State of the Art: An Update on Adult Burn Resuscitation
Abstract
1. Introduction
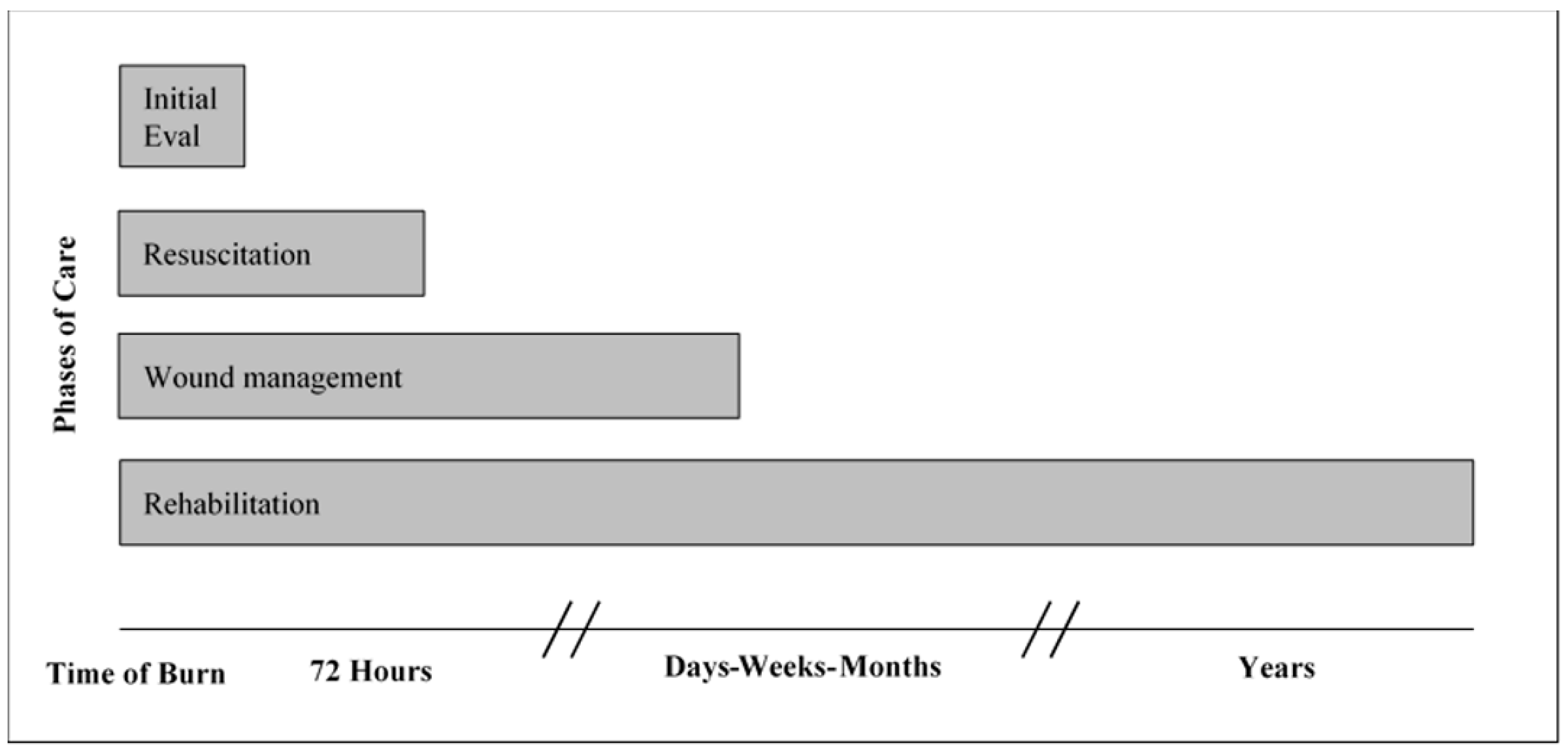
2. Overlapping Phases of Care
3. Pathophysiology of Burn Shock
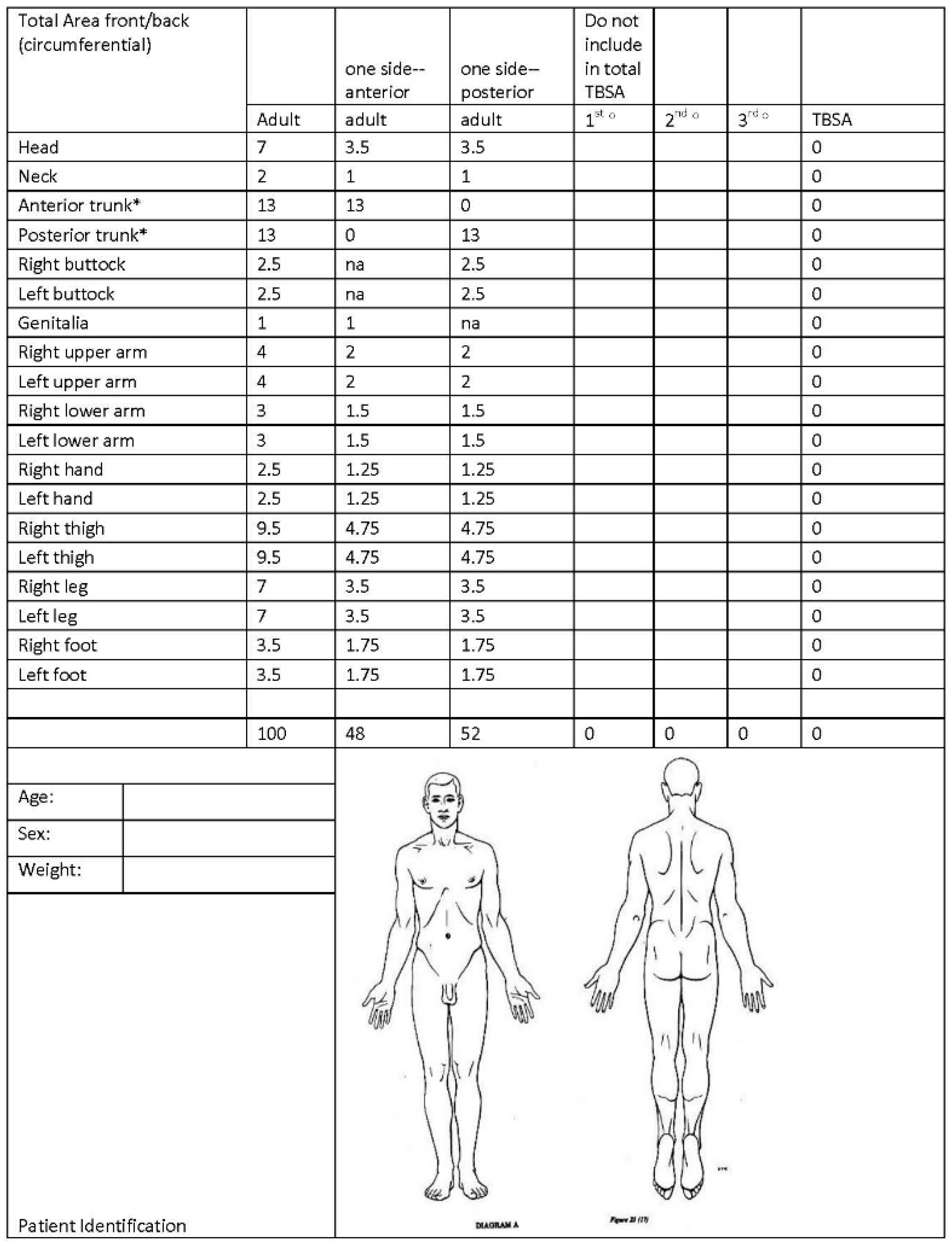
4. Initial Assessment
5. Resuscitation Calculations
6. Endpoints of Resuscitation
7. Adjuncts to Resuscitation
8. Complications of Resuscitation
9. Pitfalls of Resuscitation
10. Analgesia and Sedation
11. Wound Management during Resuscitation
12. Rehabilitation during Resuscitation
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pruitt, B.A. The effectiveness of fluid resuscitation. J. Trauma 1979, 19, S868–S870. [Google Scholar]
- Haberal, M.; Abali, A.E.S.; Karakayali, H. Fluid management in major burn injuries. Indian J. Plast. Surg. 2010, 43, 29–36. [Google Scholar] [CrossRef]
- Rowan, M.P.; Cancio, L.C.; Elster, E.A.; Burmeister, D.M.; Rose, L.F.; Natesan, S.; Chan, R.K.; Christy, R.J.; Chung, K.K. Burn wound healing and treatment: Review and advancements. Crit. Care 2015, 19, 243. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.K.; Bacomo, F.K. A primer on burn resuscitation. J. Emergencies Trauma Shock 2011, 4, 109–113. [Google Scholar] [CrossRef]
- Snell, J.A.; Loh, N.-H.W.; Mahambrey, T.; Shokrollahi, K. Clinical review: The critical care management of the burn patient. Crit. Care 2013, 17, 1–10. [Google Scholar] [CrossRef]
- Horton, J.W. Left ventricular contractile dysfunction as a complication of thermal injury. Shock 2004, 22, 495–507. [Google Scholar] [CrossRef]
- Woodcock, T.E. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: An improved paradigm for prescribing intravenous fluid therapy. Br. J. Anaesth. 2012, 108, 384–394. [Google Scholar] [CrossRef]
- Gurney, J.M.; Kozar, R.A.; Cancio, L.C. Plasma for burn shock resuscitation: Is it time to go back to the future? Transfusion 2019, 59, 1578–1586. [Google Scholar] [CrossRef]
- Purdue, G.F.; Hunt, J.L. Multiple trauma and the burn patient. Am. J. Surg. 1989, 158, 536–539. [Google Scholar] [CrossRef]
- Pruitt, L.B.A. Management of Burns in the Multiple Injury Patient. Surg. Clin. N. Am. 1970, 50, 1283–1300. [Google Scholar] [CrossRef]
- Dougherty, W.; Waxman, K. The complexities of managing severe burns with associated trauma. Surg. Clin. N. Am. 1996, 76, 923–958. [Google Scholar] [CrossRef]
- Santaniello, J.M.; Luchette, F.; Esposito, T.J.; Gunawan, H.; Reed, R.L.; Davis, K.A.; Gamelli, R.L. Ten Year Experience of Burn, Trauma, and Combined Burn/Trauma Injuries Comparing Outcomes. J. Trauma Inj. Infect. Crit. Care 2004, 57, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Brandt, C.P.; Yowler, C.J.; Fratianne, R.B. Burns with multiple trauma. Am. Surg. 2002, 68, 240–244. [Google Scholar]
