Sustainable Primary Cell Banking for Topical Compound Cytotoxicity Assays: Protocol Validation on Novel Biocides and Antifungals for Optimized Burn Wound Care
Abstract
1. Introduction
2. Materials and Methods
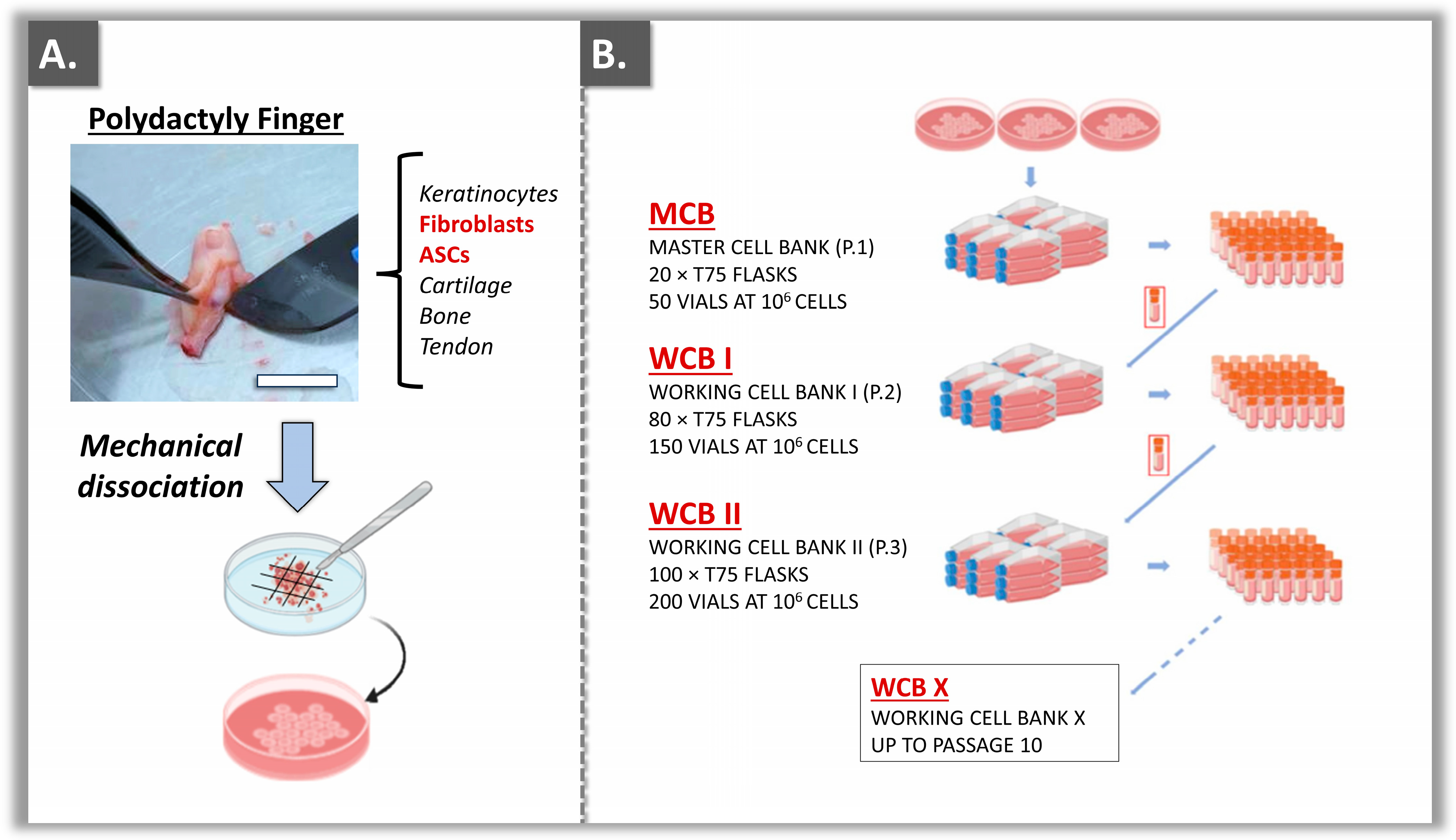
2.1. Primary Dermal Fibroblast and ASC Source Establishment
2.2. Primary Cell Subcultures and Tiered Biobanking Model Establishment
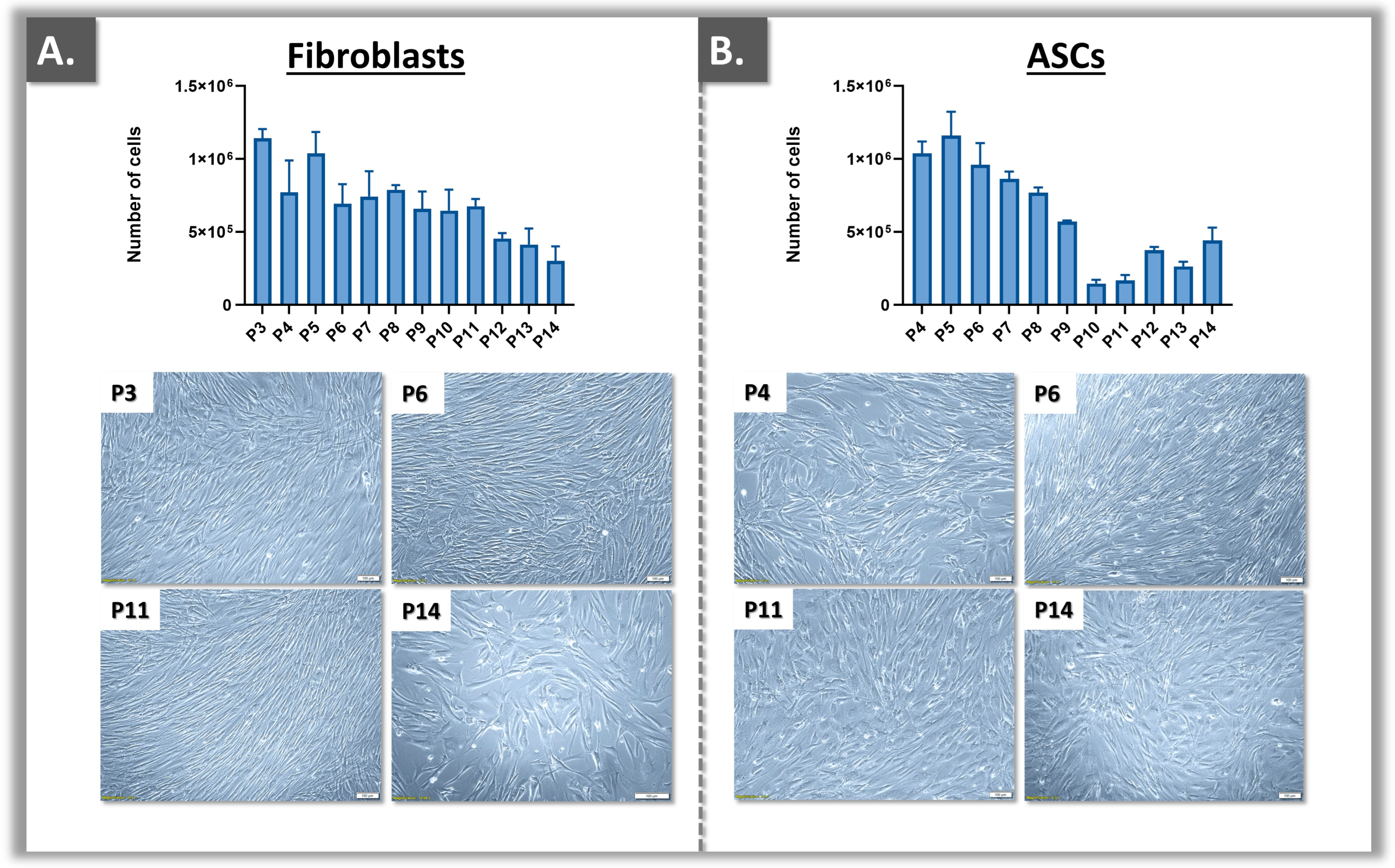
2.3. Primary Cell Proliferation Assessment and End-of-Passage Determination
2.4. Primary ASC-Type Qualification: Adipogenesis Assays
2.4.1. ASC Expansion and Adipogenic Induction
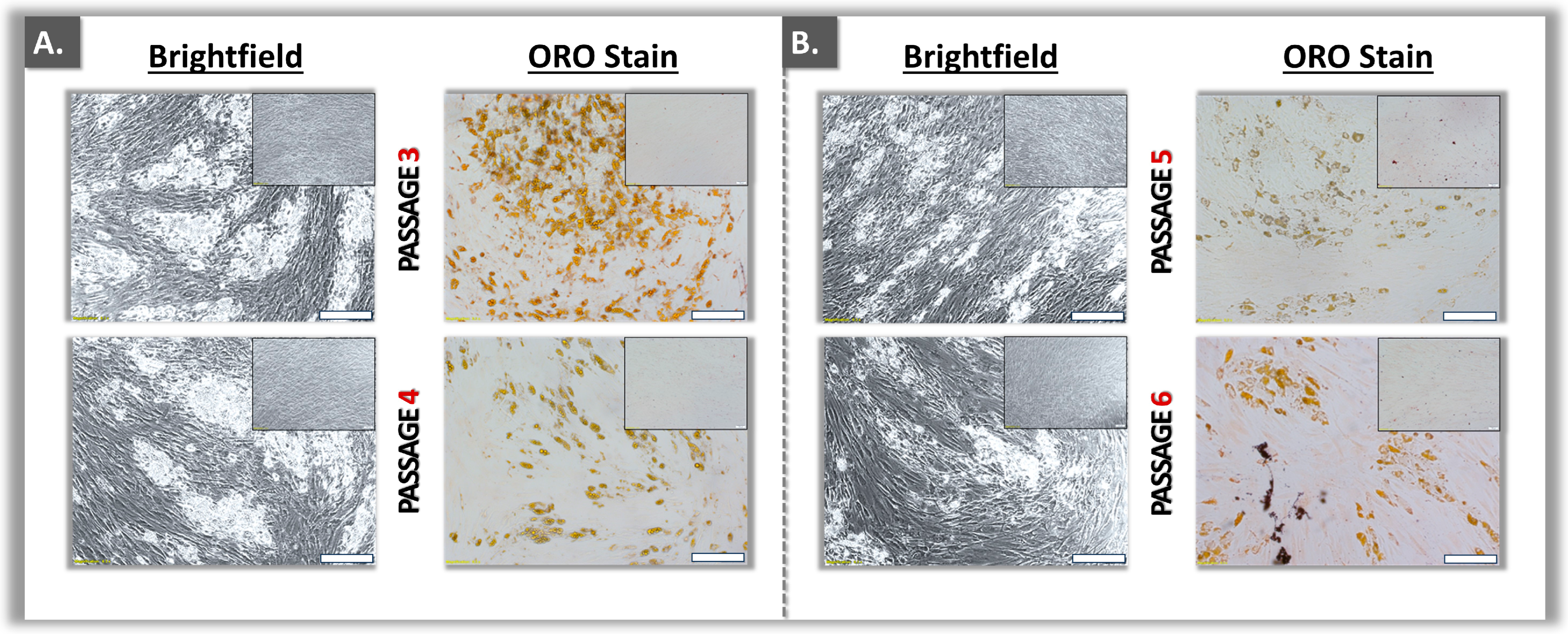
2.4.2. Induced ASC Lipid Droplet Analysis: Oil Red O Staining and Quantification
2.5. Cytotoxicity Evaluation of Biocidal and Antifungal Compounds
2.5.1. Biocides and Antifungal Compound Sources
2.5.2. Sample Preparation for In Vitro Cytotoxicity Assays
2.5.3. Validation of the Cytotoxicity Model in 24-Well Plates
2.5.4. Cytotoxicity Model Optimization in 96-Well Plates
2.6. Statistical Analysis and Data Presentation
3. Results
3.1. Primary Cell Bank Establishment and Cell Source Sustainability Evaluation
3.2. Functional Qualification of Polydactyly ASC Sources
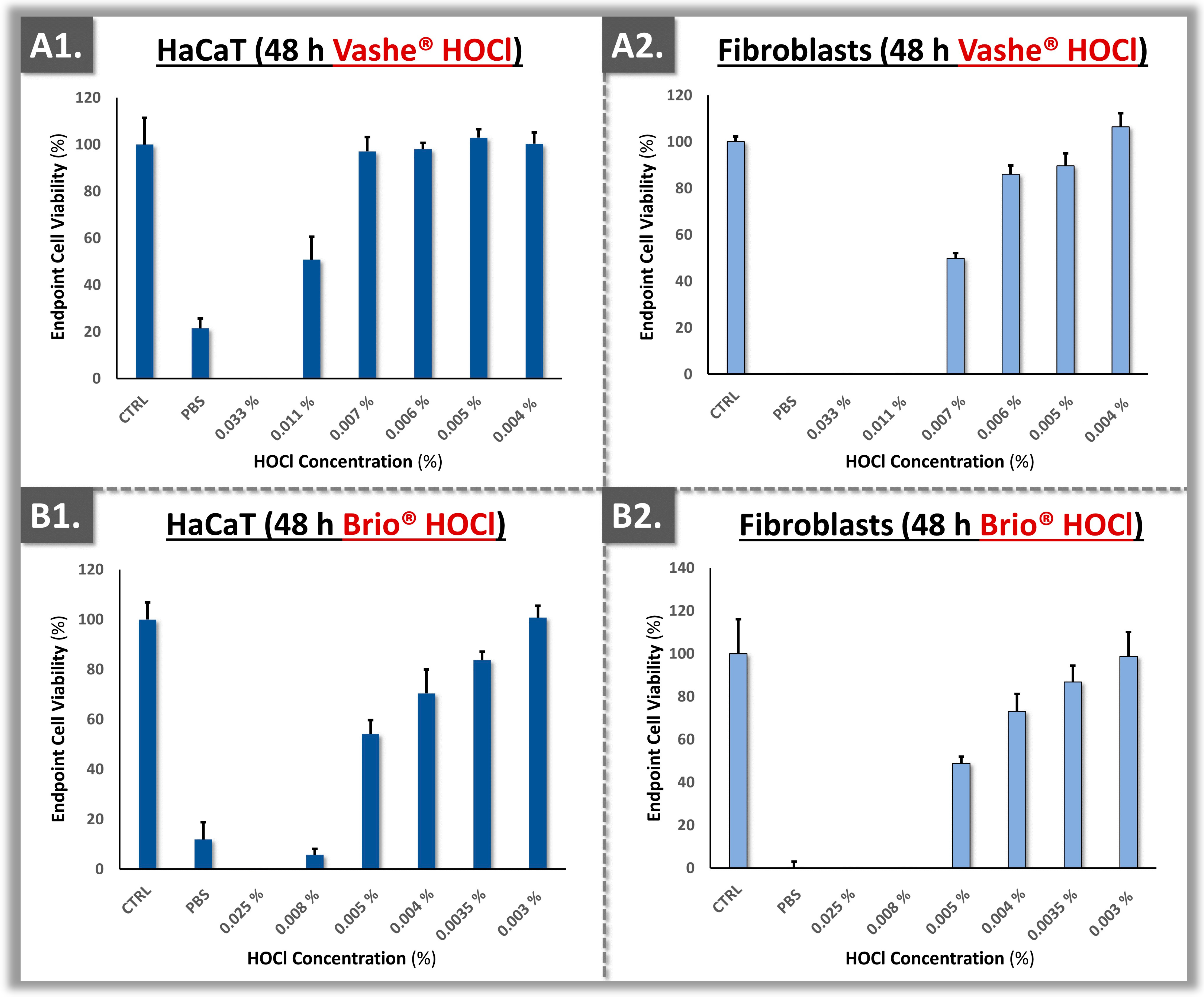
3.3. Cytotoxicity Model Validation Using Primary Polydactyly-Derived Dermal Fibroblasts
3.4. Cytotoxicity Model Validation Using Primary Polydactyly ASCs
4. Discussion
4.1. Available Cellular Models for In Vitro Topical Compound Cytotoxicity Assays
4.2. Suboptimal Safety Profile of CLX as a Topical Antiseptic in Burn Care
4.3. Topical HOCl Constitutes a Safer Alternative to CLX in Burn Care
4.4. Primary Cells for Highly Sustainable and Biologically Relevant In Vitro Cytotoxicity Assays
4.5. Pertinence of Cell-Based Cytotoxicity Assay Readouts
4.6. Determination of Topical Cytotoxic Doses for the AR-12 Compound
4.7. Study Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| AM | Adipogenic medium |
| AR-12 | Antifungal compound |
| ASC | Adipose-derived stem cells |
| ATCC | American Type Culture Collection |
| ATP | Adenosine triphosphate |
| CE | EU mark of conformity |
| CHO | Chinese hamster ovary |
| CHUV | Lausanne University Hospital |
| CLX | Chlorhexidine |
| DMEM | Dulbecco’s modified Eagle medium |
| DMSO | Dimethyl sulfoxide |
| DNA | Deoxyribonucleic acid |
| EOP | End-of-passage |
| EU | European Union |
| FBS | Fetal bovine serum |
| HaCaT | Immortalized human keratinocytes |
| HEK | Human embryonic kidney cells |
| hESC | Human embryonic stem cells |
| HOCl | Hypochlorous acid |
| HPL | Human platelet lysate |
| IBMX | 3-isobutyl-1-methylxanthine |
| IC50 | Dose inhibitory of one-half of the response |
| ISO | International standards organization |
| MCB | Master cell bank |
