Abstract
The use of multiple sampling instruments during ichthyoplankton surveys could require a significant amount of time for sample collection procedures; moreover, their use is highly dependent on weather conditions. During surveys aimed at the application of the Daily Egg Production Method (DEPM), two different kinds of sampler are employed: PairoVET and Bongo 40. The possibility of using only one of these samplers may allow for a reduction in the sampling time on board and the subsequent analyses; thus, a study was carried out to test the use of the Bongo 40 sampler alone (i.e., without PairoVET sampling) in DEPM application. Samples collected during five ichthyoplankton surveys (from 2007 to 2010 and in 2014) were analyzed to compare the efficiency in anchovy egg collection. Although the compared ichthyoplankton samplers provided differences in the collected number of eggs, as well as in the filtered volume, non-significant differences between them were recorded in the egg density. An ANCOVA revealed a significant relationship (F1,5 = 606.80; p < 0.001) between the density estimates from the two nets, but no differences were recorded among years (F1,5 = 0.99; p < 1). The slopes of the linear regressions for the two datasets were close to 1, suggesting a substantial equivalence of the two samplers in estimating the egg density. Finally, since only a few sampling stations showed densities higher than 20 ind. m−2, conversion equations were provided to estimate the PairoVET density from the Bongo 40 data at different density levels (i.e., 20 ind. m−2 or higher).
1. Introduction
Onboard surveys are essential tools for studying the early life stages of fish, whether the goal is to observe recruitment variability or to estimate spawning stock abundance using ichthyoplankton methods [1,2,3,4]. Conventional methods, such as towed nets at preset sampling stations, have limitations in their accuracy, precision, and sensitivity and are labor-intensive and expensive [5]. Furthermore, these sampling surveys require the dedicated use of a research vessel, often hindered by severe weather conditions, and produce numerous samples that need to be analyzed ashore. Difficulties in obtaining representative ichthyoplankton samples also arise when the study areas are very heterogeneous and less productive, such as the eastern and central-southern Mediterranean Sea [6,7].
The daily egg production method (DEPM [8]) is one of the main tools used to study the reproductive ecology of small pelagic species and to estimate the spawning biomass of fish stocks. In the Strait of Sicily, DEPM surveys have been performed annually on anchovy stock since 2005 [7], because these surveys have provided robust data on reproductive traits, such as spawning fraction, batch fecundity, sex ratio, maturation cycle, and gonad atresia [7,9,10,11]. Moreover, onboard surveys are well known to produce useful information on the relationship between habitat and the reproductive biology and ecology of monitored small pelagic stocks, for both adult [10,12,13] and early life stages [14,15].
Generally, plankton sampling includes the 0–100 m layer, and the distribution of anchovy eggs and larvae is restricted to the top of the water column, where concentration peaks occur in the upper 20 m, though distinct subsurface peaks often depend on local physical conditions ([6] and references therein). The vertical CalVET or PairoVET nets are sampling tools specially developed for the purposes of DEPM application to Californian anchovy populations, and they are still employed [16,17]. However, the Bongo 40 (B40; oblique operating) or Working Party 2 (WP2; vertical operating) nets were successfully used to obtain adequate samples for DEPM applications to different species and areas [18,19], including in oligotrophic waters in the Mediterranean Sea, where anchovy egg production is lower than in the oceanic upwelling areas [6,20].
European anchovy, Engraulis encrasicolus (Linneus, 1758), belonging to the Engraulidae family, is one of the highly exploited resources [21] and accounts for significant biomass in the Mediterranean Sea, playing a key role in maintaining ecological processes in pelagic ecosystems [7,22,23], due to its essential intermediate trophic level in food webs [24]. This species is an indeterminate spawner that releases pelagic eggs during a protracted spawning season, which in the Strait of Sicily ranges from April to October, with a peak during summer [7]. The European anchovy is assessed within the EU data collection framework [25], to produce management plans for sustainable stock exploitation by predictive models, accounting for several biological parameters such as population structure, growth, and size at maturity [26,27,28]. To this aim, anchovy stocks are monitored along European coasts by both acoustic and DEPM surveys [7,10,29].
