Diagnosis and Management of Invasive Fungal Wound Infections in Burn Patients
Abstract
1. Introduction
2. Causative Organisms
3. Diagnosis
4. Treatment
4.1. Surgical Management
4.2. Systemic Antifungals
4.2.1. Amphotericin B
4.2.2. Triazole Antifungals
4.2.3. Echinocandins
4.3. Topical Antifungals
4.3.1. Sulfamylon + Amphotericin B (SMAT)
4.3.2. Nystatin
4.3.3. Voriconazole
4.3.4. Manuka Honey
4.3.5. Silver
4.3.6. Dakin’s Solution
4.3.7. Cerium
4.4. Nonpharmacologic Treatments
4.4.1. Hyperbaric Oxygen Therapy
4.4.2. Ultraviolet-C Light
4.5. Immune-Enhancing Treatments
5. Prophylaxis
6. Limitations
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Barret, J.P.; Ramzy, P.I.; Heggers, J.P.; Villareal, C.; Herndon, D.N.; Desai, M.H. Topical nystatin powder in severe burns: A new treatment for angioinvasive fungal infections refractory to other topical and systemic agents. Burns 1999, 25, 505–508. [Google Scholar] [CrossRef]
- Dean, D.A.; Burchard, K.W. Surgical perspective on invasive Candida infections. World J. Surg. 1998, 22, 127–134. [Google Scholar] [CrossRef]
- Codish, S.D.; Sheridan, I.D.; Monaco, A.P. Mycotic wound infections. A new challenge of the surgeon. Arch. Surg. 1979, 114, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Blyth, D.M.; Chung, K.K.; Cancio, L.C.; King, B.T.; Murray, C.K. Clinical utility of fungal screening assays in adults with severe burns. Burns 2013, 39, 413–419. [Google Scholar] [CrossRef] [PubMed]
- D’Avignon, L.C.; Chung, K.K.; Saffle, J.R.; Renz, E.M.; Cancio, L.C. Prevention of infections associated with combat-related burn injuries. J. Trauma 2011, 71 (Suppl. S2), S282–S289. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.B.; Lam, K.; Hansen, D.; Burrell, R.E. Efficacy of topical silver against fungal burn wound pathogens. Am. J. Infect. Control 1999, 27, 344–350. [Google Scholar] [CrossRef]
- Tribble, D.R.; Rodriguez, C.J. Combat-Related Invasive Fungal Wound Infections. Curr. Fungal Infect. Rep. 2014, 8, 277–286. [Google Scholar] [CrossRef][Green Version]
- Burchard, K.W. Fungal sepsis. Infect. Dis. Clin. N. Am. 1992, 6, 677–692. [Google Scholar] [CrossRef]
- Sarabahi, S.; Tiwari, V.K.; Arora, S.; Capoor, M.R.; Pandey, A. Changing pattern of fungal infection in burn patients. Burns 2012, 38, 520–528. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Gibas, C.F.C. From the Clinical Mycology Laboratory: New Species and Changes in Fungal Taxonomy and Nomenclature. J. Fungi 2018, 4, 138. [Google Scholar] [CrossRef]