| MIC | Minimum inhibitory concentration |
| NRU | Neutral red uptake |
| OECD | Organization for Economic Co-operation and Development |
| ORO | Oil Red O |
| PAF | Paraformaldehyde |
| PBS | Phosphate-buffered saline |
| PCB | Parental cell bank |
| Ph. Eur. | European Pharmacopoeia |
| ROS | Reactive oxygen species |
| rpm | Rotations per minute |
| SD | Standard deviation |
| USA | United States of America |
| WCB | Working cell bank |
| 3T3 | Murine fibroblasts |
References
- Palackic, A.; Popp, D.; Tapking, C.; Houschyar, K.S.; Branski, L.K. Fungal infections in burn patients. Surg. Inf. 2021, 22, 83–87. [Google Scholar] [CrossRef]
- Norbury, W.; Herndon, D.N.; Tanksley, J.; Jeschke, M.G.; Finnerty, C.C. Infection in burns. Surg. Inf. 2016, 17, 250–255. [Google Scholar] [CrossRef]
- Hauc, S.C.; Stögner, V.A.; Ihnat, J.M.; Hosseini, H.; Huelsboemer, L.; Kauke-Navarro, M.; Rivera, J.C.; Williams, M.; Glahn, J.Z.; Savetamal, A.; et al. Understanding the drivers of cost and length of stay in a cohort of 21,875 patients with severe burn. J. Burn Care Res. 2024, 45, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Slaviero, L.; Avruscio, G.; Vindigni, V.; Tocco-Tussardi, I. Antiseptics for burns: A review of the evidence. Ann. Burns Fire Dis. 2018, 31, 198–203. [Google Scholar]
- Hemmati, J.; Azizi, M.; Asghari, B.; Arabestani, M.R. Multidrug-resistant pathogens in burn wound, prevention, diagnosis, and therapeutic approaches (conventional antimicrobials and nanoparticles). Can. J. Infect. Dis. Med. Microbiol. 2023, 2023, 8854311. [Google Scholar] [CrossRef]
- Ortega-Llamas, L.; Quiñones-Vico, M.I.; García-Valdivia, M.; Fernández-González, A.; Ubago-Rodríguez, A.; Sanabria-de la Torre, R.; Arias-Santiago, S. Cytotoxicity and wound closure evaluation in skin cell lines after treatment with common antiseptics for clinical use. Cells 2022, 11, 1395. [Google Scholar] [CrossRef]
- Rueda-Fernández, M.; Melguizo-Rodríguez, L.; Costela-Ruiz, V.J.; de Luna-Bertos, E.; Ruiz, C.; Ramos-Torrecillas, J.; Illescas-Montes, R. Effect of the most common wound antiseptics on human skin fibroblasts. Clin. Exp. Dermatol. 2022, 47, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Sayed, P.; Tornay, D.; Hirt-Burri, N.; de Buys Roessingh, A.; Raffoul, W.; Laurent, L.A. Implications of chlorhexidine use in burn units for wound healing. Burns 2020, 46, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- International Wound Infection Institute (IWII). Wound Infection in Clinical Practice; Wounds International: London, UK, 2022. [Google Scholar]
