Irradiance Level Only Moderately Affects Thermal Bleaching in the Stony Coral Stylophora pistillata
Abstract
1. Introduction
2. Materials and Methods
2.1. Corals and Coral Maintenance
2.2. Determination of Symbiont Identity
2.3. Experimental Aquarium System and Study Design
2.4. Measurement of Photochemical Yield
2.5. Determination of Symbiont Numbers and Chlorophyll-a
2.6. Data Analysis
3. Results
3.1. Effect of Heat and Irradiance on PSII Apparent Yield
3.2. Effect of Heat and Irradiance on Symbiont Density and Chlorophyll-a Content
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GLMM | Generalized Linear Mixed Model |
| GoA | Gulf of Aqaba |
| ROS | Reactive Oxygen Species |
| HLH | High Light Heat treatment |
| HLC | High Light Control treatment |
| LLH | Low Light Heat treatment |
| LLC | Low Light Control treatment |
References
- Hoegh-Guldberg, O.; Skirving, W.; Dove, S.G.; Spady, B.L.; Norrie, A.; Geiger, E.F.; Liu, G.; De La Cour, J.L.; Manzello, D.P. Coral reefs in peril in a record breaking year. Science 2023, 382, 1238–1240. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Anderson, K.D.; Connolly, S.R.; Heron, S.F.; Kerry, J.T.; Lough, J.M.; Baum, J.K.; Berumen, M.L.; Bridge, T.C.; Claar, D.C.; et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 2018, 359, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Reimer, J.D.; Peixoto, R.S.; Davies, S.W.; Traylor-Knowles, N.; Short, M.L.; Cabral-Tena, R.A.; Burt, J.A.; Pessoa, I.; Banaszak, A.T.; Winters, R.S.; et al. The Fourth Global Coral Bleaching Event: Where do we go from here? Coral Reefs 2024, 43, 1121–1125. [Google Scholar] [CrossRef]
- Van Oppen, M.J.H.; Lough, J.M. Introduction: Coral Bleaching–Patterns, Processes, Causes and Consequences. In Coral Bleaching–Patterns, Processes, Causes and Consequences, 2nd ed.; Van Oppen, M.J.H., Lough, J.M., Eds.; Ecological Studies; Springer: Cham, Switzerland, 2018; Volume 233, pp. 1–8. [Google Scholar]
- Lesser, M.P. Oxidative stress causes coral bleaching during exposure to elevated temperatures. Coral Reefs 1997, 16, 187–192. [Google Scholar] [CrossRef]
- Weis, V.M. Cellular mechanisms of Cnidarian bleaching: Stress causes the collapse of symbiosis. J. Exp. Biol. 2008, 211, 3059–3066. [Google Scholar] [CrossRef]