European anchovy egg occurrence and oceanographic data from summer surveys were examined to understand the mechanisms that control the spatial distribution of anchovy spawning habitat in the Strait of Sicily [30]. Ichthyoplankton distribution shows a high degree of dispersal and patchiness that may create significant challenges when trying to obtain a representative sample of a population. The sampling scheme must be designed to resolve the main physical features that govern the spatial distribution of the organisms of interest and be carried out quickly enough to avoid significant changes in distribution over the course of the survey [5]. The DEPM sampling should obtain representative samples of eggs, but also of larvae, of the target species, as well as the associated planktonic community where these eggs develop and hatch. However, often during ichthyoplankton sampling at sea, there is the need to reduce the sampling effort while safeguarding the quality and the representativeness of collected samples. The use of both samplers (Bongo 40 and PairoVET) within the sampling station grid is recommended for DEPM applications, even though the greater information obtainable from oblique plankton tows may favor the use of the Bongo net. It is well known that the B40 net is more efficient for sampling fish larvae as well, while CalVET and PairoVET (PV) nets are usually employed for fish eggs [16]. The main difference between these samplers lies in their trajectory in the water column. In the present study, vertical tows are carried out with the PV and oblique tows are carried out with the B40 at a speed of 2 knots. Finally, another difference is represented by the areas covered by the two nets. The samples provided by these nets are affected in different ways by the distribution of the planktonic organisms, with the PV catches being more sensitive to small and fine-scale patchiness. Despite previous tests on the performance of different plankton nets [31,32,33], very few studies have provided detailed information to compare these two plankton samplers (e.g., [34]). Further, the great heterogeneity and habitat variability within the Mediterranean Sea strongly suggest to compare their performances in specific areas, where DEPM is routinely applied, as in the case of the Strait of Sicily [7]. To fulfill this gap, the present study analyzed a time series of anchovy egg data collected simultaneously by two different plankton samplers, the PV and the B40. Particularly, the comparability of egg density data from these two nets was assessed, in order to compare their accuracy for sampling in oligotrophic waters.
2. Material and Methods
2.1. Sampling and Analysis of Eggs
Multidisciplinary surveys took place on the continental shelf (depth < 200 m) of the northern part of the Strait of Sicily (Central Mediterranean Sea; Figure 1) during the summer period (July–August), when anchovy reproductive season reaches its peak [7]. Data on egg density and water volume were collected during five years (2007, 2008, 2009, 2010, and 2014) by two types of plankton nets (PV and B40), and each gear was equipped with two conical nets. Each mouth was instrumented with a calibrated General Oceanics mechanical flowmeter, balanced for dynamic stability and working with a high-resolution rotor for low-speed applications, as in the case of the present sampling. The sampling was carried out at the same locations, i.e., each PV sampling station represents the starting point for each B40 sample. Ichthyoplankton was collected with the B40 net towed obliquely from the surface to 100 m (where possible, according to the depth) and back to the surface, with a vessel speed of 2 knots. The ideal operational angle between the cable supporting the gear and the sea surface was 45° for optimizing the sampling collection by the B40 net. This angle was continuously monitored by expert operators working onboard, during the sampling station period, and eventually corrected if needed. This gear has two mouths with an opening diameter of 40 cm and a 200 μm filter mesh. In each station of the sampling grid, with the vessel stationary, the PV net was brought vertically from the surface to the maximum depth (i.e., 100 m where possible or close to the bottom). The PV conical nets have a filtering mesh of 150 µm, the diameter of the two mouths is 25 cm, and the descent speed is about 1 m/s, which was constantly monitored by the research vessel crew operating with a speed-controlled winch. The biological sample was collected only during the ascent phase from maximum depth to the surface.
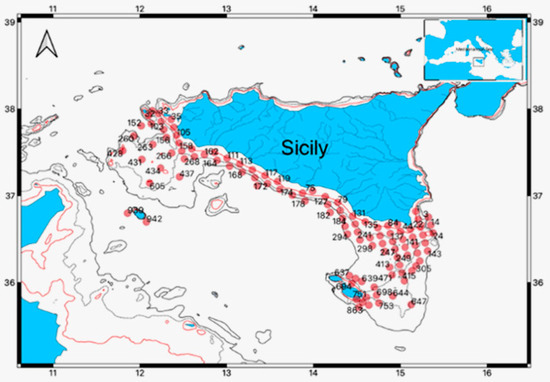
Figure 1.
The study area, over the continental shelf of the Strait of Sicily. The red dots represent the sampling stations for both nets, Bongo 40 and PairoVET.
Over the study period, biological samples collected at 493 stations were analyzed; their distribution per survey is presented in Table 1. Samples from both samplers were fixed in 4% buffered formalin for subsequent laboratory analyses, where they were observed under a stereomicroscope for identifying eggs of the target species. For both PV and B40, the egg catches were standardized to m2 by estimating the egg density in each sample according to Houde [2]:

Table 1.
Information on the samples collected by the two samplers (PairoVET, PV, and Bongo 40, B40) during the study period (2007, 2008, 2009, 2010, 2014).
2.2. Statistical Analysis
In order to compare both filtered water volume and egg density between the two samplers, data were fitted using linear regression models. The negative stations (i.e., stations in which no anchovy eggs were caught by either net) were not included in these models [34].
Outliers in the data can distort predictions and compromise accuracy, especially in regression models, if they are not detected and appropriately handled. A multivariate approach was adopted for egg densities in each year to identify possible outliers. The advantage of a multivariate approach was linked to the use of Cook’s distance (threshold 0.5). This method allowed for the detection of outliers, specifically, by assessing how much each observation affected the fitted values and identifying those that might alter the fitting linearity. Therefore, the regression analysis was performed on stations with an egg count greater than 0 (#egg > 0) in at least one of the samplers, after excluding the identified outliers.