- Moore, E.C.; Padiglione, A.A.; Wasiak, J.; Paul, E.; Cleland, H. Candida in burns: Risk factors and outcomes. J. Burn Care Res. 2010, 31, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.K.; Loo, F.L.; Hospenthal, D.R.; Cancio, L.C.; Jones, J.A.; Kim, S.H.; Holcomb, J.B.; Wade, C.E.; Wolf, S.E. Incidence of systemic funcal infection and related mortality following severe burns. Burns 2008, 34, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, M.; Anagnostou, T.; Desalermos, A.; Kourkoumpetis, T.K.; Carneiro, H.A.; Glavis-Bloom, J.; Coleman, J.J.; Mylonakis, E. Fusarium infection: Report of 26 cases and review of 97 cases from the literature. Medicine 2013, 92, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Cawley, M.J.; Braxton, G.R.; Haith, L.R.; Reilly, K.J.; Guilday, R.E.; Patton, M.L. Trichosporon beigelii infection: Experience in a regional burn center. Burns 2000, 26, 483–486. [Google Scholar] [CrossRef]
- Mitchell, T.A.; Hardin, M.O.; Murray, C.K.; Ritchie, J.D.; Cancio, L.C.; Renz, E.M.; White, C.E. Mucormycosis attributed mortality: A seven-year review of surgical and medical management. Burns 2014, 40, 1689–1695. [Google Scholar] [CrossRef]
- dela Cruz, W.P.; Calvano, T.P.; Griffith, M.E.; White, C.E.; Kim, S.H.; Sutton, D.A.; Thompson, E.H.; Fu, J.; Wickes, B.L.; Guarro, J.; et al. Invasive Apophysomyces variabilis infection in a burn patient. J. Clin. Microbiol. 2012, 50, 2814–2817. [Google Scholar] [CrossRef]
- Hospenthal, D.R.; Chung, K.K.; Lairet, K.; Thompson, E.H.; Guarro, J.; Renz, E.M.; Sutton, D.A. Saksenaea erythrospora infection following combat trauma. J. Clin. Microbiol. 2011, 49, 3707–3709. [Google Scholar] [CrossRef]
- Kucan, J.O.; Hall, S. Alternaria burn wound sepsis. J. Burn Care Rehabil. 1985, 6, 501–502. [Google Scholar] [CrossRef]
- Schofield, C.M.; Murray, C.K.; Horvath, E.E.; Cancio, L.C.; Kim, S.H.; Wolf, S.E.; Hospenthal, D.R. Correlation of culture with histopathology in fungal burn wound colonization and infection. Burns 2007, 33, 341–346. [Google Scholar] [CrossRef]
- Beckett, A.R.; Kahn, S.A.; Seay, R.; Lintner, A.C. Invasive Curvularia infections in burn patients: A case series. Surg. Infect. Case Rep. 2017, 2, 76–79. [Google Scholar] [CrossRef]
- Marcus, J.E.; Piper, L.C.; Ainsworth, C.R.; Sams, V.G.; Batchinsky, A.; Okulicz, J.F.; Barsoumian, A.E. Infections in patients with burn injuries receiving extracorporeal membrane oxygenation. Burns 2019, 45, 1880–1887. [Google Scholar] [CrossRef] [PubMed]
- Maurel, V.; Denis, B.; Camby, M.; Jeanne, M.; Cornesse, A.; Glavnik, B.; Alanio, A.; Rousseau, A.F.; Lefloch, R.; Lagrange-Xelot, M.; et al. Outcome and characteristics of invasive fungal infections in critically ill burn patients: A multicenter retrospective study. Mycoses 2020, 63, 535–542. [Google Scholar] [CrossRef]
- Van Bang, B.N.; Thanh Xuan, N.; Xuan Quang, D.; Ba Loi, C.; Thai Ngoc Minh, N.; Nhu Lam, N.; Ngoc Anh, D.; Thi Thu Hien, T.; Xuan Su, H.; Tran-Anh, L. Prevalence, species distribution, and risk factors of fungal colonization and infection in patients at a burn intensive care unit in Vietnam. Curr. Med. Mycol. 2020, 6, 42–49. [Google Scholar]