- Warner, M.E.; Fitt, W.K.; Schmidt, G.W. The effects of elevated temperature on the photosynthetic efficiency of zooxanthellae in hospite from four different species of reef coral: A novel approach. Plant Cell Environ. 1996, 19, 291–299. [Google Scholar] [CrossRef]
- Nielsen, D.A.; Petrou, K.; Gates, R.D. Coral bleaching from a single cell perspective. ISME J. 2018, 12, 6. [Google Scholar] [CrossRef]
- Saragosti, E.; Tchernov, D.; Katsir, A.; Shaked, Y. Extracellular Production and Degradation of Superoxide in the Coral Stylophora pistillata and Cultured Symbiodinium. PLoS ONE 2010, 5, e12508. [Google Scholar] [CrossRef]
- Aro, E.M.; Virgin, I.; Andersson, B. Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta Bioenergy 1993, 1143, 113–134. [Google Scholar] [CrossRef]
- Lesser, M.P. Phylogenetic signature of light and thermal stress for the endosymbiotic dinoflagellates of corals (Family Symbiodiniaceae). Limnol. Oceanogr. 2019, 64, 1852–1863. [Google Scholar] [CrossRef]
- Nanba, O.; Satoh, K. Isolation of a photosystem II reaction center consisting of D-1 andD-2 polypeptides and cytochrome b-559. Proc. Nat. Acad. Sci. USA 1987, 84, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Fitt, W.K.; Brown, B.E.; Warner, M.E.; Dunne, R.P. Coral bleaching: Interpretation of thermal tolerance limits and thermal thresholds in tropical corals. Coral Reefs 2001, 20, 51–65. [Google Scholar] [CrossRef]
- Sweet, M.E.; Brown, B.E. Coral responses to anthropogenic stress in the twenty-first century: An ecophysiological perspective. Oceanogr. Mar. Biol. Ann. Rev. 2016, 54, 271–314. [Google Scholar]
- Tagliafico, A.; Baker, P.; Kelaher, B.; Ellis, S.; Harrison, D. The effects of shade and light on corals in the context of coral bleaching and shading technologies. Front. Mar. Sci. 2022, 9, 919382. [Google Scholar] [CrossRef]
- Brown, B.E.; Downs, C.A.; Dunne, R.P.; Gibb, S.W. Exploring the basis of thermotolerance in the reef coral Goniastrea aspera. Mar. Ecol. Prog. Ser. 2002, 242, 119–129. [Google Scholar] [CrossRef]
- Dunne, R.P.; Brown, B.E. The influence of solar radiation on bleaching of shallow water reef corals in the Andaman Sea, 1993–1998. Coral Reefs 2001, 20, 201–210. [Google Scholar] [CrossRef]
- Bhagooli, R.; Hidaka, M. Comparison of stress susceptibility of in hospite and isolated zooxanthellae among five coral species. J. Exp. Mar. Biol. Ecol. 2003, 291, 181–197. [Google Scholar] [CrossRef]