- Hawkins, A.; MacLennan, P.A.; McGwin, G.; Cross, J.M.; Rue, L.W. The Impact of Combined Trauma and Burns on Patient Mortality. J. Trauma Inj. Infect. Crit. Care 2005, 58, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Battaloglu, E.; Iniguez, M.F.; Lecky, F.; Porter, K. Incidence of combined burns and major trauma in England and Wales. Trauma 2018, 22, 51–55. [Google Scholar] [CrossRef]
- Purdue, G.F.; Hunt, J.L.; Layton, T.R.; Copeland, C.E.; Delmundo, A.G.; Baxter, C.R. Burns in Motor Vehicle Accidents. J. Trauma Inj. Infect. Crit. Care 1985, 25, 216–219. [Google Scholar] [CrossRef]
- Wolf, S.E.; Kauvar, D.S.; Wade, C.E.; Cancio, L.C.; Renz, E.P.; Horvath, E.E.; White, C.E.; Park, M.S.; Wanek, S.; Albrecht, M.A.; et al. Comparison Between Civilian Burns and Combat Burns from Operation Iraqi Freedom and Operation Enduring Freedom. Ann. Surg. 2006, 243, 786–795. [Google Scholar] [CrossRef]
- Harrington, D.T. Complicated Burn Resuscitation. Crit. Care Clin. 2016, 32, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Clarey, A.; Trainor, D. Critical care management of severe burns and inhalational injury. Anaesth. Intensiv. Care Med. 2017, 18, 395–400. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Prim. 2020, 6, 11. [Google Scholar] [CrossRef]
- Woodson, L.C.; Branski, L.K.; Enkhbaatar, P.; Talon, M. Diagnosis and treatment of inhalation injury. In Total Burn Care, 5th ed.; Hernon, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 184–194. [Google Scholar]
- Douin, D.; Schauer, S.G.; Anderson, E.L.; Jones, J.; DeSanto, K.; Cunningham, C.W.; Bebarta, V.S.; Ginde, A.A. Systematic review of oxygenation and clinical outcomes to inform oxygen targets in critically ill trauma patients. J. Trauma Acute Care Surg. 2019, 87, 961–977. [Google Scholar] [CrossRef]
- Wachtel, T.L.; Berry, C.C.; Wachtel, E.E.; Frank, H.A. The inter-rater reliability of estimating the size of burns from various burn area chart drawings. Burns 2000, 26, 156–170. [Google Scholar] [CrossRef]
- Giretzlehner, M.; Ganitzer, I.; Haller, H. Technical and Medical Aspects of Burn Size Assessment and Documentation. Medicina 2021, 57, 242. [Google Scholar] [CrossRef]
- Parvizi, D.; Kamolz, L.-P.; Giretzlehner, M.; Haller, H.L.; Trop, M.; Selig, H.; Nagele, P.; Lumenta, D.B. The potential impact of wrong TBSA estimations on fluid resuscitation in patients suffering from burns: Things to keep in mind. Burns 2014, 40, 241–245. [Google Scholar] [CrossRef]
- Murari, A.; Singh, K.N. Lund and Browder chart—modified versus original: A comparative study. Acute Crit. Care 2019, 34, 276–281. [Google Scholar] [CrossRef]
- Parvizi, D.; Giretzlehner, M.; Wurzer, P.; Klein, L.D.; Shoham, Y.; Bohanon, F.J.; Haller, H.L.; Tuca, A.; Branski, L.K.; Lumenta, D.B.; et al. BurnCase 3D software validation study: Burn size measurement accuracy and inter-rater reliability. Burns 2016, 42, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.-B.; Zeng, D.; Wan, Y.; Yao, L.; Tang, H.-T.; Xia, Z.-F. BurnCalc assessment study of computer-aided individual three-dimensional burn area calculation. J. Transl. Med. 2014, 12, 242. [Google Scholar] [CrossRef][Green Version]