- Khan, A.; Nikhil, V.; Pandey, A.; Chaturvedi, P. Effectiveness of polyhexamethylene biguanide, chlorhexidine, and calcium hydroxide intracanal medicament against intraradicular mature polymicrobial biofilm: A microbiological study. J. Conserv. Dent. 2022, 25, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, E.; Liu, P.; Schultz, G.S.; Martins-Green, M.M.; Tanaka, R.; Weir, D.; Gould, L.J.; Armstrong, D.G.; Gibbons, G.W.; Wolcott, R.; et al. Chronic wounds: Treatment consensus. Wound Rep. Regen. 2022, 30, 156–171. [Google Scholar] [CrossRef]
- Gold, M.H.; Andriessen, A.; Bhatia, A.C.; Bitter, P., Jr.; Chilukuri, S.; Cohen, J.L.; Robb, C.W. Topical stabilized hypochlorous acid: The future gold standard for wound care and scar management in dermatologic and plastic surgery procedures. J. Cosmet. Dermatol. 2020, 19, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Sakarya, S.; Gunay, N.; Karakulak, M.; Ozturk, B.; Ertugrul, B. Hypochlorous acid: An ideal wound care agent with powerful microbicidal, antibiofilm, and wound healing potency. Wounds 2014, 26, 342–350. [Google Scholar] [PubMed]
- OECD. Guidance Document on Using Cytotoxicity Tests to Estimate Starting doses for Acute Oral Systemic Toxicity Tests, No. 129; OECD: Paris, France, 2010. [Google Scholar]
- OECD. Guidance Document for the Testing of Chemicals: In Vitro Skin Sensitization, No. 442D; OECD: Paris, France, 2022. [Google Scholar]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices. International Standards Organization: Geneva, Switzerland, 2022.
- Garg, S.; Huifu, H.; Kaul, S.C.; Wadhwa, R. Integration of conventional cell viability assays for reliable and reproducible read-outs: Experimental evidence. BMC Res. Notes 2018, 11, 403. [Google Scholar] [CrossRef] [PubMed]
- Olschläger, V.; Schrader, A.; Hockertz, S. Comparison of primary human fibroblasts and keratinocytes with immortalized cell lines regarding their sensitivity to sodium dodecyl sulfate in a neutral red uptake cytotoxicity assay. Arzneimittelforschung 2009, 59, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Abud, A.P.; Zych, J.; Reus, T.L.; Kuligovski, C.; de Moraes, E.; Dallagiovanna, B.; De Aguiar, A.M. The use of human adipose-derived stem cells-based cytotoxicity assay for acute toxicity test. Regul. Toxicol. Pharmacol. 2015, 73, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Xu, T.; Strickland, J.; Zhang, L.; Fang, Y.; Tao, D.; Simeonov, A.; Huang, R.; Kleinstreuer, N.C.; Xia, M. Use of in vitro methods combined with in silico analysis to identify potential skin sensitizers in the Tox21 10K compound library. Front. Toxicol. 2024, 6, 1321857. [Google Scholar] [CrossRef]
- Kanďárová, H.; Pôbiš, P. The “Big Three” in biocompatibility testing of medical devices: Implementation of alternatives to animal experimentation-are we there yet? Front. Toxicol. 2024, 5, 1337468. [Google Scholar] [CrossRef] [PubMed]