- Brown, B.E.; Dunne, R.P. Solar radiation modulates bleaching and damage protection in a shallow water coral. Mar. Ecol. Prog. Ser. 2008, 362, 99–107. [Google Scholar] [CrossRef]
- Fournie, J.W.; Vivian, D.N.; Yee, S.H.; Courtney, L.A.; Barron, M.G. Comparative sensitivity of six scleractinian corals to temperature and solar radiation. Dis. Aquat. Organ. 2012, 99, 85–93. [Google Scholar] [CrossRef]
- Lesser, M.P.; Farrell, J.H. Exposure to solar radiation increases damage to both host tissues and algal symbionts of corals during thermal stress. Coral Reefs 2004, 23, 367–377. [Google Scholar] [CrossRef]
- Wijgerde, T.; van Ballegooijen, M.; Nijland, R.; van der Loos, L.; Kwadijk, C.; Osinga, R.; Murk, A.J.; Slijkerman, D. Adding insult to injury: Effects of chronic oxybenzone exposure and elevated temperature on two reef-building corals. Sci. Total Environ. 2020, 733, 139030. [Google Scholar] [CrossRef] [PubMed]
- Hume, B.C.C.; Ziegler, M.; Poulain, J.; Pochon, X.; Romac, S.; Boissin, E.; de Vargas, C.; Planes, S.; Wincker, P.; Voolstra, C.R. An improved primer set and amplification protocol with increased specificity and sensitivity targeting the Symbiodinium ITS2 region. PeerJ 2018, 6, e4816. [Google Scholar] [CrossRef] [PubMed]
- Wiedenmann, J.; D’Angelo, C.; Smith, E.G.; Hunt, A.N.; Legiret, F.E.; Postle, A.D.; Achterberg, E.P. Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nat. Clim. Change 2013, 3, 160–164. [Google Scholar] [CrossRef]
- Fernandes de Barros Marangoni, L.; Ferrier-Pagès, C.; Rottier, C.; Bianchini, A.; Grover, R. Unravelling the different causes of nitrate and ammonium effects on coral bleaching. Sci. Rep. 2020, 10, 11975. [Google Scholar] [CrossRef]
- Tilstra, A.; Wijgerde, T.; Dini-Andreote, F.; Eriksson, B.K.; Falcao Salles, J.; Pen, I.; Osinga, R.; Wild, C. Light induced intraspecific variability in response to thermal stress in the hard coral Stylophora pistillata. PeerJ 2017, 5, e3802. [Google Scholar] [CrossRef]
- Titlyanov, E.A.; Titlyanova, E.V.; Yamazato, K.; Van Woesik, R. Photo-acclimation dynamics of the coral Stylophora pistillata to low and extremely low light. J. Exp. Mar. Biol. Ecol. 2001, 263, 211–225. [Google Scholar] [CrossRef]