- Kaur, R.; Bala, K.; Ahuja, R.B.; Srivastav, P.; Bansal, U. Primary cutaneous mucormycosis in a patient with burn wounds due to Lichtheimia ramosa. Mycopathologia 2014, 178, 291–295. [Google Scholar] [CrossRef]
- Moon, P.; Jithendran, N. Invasive Fungal Infection with Absidia Corymbifera in Immunocompetent Patient with Electrical Scalp Burn. World J. Plast. Surg. 2018, 7, 249–252. [Google Scholar]
- Farmer, A.R.; Murray, C.K.; Driscoll, I.R.; Wickes, B.L.; Wiederhold, N.; Sutton, D.A.; Sanders, C.; Mende, K.; Enniss, B.; Feig, J.; et al. Combat-Related Pythium aphanidermatum Invasive Wound Infection: Case Report and Discussion of Utility of Molecular Diagnostics. J. Clin. Microbiol. 2015, 53, 1968–1975. [Google Scholar] [CrossRef]
- Tamayo Lomas, L.; Domínguez-Gil González, M.; Martín Luengo, A.I.; Eiros Bouza, J.M.; Piqueras Pérez, J.M. Nosocomial infection due to Trichosporon asahii in a critical burned patient. Rev. Iberoam. Micol. 2015, 32, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Tram, Q.A.; Minh, N.T.N.; Anh, D.N.; Lam, N.N.; Dung, T.N.; Thi Minh Chau, N.; Tran-Anh, L. A Rare Case of Fungal Burn Wound Infection Caused by Fusarium solani in Vietnam. J. Investig. Med. High Impact Case Rep. 2020, 8, 2324709620912122. [Google Scholar] [CrossRef] [PubMed]
- Que, A.T.; Nguyen, N.T.; Do, N.A.; Nguyen, N.L.; Tran, N.D.; Le, T.A. Infection of burn wound by Aspergillus fumigatus with gross appearance of fungal colonies. Med. Mycol. Case Rep. 2019, 24, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Akers, K.S.; Rowan, M.P.; Niece, K.L.; Graybill, J.C.; Mende, K.; Chung, K.K.; Murray, C.K. Antifungal wound penetration of amphotericin and voriconazole in combat-related injuries: Case report. BMC Infect. Dis. 2015, 15, 184. [Google Scholar] [CrossRef] [PubMed]
- Hoenigl, M.; Strenger, V.; Buzina, W.; Valentin, T.; Koidl, C.; Wölfler, A.; Seeber, K.; Valentin, A.; Strohmeier, A.T.; Zollner-Schwetz, I.; et al. European Organization for the Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) host factors and invasive fungal infections in patients with haematological malignancies. J. Antimicrob. Chemother. 2012, 67, 2029–2033. [Google Scholar] [CrossRef] [PubMed]
- Patterson, T.F.; Thompson, G.R., 3rd; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef] [PubMed]
- Ladhani, H.A.; Yowler, C.J.; Claridge, J.A. Burn Wound Colonization, Infection, and Sepsis. Surg. Infect. 2021, 22, 44–48. [Google Scholar] [CrossRef]
- Pruitt, B.A., Jr.; McManus, A.T.; Kim, S.H.; Goodwin, C.W. Burn wound infections: Current status. World J. Surg. 1998, 22, 135–145. [Google Scholar]
- Heaton, S.M.; Weintrob, A.C.; Downing, K.; Keenan, B.; Aggarwal, D.; Shaikh, F.; Tribble, D.R.; Wells, J. Histopathological techniques for the diagnosis of combat-related invasive fungal wound infections. BMC Clin. Pathol. 2016, 16, 11. [Google Scholar] [CrossRef]