Finally, to test for possible secondary effects on the linear regression model, an ANCOVA was run on the density values from the B40 and PV, including year as a factor and the interaction terms as the main effects. The regression analysis and all other computations were performed in the R statistical environment [35].
3. Results
Results for the analyzed plankton time series are summarized in Table 2, providing various descriptive statistics, including Pennington’s estimators of the mean, the variance, and the variance of the mean, which are efficient descriptors for fish and plankton surveys [36].

Table 2.
Basic statistics of the Bongo 40 (B40) and PairoVET (PV) samples used in the regression analysis excluding double 0 stations and outliers (256 stations). EggPV: number of eggs collected by PairoVET. EggB40: number of eggs collected by Bongo 40. VolPV: volume of water (m3) filtered by PairoVET. VolB40: volume of water (m3) filtered by Bongo 40. DepthPV: depth reached during sampling by PairoVET. DepthB40: depth reached during sampling by Bongo 40. densPV: density of eggs in PairoVET samples. densB40: density of eggs in Bongo 40 samples.
The ichthyoplankton samplers we used showed differences in the absolute number of eggs collected, as well as in the volume filtered, while the mean depth was similar (Table 2, Figure 2). The comparison of tow depth data showed a similar depth range reached by both samplers (Table 2).
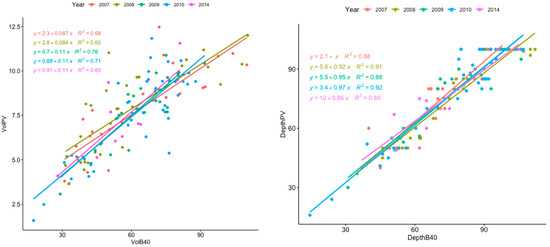
Figure 2.
Relationship between the filtered volumes (left panel) and the achieved depth (right panel) of the two nets (PairoVET, PV, and Bongo 40, B40) per year. The graphs show the data (points) and linear regression for each study year, as well as the relative equation and R2 value (p < 0.001). The legend shows the different colors according to the year of sampling.
Filtered volumes by the two samplers showed a great difference in values; generally, the mean B40 values were at least seven times higher than the volume sampled by the PV (Table 2). As expected, while the average filtered volumes differed between PV and B40, the standardized square meter densities were quite similar (Table 2, Figure 2). Interannual variability of linear regression models showed some differences in 2007 and 2008 (Figure 2), although the ANOVA revealed that such differences were not significant (p < 0.1; Table 3). In contrast, interannual variability with depth of the linear regression models appeared significant (Figure 2), as confirmed by the ANOVA test (p < 0.01; Table 3).

Table 3.
Analysis of variance (ANOVA) to test for variability in (A) volumes of water filtered (Vol); (B) sampling depths achieved (Depth).
Over the study period, the stations where at least one of the two samplers collected anchovy eggs were 256 out of 493 samples (Table 1 and Table 2). As previously mentioned, those stations with 0 anchovy eggs in both samplers were excluded (n = 111), since they cannot be used as an element of similarity. Therefore, the dataset used for the regression analyses included 145 samples. The ANCOVA performed on egg density between the two gears, with PV as the response variable and B40 as the predictor, showed a significant relationship but no differences between years (Table 4). Since the intercept was not significantly different from 0, it was set to 0 in the final linear regression model. Moreover, since the Cook’s distance index for influential observations presented values higher than the chosen threshold (i.e., 0.5), mainly for those stations with a density higher than 20 ind. m2, and considering these were very few stations (i.e., 7) (Figure 3A,C), a new subset was obtained by excluding the higher density stations (Figure 3B,D). The regression models obtained by each dataset (overall and low-density subset—i.e., <20 ind. m2) were both statistically significant, and the slopes of the estimated linear models were 1.17 ± 0.05 (t = 23.9, p < 0.001) overall and 0.97 ± 0.07 (t = 14.1, p < 0.001) for stations with densities lower than 20 ind. m−2, explaining 76% and 64% of the total variance, respectively (see Table 5 and its caption for more details).

Table 4.
ANCOVA on egg density (dens) between the two gears, PairoVET (PV) and Bongo 40 (B40): densPV = response variable; densB40 = predictor; Year = factor. Intercept set to 0.