- Enriquez, S.; Borowitzka, M. The Use of the Fluorescence Signal in Studies of Seagrasses and Macroalgae. In Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications; Sugget, D.J., Prasil, O., Borowitzka, M., Eds.; Springer: Dordrecht, The Netherlands, 2009; Developments in Applied Phycology 4; pp. 187–208. [Google Scholar]
- Niyogi, K.K. Safety valves for photosynthesis. Curr. Opin. Plant Biol. 2000, 3, 455–460. [Google Scholar] [CrossRef]
- Ralph, P.J.; Gademann, R.; Larkum, A.W.D. Zooxanthellae expelled from bleached corals at 33_C are photosynthetically competent. Mar. Ecol. Prog. Ser. 2001, 220, 163–168. [Google Scholar] [CrossRef]
- Warner, M.E.; Fitt, W.K.; Schmidt, G.W. Damage to Photosystem II in symbiotic dinoflagellates: A determinant of coral bleaching. Proc. Nat. Acad. Sci. USA 1999, 96, 8007–8012. [Google Scholar] [CrossRef]
- Rosic, N.; Rémond, C.; Mello-Athayde, M.A. Differential impact of heat stress on reef-building corals under different light conditions. Mar. Environ. Res. 2020, 158, 104947. [Google Scholar] [CrossRef]
- Tolleter, D.; Seneca, F.O.; DeNofrio, J.C.; Krediet, C.J.; Palumbi, S.R.; Pringle, J.R.; Grossman, A.R. Coral Bleaching Independent of Photosynthetic Activity. Curr. Biol. 2013, 23, 1782–1786. [Google Scholar] [CrossRef] [PubMed]
- Yamane, Y.; Kashino, Y.; Koike, H.; Satoh, K. Effects of high temperatures on the photosynthetic systems in spinach: Oxygen-evolving activities, fluorescence characteristics and the denaturation process. Photosynth. Res. 1998, 57, 51–59. [Google Scholar] [CrossRef]
- Tchernov, D.; Gorbunov, M.Y.; de Vargas, C.; Yadav, S.N.; Milligan, A.J.; Häggblom, M.; Falkowski, P.G. Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc. Nat. Acad. Sci. USA 2004, 101, 13531–13535. [Google Scholar] [CrossRef] [PubMed]
- Oakley, C.A.; Davy, S.K. Cell biology of coral bleaching. In Coral Bleaching–Patterns, Processes, Causes and Consequences, 2nd ed.; Van Oppen, M.J.H., Lough, J.M., Eds.; Ecological Studies; Springer: Cham, Switzerland, 2018; Volume 233, pp. 189–211. [Google Scholar]
- McLachlan, R.H.; Price, J.T.; Solomon, S.L.; Grottoli, A.G. Thirty years of coral heat-stress experiments: A review of methods. Coral Reefs 2020, 39, 885–902. [Google Scholar] [CrossRef]