- Horvath, E.E.; Murray, C.K.; Vaughan, G.M.; Chung, K.K.; Hospenthal, D.R.; Wade, C.E.; Holcomb, J.B.; Wolf, S.E.; Mason, A.D., Jr.; Cancio, L.C. Fungal wound infection (not colonization) is independently associated with mortality in burn patients. Ann. Surg. 2007, 245, 978–985. [Google Scholar] [CrossRef]
- Ganesan, A.; Wells, J.; Shaikh, F.; Peterson, P.; Bradley, W.; Carson, M.L.; Petfield, J.L.; Klassen-Fischer, M.; Akers, K.S.; Downing, K.; et al. Molecular Detection of Filamentous Fungi in Formalin-Fixed Paraffin-Embedded Specimens in Invasive Fungal Wound Infections Is Feasible with High Specificity. J. Clin. Microbiol. 2019, 58, 58. [Google Scholar] [CrossRef]
- Guarner, J.; Brandt, M.E. Histopathologic diagnosis of fungal infections in the 21st century. Clin. Microbiol. Rev. 2011, 24, 247–280. [Google Scholar] [CrossRef]
- Son, H.J.; Song, J.S.; Choi, S.; Jung, J.; Kim, M.J.; Chong, Y.P.; Lee, S.O.; Choi, S.H.; Kim, Y.S.; Woo, J.H.; et al. A comparison of histomorphologic diagnosis with culture- and immunohistochemistry-based diagnosis of invasive aspergillosis and mucormycosis. Infect. Dis. 2020, 52, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Hayden, R.T.; Isotalo, P.A.; Parrett, T.; Wolk, D.M.; Qian, X.; Roberts, G.D.; Lloyd, R.V. In situ hybridization for the differentiation of Aspergillus, Fusarium, and Pseudallescheria species in tissue section. Diagn. Mol. Pathol. Am. J. Surg. Pathol. Part B 2003, 12, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Shupp, J.W.; Petraitiene, R.; Jaskille, A.D.; Pavlovich, A.R.; Matt, S.E.; Nguyen do, T.; Kath, M.A.; Jeng, J.C.; Jordan, M.H.; Finkelman, M.; et al. Early serum (1→3)-β-D-glucan levels in patients with burn injury. Mycoses 2012, 55, 224–227. [Google Scholar] [CrossRef]
- Kaita, Y.; Tarui, T.; Otsu, A.; Tanaka, Y.; Suzuki, J.; Yoshikawa, K.; Yamaguchi, Y. The Clinical Significance of Serum 1,3-β-D-Glucan For the Diagnosis of Candidemia in Severe Burn Patients. J. Burn Care Res. 2019, 40, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Kaide, C.G.; Khandelwal, S. Hyperbaric oxygen: Applications in infectious disease. Emerg. Med. Clin. N. Am. 2008, 26, 571–595. [Google Scholar] [CrossRef]
- Blum, G.; Hörtnagl, C.; Jukic, E.; Erbeznik, T.; Pümpel, T.; Dietrich, H.; Nagl, M.; Speth, C.; Rambach, G.; Lass-Flörl, C. New insight into amphotericin B resistance in Aspergillus terreus. Antimicrob. Agents Chemother. 2013, 57, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, W.J.; Benjamin, D.K., Jr.; Kontoyiannis, D.P.; Perfect, J.R.; Lutsar, I.; Marr, K.A.; Lionakis, M.S.; Torres, H.A.; Jafri, H.; Walsh, T.J. Infections due to Aspergillus terreus: A multicenter retrospective analysis of 83 cases. Clin. Infect. Dis. 2004, 39, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Warkentien, T.; Rodriguez, C.; Lloyd, B.; Wells, J.; Weintrob, A.; Dunne, J.R.; Ganesan, A.; Li, P.; Bradley, W.; Gaskins, L.J. Invasive