952. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, B.; Kenngott, T.; Fischer, S.; Hundeshagen, G.; Hartmann, B.; Horter, J.; Münzberg, M.; Kneser, U.; Hirche, C. Early hypothermia as risk factor in severely burned patients: A retrospective outcome study. Burns 2019, 45, 1895–1900. [Google Scholar] [CrossRef]
- Mullhi, R.; Ewington, I.; Chipp, E.; Torlinski, T. A descriptive survey of operating theatre and intensive care unit temperature management of burn patients in the United Kingdom. Int. J. Burns Trauma 2021, 11, 136–144. [Google Scholar] [PubMed]
- Pruskowski, K.A.; Rizzo, J.A.; Shields, B.A.; Chan, R.K.; Driscoll, I.R.; Rowan, M.P.; Chung, K.K. A Survey of Temperature Management Practices Among Burn Centers in North America. J. Burn Care Res. 2018, 39, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Bindu, B.; Bindra, A.; Rath, G. Temperature management under general anesthesia: Compulsion or option. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 306–316. [Google Scholar]
- Schey, B.M.; Williams, D.Y.; Bucknall, T. Skin temperature and core-peripheral temperature gradient as markers of hemodynamic status in critically ill patients: A review. Heart Lung. 2010, 39, 27–40. [Google Scholar] [CrossRef]
- Isben, B. Treatment of Shock with Vasodilators Measuring Skin Temperature on the Big Toe: Ten Years’ Experience in 150 Cases. Dis. Chest 1967, 52, 424–429. [Google Scholar]
- Joly, H.R.; Weil, M.H. Temperature of the great toe as an indication of the severity of shock. Circulation 1969, 39, 131–138. [Google Scholar] [CrossRef]
- Amson, H.; Vacheron, C.H.; Thiolliere, F.; Piriou, V.; Magnin, M.; Allaouchiche, B. Core-to-skin temperature gradient measured by thermography predicts day-8 mortality in septic shock: A prospective observational study. J. Crit. Care 2020, 60, 294–299. [Google Scholar] [CrossRef]
- Woods, I.; Wilkins, R.G.; Edwards, J.D.; Martin, P.D.; Faragher, E.B. Danger of using core/peripehral temperature gradient as a guide to therapy in shock. Crit. Care Med. 1987, 15, 850–852. [Google Scholar] [CrossRef]
- Yeong Brigitte, W.F.; Childs, C. A systematic review on the role of extremity skin temperature as a non-invasive marker for hypoperfusion in critically ill adults in the intensive care setting. JBI Libr. Syst. Rev. 2012, 10, 1504–1548. [Google Scholar]
- Renshaw, A.; Childs, C. The significance of peripheral skin temperature measurement during the acute phase of burn injury: An illustrative case report. Burns 2000, 26, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Ross, B.; Brock, L.; Aynsley-Green, A. Observations on central and peripheral temperatures in the understanding and management of shock. Br. J. Surg. 1969, 56, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Aynsley-Green, A.; Pickering, D. Use of central and peripheral temperature measurements in care of the critically ill child. Arch. Dis. Child. 1974, 49, 477–481. [Google Scholar] [CrossRef]
- Snell, J.A.; Loh, N.H.; Mahambrey, T.; Shokrollahi, K. Clinical review: The critical care management of the burn patient. Crit. Care. 2013, 17, 241. [Google Scholar] [CrossRef] [PubMed]
- Kamolz, L.P.; Andel, H.; Schramm, W.; Meissl, G.; Herndon, D.N.; Frey, M. Lactate: Early predictor of morbidity and mortality in patients with severe burns. Burns 2005, 31, 986–990. [Google Scholar] [CrossRef]
- Jeng, J.C.; Lee, K.; Jablonski, K.; Jordan, M.H. Serum lactate and base deficit suggest inadequate resuscitation of patients with burns injuries: Application of point-of-care laboratory instrument. J. Burn Care Rehabil. 1997, 18, 402–405. [Google Scholar] [CrossRef]
- Hostler, D.; Weaver, M.D.; Ziembicki, J.A.; Kowger, H.L.; McEntire, S.J.; Rittenberger, J.C.; Callaway, C.W.; Patterson, P.D.; Corcos, A.C. Admission temperature and survival in patients admitted to burn centers. J. Burn Care Res. 2013, 34, 498–506. [Google Scholar] [CrossRef]
- Jeschke, M.G.; van Baar, M.E.; Chaudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 11. [Google Scholar] [CrossRef]
- Sherren, P.B.; Hussey, J.; Martin, R.; Kundishora, T.; Parker, M.; Emerson, B. Lethal triad in severe burns. Burns 2014, 40, 1492–1496. [Google Scholar] [CrossRef]