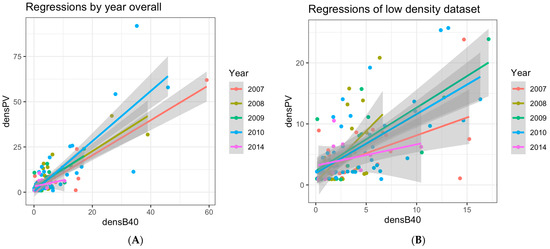
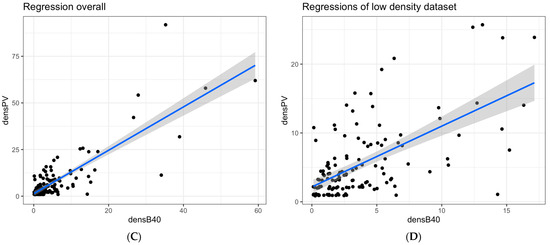
Figure 3.
Scatterplot of egg density (#eggs m−2) values in the PairoVET (densPV) and Bongo40 (densB40) with their confidence intervals superimposed. (A) Scatterplot of egg density from the whole dataset by sampling year (2007, 2008, 2009, 2010, 2014). (B) Scatterplot of egg density from the stations with density less than 20 ind. m−2 by sampling year (2007, 2008, 2009, 2010, 2014); the legend for panels A and B shows the different colors corresponding to the sampling year. (C) Scatterplot of egg density from the whole dataset for all years combined. (D) Scatterplot of egg density from the stations with density lower than 20 ind. m−2 for all years combined. Within all the four panels, the gray areas (i.e., the shaded areas) represent the confidence interval.

Table 5.
(A) Regression parameter estimation of egg density estimates for all the positive (#egg > 0) Bongo 40 stations (densB40) [Residual standard error: 5.364 with 250 degrees of freedom; adjusted R-squared: 0.7505; F-statistic: 129.4; p-value: <2.2 × 10−16]. (B) Regression parameter estimation for the subset of stations with Bongo 40 density (densB4020) values less than 20 ind. m−2 [Residual standard error: 3.475 with 243 degrees of freedom; adjusted R-squared: 0.6253; F-statistic: 70.27; p-value: <2.2 × 10−16].
4. Discussion
Monitoring anchovy populations has been regularly carried out during scientific expeditions in the Mediterranean Sea and Atlantic Ocean to provide spatio-temporal insights into the occurrences and abundances of anchovy adults and early life stages. This monitoring may shed light on the recently recorded diminished reproductive potential [37]. The study results highlighted a general interannual variability and a negative trend in egg and larva abundance, which may be linked to both overexploitation [38] and environmental variability experienced by spawning adults and early life stages [7,39,40]. For the purposes of this study, the sampling period took place during the peak of the anchovy reproductive season [7,12] for a dual advantage. Indeed, when the DEPM is applied during the spawning period, the estimates of spawning stock biomass are considered representative of the entire stock biomass, since almost the entire population is involved in reproduction. Secondly, the reproductive peak results in a higher concentration of spawned eggs in the water column, allowing for optimal sampling by any type of ichthyoplankton sampler [1]. The comparison between PV vertical and B40 oblique tows carried out in the study area showed no significant differences in the mean density of anchovy eggs, with a small effect on this relationship related to the year of sampling. However, the interannual variance in egg production recorded during the study period did not affect the relationship between the two samplers, although some fluctuations among years could be introduced by the intrinsic variability in field sampling due to sea weather conditions, navigation requirements and similar tasks, as well as the ability of fish populations to modify their spatial distribution depending on ecosystem characteristics and density-dependent behavior ([29] and references therein).
Comparisons of several kinds of plankton samplers have been carried out for different purposes in recent decades. The sampling nets were tested for quantitative and/or qualitative efficiency regarding mesh size, species representativeness, species composition, and larvae size [41,42,43,44,45]. Hydrodynamic experiments, as well as comparisons of sampler structure efficiency, were carried out to enhance stability and accuracy in ichthyoplankton surveys [46,47]. Nevertheless, few comparisons are available on ichthyoplankton sampling for DEPM, although an application on Baltic cod (Gadus morhua) used data from both a Bongo net and a vertical net (i.e., IKS-80 net), as no differences in catchability between these nets were previously proven [18]. The slopes of the linear regressions for both datasets (overall and lower densities) examined here were close to 1, suggesting a substantial equivalence between the two samplers in obtaining egg density values, particularly for the lower density range (0–20 ind. m−2). This suggests that the data could be used interchangeably to estimate egg production and the related parameters needed for DEPM application, despite the differences that occurred between the two nets in other parameters, such as filtered volume. The efficiency of the B40 in filtering a larger volume of water than the vertical ichthyoplankton nets has already been documented in the literature [6]. The oblique trajectories of the Bongo net could also explain the difference in depth reached. These aspects of variability were minimized by the standardization per unit area (m2) of the number of sampled eggs and by considering that both types of sampling are performed in the upper water layers (i.e., in the egg concentration zone of the target species) [6,41]. Moreover, the present results yielded statistically significant regression models, which explain much of the variability between the two samplers and, therefore, should be used to make spawning biomass estimates by DEPM comparable, when employing these two sampling tools (Table 5).