- Mannerström, M.; Toimela, T.; Sarkanen, J.R.; Heinonen, T. Human BJ fibroblasts is an alternative to mouse BALB/c 3T3 cells in in vitro neutral red uptake assay. Basic Clin. Pharmacol. Toxicol. 2017, 121, 109–115. [Google Scholar] [CrossRef]
- Klemola, K.; Pearson, J.; von Wright, A.; Liesivuori, J. Evaluating the toxicity of reactive dyes and dyed fabrics with the HaCaT cytotoxicity test. Autex Res. J. 2007, 7, 224–230. [Google Scholar] [CrossRef]
- Rusanov, A.L.; Luzgina, N.G.; Lisitsa, A.V. Sodium dodecyl sulfate cytotoxicity towards HaCaT keratinocytes: Comparative analysis of methods for evaluation of cell viability. Bull. Exp. Biol. Med. 2017, 163, 284–288. [Google Scholar] [CrossRef]
- Almeida, A.; Sarmento, B.; Rodrigues, F. Insights on in vitro models for safety and toxicity assessment of cosmetic ingredients. Int. J. Pharm. 2017, 519, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Zerbinati, N.; Sommatis, S.; Maccario, C.; Di Francesco, S.; Capillo, M.C.; Grimaldi, G.; Rauso, R.; Herrera, M.; Bencini, P.L.; Mocchi, R. A practical approach for the in vitro safety and efficacy assessment of an anti-ageing cosmetic cream enriched with functional compounds. Molecules 2021, 26, 7592. [Google Scholar] [CrossRef] [PubMed]
- Abud, A.P.R.; Paschoal, A.C.C.; Kuligovski, C.; Caruso, R.R.B.; Dallagiovanna, B.; de Aguiar, A.M. Using inhibition of the adipogenesis of adipose-derived stem cells in vitro for toxicity prediction. MethodsX 2021, 8, 101515. [Google Scholar] [CrossRef] [PubMed]
- OECD. Guidance Document on the Validation and International Acceptance of New or Updated Test Methods for Hazard Assessment; OECD Series on Testing and Assessment, No. 34. OECD: Paris, France, 2005. [Google Scholar]
- Krähenbühl, S.M.; Grognuz, A.; Michetti, M.; Raffoul, W.; Laurent, L.A. Enhancement of human adipose-derived stem cell expansion and stability for clinical use. Int. J. Stem Cell Res. Ther. 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Lynch, S.; Pridgeon, C.S.; Duckworth, C.A.; Sharma, P.; Park, B.K.; Goldring, C.E.P. Stem cell models as an in vitro model for predictive toxicology. Biochem. J. 2019, 476, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.; Abdel-Sayed, P.; Hirt-Burri, N.; Scaletta, C.; Michetti, M.; de Buys Roessingh, A.; Raffoul, W.; Applegate, L.A. Evolution of diploid progenitor lung cell applications: From optimized biotechnological substrates to potential active pharmaceutical ingredients in respiratory tract regenerative medicine. Cells 2021, 10, 2526. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, E.; Levinson, C.; Hertl, M.; Broguiere, N.; Brück, O.; Mustjoki, S.; Gerstenberg, A.; Weber, D.; Salzmann, G.; Steinwachs, M.; et al. Characterization of polydactyly chondrocytes and their use in cartilage engineering. Sci. Rep. 2019, 9, 4275. [Google Scholar] [CrossRef] [PubMed]