- Prieto, M.F.; Acha, B.; Gomez-Cia, T.; Fondón, I.; Serrano, C. A system for 3D representation of burns and calculation of burnt skin area. Burns 2011, 37, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, D.G. Burn resuscitation: The results of the ISBI/ABA survey. Burns 2010, 36, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Blumetti, J.; Hunt, J.L.; Arnoldo, B.D.; Parks, J.K.; Purdue, G.F. The Parkland formula under fire: Is the criticism justified? J. Burn Care Res. 2008, 29, 180–186. [Google Scholar] [CrossRef]
- Bhat, S.; Humphries, Y.M.; Gulati, S.; Rylah, B.; Olson, W.E.; Twomey, J. The problems of burn resuscitation formulas: A need for a simplified guideline. J. Burn. Wounds 2004, 3, 7. [Google Scholar]
- Boehm, D.; Menke, H. A History of Fluid Management—From “One Size Fits All” to an Individualized Fluid Therapy in Burn Resuscitation. Medicina 2021, 57, 187. [Google Scholar] [CrossRef]
- Al-Benna, S.; Leary, J.; Sheikh, Z.; Rodrigues, J. A survey of fluid resuscitation protocols of burns in intensive care units of the United Kingdom and Ireland. Burns 2009, 35, S14–S15. [Google Scholar] [CrossRef]
- Pham, T.N.; Cancio, L.C.; Gibran, N. American Burn Association Practice Guidelines Burn Shock Resuscitation. J. Burn Care Res. 2008, 29, 257–266. [Google Scholar] [CrossRef]
- Csontos, C.; Foldi, V.; Fischer, T.; Bogar, L. Factors affecting fluid requirement on the first day after severe burn trauma. ANZ J. Surg. 2007, 77, 745–748. [Google Scholar] [CrossRef]
- Klein, M.B.; Hayden, D.; Elson, C.; Nathens, A.B.; Gamelli, R.L.; Gibran, N.S.; Herndon, D.N.; Arnoldo, B.; Silver, G.; Schoenfeld, D.; et al. The Association Between Fluid Administration and Outcome Following Major Burn. Ann. Surg. 2007, 245, 622–628. [Google Scholar] [CrossRef] [PubMed]
- American Burn Association. Disaster Management. 2019. Available online: https://ameriburn.org/quality-care/disaster-response/disaster-management/ (accessed on 25 June 2021).
- Chung, K.K.; Salinas, J.; Renz, E.M.; Alvarado, R.A.; King, B.T.; Barillo, D.J.; Cancio, L.C.; Wolf, S.; Blackbourne, L.H. Simple Derivation of the Initial Fluid Rate for the Resuscitation of Severely Burned Adult Combat Casualties: In Silico Validation of the Rule of 10. J. Trauma Inj. Infect. Crit. Care 2010, 69, S49–S54. [Google Scholar] [CrossRef]
- Gueugniaud, P.Y.; Carsin, H.; Bertin-Maghit, M.; Petit, P. Current advances in the initial management of major thermal burns. Intensive Care Med. 2000, 26, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, I.R.; Gurney, J.; Stockinger, Z.T.; Renz, E.M.; Cancio, L.C.; Chung, K.K.; King, B.T.; Rizzo, J.A.; Graybill, J.C.; Peterson, W.C.; et al. Burn Care Clinical Practice Guideline. Available online: https://jts.amedd.army.mil/index.cfm/PI_CPGs/cpgs (accessed on 25 June 2021).