The CalVET net was specially designed to minimize sampling effort and post-processing time, reducing the standard error of DEPM estimates in a highly productive system, as occurs in California waters [16]. In the Bay of Biscay, for DEPM applications in European oceanic waters, the vertical sampler was changed from a CalVET (one sampling mouth) to a PairoVET (two sampling mouths) because doubling the effective sampling reduced errors, and its applications still proved good efficiency [17], also compared with a Continuous Underway Fish Egg Sample (CUFES) [48]. Similarly, it could be expected that in low-productivity regions, like several Mediterranean areas [49], the PV may produce a sampling bias in bordering the spawning ground, as well as in the estimate of the egg mortality curve, which could be reduced by the B40. In the present study, the B40 was observed to detect anchovy eggs at many more stations compared to the PV at the same location and time (Table 2).
Oblique B40 tows have been criticized in terms of their suitability for DEPM application [33] because of the vertical distribution of anchovy eggs and their high concentrations in the upper layers (sub-surface); thus, oblique tows may result in oversampling compared to vertical nets. However, a horizontal sampler proved more efficient than a vertical net in a recent study on ichthyoplankton assemblages in the Aegean Sea [50]. The distribution of anchovy early life stages in the Mediterranean Sea, and particularly eggs in the study area, appeared to be mainly concentrated in the upper 20 m depth layer [51,52], suggesting that both nets covered the layer of egg distribution, although they may reach different sampling depths. Moreover, previous DEPM applications in Mediterranean waters adopted the B40, observing that both vertical and oblique tows yielded consistent, compatible estimates of ichthyoplankton production [6,34].
In the present study, the B40 showed higher anchovy egg abundance and reduced variance in the samples (Table 1), and it allowed for better delimitation of the spawning area, since the number of stations with 0 values (i.e., no egg found) was lower than with the PV throughout the study period and overall (Table 1 and Table 2). This evidence is in agreement with a previous study that used the B40 to predict the anchovy spawning habitat [14]. Moreover, the samples were affected differently by the distribution of the planktonic organisms, with the PairoVET catches being more sensitive to small and fine-scale patchiness [6], which is the usual ichthyoplankton distribution [5]. Regarding the filtration efficiency of the PV, the cylindrical portion reduces the probability of net clogging during a single tow compared to the B40, but when equipped with an accurate flowmeter, such a problem could be avoided.
The B40 may be preferred, especially in areas with lower egg abundances compared to oceanic waters or in years with reduced egg densities. However, the years with higher egg densities (i.e., 2007 and 2010) also showed a higher proportion of positive stations with the B40, highlighting an overall performance 19% higher than the PV in catching anchovy eggs (Table 1). Although no larva data were available in the present study, it is well known that the B40 is more efficient than the PV for sampling fish larvae. Thus, the Bongo net allows for the sampling of early-hatched larvae, which could be used to obtain a more accurate mortality curve for eggs, substantially improving the precision of daily egg production estimate [6].
5. Conclusions
The results of this comparative experiment showed an agreement between the egg density data provided by the two samplers, suggesting the use of the Bongo 40 instead of PairoVET for DEPM applications, when only one plankton sampler is deployed. Especially in oligotrophic areas such as the Mediterranean Sea, where egg densities are lower than in oceanic waters, the B40 allows for an increase in the probability of capturing anchovy eggs, providing more data on the boundaries of the spawning area and the daily egg production.