- Maehara, M.; Sato, M.; Toyoda, E.; Takahashi, T.; Okada, E.; Kotoku, T.; Watanabe, M. Characterization of polydactyly-derived chondrocyte sheets versus adult chondrocyte sheets for articular cartilage repair. Inflamm. Regen. 2017, 37, 22. [Google Scholar] [CrossRef] [PubMed]
- Kehe, K.; Abend, M.; Kehe, K.; Ridi, R.; Peter, R.U.; van Beuningen, D. Tissue engineering with HaCaT cells and a fibroblast cell line. Arch. Dermatol. Res. 1999, 291, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Balin, A.K.; Fisher, A.J.; Anzelone, M.; Leong, I.; Allen, R.G. Effects of establishing cell cultures and cell culture conditions on the proliferative life span of human fibroblasts isolated from different tissues and donors of different ages. Exp. Cell Res. 2002, 274, 275–287. [Google Scholar] [CrossRef]
- Melichercíková, V.; Urban, J.; Goroncy-Bermes, P. Residual effect of antiseptic substances on human skin. J. Hosp. Inf. 2010, 75, 238–239. [Google Scholar] [CrossRef] [PubMed]
- Edmiston, C.E.; Bruden, B.; Rucinski, M.C.; Henen, C.; Graham, M.B.; Lewis, B.L. Reducing the risk of surgical site infections: Does chlorhexidine gluconate provide a risk reduction benefit? Am. J. Inf. Contr. 2013, 41, S49–S55. [Google Scholar] [CrossRef] [PubMed]
- Raines, K.; Rosen, K. The effect of chlorhexidine bathing on rates of nosocomial infections among the critically ill population: An analysis of current clinical research and recommendations for practice. Dim. Crit. Care Nurs. 2016, 35, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Severing, A.L.; Rembe, J.D.; Koester, V.; Stuermer, E.K. Safety and efficacy profiles of different commercial sodium hypochlorite/hypochlorous acid solutions (NaClO/HClO): Antimicrobial efficacy, cytotoxic impact, and physicochemical parameters in vitro. J. Antimicrob. Chemother. 2019, 74, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Peña, S.; Hidalgo-González, C.; Robson, M.C.; Krötzsch, E. In vitro microbicidal, anti-biofilm and cytotoxic effects of different commercial antiseptics. Int. Wound J. 2017, 14, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Murakami, K.; Fukuda, K.; Nakamura, S.; Kuwabara, M.; Hattori, H.; Fujita, M.; Kiyosawa, T.; Yokoe, H. Stability of weakly acidic hypochlorous acid solution with microbicidal activity. Biocontrol. Sci. 2017, 22, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Krynicka, K.; Trzeciak, M. The role of sodium hypochlorite in atopic dermatitis therapy: A narrative review. Int. J. Derm. 2022, 61, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Majewski, S.; Bhattacharya, T.; Asztalos, M.; Bohaty, B.; Durham, K.C.; West, D.P.; Hebert, A.A.; Paller, A.S. Sodium hypochlorite body wash in the management of Staphylococcus aureus-colonized moderate-to-severe atopic dermatitis in infants, children, and adolescents. Ped. Dermatol. 2019, 36, 442–447. [Google Scholar] [CrossRef]