- Keren, N.; Krieger-Liszkay, A. Photoinhibition: Molecular mechanisms and physiological significance. Physiol. Plant. 2011, 141, 1–5. [Google Scholar] [CrossRef]
- Courtial, L.; Roberty, S.; Shick, J.M.; Houlbrèque, F.; Ferrier-Pagès, C. Interactive Effects of Ultraviolet Radiation and Thermal Stress on Two Reef-Building Corals. Limnol. Oceanogr. 2017, 62, 1000–1013. [Google Scholar] [CrossRef]
- Banaszak, A.T.; Lesser, M.P. Effects of Solar Ultraviolet Radiation on Coral Reef Organisms. Photochem. Photobiol. Sci. 2009, 8, 1276–1294. [Google Scholar] [CrossRef]
- Santoro, E.P.; Cárdenas, A.; Villela, H.D.M.; Vilela, C.L.S.; Ghizelini, A.M.; Duarte, G.A.S.; Perna, G.; Saraiva, J.P.; Thomas, T.; Voolstra, C.R.; et al. Inherent differential microbial assemblages and functions associated with corals exhibiting different thermal phenotypes. Sci. Adv. 2025, 11, eadq2583. [Google Scholar] [CrossRef]
- Fine, M.; Gildor, H.; Genin, A. A coral reef refuge in the Red Sea. Glob. Change Biol. 2013, 19, 3640–3647. [Google Scholar] [CrossRef]
- Strong, A.; Liu, G.; Skirving, W.; Eakin, C.M. NOAA’s Coral Reef Watch program from satellite observations. Ann. GIS 2011, 17, 83–92. [Google Scholar] [CrossRef]
- Bhagooli, R.; Hidaka, M. Photoinhibition, bleaching susceptibility and mortality in two scleractinian corals, Platygyra ryukyuensis and Stylophora pistillata, in response to thermal and light stresses. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 137, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.N.; Cunning, R.; Baker, A.C. Change in algal symbiont communities after bleaching, not prior heat exposure, increases heat tolerance of reef corals. Glob. Change Biol. 2015, 21, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Lampert-Karako, S.; Stambler, N.; Katcoff, D.J.; Achituv, Y.; Dubinsky, Z. Effects of depth and eutrophication on the zooxanthella clades of Stylophora pistillata from the Gulf of Eilat (Red Sea). Aquat. Cons. 2008, 18, 1039–1045. [Google Scholar] [CrossRef]

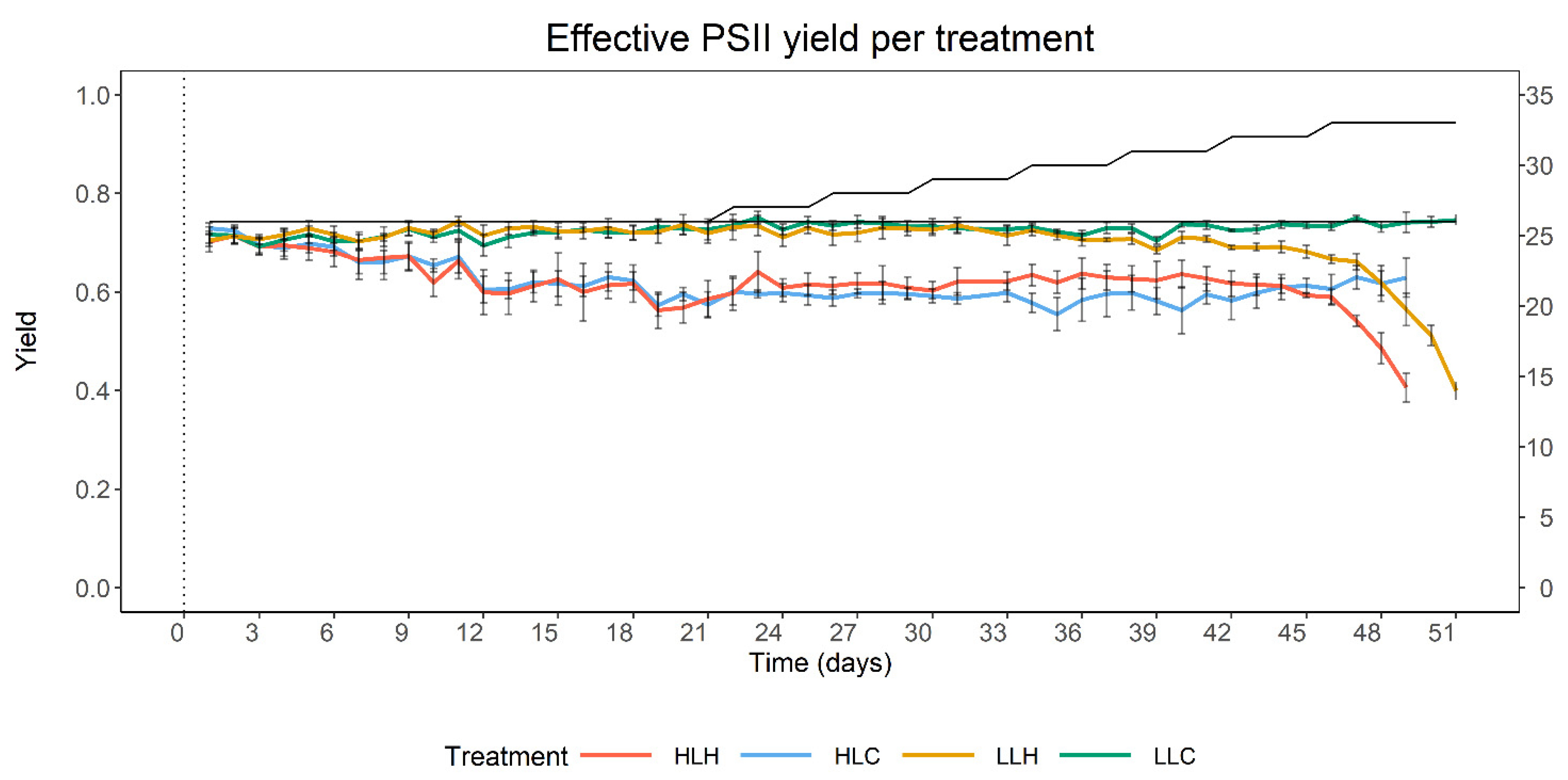
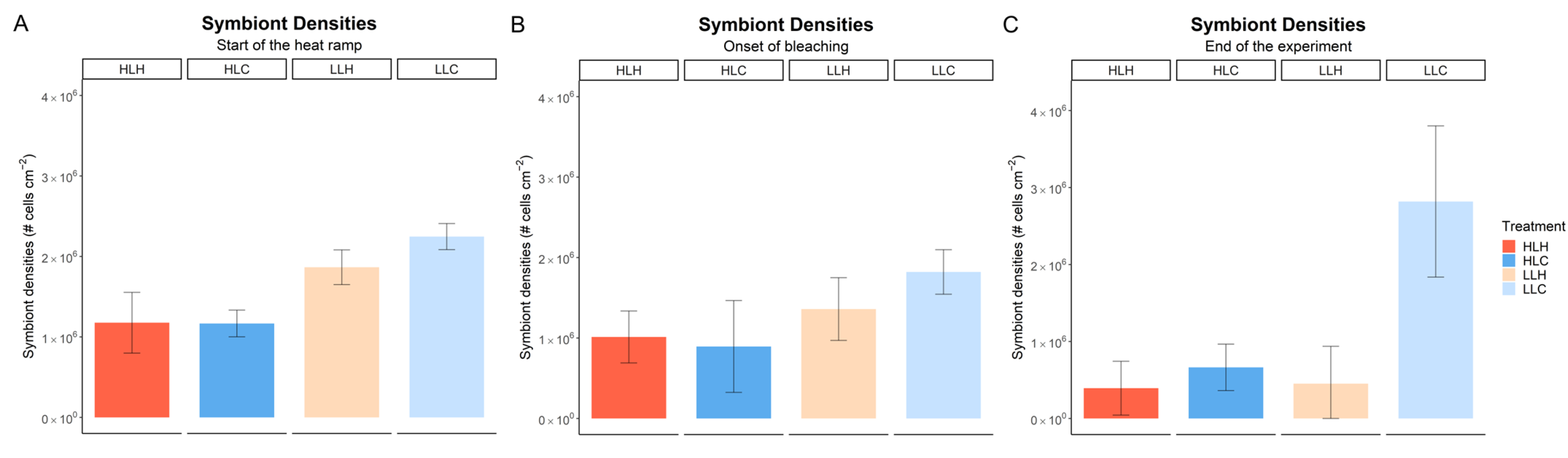
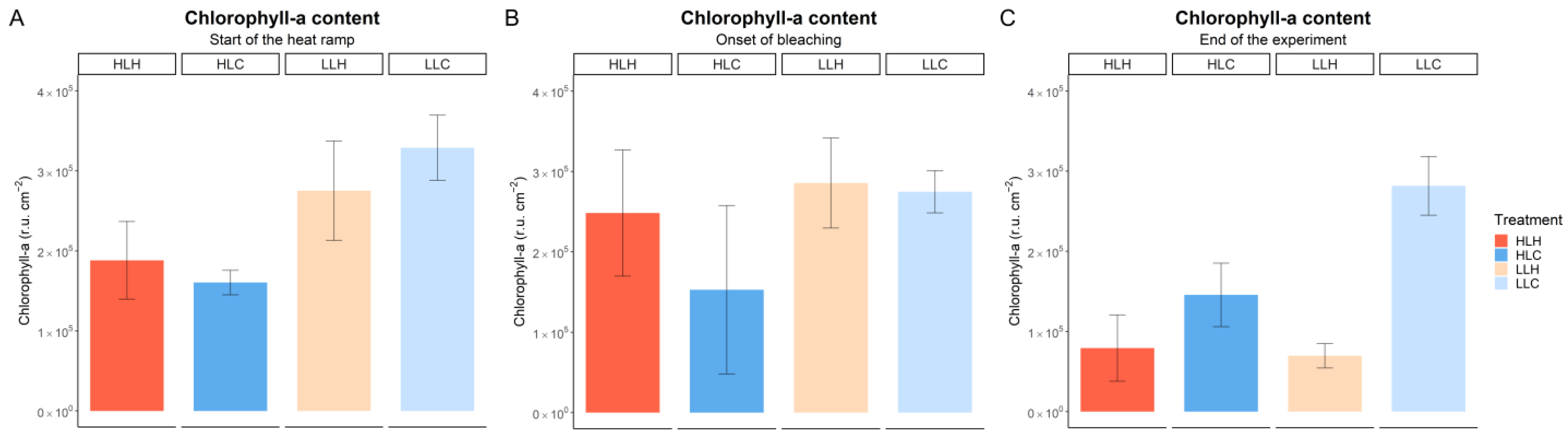
| GLMM | Estimate | std. Error | DF | t-Value | p-Value |
|---|---|---|---|---|---|
| (intercept) | 7.11 × 10−1 | 9.59 × 10−3 | 2.07 × 10−1 | 74.12 | <2 × 10−16 *** |
| time | 5.87 × 10−4 | 1.82 × 10−4 | 7.64 × 10−2 | 3.22 | 0.00134 ** |