mold infections following combat-related injuries. Clin. Infect. Dis. 2012, 55, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.J.; Tribble, D.R.; Malone, D.L.; Murray, C.K.; Jessie, E.M.; Khan, M.; Fleming, M.E.; Potter, B.K.; Gordon, W.T.; Shackelford, S.A. Treatment of Suspected Invasive Fungal Infection in War Wounds. Mil. Med. 2018, 183 (Suppl. S2), 142–146. [Google Scholar] [CrossRef] [PubMed]
- Mayr, A.; Lass-Flörl, C. Epidemiology and antifungal resistance in invasive Aspergillosis according to primary disease: Review of the literature. Eur. J. Med. Res. 2011, 16, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Snelders, E.; van der Lee, H.A.; Kuijpers, J.; Rijs, A.J.; Varga, J.; Samson, R.A.; Mellado, E.; Donders, A.R.; Melchers, W.J.; Verweij, P.E. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 2008, 5, e219. [Google Scholar] [CrossRef]
- Schlotman, T.; Akers, K. 381 Pharmacokinetics and pharmacodynamics of voriconazole in burn patients: A case series. J. Burn Care Res. 2018, 39 (Suppl. S1), S161. [Google Scholar] [CrossRef]
- Rizzo, J.A.; Martini, A.K.; Pruskowski, K.A.; Rowan, M.P.; Niece, K.L.; Akers, K.S. Thermal stability of mafenide and amphotericin B topical solution. Burns 2018, 44, 475–480. [Google Scholar] [CrossRef]
- Heggers, J.P.; Robson, M.C.; Herndon, D.N.; Desai, M.H. The efficacy of nystatin combined with topical microbial agents in the treatment of burn wound sepsis. J. Burn Care Rehabil. 1989, 10, 508–511. [Google Scholar] [CrossRef]
- Klein, K.C.; Blackwood, R.A. Topical voriconazole solution for cutaneous aspergillosis in a pediatric patient after bone marrow transplant. Pediatrics 2006, 118, e506–e508. [Google Scholar] [CrossRef]
- Juliano, C.; Magrini, G.A. Methylglyoxal, the major antibacterial factor in manuka honey: An alternative to preserve natural cosmetics? Cosmetics 2019, 6, 1. [Google Scholar] [CrossRef]
- Yabes, J.M.; White, B.K.; Murray, C.K.; Sanchez, C.J.; Mende, K.; Beckius, M.L.; Zera, W.C.; Wenke, J.C.; Akers, K.S. In Vitro activity of Manuka Honey and polyhexamethylene biguanide on filamentous fungi and toxicity to human cell lines. Med. Mycol. 2017, 55, 334–343. [Google Scholar]
- Atiyeh, B.S.; Costagliola, M.; Hayek, S.N.; Dibo, S.A. Effect of silver on burn wound infection control and healing: Review of the literature. Burns 2007, 33, 139–148. [Google Scholar] [CrossRef]
- Cancio, L.C. Topical Antimicrobial Agents for Burn Wound Care: History and Current Status. Surg. Infect. 2021, 22, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Barsoumian, A.; Sanchez, C.J.; Mende, K.; Tully, C.C.; Beckius, M.L.; Akers, K.S.; Wenke, J.C.; Murray, C.K. In vitro toxicity and activity of Dakin’s solution, mafenide acetate, and amphotericin B on filamentous fungi and human cells. J. Orthop. Trauma 2013, 27, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Farias, I.A.P.; Dos Santos, C.C.L.; Sampaio, F.C. Antimicrobial Activity of Cerium Oxide Nanoparticles on Opportunistic Microorganisms: A Systematic Review. BioMed Res. Int. 2018, 2018, 1923606. [Google Scholar] [CrossRef] [PubMed]