- Jull, A.B.; Cullum, N.; Dumville, J.C.; Westby, M.J.; Deshpande, S.; Walker, N. Honey as a topical treatment for wounds. Cochrane Data Syst. Rev. 2015, 2015, CD005083. [Google Scholar] [CrossRef]
- Block, M.S.; Rowan, B.G. Hypochlorous acid: A review. J. Oral Maxillofac. Surg. 2020, 78, 1461–1466. [Google Scholar] [CrossRef]
- Fukuzaki, S. Uses of gaseous hypochlorous acid for controlling microorganisms in indoor spaces. J. Microorg. Contr. 2023, 28, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Tazawa, K.; Jadhav, R.; Azuma, M.M.; Fenno, J.C.; McDonald, N.J.; Sasaki, H. Hypochlorous acid inactivates oral pathogens and a SARS-CoV-2-surrogate. BMC Oral Health 2023, 23, 111. [Google Scholar] [CrossRef] [PubMed]
- Kubota, A.; Goda, T.; Tsuru, T.; Yonekura, T.; Yagi, M.; Kawahara, H.; Yoneda, A.; Tazuke, Y.; Tani, G.; Ishii, T.; et al. Efficacy and safety of strong acid electrolyzed water for peritoneal lavage to prevent surgical site infection in patients with perforated appendicitis. Surg. Today 2015, 45, 876–879. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Matsuda, S.; Jamsransuren, D.; Ogawa, H. Comparison of the SARS-CoV-2-inactivating activities of the differently manufactured hypochlorous acid water products with various pH. J. Water Health 2021, 19, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Briotech. Instructions for Use of the Brio® Product; Briotech: Everett, WA, USA, 2022; unpublished work. [Google Scholar]
- Urgo Medical. Instructions for Use of the Vashe® Product; Urgo Medical: Shepshed, UK, 2022; unpublished work. [Google Scholar]
- Repetto, G.; del Peso, A.; Zurita, J. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Koselny, K.; Green, J.; Favazzo, L.; Glazier, V.E.; DiDone, L.; Ransford, S.; Krysan, D.J. Antitumor/antifungal celecoxib derivative AR-12 is a non-nucleoside inhibitor of the ANL-family adenylating enzyme acetyl CoA synthetase. ACS Infect. Dis. 2016, 2, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Koselny, K.; Green, J.; DiDone, L.; Halterman, J.P.; Fothergill, A.W.; Wiederhold, N.P.; Patterson, T.F.; Cushion, M.T.; Rappelye, C.; Wellington, M.; et al. The celecoxib derivative AR-12 has broad-spectrum antifungal activity in vitro and improves the activity of fluconazole in a murine model of cryptococcosis. Antimicrob. Agents Chemother. 2016, 60, 7115–7127. [Google Scholar] [CrossRef] [PubMed]