Author Contributions
Conceptualization, G.B.; Software, S.A.; Formal analysis, G.B.; Investigation, A.B. and S.G.; Data curation, R.F.; Writing—original draft, G.B. and R.F.; Writing—review and editing, A.B., S.G. and S.A.; Supervision, R.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data analyzed in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank the crew of the research vessels “Urania”, for the logistic support and assistance during the surveys. The authors would also like to thank the technicians of CNR laboratory in Capo Granitola for the careful work done in sample analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stratoudakis, Y.; Bernal, M.; Ganias, K.; Uriarte, A. The Daily Egg Production Method: Recent advances, current applications and future challenges. Fish Fish. 2006, 7, 35–57. [Google Scholar] [CrossRef]
- Houde, E.D. Emerging from Hjort’s Shadow. J. Northw. Atl. Fish. Sci. 2008, 41, 53–70. [Google Scholar] [CrossRef]
- Diaz Conde, P.; Carrera Lopez, P.; Sanz Pinilla, L.; Tel, E. Sardine and Anchovy Eggs Abundance from PELACUS Surveys in the Northeast Atlantic Waters (Spring); SEANOE: Plouzané, France, 2024. [Google Scholar] [CrossRef]
- ICES. Working group on Southern Horse mackerel, anchovy and sardine (WGHANSA). ICES Sci. Rep. 2024, 6, 738. [Google Scholar]
- Pepin, P.; Helbig, J.A. Sampling variability of ichthyoplankton surveys—Exploring the roles of scale and resolution on uncertainty. Fish. Res. 2012, 117–118, 137–145. [Google Scholar] [CrossRef]
- Somarakis, S.; Palomera, I.; Garcia, A.; Quintanilla, L.; Koutsikopoulos, C.; Uriarte, A.; Motos, L. Daily Egg Production of anchovy in European waters. ICES J. Mar. Sci. 2004, 61, 944–958. [Google Scholar] [CrossRef]
- Basilone, G.; Ferreri, R.; Barra, M.; Bonanno, A.; Pulizzi, M.; Gargano, A.; Fontana, I.; Giacalone, G.; Rumolo, P.; Mazzola, S.; et al. Spawning eology of the European anchovy (Engraulis encrasicolus) in the Strait of Sicily: Linking variations of zooplankton prey, fish density, growth, and reproduction in an upwelling system. Progr. Oceanogr. 2020, 184, 102330. [Google Scholar] [CrossRef]
- Parker, K. A direct method for estimating Northern anchovy, Engraulis mordax, spawning biomass. Fish. Bull. 1980, 78, 541–544. [Google Scholar]
- Kurita, Y. Oocyte growth and fecundity regulation by atresia of Atlantic herring (Clupea harengus) in relation to body condition throughout the maturation cycle. J. Sea Res. 2003, 49, 203–219. [Google Scholar] [CrossRef]
- Somarakis, S.; Schismenou, E.; Siapatis, A.; Giannoulaki, M.; Kallianiotis, A.; Machias, A. High variability in the Daily Egg Production method parameters of an Eastern Mediterranean anchovy stock: Influence of environmental factors, fish condition and population density. Fish. Res. 2012, 117–118, 12–21. [Google Scholar] [CrossRef]
- Uriarte, A.; Alday, A.; Santos, M.; Motos, L. A re-evaluation of the spawning fraction estimation procedures for Bay of Biscay anchovy, a species with short interspawning intervals. Fish. Res. 2012, 117–118, 96–111. [Google Scholar] [CrossRef]
- Basilone, G.; Ganias, K.; Ferreri, R.; D’Elia, M.; Quinci, E.M.; Mazzola, S.; Bonanno, A. Application of GAMs and multinomial models to assess the spawning pattern of fishes with daily spawning synchronicity: A case study in the European anchovy (Engraulis encrasicolus) in the central Mediterranean Sea. Fish. Res. 2015, 167, 92–100. [Google Scholar] [CrossRef]
- Palermino, A.; De Felice, A.; Canduci, G.; Biagiotti, I.; Costantini, I.; Centurelli, M.; Menicucci, S.; Gašparević, D.; Tičina, V.; Leonori, I. Modeling of the habitat suitability of European sprat (Sprattus sprattus, L.) in the Adriatic Sea under several climate change scenarios. Front. Mar. Sci. 2024, 11, 1383063. [Google Scholar] [CrossRef]
- Quinci, E.M.; Torri, M.; Cuttitta, A.; Patti, B. Predicting potential spawning habitat by ensemble species distribution models: The case study of European anchovy (Engraulis encrasicolus) in the Strait of Sicily. Water 2022, 14, 1400. [Google Scholar] [CrossRef]
- Xiang, X.; Zhang, X.; Bian, X.; Lou, Q.; Xiong, Y. HSI model for early life stages of anchovy considering transport processes in Laizhou Bay. Front. Mar. Sci. 2022, 9, 946114. [Google Scholar] [CrossRef]