- Silva-Dias, A.; Miranda, I.M.; Branco, J.; Cobrado, L.; Monteiro-Soares, M.; Pina-Vaz, C.; Rodrigues, A.G. In vitro antifungal activity and in vivo antibiofilm activity of cerium nitrate against Candida species. J. Antimicrob. Chemother. 2015, 70, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Memar, M.Y.; Yekani, M.; Alizadeh, N.; Baghi, H.B. Hyperbaric oxygen therapy: Antimicrobial mechanisms and clinical application for infections. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 109, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Segal, E.; Menhusen, M.J.; Shawn, S. Hyperbaric oxygen in the treatment of invasive fungal infections: A single-center experience. Isr. Med Assoc. J. IMAJ 2007, 9, 355–357. [Google Scholar] [PubMed]
- Andresen, D.; Donaldson, A.; Choo, L.; Knox, A.; Klaassen, M.; Ursic, C.; Vonthethoff, L.; Krilis, S.; Konecny, P. Multifocal cutaneous mucormycosis complicating polymicrobial wound infections in a tsunami survivor from Sri Lanka. Lancet 2005, 365, 876–878. [Google Scholar] [CrossRef]
- Dai, T.; Kharkwal, G.B.; Zhao, J.; St Denis, T.G.; Wu, Q.; Xia, Y.; Huang, L.; Sharma, S.K.; d’Enfert, C.; Hamblin, M.R. Ultraviolet-C light for treatment of Candida albicans burn infection in mice. Photochem. Photobiol. 2011, 87, 342–349. [Google Scholar] [CrossRef]
- Jun, S.; Irudayaraj, J.; Demirci, A.; Geiser, D. Pulsed UV-light treatment of corn meal for inactivation of Aspergillus niger spores. Int. J. Food Sci. Technol. 2003, 38, 883–888. [Google Scholar] [CrossRef]
- Córdova-Alcántara, I.M.; Venegas-Cortés, D.L.; Martínez-Rivera, M.; Pérez, N.O.; Rodriguez-Tovar, A.V. Biofilm characterization of Fusarium solani keratitis isolate: Increased resistance to antifungals and UV light. J. Microbiol. 2019, 57, 485–497. [Google Scholar] [CrossRef]
- Unsinger, J.; Burnham, C.A.; McDonough, J.; Morre, M.; Prakash, P.S.; Caldwell, C.C.; Dunne, W.M., Jr.; Hotchkiss, R.S. Interleukin-7 ameliorates immune dysfunction and improves survival in a 2-hit model of fungal sepsis. J. Infect. Dis. 2012, 206, 606–616. [Google Scholar] [CrossRef]
- Turnbull, I.R.; Mazer, M.B.; Hoofnagle, M.H.; Kirby, J.P.; Leonard, J.M.; Mejia-Chew, C.; Spec, A.; Blood, J.; Miles, S.M.; Ransom, E.M.; et al. IL-7 Immunotherapy in a Nonimmunocompromised Patient With Intractable Fungal Wound Sepsis. Open Forum Infect. Dis. 2021, 8, ofab256. [Google Scholar] [CrossRef]
- Grimaldi, D.; Pradier, O.; Hotchkiss, R.S.; Vincent, J.L. Nivolumab plus interferon-γ in the treatment of intractable mucormycosis. Lancet Infect. Dis. 2017, 17, 18. [Google Scholar] [CrossRef]