- Abt, E.R.; Rosser, E.W.; Durst, M.A.; Lok, V.; Poddar, S.; Le, T.M.; Cho, A.; Kim, W.; Wei, L.; Song, J.; et al. Metabolic modifier screen reveals secondary targets of protein kinase inhibitors within nucleotide metabolism. Cell Chem. Biol. 2020, 27, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Dolatyari, M.; Rostami, A. Strong anti-viral nano biocide based on Ag/ZnO modified by amodiaquine as an antibacterial and antiviral composite. Sci. Rep. 2022, 12, 19934. [Google Scholar] [CrossRef]
- Mast, J.; Van Miert, E.; Siciliani, L.; Cheyns, K.; Blaude, M.N.; Wouters, C.; Waegeneers, N.; Bernsen, R.; Vleminckx, C.; Van Loco, J.; et al. Application of silver-based biocides in face masks intended for general use requires regulatory control. Sci. Tot. Environ. 2023, 870, 161889. [Google Scholar] [CrossRef]
- El Sayed, M.T.; El-Sayed, A.S.A. Biocidal activity of metal nanoparticles synthesized by Fusarium solani against multidrug-resistant bacteria and mycotoxigenic fungi. J. Microbiol. Biotechnol. 2020, 30, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Hasanin, M.; Elbahnasawy, M.A.; Shehabeldine, A.M.; Hashem, A.H. Ecofriendly preparation of silver nanoparticles-based nanocomposite stabilized by polysaccharides with antibacterial, antifungal and antiviral activities. Biometals 2021, 34, 1313–1328. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Analysis Methods | Targets | Cumulative Acceptance Criteria 1 | Application Levels | Application Timepoints 2 |
|---|---|---|---|---|---|
| Cellular morphology | Microscopic operator assessment | Fibroblastic morphology | Fibroblastic morphology maintenance | MCB; WCB I; WCB II | In-process; before medium exchanges |
| Cell monolayer confluency level | Microscopic operator assessment | Endpoint confluency level 95–100% | Increasing confluency level throughout expansion; absolute value > 95% at harvest | MCB; WCB I; WCB II | In-process; before medium exchanges |
| Cell proliferation in culture | Microscopic operator assessment | At least 2.5 population doublings in expansion | At least 2.5 population doublings at harvest | MCB; WCB I; WCB II | In-process; after harvest |
| Cell monolayer homogeneity | Microscopic operator assessment | Monolayer homogeneity | Monolayer homogeneity maintenance | MCB; WCB I; WCB II | In-process; before medium exchanges |
| Cell population homogeneity | Microscopic operator assessment | No cell population contamination | Absence of a contaminant cell population | MCB; WCB I; WCB II | In-process; before medium exchanges |
| Cell proliferation medium aspect | Macroscopic operator assessment | No adventitious contamination | Absence of contamination indication (e.g., yellowing, turbidity) | MCB; WCB I; WCB II | In-process; before medium exchanges |
| Cellular viability at harvest | Manual enumeration with Trypan Blue dye | Harvest cell viability 85–100% | Viability level ≥ 85% | MCB; WCB I; WCB II | In-process; after harvest |