- Benicke, M.; Perbix, W.; Lefering, R.; Knam, F.; Ipaktchi, K.R.; Tannapfel, A.; Neugebauer, E.A.; Spilker, G. New multifactorial burn resuscitation formula offers superior predictive reliability in comparison to established algorithms. Burns 2009, 35, 30–35. [Google Scholar] [CrossRef]
- Rosenthal, J.; Clark, A.; Campbell, S.; McMahon, M.; Arnoldo, B.; Wolf, S.E.; Phelan, H. Effects of obesity on burn resuscitation. Burns 2018, 44, 1947–1953. [Google Scholar] [CrossRef]
- Rae, L.; Pham, T.N.; Carrougher, G.; Honari, S.; Gibran, N.S.; Arnoldo, B.D.; Gamelli, R.L.; Tompkins, R.G.; Herndon, D.N. Differences in Resuscitation in Morbidly Obese Burn Patients May Contribute to High Mortality. J. Burn Care Res. 2013, 34, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, L.; Purvis, M.; Miles, D.; Lintner, A.; Scott, V.; McGinn, K.; Bright, A.; Kahn, S. An Adjusted Ideal Body Weight Index Formula with Fresh Frozen Plasma (FFP) Rescue Decreases Fluid Creep During Burn Resuscitation. Ann. Burn. Fire Disasters 2020, 33, 216–223. [Google Scholar]
- Liu, N.T.; Fenrich, C.A.; Serio-Melvin, M.L.; Peterson, W.C.; Cancio, L.C.; Salinas, J. The impact of patient weight on burn resuscitation. J. Trauma Acute Care Surg. 2017, 83, S112–S119. [Google Scholar] [CrossRef] [PubMed]
- Gus, E.; Cleland, H. Burn fluid resuscitation formulae: Concept and misconception. Injury 2021, 52, 780–781. [Google Scholar] [CrossRef]
- Alvarado, R.; Chung, K.K.; Cancio, L.C.; Wolf, S.E. Burn resuscitation. Burns 2009, 35, 4–14. [Google Scholar] [CrossRef]
- Leclerc, T.; Potokar, T.; Hughes, A.; Norton, I.; Alexandru, C.; Haik, J.; Moiemen, N.; Almeland, S.K. A simplified fluid resuscitation formula for burns in mass casualty scenarios: Analysis of the consensus recommendation from the WHO Emergency Medical Teams Technical Working Group on Burns. Burns 2021. [Google Scholar] [CrossRef] [PubMed]
- Cancio, L.C. Initial Assessment and Fluid Resuscitation of Burn Patients. Surg. Clin. N. Am. 2014, 94, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Herndon, D.N.; Culnan, D.M.; Sherman, W.C.; Chung, K.K.; Wolf, S.E. Critical Care in the Severely Burned: Organ Support and Management. In Total Burn Care; Elsevier: Amsterdam, The Netherlands, 2018; pp. 328–354. [Google Scholar]
- Diver, A.J. The evolution of burn fluid resuscitation. Int. J. Surg. 2008, 6, 345–350. [Google Scholar] [CrossRef]
- Holm, C.; Mayr, M.; Tegeler, J.; Hörbrand, F.; von Donnersmarck, G.H.; Mühlbauer, W.; Pfeiffer, U. A clinical randomized study on the effects of invasive monitoring on burn shock resuscitation. Burns 2004, 30, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Tricklebank, S. Modern trends in fluid therapy for burns. Burns 2009, 35, 757–767. [Google Scholar] [CrossRef]
- Hodgman, E.I.; Subramanian, M.; Arnoldo, B.D.; Phelan, H.A.; Wolf, S.E. Future Therapies in Burn Resuscitation. Crit. Care Clin. 2016, 32, 611–619. [Google Scholar] [CrossRef]
- Paratz, J.D.; Stockton, K.; Paratz, E.D.; Blot, S.; Muller, M.; Lipman, J.; Boots, R. Burn Resuscitation—Hourly Urine Output Versus Alternative Endpoints. Shock 2014, 42, 295–306. [Google Scholar] [CrossRef]
- Andel, D.; Kamolz, L.-P.; Roka, J.; Schramm, W.; Zimpfer, M.; Frey, M.; Andel, H. Base deficit and lactate: Early predictors of morbidity and mortality in patients with burns. Burns 2007, 33, 973–978. [Google Scholar] [CrossRef]
- Kamolz, L.-P.; Andel, H.; Schramm, W.; Meissl, G.; Herndon, D.; Frey, M. Lactate: Early predictor of morbidity and mortality in patients with severe burns. Burns 2005, 31, 986–990. [Google Scholar] [CrossRef]
- Muthukumar, V.; Arumugam, P.K.; Narasimhan, A.; Kumar, S.; Sharma, U.; Sharma, S.; Kain, R. Blood Lactate and Lactate Clearance: Refined Biomarker and Prog-nostic Marker in Burn Resuscitation. Ann. Burn. Fire Disasters 2020, 33, 293–298. [Google Scholar]
- Sterling, S.A.; Puskarich, M.; Jones, A.E. The effect of liver disease on lactate normalization in severe sepsis and septic shock: A cohort study. Clin. Exp. Emerg. Med. 2015, 2, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Luft, F.C. Lactic Acidosis Update for Critical Care Clinicians. J. Am. Soc. Nephrol. 2001, 12, S15–S19. [Google Scholar] [CrossRef]