- Rauseo, A.M.; Coler-Reilly, A.; Larson, L.; Spec, A. Hope on the Horizon: Novel Fungal Treatments in Development. Open Forum Infect. Dis. 2020, 7, ofaa016. [Google Scholar] [CrossRef]
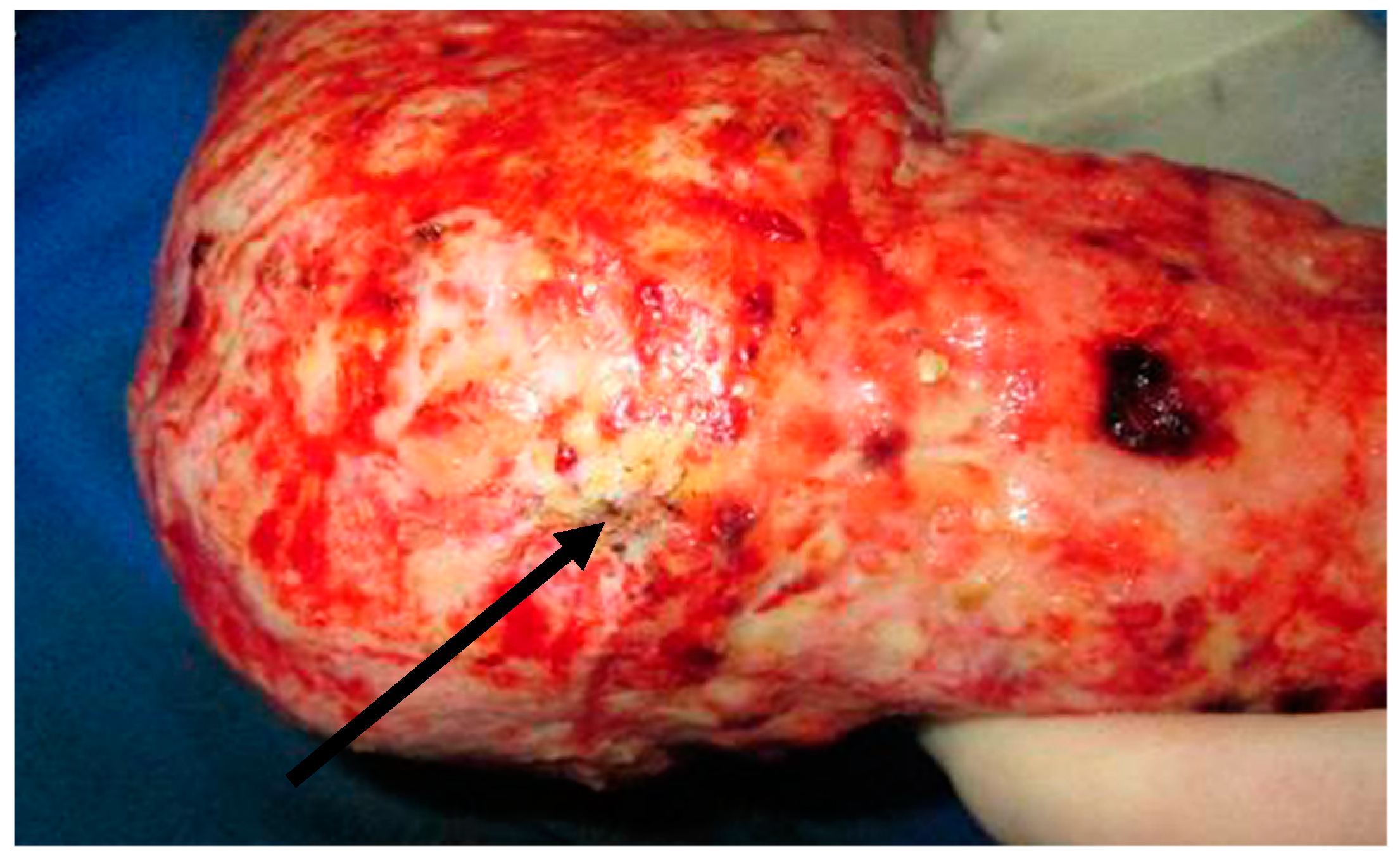
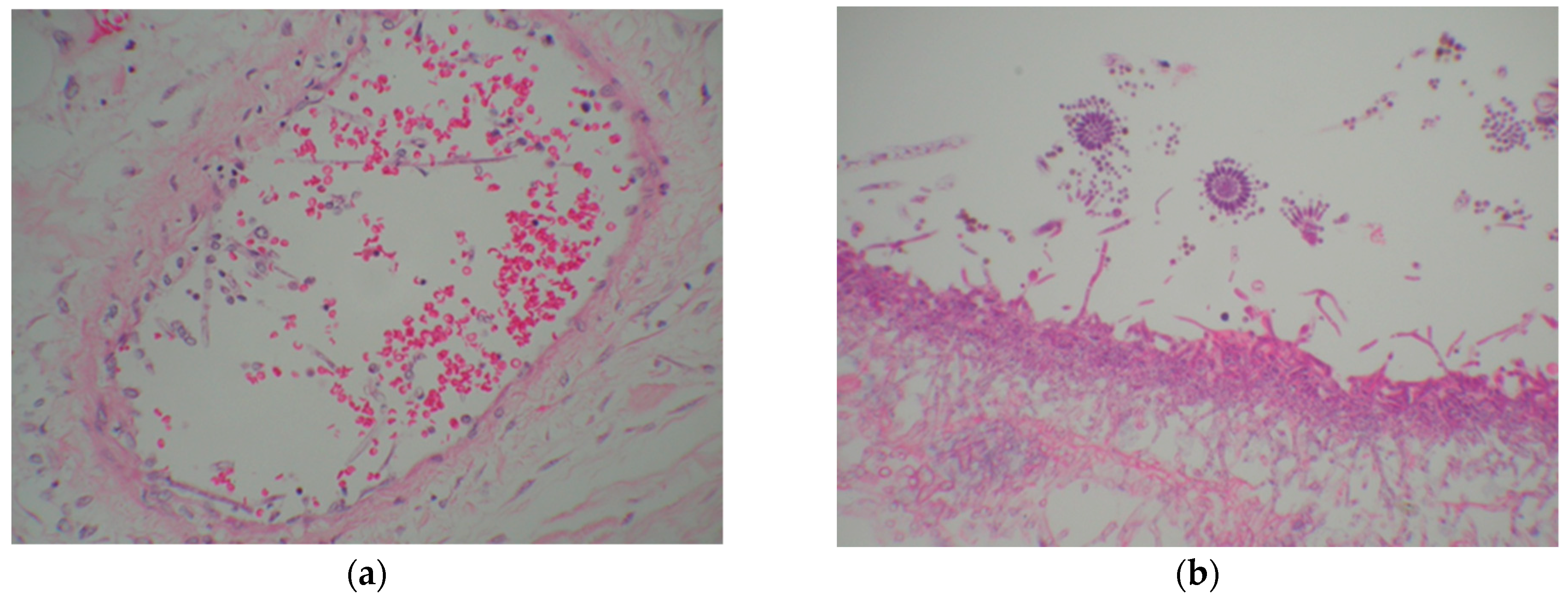
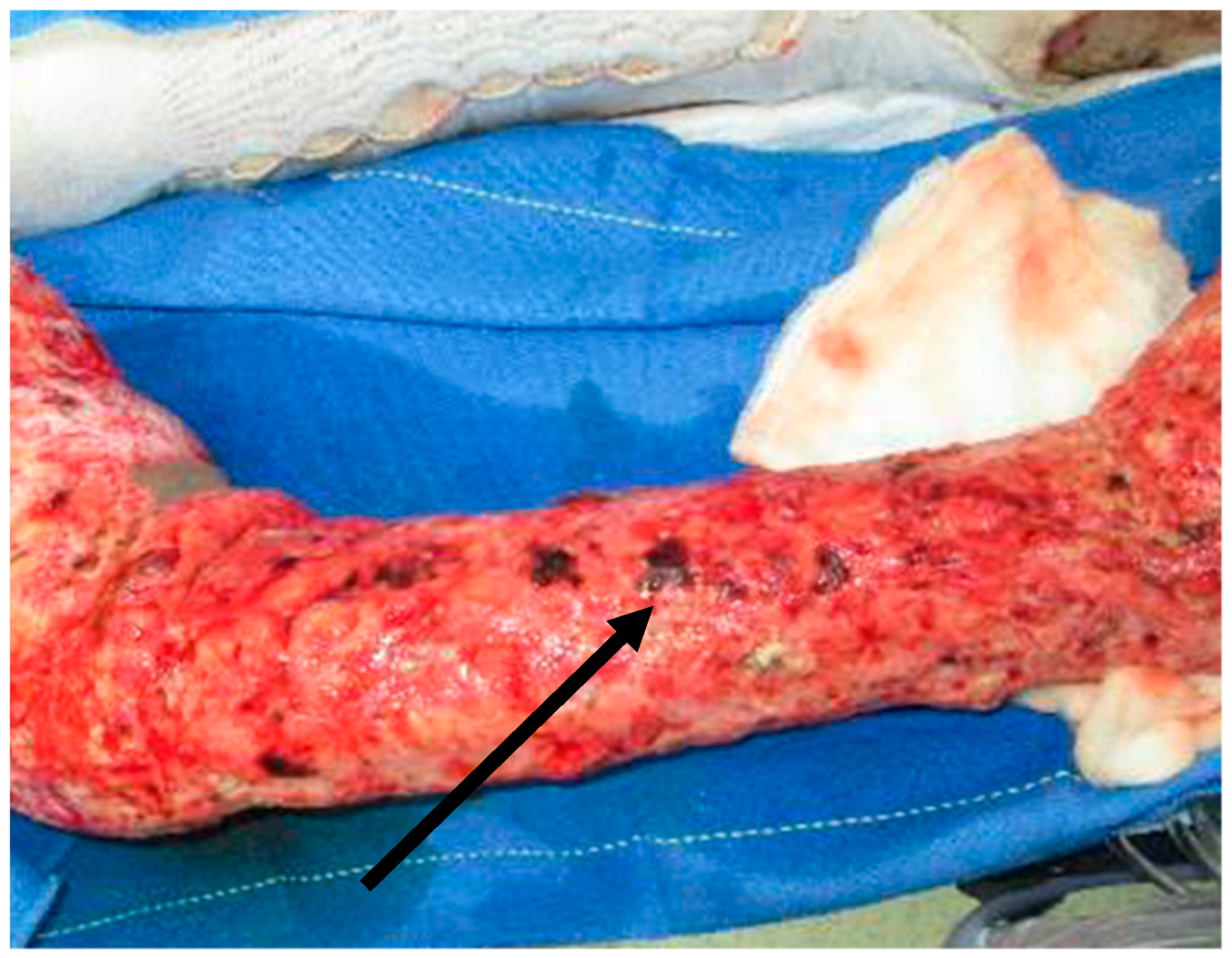
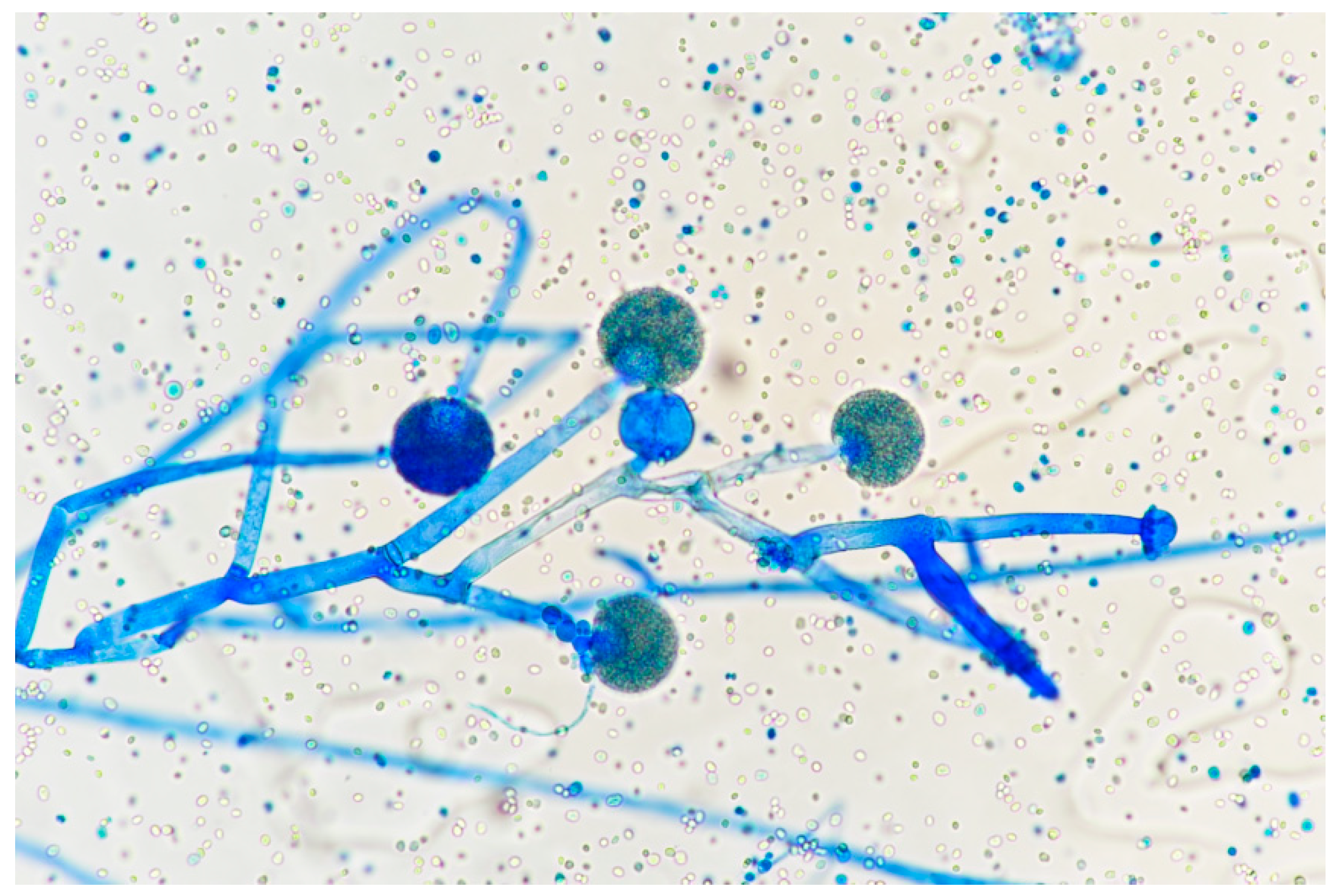
| Risk Factor | |
|---|---|
| Burn-related | >60% TBSA burns |
| Full-thickness burns | |
| Total parenteral nutrition (TPN) | |
| Multisystem organ failure (MSOF) | |
| Serum glucose > 200 mg/dL | |
| >7 days of systemic antibiotic therapy |
| Group | Syndrome | Pathophysiology | Morphology | References |
|---|---|---|---|---|
| Candida spp. | Candidiasis | Frequent colonizer but infrequent invader of burn wounds; increasingly non-albicans species | Budding yeasts or rounded, yeast-like structures, with or without septa or pseudohyphae | [11] |
| Hyaline, septate molds (Aspergillus, Fusarium spp.) | Aspergillosis; hyalohyphomycosis (non-Aspergillus infections) | Aspergillus is the most common cause of lethal FWI in the modern era; Fusarium has a propensity to enter bloodstream | Thin (3–12 μm), septate, acute-angle (45°) or dichotomous branching hyphae; nonpigmented | [12,13,14] |
| Mucorales order (Apophysomyces, Mucor, Rhizomucor, Rhizopus, Saksenaea spp.) | Mucormycosis (previously zygomycosis, Phyto mycosis) | Most aggressive invader; causes edema then angioinvasion, thrombosis, and necrosis | Broad (5–20 μm), pauciseptate, thin-walled, right-angle-branching, ribbon-like, folded or crinkled hyphae | [15,16,17] |
| Dematiaceous fungi (Alternaria, Bipolaris, Curvularia spp.) | Phaeohyphomycosis | Rare in burn patients | Pigmented hyphae | [18,19,20] |
| Authors | Study Type | Organisms Isolated | Type of Burn | Surgical Intervention | Outcomes |
|---|---|---|---|---|---|
| Marcus et al. [21] | Retrospective review | Candida albicans, Candida rugosa, Mucor sp., and Fusarium sp. | Thermal burns, electrical injury, inhalation injury only, toxic epidermal necrolysis | Not reported | In-hospital mortality: 40% |
| Maurel et al. [22] | Retrospective review | Candida albicans, C. parapsilosis, C. glabrata, C. krusei, C. tropicalis, Trichosporon, Aspergillus fumigatus, A. flavus, A. terreus Mucor sp., M. circinelloides, Rhizopus, Rhizomucor, Lichteimia (Absidia), Fusarium sp., Scedosporium | Thermal burn, electrical injury | Not reported | 90-day mortality: 37.2% |
| Sarabahi et al. [9] | Prospective observational | Non-albicans Candida sp., C. albicans, Aspergillus sp. | Not reported | Not reported | 43% mortality |
| Van Bang et al. [23] | Prospective observational | Candida albicans, C. tropicalis, C. parapsilosis, C. duobushaemulonis, Kodameae ohmeri, Aspergillus fumigatus, A. flavus, A. oryzae, A. chevalieri, A. nominus, Fusarium solium | Not reported | Not reported | 19.5% mortality |