- Smith, P.E.; Flerx, W.; Hewitt, R.P. The CalCOFI vertical egg tow (CalVET) Net. In An Egg Production Method for Estimating Spawning Biomass of Pelagic Fish: Application to the Northern Anchovy (Engraulis mordax); Lasker, R., Ed.; NOAA Technical Report NMFS; U.S. Department of Commerce: Washington, DC, USA, 1985; Volume 36, pp. 27–32. [Google Scholar]
- Citores, L.; Ibaibarriaga, L.; Santos, M.; Uriarte, A. A Bayesian spatially explicit estimation of Daily Egg Production: Application to anchovy in the Bay of Biscay. Can. J. Fish. Aquat. Sci. 2024, 81, 1013–1028. [Google Scholar] [CrossRef]
- Köster, F.W.; Huwer, B.; Kraus, G.; Diekmann, R.; Eero, M.; Makarchouk, A.; Örey, S.; Dierking, J.; Margonski, P.; Herrmann, J.P.; et al. Egg production methods applied to eastern Baltic cod provide indices of spawning stock dynamics. Fish. Res. 2020, 227, 105553. [Google Scholar] [CrossRef]
- Ward, T.M.; Wolfe, B.W.; Grammer, G.L.; Ivey, A.R.; King, E.; Schiller, A.; McDonald, K.S.; Dambacher, J.M. Large sardine resource discovered off South-Eastern Australia: Potential risks, challenges and benefits of establishing a new fishery. Mar. Policy 2023, 155, 105739. [Google Scholar] [CrossRef]
- Schismenou, E.; Giannoulaki, M.; Valavanis, V.D.; Somarakis, S. Modeling and predicting potential spawning habitat of anchovy (Engraulis encrasicolus) and Round sardinella (Sardinella aurita) based on satellite environmental information. Hydrobiologia 2008, 612, 201–214. [Google Scholar] [CrossRef]
- FAO. The State of Mediterranean and Black Sea Fisheries 2023; FAO: Rome, Italy, 2023; ISBN 978-92-5-138411-4. [Google Scholar]
- Cury, P. Small pelagics in upwelling systems: Patterns of interaction and structural changes in “Wasp-Waist” ecosystems. ICES J. Mar. Sci. 2000, 57, 603–618. [Google Scholar] [CrossRef]
- Valenti, D.; Denaro, G.; Conversano, F.; Brunet, C.; Bonanno, A.; Basilone, G.; Mazzola, S.; Spagnolo, B. The role of noise on the steady state distributions of phytoplankton populations. J. Stat. Mech. 2016, 2016, 054044. [Google Scholar] [CrossRef]
- Rumolo, P.; Bonanno, A.; Barra, M.; Fanelli, E.; Calabrò, M.; Genovese, S.; Ferreri, R.; Mazzola, S.; Basilone, G. Spatial variations in feeding habits and trophic levels of two small pelagic fish species in the central Mediterranean Sea. Mar. Environ. Res. 2016, 115, 65–77. [Google Scholar] [CrossRef] [PubMed]
- European Union. Regulation (EU) Regulation (EU) 2017/1004 of the European Parliament and of the Council of 17 May 2017 on the Establishment of a Union Framework for the Collection, Management and Use of Data in the Fisheries Sector and Support for Scientific Advice Regarding the Common Fisheries Policy and Repealing Council Regulation (EC) No 199/2008 (Recast); OJEU L 157; European Union: Brussels, Belgium, 2017; pp. 1–121.
- ICES. Workshop 3 on age estimation of European anchovy (Engraulis encrasicolus) (WKARA3; Outputs from 2021 Meeting). ICES Sci. Rep. 2023, 5, 59. [Google Scholar]
- Lleonart, J.; Maynou, F. Fish stock assessments in the Mediterranean: State of the art. Sci. Mar. 2003, 67, 37–49. [Google Scholar] [CrossRef]
- Ferreri, R.; Genovese, S.; Barra, M.; Biagiotti, I.; Bourdeix, J.-H.; De FELICE, A.; Gašparević, D.; Hattab, T.; Iglesias, M.; Juretić, T.; et al. Variability in size at maturity of the European anchovy (Engraulis encrasicolus) in the Mediterranean Sea. Medit. Mar. Sci. 2021, 22, 858. [Google Scholar] [CrossRef]
- Barra, M.; Bonanno, A.; Hattab, T.; Saraux, C.; Iglesias5, M.; Leonori, I.; Tičina, V.; Basilone, G.; De Felice, A.; Ferreri, R.; et al. Effects of sampling intensity and biomass levels on the precision of acoustic surveys in the Mediterranean Sea. Medit. Mar. Sci. 2021, 22, 769. [Google Scholar] [CrossRef]
- Basilone, G.; Bonanno, A.; Patti, B.; Mazzola, S.; Barra, M.; Cuttitta, A.; McBride, R. Spawning site selection by European anchovy (Engraulis encrasicolus) in relation to oceanographic conditions in the Strait of Sicily. Fish. Oceanogr. 2013, 22, 309–323. [Google Scholar] [CrossRef]
- Lo, N.C.H.; Green Ruiz, Y.A.; Cervantes, M.J.; Moser, H.G.; Lynn, R.J. Egg production and spawning biomass of Pacific sardine (Sardinops sagax) in 1994, determined by the Daily Egg Production Method. CalCOFl Rep. 1996, 37, 160–174. [Google Scholar]
- Checkley, D.M., Jr.; Ortner, P.B.; Settle, L.R.; Cummings, S.R. A Continuous, underway fish egg sampler. Fish. Oceanogr. 1997, 6, 58–73. [Google Scholar] [CrossRef]