- Baud, F.J.; Borron, S.W.; Mégarbane, B.; Trout, H.; Lapostolle, F.; Vicaut, E.; Debray, M.; Bismuth, C. Value of lactic acidosis in the assessment of the severity of acute cyanide poisoning. Crit. Care Med. 2002, 30, 2044–2050. [Google Scholar] [CrossRef]
- Zhao, L.-P.; Huang, Y.-S.; He, T.; Hu, X.-G. Clinical study on hematocrit used as a predictor for evaluation of resuscitation effect in the early shock stage after burn. Zhonghua Shao Shang Za Zhi Zhonghua Shaoshang Zazhi Chin. J. Burn. 2013, 29, 235–238. [Google Scholar]
- Held, J.M.; Litt, J.; Kennedy, J.D.; McGrane, S.; Gunter, O.L.; Rae, L.; Kahn, S.A. Surgeon-Performed Hemodynamic Transesophageal Echo-cardiography in the Burn Intensive Care Unit. J. Burn Care Res. 2016, 37, e63–e68. [Google Scholar] [CrossRef]
- Davenport, L.; Dobson, G.P.; Letson, H.L. The role of invasive monitoring in the resuscitation of major burns: A systematic review and meta-analysis. Int. J. Burn. Trauma 2019, 9, 28–40. [Google Scholar]
- Holm, C.; Melcer, B.; Hörbrand, F.; Wörl, H.; Von Donnersmarck, G.H.; Mühlbauer, W. Intrathoracic Blood Volume as an End Point in Resuscitation of the Severely Burned: An Observational Study of 24 Patients. J. Trauma Inj. Infect. Crit. Care 2000, 48, 728–734. [Google Scholar] [CrossRef]
- Arlati, S.; Storti, E.; Pradella, V.; Bucci, L.; Vitolo, A.; Pulici, M. Decreased fluid volume to reduce organ damage: A new approach to burn shock resuscitation? A preliminary study. Resuscitation 2007, 72, 371–378. [Google Scholar] [CrossRef]
- Sánchez, M.; García-De-Lorenzo, A.; Herrero, E.; Lopez, T.; Galvan, B.; Asensio, M.J.; Cachafeiro, L.; Casado, C. A protocol for resuscitation of severe burn patients guided by transpulmonary thermodilution and lactate levels: A 3-year prospective cohort study. Crit. Care 2013, 17, R176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, X.; Ye, S.; Xu, L. Ultrasonographic Measurement of the Respiratory Variation in the Inferior Vena Cava Diameter Is Predictive of Fluid Responsiveness in Critically Ill Patients: Systematic Review and Meta-analysis. Ultrasound Med. Biol. 2014, 40, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Comish, P.; Walsh, M.; Castillo-Angeles, M.; Kuhlenschmidt, K.; Carlson, D.; Arnoldo, B.; Kubasiak, J. Adoption of rescue colloid during burn resuscitation decreases fluid administered and restores end-organ perfusion. Burns 2021. [Google Scholar] [CrossRef]
- Navickis, R.J.; Greenhalgh, D.G.; Wilkes, M.M. Albumin in Burn Shock Resuscitation. J. Burn Care Res. 2016, 37, e268–e278. [Google Scholar] [CrossRef]
- Vlachou, E.; Gosling, P.; Moiemen, N. Hydroxyethylstarch supplementation in burn resuscitation—A prospective randomised controlled trial. Burns 2010, 36, 984–991. [Google Scholar] [CrossRef]
- Tanaka, H.; Matsuda, T.; Miyagantani, Y.; Yukioka, T.; Matsuda, H.; Shimazaki, S. Reduction of Resuscitation Fluid Volumes in Severely Burned Patients Using Ascorbic Acid Administration. Arch. Surg. 2000, 135, 326–331. [Google Scholar] [CrossRef]
- Kahn, S.A.; Beers, R.J.; Lentz, C.W. Resuscitation after Severe Burn Injury Using High-Dose Ascorbic Acid: A Retrospective Review. J. Burn Care Res. 2011, 32, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Falwell, S.; Greenhalgh, D.; Palmieri, T.; Sen, S. High-Dose Ascorbic Acid for Burn Shock Resuscitation May Not Improve Outcomes. J. Burn Care Res. 2018, 39, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.V.; Carney, B.C.; Luker, J.N.; Monger, K.W.; Vazquez, J.S.; Moffatt, L.T.; Johnson, L.S.; Shupp, J.W. Plasma Ameliorates Endothelial Dysfunction in Burn Injury. J. Surg. Res. 2018, 233, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, B.A., Jr.; Mason, A.D., Jr.; Moncrief, J.A. Hemodynamic changes in the early postburn patient: The influence of fluid admin-istration and of a vasodilator (hydralazine). J. Trauma 1971, 11, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, C.W.; Dorethy, J.; Lam, V.; Pruitt, B.A. Randomized Trial of Efficacy of Crystalloid and Colloid Resuscitation on Hemodynamic Response and Lung Water Following Thermal Injury. Ann. Surg. 1983, 197, 520–531. [Google Scholar] [CrossRef]
- Demling, R.H.; Smith, M.; Bodai, B.; Harms, B.; Gunther, R.; Kramer, G. Comparison of Postburn Capillary Permeability in Soft Tissue and Lung. J. Burn. Care Rehabil. 1981, 2, 86–93. [Google Scholar] [CrossRef]