| Kaur et al. [24] | Case report | Lichtheimia ramosa | Flame burn | Daily surgical debridement | Survived |
| Mitchell et al. [15] | Retrospective study | Mucor circinelloides, Saksenaea vasoformis, S. erythroospora, Pythium aphanidermatum | Blast injury, motor vehicle collision) | Average of 2.5 operative procedures 83% had an amputation | 92% mortality |
| Dela Cruz et al. [16] | Case report | Apophysomyces variabilis | Flame burn | Aggressive debridement of infected tissue after FWI suspicion | Died |
| Moon et al. [25] | Case report | Absidia corymbifera | High-voltage electrical injury | Wide debridement after clinical concern for FWI, then anterior lateral thigh flap | Survived |
| Farmer et al. [26] | Case report | Alternaria sp., Aspergillus sp., C. elegans, Geotrichum sp., and Pythium aphanidermatum | Blast injury | Serial irrigation and debridement | Died |
| Tamayo Lomas et al. [27] | Case report | Trichosporon asahii | Not reported | Six surgical procedures including escaharotomies, debridements, and autografts | Survived |
| Tram et al. [28] | Case report | Fusarium solani | Flame burn | Surgical measures for debridement and skin transplantation | Died |
| Que et al. [29] | Case report | Aspergillus fumigatus | Not reported | Not reported | Died |
| Colonization | |
| Stage 1A | Microorganisms present on wound surface |
| Stage 1B | Microbial penetration of eschar |
| Stage 1C | Proliferation of microorganisms at interface between viable and nonviable tissue (subeschar space) |
| Infection | |
| Stage IIA | Foci of microinvasion in uppermost viable tissue |
| Stage IIB | Penetration of microbes to variable depth within viable tissue |
| Stage IIC | Angioinvasion (microorganisms within small blood vessels and/or lymphatics) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pruskowski, K.A.; Mitchell, T.A.; Kiley, J.L.; Wellington, T.; Britton, G.W.; Cancio, L.C. Diagnosis and Management of Invasive Fungal Wound Infections in Burn Patients. Eur. Burn J. 2021, 2, 168-183. https://doi.org/10.3390/ebj2040013
Pruskowski KA, Mitchell TA, Kiley JL, Wellington T, Britton GW, Cancio LC. Diagnosis and Management of Invasive Fungal Wound Infections in Burn Patients. European Burn Journal. 2021; 2(4):168-183. https://doi.org/10.3390/ebj2040013
Chicago/Turabian StylePruskowski, Kaitlin A., Thomas A. Mitchell, John L. Kiley, Trevor Wellington, Garrett W. Britton, and Leopoldo C. Cancio. 2021. "Diagnosis and Management of Invasive Fungal Wound Infections in Burn Patients" European Burn Journal 2, no. 4: 168-183. https://doi.org/10.3390/ebj2040013
APA StylePruskowski, K. A., Mitchell, T. A., Kiley, J. L., Wellington, T., Britton, G. W., & Cancio, L. C. (2021). Diagnosis and Management of Invasive Fungal Wound Infections in Burn Patients. European Burn Journal, 2(4), 168-183. https://doi.org/10.3390/ebj2040013






