Baleen–Plastic Interactions Reveal High Risk to All Filter-Feeding Whales from Clogging, Ingestion, and Entanglement
Abstract
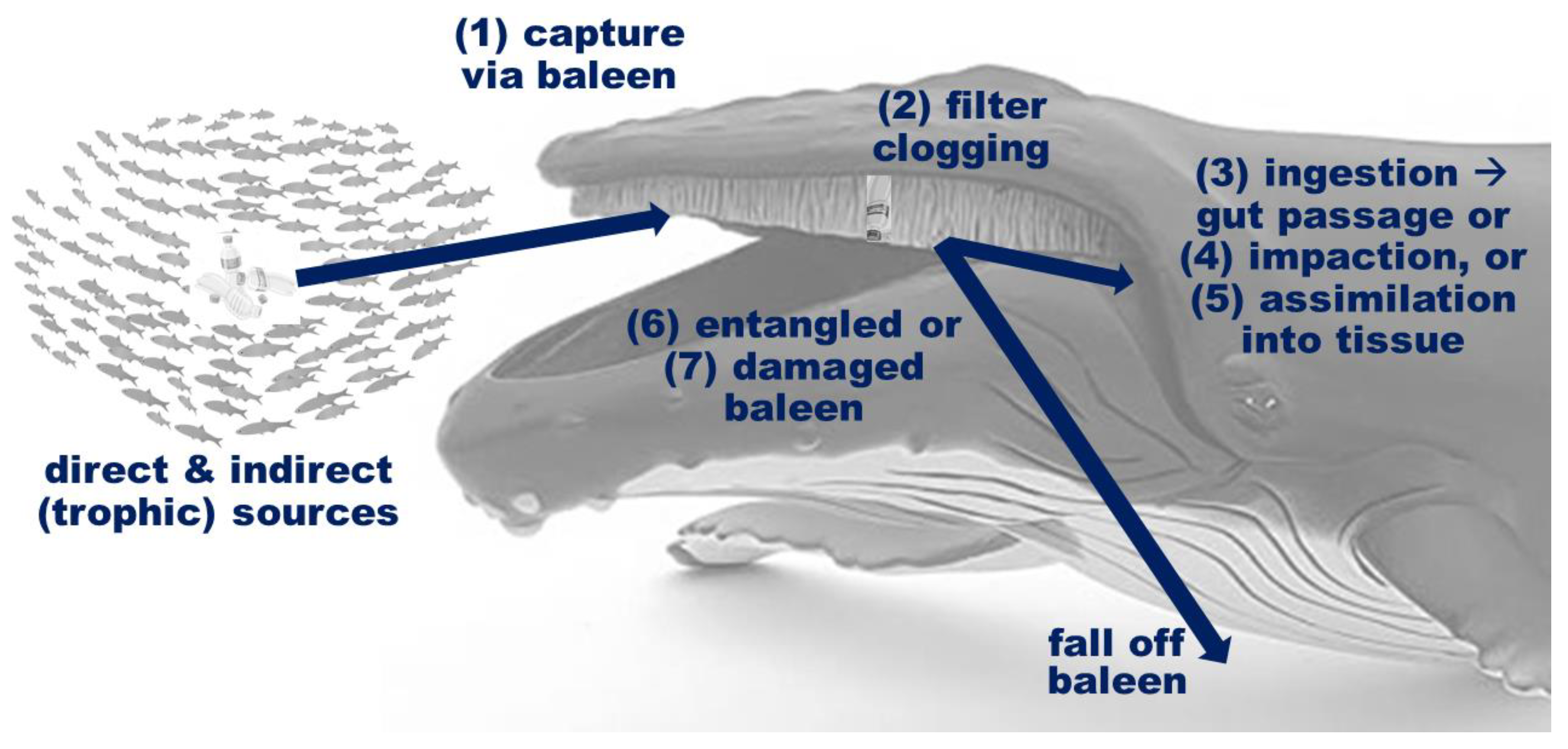
1. Introduction