- O’Mara, M.; Slater, H.; Goldfarb, I.W.; Caushaj, P.F. A Prospective, Randomized Evaluation of Intra-abdominal Pressures with Crystalloid and Colloid Resuscitation in Burn Patients. J. Trauma Inj. Infect. Crit. Care 2005, 58, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.; Loh, E.; Hsu, C.; Lin, H.; Huang, C.; Chou, Y.; Lien, C.; Tam, K. Fluid Resuscitation in Patients with Severe Burns: A Meta-analysis of Randomized Controlled Trials. Acad. Emerg. Med. 2018, 25, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Eljaiek, R.; Heylbroeck, C.; Dubois, M.-J. Albumin administration for fluid resuscitation in burn patients: A systematic review and meta-analysis. Burns 2017, 43, 17–24. [Google Scholar] [CrossRef]
- Cartotto, R.; Greenhalgh, D. Colloids in Acute Burn Resuscitation. Crit. Care Clin. 2016, 32, 507–523. [Google Scholar] [CrossRef]
- Kramer, G.C.; Michell, M.W.; Oliveira, H.; Brown, T.L.H.; Herndon, D.; Baker, R.D.; Muller, M. Oral and Enteral Resuscitation of Burn Shock the Historical Record and Implications for Mass Casualty Care. Eplasty 2010, 10, e56. [Google Scholar]
- Moghazy, A.; Adly, O.; Elbadawy, M.; Hashem, R. Evaluation of who oral rehydration solution (ORS) and salt tablets in resuscitating adult patients with burns covering more than 15% of total body surface area (TBSA). Ann. Burn. Fire Disasters 2016, 29, 43–47. [Google Scholar]
- Brownson, E.G.; Pham, T.N.; Chung, K.K. How to Recognize a Failed Burn Resuscitation. Crit. Care Clin. 2016, 32, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Cancio, L.C.; Bohanon, F.J.; Kramer, G.C. Burn Resuscitation. In Total Burn Care, 5th ed.; Elsevier: Edinburgh, UK, 2018; pp. 77–86. [Google Scholar]
- Adibfar, A.; Camacho, F.; Rogers, A.D.; Cartotto, R. The use of vasopressors during acute burn resuscitation. Burns 2021, 47, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Payen, D.; Mateo, J.; Cavaillon, J.M.; Fraisse, F.; Floriot, C.; Vicaut, E. Impact of continuous venovenous hemofiltration on organ failure during the early phase of severe sepsis: A randomized controlled trial. Crit. Care Med. 2009, 37, 803–810. [Google Scholar] [CrossRef]
- Gaudry, S.; Hajage, D.; Schortgen, F.; Martin-Lefevre, L.; Pons, B.; Boulet, E.; Boyer, A.; Chevrel, G.; Lerolle, N.; Carpentier, D.; et al. Initiation Strategies for Renal-Replacement Therapy in the Intensive Care Unit. N. Engl. J. Med. 2016, 375, 122–133. [Google Scholar] [CrossRef]
- Chung, K.K.; Lundy, J.B.; Matson, J.R.; Renz, E.M.; White, C.E.; King, B.T.; Barillo, D.J.; Jones, J.A.; Cancio, L.C.; Blackbourne, L.H.; et al. Continuous venovenous hemofiltration in severely burned patients with acute kidney injury: A cohort study. Crit. Care 2009, 13, R62. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.K.; Coates, E.C.; Smith, D.J.; Karlnoski, R.A.; Hickerson, W.L.; Arnold-Ross, A.L.; Mosier, M.J.; Halerz, M.; Sprague, A.M.; Mullins, R.F.; et al. High-volume hemofiltration in adult burn patients with septic shock and acute kidney injury: A multicenter randomized controlled trial. Crit. Care 2017, 21, 289. [Google Scholar] [CrossRef]
- You, B.; Zhang, Y.L.; Luo, G.X.; Dang, Y.M.; Jiang, B.; Huang, G.T.; Liu, X.Z.; Yang, Z.C.; Chen, Y.; Chen, J.; et al. Early application of continuous high-volume haemofiltration can reduce sepsis and improve the prognosis of patients with severe burns. Crit. Care 2018, 22, 173. [Google Scholar] [CrossRef]
- Salinas, J.; Chung, K.K.; Mann, E.A.; Cancio, L.C.; Kramer, G.C.; Serio-Melvin, M.L.; Renz, E.M.; Wade, C.E.; Wolf, S. Computerized decision support system improves fluid resuscitation following severe burns: An original study. Crit. Care Med. 2011, 39, 2031–2038. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.R.; Ahmadi, A.J.; Singh, C.N.; Sires, B.S.; Engrav, L.H.; Gibran, N.S.; Heimbach, D.M.; Klein, M.B. Elevated Orbital Pressure: Another Untoward Effect of Massive Resuscitation after Burn Injury. J. Trauma Inj. Infect. Crit. Care 2006, 60, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hughes, P.G. Escharotomy; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482120/ (accessed on 21 January 2021).