- Somarakis, S.; Tsimenides, N. A Daily Egg Production biomass estimate of the Northern Aegean Sea anchovy stock. Ozeanografika 1997, 2, 133–148. [Google Scholar]
- Machias, A.; Somarakis, S.; Tsimenides, N.; Magoulas, A.; Koutsikopoulos, C. Evaluation of the Southern Greek Anchovy Stocks; Project, No. 97-0048; Institute of Marine Biology of Crete: Gournes, Greece, 2000; p. 105. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Pennington, M. Efficient estimators of abundance, for fish and plankton surveys. Biometrics 1983, 39, 281. [Google Scholar] [CrossRef]
- ICES. Working Group on Biological Parameters (WGBIOP). ICES Sci. Rep. 2024, 5, 372. [Google Scholar]
- GFCM. Report of the Working Group on Stock Assessment of Small Pelagic Species (WGSASP); GFCM: Rome, Italy, 12–17 December 2022; p. 65. [Google Scholar]
- Džoić, T.; Zorica, B.; Matić, F.; Šestanović, M.; Čikeš Keč, V. Cataloguing environmental influences on the spatiotemporal variability of Adriatic anchovy early life stages in the Eastern Adriatic Sea using an artificial neural network. Front. Mar. Sci. 2022, 9, 997937. [Google Scholar] [CrossRef]
- Fernández-Corredor, E.; Albo-Puigserver, M.; Pennino, M.G.; Bellido, J.M.; Coll, M. Influence of environmental factors on different life stages of European anchovy (Engraulis encrasicolus) and European sardine (Sardina pilchardus) from the Mediterranean Sea: A literature review. Reg. Stud. Mar. Sci. 2021, 41, 101606. [Google Scholar] [CrossRef]
- Somarakis, S.; Catalano, B.; Tsimenides, N. Catchability and retention of larval European anchovy, Engraulis encrasicolus, with Bongo nets. Fish. Bull. 1998, 96, 917–925. [Google Scholar]
- Cada, G.F.; Loar, J.M. Relative effectiveness of two ichthyoplankton sampling techniques. Can. J. Fish. Aquat. Sci. 1982, 39. [Google Scholar] [CrossRef]
- Habtes, S.; Muller-Karger, F.E.; Roffer, M.A.; Lamkin, J.T.; Muhling, B.A. A comparison of sampling methods for larvae of medium and large epipelagic fish species during spring SEAMAP ichthyoplankton surveys in the Gulf of Mexico: Gulf of Mexico larval fish sampling gear. Limnol. Oceanogr. Methods 2014, 12, 86–101. [Google Scholar] [CrossRef]
- Hernandez, F.J.; Carassou, L.; Muffelman, S.; Powers, S.P.; Graham, W.M. Comparison of two plankton net mesh sizes for ichthyoplankton collection in the Northern Gulf of Mexico. Fish. Res. 2011, 108, 327–335. [Google Scholar] [CrossRef]
- Gutkowska, A.; Paturej, E.; Kowalska, E. Qualitative and quantitative methods for sampling zooplankton in shallow coastal estuaries. Ecohydrol. Hydrobiol. 2012, 12, 253–263. [Google Scholar] [CrossRef]
- Zhuang, X.; Guo, G.; You, X.; Hu, F. Sinking performance of new biplane depressor for constant-depth ichthyoplankton sampler at horizonal sampling. Aquac. Fish. 2024, S2468550X24001539. [Google Scholar] [CrossRef]
- Guo, G.; You, X.; Zhuang, X.; Hu, F.; Wang, H.; He, S.; Huang, L. Drag force and filtration performance of square-frame ichthyoplankton sampling net. Ocean Eng. 2024, 310, 118662. [Google Scholar] [CrossRef]
- ICES; WGACEGG. Working Group on Acoustic and Egg Surveys for Small Pelagic Fish in Northeast Atlantic (WGACEGG.; Outputs from 2023 Meeting). ICES Sci. Rep. 2024, 6, 227. [Google Scholar]
- Pinardi, N.; Zavatarelli, M.; Adani, M.; Coppini, G.; Fratianni, C.; Oddo, P.; Simoncelli, S.; Tonani, M.; Lyubartsev, V.; Dobricic, S.; et al. Mediterranean Sea large-scale low-frequency ocean variability and water mass formation rates from 1987 to 2007: A retrospective analysis. Progr. Oceanogr. 2015, 132, 318–332. [Google Scholar] [CrossRef]
- Uygun, O.; Hoşsucu, B. Temporal dynamics of the ichthyoplankton assemblages in the central Aegean Sea during one year and the effects of ecological factors. Reg. Stud. Mar. Sci. 2024, 72, 103439. [Google Scholar] [CrossRef]
- Patti, B.; Zarrad, R.; Jarboui, O.; Cuttitta, A.; Basilone, G.; Aronica, S.; Placenti, F.; Tranchida, G.; Armeri, G.M.; Buffa, G.; et al. Anchovy (Engraulis encrasicolus) early life stages in the central Mediterranean Sea: Connectivity issues emerging among adjacent sub-areas across the Strait of Sicily. Hydrobiologia 2018, 821, 25–40. [Google Scholar] [CrossRef]
- García, A.; Palomera, I. Anchovy early life history and its relation to its surrounding environment in the Western Mediterranean basin. Sci. Mar. 1996, 60, 155–166. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).