2. Material and Methods
2.1. Biological Specimens
2.2. Flow Tank Testing
2.3. Experimental Design and Statistical Power Analysis
2.4. Video Analysis
2.5. Plastic Pollutants Used
2.5.1. Plastic Shapes
2.5.2. Plastic Sizes
2.5.3. Plastic Resin/Polymer Types
2.5.4. Plastic Residues
3. Results
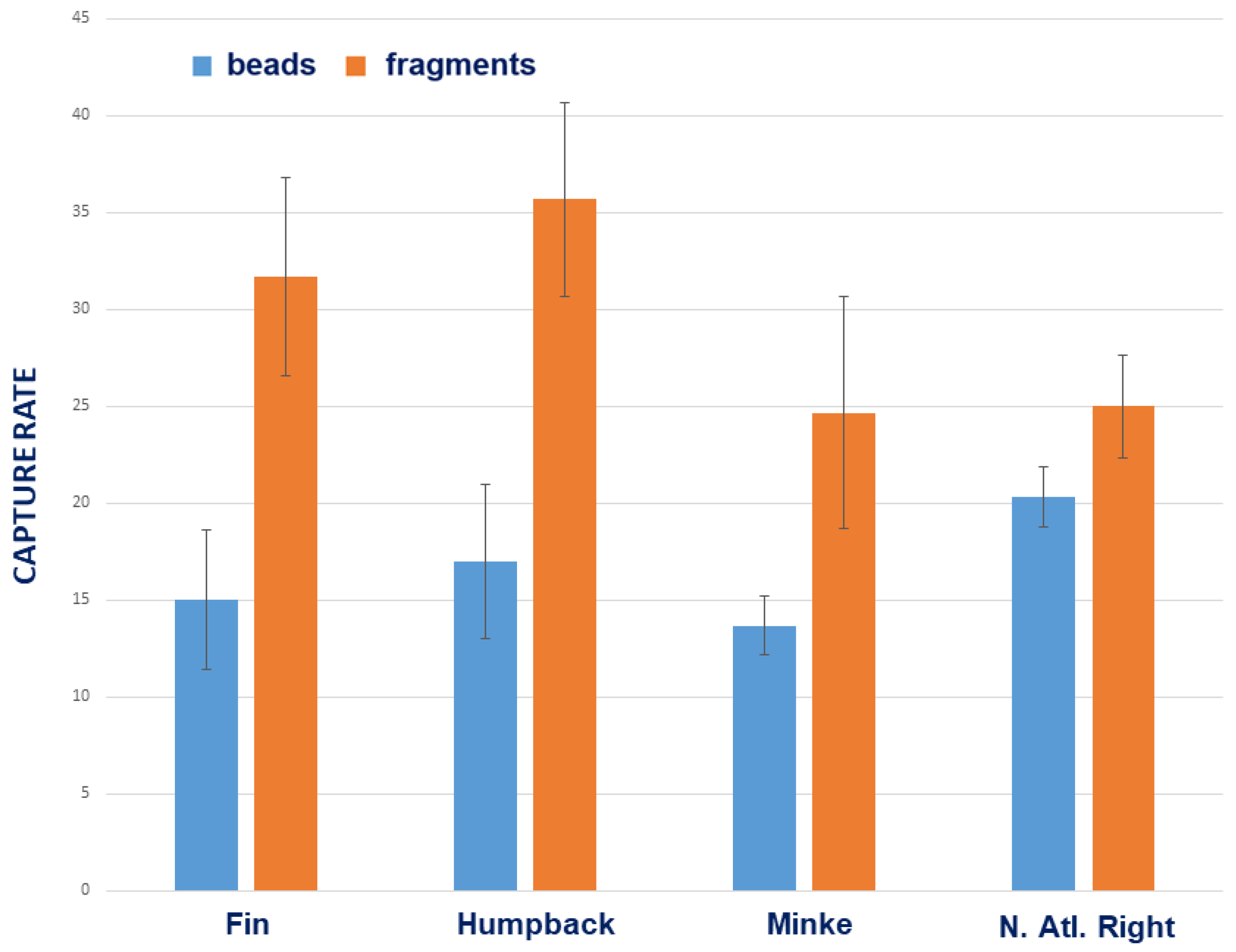
3.1. Comparative Species Results