| Cellular viability at initiation from cryostorage | Manual enumeration with Trypan Blue dye | Initiation cell viability 85–100% | Viability level ≥ 85% | MCB; WCB I; WCB II | Post-process; after initiation from storage |
| Cellular recovery at initiation from cryostorage | Manual enumeration with Trypan Blue dye | Homogeneous cell bank lot (cell quantity/vial) | Cell quantity at filling ± 15% 3 | MCB; WCB I; WCB II | Post-process; after initiation from storage |
| Passage Level | FBS-Based Medium | HPL-Based Medium | ||
|---|---|---|---|---|
| Lipid Droplet Density | ORO Quantification | Lipid Droplet Density | ORO Quantification | |
| Passage level 3 | +++ | +++ | ++ | ++ |
| Passage level 4 | +++ | +++ | ++ | ++ |
| Passage level 5 | +++ | +++ | ++ | ++ |
| Passage level 6 | ++ | ++ | ++ | ++ |
| Product | Original Product Concentration 1 | Working Concentration Range |
|---|---|---|
| Chlorhexidine CHUV | 0.100% | 0.100–0.00001% |
| Brio® HOCl | 0.025% | 0.025–0.003% |
| Vashe® HOCl | 0.033% | 0.033–0.004% |
| AR-12 | 10 mg/mL stock solution | 64.0–0.125 μg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Z.; Laurent, N.; Hirt-Burri, N.; Scaletta, C.; Abdel-Sayed, P.; Raffoul, W.; Luo, S.; Krysan, D.J.; Laurent, A.; Applegate, L.A. Sustainable Primary Cell Banking for Topical Compound Cytotoxicity Assays: Protocol Validation on Novel Biocides and Antifungals for Optimized Burn Wound Care. Eur. Burn J. 2024, 5, 249-270. https://doi.org/10.3390/ebj5030024
Liao Z, Laurent N, Hirt-Burri N, Scaletta C, Abdel-Sayed P, Raffoul W, Luo S, Krysan DJ, Laurent A, Applegate LA. Sustainable Primary Cell Banking for Topical Compound Cytotoxicity Assays: Protocol Validation on Novel Biocides and Antifungals for Optimized Burn Wound Care. European Burn Journal. 2024; 5(3):249-270. https://doi.org/10.3390/ebj5030024
Chicago/Turabian StyleLiao, Zhifeng, Nicolas Laurent, Nathalie Hirt-Burri, Corinne Scaletta, Philippe Abdel-Sayed, Wassim Raffoul, Shengkang Luo, Damian J. Krysan, Alexis Laurent, and Lee Ann Applegate. 2024. "Sustainable Primary Cell Banking for Topical Compound Cytotoxicity Assays: Protocol Validation on Novel Biocides and Antifungals for Optimized Burn Wound Care" European Burn Journal 5, no. 3: 249-270. https://doi.org/10.3390/ebj5030024
APA StyleLiao, Z., Laurent, N., Hirt-Burri, N., Scaletta, C., Abdel-Sayed, P., Raffoul, W., Luo, S., Krysan, D. J., Laurent, A., & Applegate, L. A. (2024). Sustainable Primary Cell Banking for Topical Compound Cytotoxicity Assays: Protocol Validation on Novel Biocides and Antifungals for Optimized Burn Wound Care. European Burn Journal, 5(3), 249-270. https://doi.org/10.3390/ebj5030024