| temperature | 4.56 × 10−2 | 1.36 × 10−2 | 2.07 × 10−1 | 3.36 | 0.00301 ** |
| irradiance | −4.32 × 10−2 | 1.36 × 10−2 | 2.10 × 10−1 | −3.18 | 0.00453 ** |
| time × irradiance | −2.79 × 10−3 | 2.58 × 10−4 | 7.64 × 10−2 | −10.83 | <2 × 10−16 *** |
| time × temperature | 2.57 × 10−3 | 2.66 × 10−4 | 7.64 × 10−2 | −9.64 | <2 × 10−16 *** |
| irradiance × temperature | 3.83 × 10−2 | 1.92 × 10−2 | 2.10 × 10−1 | −1.99 | 0.05981 |
| time × temperature × irradiance | −2.52 × 10−3 | 3.76 × 10−4 | 7.64 × 10−2 | 6.70 | 4.06 × 10−11 *** |
| Treatment | RC Pre-Onset | RC Post-Onset | p-Value |
|---|---|---|---|
| LLH | 0.007 ± 0.004 | 0.063 ± 0.003 | >0.0000001 |
| HLH | 0.01 ± 0.004 | 0.061 ± 0.008 | >0.00001 |
| Sampling Point: | Start of Heat Ramp | Onset of Bleaching | End of Experiment |
|---|---|---|---|
| irradiance | 1.27 × 10−5 *** | 0.0174 * | 0.0092 ** |
| temperature | x | 0.7175 | 0.0001 *** |
| irradiance × temperature | x | 0.2141 | 0.0263 * |
| Sampling Point: | Start of Heat Ramp | Onset of Bleaching | End of Experiment |
|---|---|---|---|
| irradiance | 9.95 × 10−5 *** | 0.0481 * | 3.82 × 10−6 *** |
| temperature | x | 0.1671 | 0.00344 ** |
| irradiance × temperature | x | 0.2653 | 0.00128 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osinga, R.; van Veenendaal, E.; Geschiere, D.S.L.; van Herpen, B.J.A.; Oosterbroek, S. Irradiance Level Only Moderately Affects Thermal Bleaching in the Stony Coral Stylophora pistillata. Oceans 2025, 6, 32. https://doi.org/10.3390/oceans6020032
Osinga R, van Veenendaal E, Geschiere DSL, van Herpen BJA, Oosterbroek S. Irradiance Level Only Moderately Affects Thermal Bleaching in the Stony Coral Stylophora pistillata. Oceans. 2025; 6(2):32. https://doi.org/10.3390/oceans6020032
Chicago/Turabian StyleOsinga, Ronald, Emma van Veenendaal, Daniëlle S. L. Geschiere, Britt J. A. van Herpen, and Saskia Oosterbroek. 2025. "Irradiance Level Only Moderately Affects Thermal Bleaching in the Stony Coral Stylophora pistillata" Oceans 6, no. 2: 32. https://doi.org/10.3390/oceans6020032
APA StyleOsinga, R., van Veenendaal, E., Geschiere, D. S. L., van Herpen, B. J. A., & Oosterbroek, S. (2025). Irradiance Level Only Moderately Affects Thermal Bleaching in the Stony Coral Stylophora pistillata. Oceans, 6(2), 32. https://doi.org/10.3390/oceans6020032