- Williams, F.N.; Herndon, D.N.; Jeschke, M.G. The hypermetabolic response to burn injury and interventions to modify this response. Clin. Plast. Surg. 2009, 36, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Driver, J.; Fielding, A.; Mullhi, R.; Chipp, E.; Torlinski, T. Temperature management of adult burn patients in intensive care: Findings from a retrospective cohort study in a tertiary centre in the United Kingdom. Anaesthesiol. Intensive Ther. 2022, 54, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Lavrentieva, A. Critical care of burn patients. New approaches to old problems. Burns 2016, 42, 13–19. [Google Scholar] [CrossRef] [PubMed]
| Mean | SD | |
|---|---|---|
| Sex: Male/Female | 45/16 (73.7%/26.2%) | |
| Age (years) | 43.1 | 16.6 |
| Inhalational burns (n, %) | 37 (60.6%) | |
| Revised Baux score | 99.2 | 27.4 |
| High Baux > 100 (n, %) | 31 (50.8%) | |
| TBSA (%) | 45.8 | 20.3 |
| High TSBA > 50% (n, %) | 25 (41%) |
| Mean | SD | |
|---|---|---|
| Core temperature (°C) | 36.6 | 1.4 |
| Peripheral temperature (°C) | 35.2 | 2.2 |
| Core-peripheral gap (°C) | 1.4 | 1.7 |
| Hypothermia (n, %) | 18 (29.5%) | |
| Core-peripheral gap > 2 °C (n, %) | 19 (31.1%) | |
| Core-peripheral gap < 0 °C (n, %) | 11 (18.0%) |
| Mean | SD | |
|---|---|---|
| Core temperature (°C) | 37.8 | 0.79 |
| Hypothermia (n, %) | 2 (3.3%) | |
| Core-peripheral gap > 2 °C (n, %) | 12 (19.7%) | |
| Core-peripheral gap < 0 °C (n, %) | 1 (1.6%) |
| Odds Ratio | Lower 95% CI | Upper 95% CI | p-Value | |
|---|---|---|---|---|
| Female | 3.091 | 0.938 | 10.188 | 0.064 |
| Age > 65 y | 0.871 | 0.153 | 4.948 | 0.876 |
| TBSA | 1.010 | 0.983 | 1.038 | 0.469 |
| Revised Baux score | 1.005 | 0.985 | 1.026 | 0.603 |
| Core temperature (°C) | 0.839 | 0.564 | 1.250 | 0.388 |
| Hypothermia (<36 °C) | 2.327 | 0.733 | 7.385 | 0.152 |
| Flame burns | 3.600 | 0.411 | 31.563 | 0.248 |
| TBSA > 50 | 1.070 | 0.356 | 3.212 | 0.905 |
| Baux score > 100 | 1.512 | 0.507 | 4.515 | 0.458 |
| External transfer | 0.236 | 0.027 | 2.039 | 0.189 |
| Odds Ratio | Lower 95% CI | Upper 95% CI | p-Value | |
|---|---|---|---|---|
| Age > 65 y | 0.652 | 0.071 | 5.988 | 0.705 |
| Female | 0.923 | 0.216 | 3.945 | 0.914 |
| TBSA | 1.030 | 0.997 | 1.064 | 0.076 |
| Revised Baux score | 1.022 | 0.995 | 1.049 | 0.111 |
| Baseline core temperature (°C) | 1.047 | 0.657 | 1.670 | 0.846 |
| 48 h core temperature (°C) | 3.214 | 1.162 | 8.890 | 0.024 |
| Hypothermia (<36 °C) | 1.250 | 0.324 | 4.826 | 0.746 |
| Flame burns | 0.698 | 0.122 | 3.983 | 0.685 |
| TBSA > 50 | 3.765 | 0.989 | 14.329 | 0.052 |
| Baux score > 100 | 3.682 | 0.888 | 15.274 | 0.073 |
| External transfer | 1.200 | 0.216 | 6.676 | 0.835 |
| Odds Ratio | Lower 95% CI | Upper 95% CI | p-Value | |
|---|---|---|---|---|
| Age > 65 y | 2.629 | 0.425 | 16.263 | 0.299 |
| Female | 1.462 | 0.319 | 6.698 | 0.625 |
| Revised Baux score | 1.033 | 0.999 | 1.068 | 0.056 |
| Baseline core temperature (°C) | 0.751 | 0.449 | 1.258 | 0.277 |
| 48 h core temperature (°C) | 0.247 | 0.080 | 0.758 | 0.015 |
| Baseline core-peripheral gap > 2 °C | 1.020 | 0.671 | 1.550 | 0.926 |
| 48 h core-peripheral gap > 2 °C | 0.350 | 0.126 | 0.970 | 0.044 |
| Hypothermia (<36 °C) | 2.114 | 0.495 | 9.027 | 0.312 |
| Flame burns | 1.000 | |||
| TBSA > 50 | 2.105 | 0.503 | 8.816 | 0.308 |
| Revised Baux score > 100 | 2.250 | 0.507 | 9.993 | 0.286 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the European Burns Association. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keohane, N.; Driver, J.; Mullhi, R.; Chipp, E.; Torlinska, B.; Torlinski, T. Mind the Gap! Core-Peripheral Temperature Gradient and Its Relationship to Mortality in Major Burns. Eur. Burn J. 2025, 6, 11. https://doi.org/10.3390/ebj6010011
Keohane N, Driver J, Mullhi R, Chipp E, Torlinska B, Torlinski T. Mind the Gap! Core-Peripheral Temperature Gradient and Its Relationship to Mortality in Major Burns. European Burn Journal. 2025; 6(1):11. https://doi.org/10.3390/ebj6010011
Chicago/Turabian StyleKeohane, Niamh, Jennifer Driver, Randeep Mullhi, Elizabeth Chipp, Barbara Torlinska, and Tomasz Torlinski. 2025. "Mind the Gap! Core-Peripheral Temperature Gradient and Its Relationship to Mortality in Major Burns" European Burn Journal 6, no. 1: 11. https://doi.org/10.3390/ebj6010011
APA StyleKeohane, N., Driver, J., Mullhi, R., Chipp, E., Torlinska, B., & Torlinski, T. (2025). Mind the Gap! Core-Peripheral Temperature Gradient and Its Relationship to Mortality in Major Burns. European Burn Journal, 6(1), 11. https://doi.org/10.3390/ebj6010011