- Boccara, D.; Lavocat, R.; Soussi, S.; Legrand, M.; Chaouat, M.; Mebazaa, A.; Mimoun, M.; Blet, A.; Serror, K. Pressure guided surgery of compartment syndrome of the limbs in burn patients. Ann. Burn. Fire Disasters 2017, 30, 193–197. [Google Scholar]
- Ivy, M.E.; Atweh, N.A.; Palmer, J.; Possenti, P.P.; Pineau, M.; D’Aiuto, M. Intra-abdominal Hypertension and Abdominal Compartment Syndrome in Burn Patients. J. Trauma Inj. Infect. Crit. Care 2000, 49, 387–391. [Google Scholar] [CrossRef]
- Singh, C.N.; Klein, M.B.; Sullivan, S.R.; Sires, B.S.; Hutter, C.M.; Rice, K.; Jian-Amadi, A. Orbital Compartment Syndrome in Burn Patients. Ophthalmic Plast. Reconstr. Surg. 2008, 24, 102–106. [Google Scholar] [CrossRef]
- Hundeshagen, G.; Lee, J.O.; Norbury, W.B.; Herndon, D.N. Care of Geriatric Patients. In Total Burn Care, 5th ed.; Hernon, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 381–385. [Google Scholar]
- Abu-Sittah, G.; Chahine, F.; Janom, H. Management of burns in the elderly. Ann. Burn. Fire Disasters 2016, 29, 249. [Google Scholar]
- Pereira, C.T.; Barrow, R.E.; Sterns, A.M.; Hawkins, H.K.; Kimbrough, C.W.; Jeschke, M.G.; Lee, J.O.; Sanford, A.P.; Herndon, D.N. Age-Dependent Differences in Survival after Severe Burns: A Unicentric Review of 1,674 Patients and 179 Autopsies over 15 Years. J. Am. Coll. Surg. 2006, 202, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Keck, M.; Lumenta, D.; Andel, H.; Kamolz, L.-P.; Frey, M. Burn treatment in the elderly. Burns 2009, 35, 1071–1079. [Google Scholar] [CrossRef]
- Rehou, S.; Shahrokhi, S.; Thai, J.; Stanojcic, M.; Jeschke, M.G. Acute Phase Response in Critically Ill Elderly Burn Patients. Crit. Care Med. 2019, 47, 201–209. [Google Scholar] [CrossRef]
- Romanowski, K.; Curtis, E.; Barsun, A.; Palmieri, T.; Greenhalgh, D.; Sen, S. The frailty tipping point: Determining which patients are targets for intervention in a burn population. Burns 2019, 45, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Purdue, G.F.; Hunt, J.L.; Lang, E.D. Obesity: A Risk Factor in the Burn Patient. J. Burn. Care Rehabil. 1990, 11, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Gottschlich, M.M.; Mayes, T.; Khoury, J.C.; Warden, G.D. Significance of obesity on nutritional, immunologic, hormonal, and clinical outcome parameters in burns. J. Am. Diet. Assoc. 1993, 93, 1261–1268. [Google Scholar] [CrossRef]
- Sheridan, R.L.; Rue, L.W.; McManus, W.F.; Pruitt, B.A. Burns in morbidly obese patients. J. Trauma Inj. Infect. Crit. Care 1992, 33, 818–820. [Google Scholar] [CrossRef]
- Ghanem, A.M.; Sen, S.; Philp, B.; Dziewulski, P.; Shelley, O. Body Mass Index (BMI) and mortality in patients with severe burns: Is there a “tilt point” at which obesity influences outcome? Burns 2011, 37, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.M.; Hollett, L.P.; Jeng, J.C.; Wu, J.; Turner, D.G.; Jordan, M.H. How Long a Shadow Does Epidemic Obesity Cast in the Burn Unit? A Dietitian’s Analysis of the Strengths and Weaknesses of the Available Data in the National Burn Repository. J. Burn Care Res. 2008, 29, 97–101. [Google Scholar] [CrossRef]
- Liodaki, E.; Senyaman, Ö.; Stollwerck, P.L.; Möllmeier, D.; Mauss, K.L.; Mailänder, P.; Stang, F. Obese patients in a burn care unit: A major challenge. Burns 2014, 40, 1738–1742. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; Finnerty, C.C.; Emdad, F.; Rivero, H.G.; Kraft, R.; Williams, F.N.; Gamelli, R.L.; Gibran, N.; Klein, M.B.; Arnoldo, B.D.; et al. Mild Obesity Is Protective After Severe Burn Injury. Ann. Surg. 2013, 258, 1119–1129. [Google Scholar] [CrossRef]
- Keshavarzi, A.; Kardeh, S.; Dehghankhalili, M.; Varahram, M.H.; Omidi, M.; Zardosht, M.; Mehrabani, D. Mortality and Body Mass Index in Burn Patients: Experience from a Tertiary Referral Burn Center in Southern Iran. World J. Plast. Surg. 2019, 8, 382–387. [Google Scholar]
- Yim, G.H.; Pujji, O.J.S.; Bharj, I.S.; Farrar, E.; La, J.S. The value of a bariatric specific chart to initiate resuscitation of adult bariatric burns. Burns 2019, 45, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Neaman, K.C.; Andres, L.A.; McClure, A.M.; Burton, M.E.; Kemmeter, P.R.; Ford, R.D. A New Method for Estimation of Involved BSAs for Obese and Normal-Weight Patients with Burn Injury. J. Burn Care Res. 2011, 32, 421–428. [Google Scholar] [CrossRef]
- Clemens, M.S.; Stewart, I.J.; Sosnov, J.A.; Howard, J.T.; Belenkiy, S.M.; Sine, C.R.; Henderson, J.L.; Buel, A.R.; Batchinsky, A.I.; Cancio, L.C.; et al. Reciprocal Risk of Acute Kidney Injury and Acute Respiratory Distress Syndrome in Critically Ill Burn Patients. Crit. Care Med. 2016, 44, e915–e922. [Google Scholar] [CrossRef] [PubMed]
- Basel, A.P.; Britton, G.W.; Chung, K.K. Acute Kidney Injury in Burns. In Handbook of Critical Care Nephrology; Koyner, J.L., Topf, J., Lerma, E., Eds.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2021. [Google Scholar]
- Chung, K.K.; Juncos, L.A.; Wolf, S.; Mann, E.E.; Renz, E.M.; White, C.E.; Barillo, D.J.; Clark, R.A.; Jones, J.A.; Edgecombe, H.P.; et al. Continuous Renal Replacement Therapy Improves Survival in Severely Burned Military Casualties with Acute Kidney Injury. J. Trauma Inj. Infect. Crit. Care 2008, 64, S179–S187. [Google Scholar] [CrossRef] [PubMed]
- Robert, I.; Oliver, J. Burn Resuscitation and Early Management: Background, Pathophysiology, Initial Evaluation and Treatment. Medscape. 2021. Available online: https://emedicine.medscape.com/article/1277360-overview (accessed on 25 June 2021).
- Mackie, D.P.; Spoelder, E.J.; Paauw, R.J.; Knape, P.; Boer, C. Mechanical Venti-lation and Fluid Retention in Burn Patients. J. Trauma Inj. Infect. Crit. Care 2009, 67, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Curley, G.F.; Laffey, J.G.; Zhang, H.; Slutsky, A.S. Biotrauma and Ventilator-Induced Lung Injury: Clinical Implications. Chest 2016, 150, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, S.K.; Wasiak, J.; Cleland, H.; Symons, J.; Hogan, L.; Hucker, T.; Mahar, P.D. A Systematic Review of Ketamine as an Analgesic Agent in Adult Burn Injuries. Pain Med. 2011, 12, 1551–1558. [Google Scholar] [CrossRef]
- Sullivan, S.R.; Friedrich, J.B.; Engrav, L.H.; Round, K.A.; Heimbach, D.M.; Heckbert, S.R.; Carrougher, G.J.; Lezotte, D.C.; Wiechman, S.A.; Honari, S.; et al. Opioid creep “is real and may be the cause of” fluid creep. Burns 2004, 30, 583–590. [Google Scholar] [CrossRef]
- Wibbenmeyer, L.; Sevier, A.; Liao, J.; Williams, I.; Light, T.; Latenser, B.; Lewis, R.; Kealey, P.; Rosenquist, R. The impact of opioid ad-ministration on resuscitation volumes in thermally injured patients. J. Burn Care Res. 2010, 31, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Owens, V.F.; Palmieri, T.L.; Comroe, C.M.; Conroy, J.M.; Scavone, J.A.; Greenhalgh, D.G. Ketamine: A safe and effective agent for painful procedures in the pediatric burn patient. J. Burn Care Res. 2006, 27, 211–216. [Google Scholar] [CrossRef]
- Miller, M.; Kruit, N.; Heldreich, C.; Ware, S.; Habig, K.; Reid, C.; Burns, B. Hemodynamic Response After Rapid Sequence Induction with Ketamine in Out-of-Hospital Patients at Risk of Shock as Defined by the Shock Index. Ann. Emerg. Med. 2016, 68, 181–188.e2. [Google Scholar] [CrossRef]
- Kim, D.; Pruskowski, K.A.; Ainsworth, C.R.; Linsenbardt, H.R.; Rizzo, J.A.; Cancio, L.C. A Review of Adjunctive Therapies for Burn Injury Pain During the Opioid Crisis. J. Burn Care Res. 2019, 40, 983–995. [Google Scholar] [CrossRef]
- Blanchet, B.; Jullien, V.; Vinsonneau, C.; Tod, M. Influence of Burns on Pharmacokinetics and Pharmacodynamics of Drugs Used in the Care of Burn Patients. Clin. Pharm. 2008, 47, 635–654. [Google Scholar] [CrossRef]
- Steele, A.N.; Grimsrud, K.N.; Sen, S.; Palmieri, T.L.; Greenhalgh, D.G.; Tran, N. Gap Analysis of Pharmacokinetics and Pharmacodynamics in Burn Patients. J. Burn Care Res. 2015, 36, e194–e211. [Google Scholar] [CrossRef]
- Serghiou, M.; Niszczak, J.; Parry, I.; Richard, R. Clinical practice recommendations for positioning of the burn patient. Burns 2016, 42, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Esselman, P.C.; Thombs, B.; Magyar-Russell, G.; Fauerbach, J.A. Burn Rehabilitation. Am. J. Phys. Med. Rehabil. 2006, 85, 383–413. [Google Scholar] [CrossRef] [PubMed]
- Cambiaso-Daniel, J.; Parry, I.; Rivas, E.; Kemp-Offenberg, J.; Sen, S.; Rizzo, J.A.; Serghiou, M.A.; Kowalske, K.; Wolf, S.E.; Herndon, D.N.; et al. Strength and Cardiorespiratory Exercise Rehabilitation for Severely Burned Patients During Intensive Care Units: A Survey of Practice. J. Burn Care Res. 2018, 39, 897–901. [Google Scholar] [CrossRef] [PubMed]


| Scheme | Postburn Hour | Cardiac Output | SVR | Plasma Volume | Urine Output | Fluid Infusion Rate |
|---|---|---|---|---|---|---|
| 1 | 0–12 | Rapidly reaches nadir | Rapidly reaches peak | Decreases at its most rapid rate | Oliguria is common | May peak at hour 8–10 |
| 2 | 12–36 | Slightly less than normal | Decreases towards normal | Nadir at hour 12–18, then slowly increases | Adequate | Slowly decreases |
| 3 | 36-beyond | Supranormal | Subnormal | Normal | Often above target | Reaches a maintenance rate |
| Resuscitation Pitfalls |
|---|
| Advanced age |
| Obesity |
| Existing cardiomyopathy |
| Renal failure |
| Osmotic diuresis (glycosuria, alcohol consumption) |
| Active hemorrhage |
| Inhalation injury |
| Mechanical Ventilation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Causbie, J.M.; Sattler, L.A.; Basel, A.P.; Britton, G.W.; Cancio, L.C. State of the Art: An Update on Adult Burn Resuscitation. Eur. Burn J. 2021, 2, 152-167. https://doi.org/10.3390/ebj2030012
Causbie JM, Sattler LA, Basel AP, Britton GW, Cancio LC. State of the Art: An Update on Adult Burn Resuscitation. European Burn Journal. 2021; 2(3):152-167. https://doi.org/10.3390/ebj2030012
Chicago/Turabian StyleCausbie, Jacqueline M., Lauren A. Sattler, Anthony P. Basel, Garrett W. Britton, and Leopoldo C. Cancio. 2021. "State of the Art: An Update on Adult Burn Resuscitation" European Burn Journal 2, no. 3: 152-167. https://doi.org/10.3390/ebj2030012
APA StyleCausbie, J. M., Sattler, L. A., Basel, A. P., Britton, G. W., & Cancio, L. C. (2021). State of the Art: An Update on Adult Burn Resuscitation. European Burn Journal, 2(3), 152-167. https://doi.org/10.3390/ebj2030012