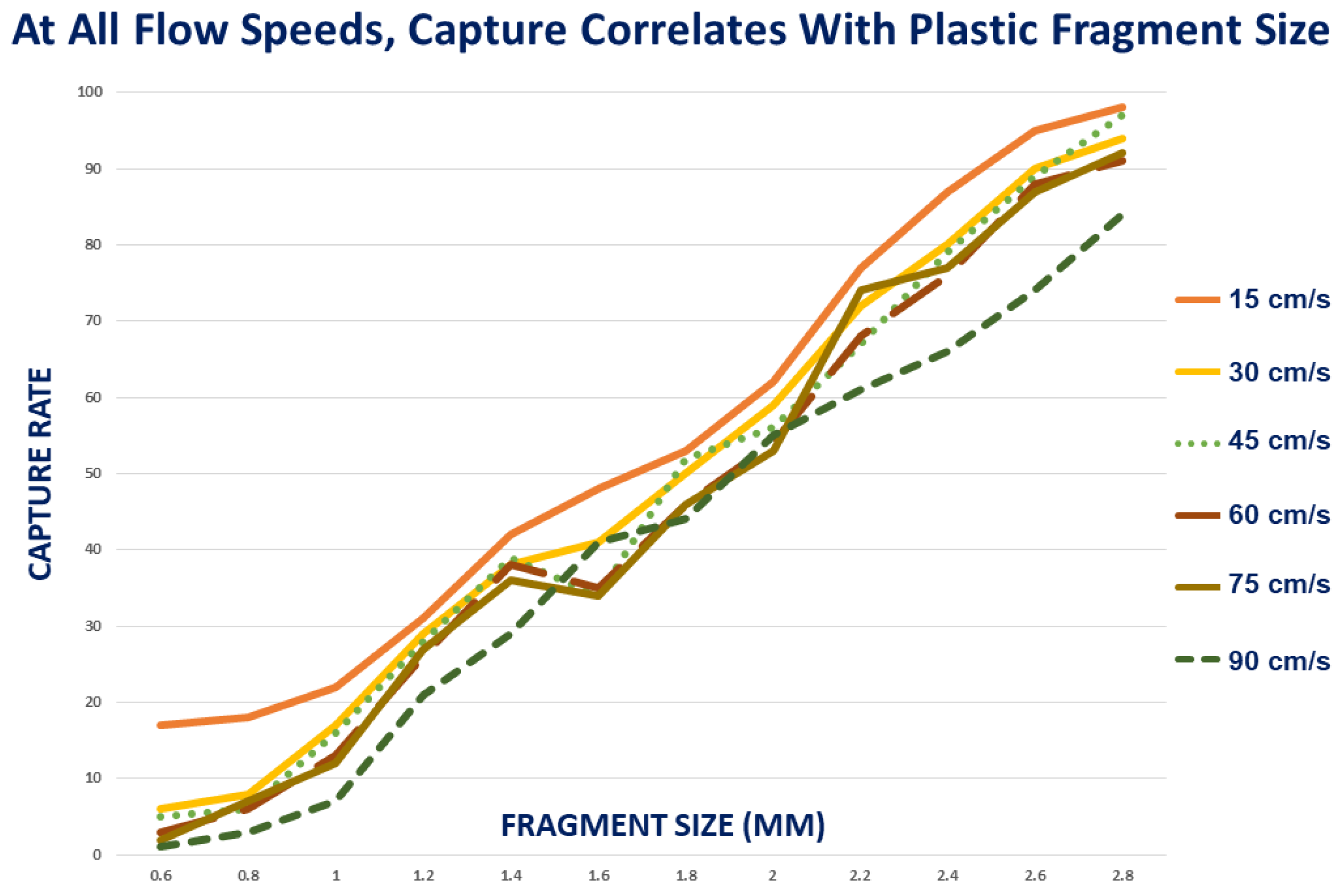
3.2. Effect of Fragment Size
3.3. Effect of Plastic Fragment Shape
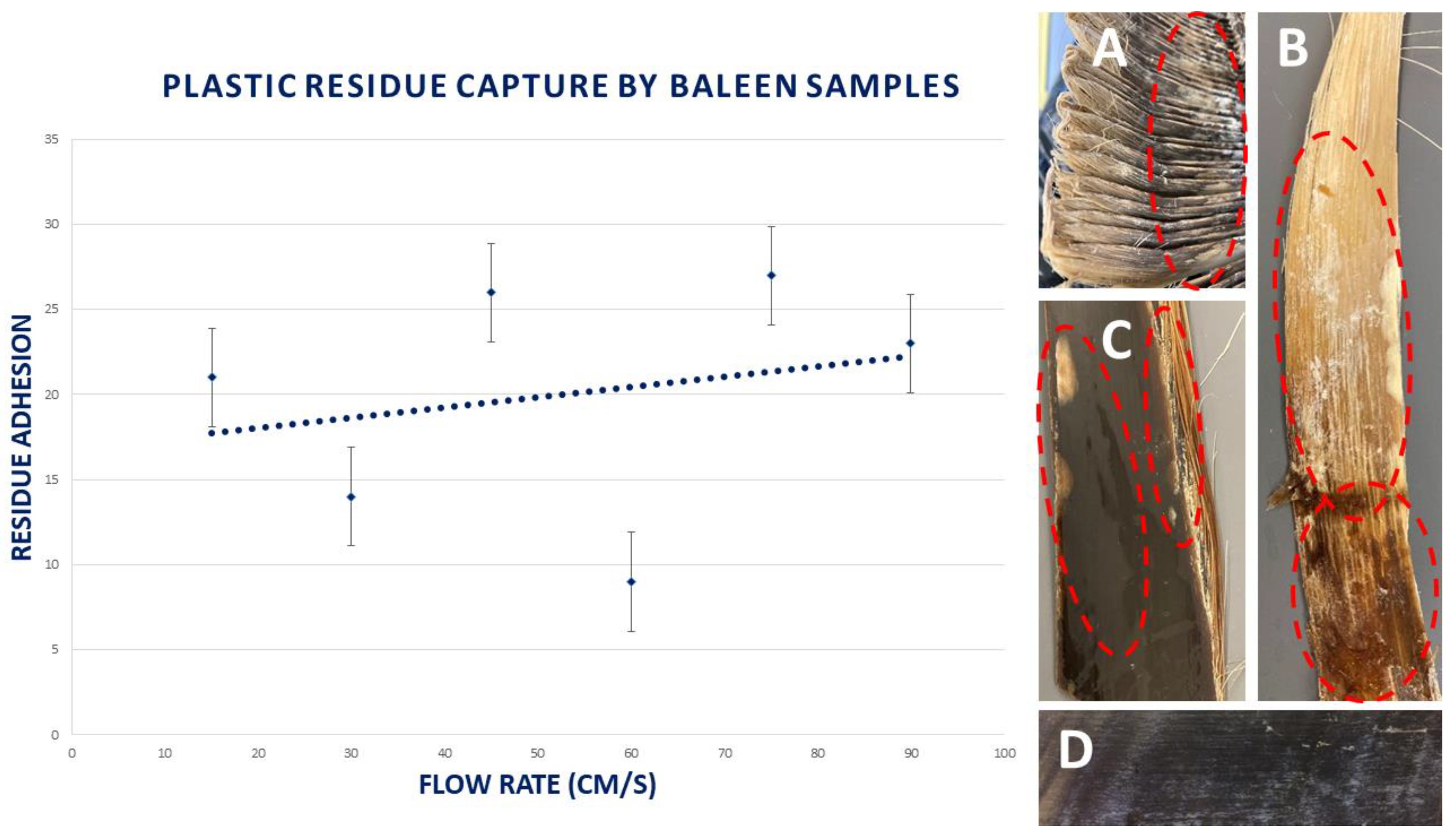
3.4. Effect of Flow Parameters
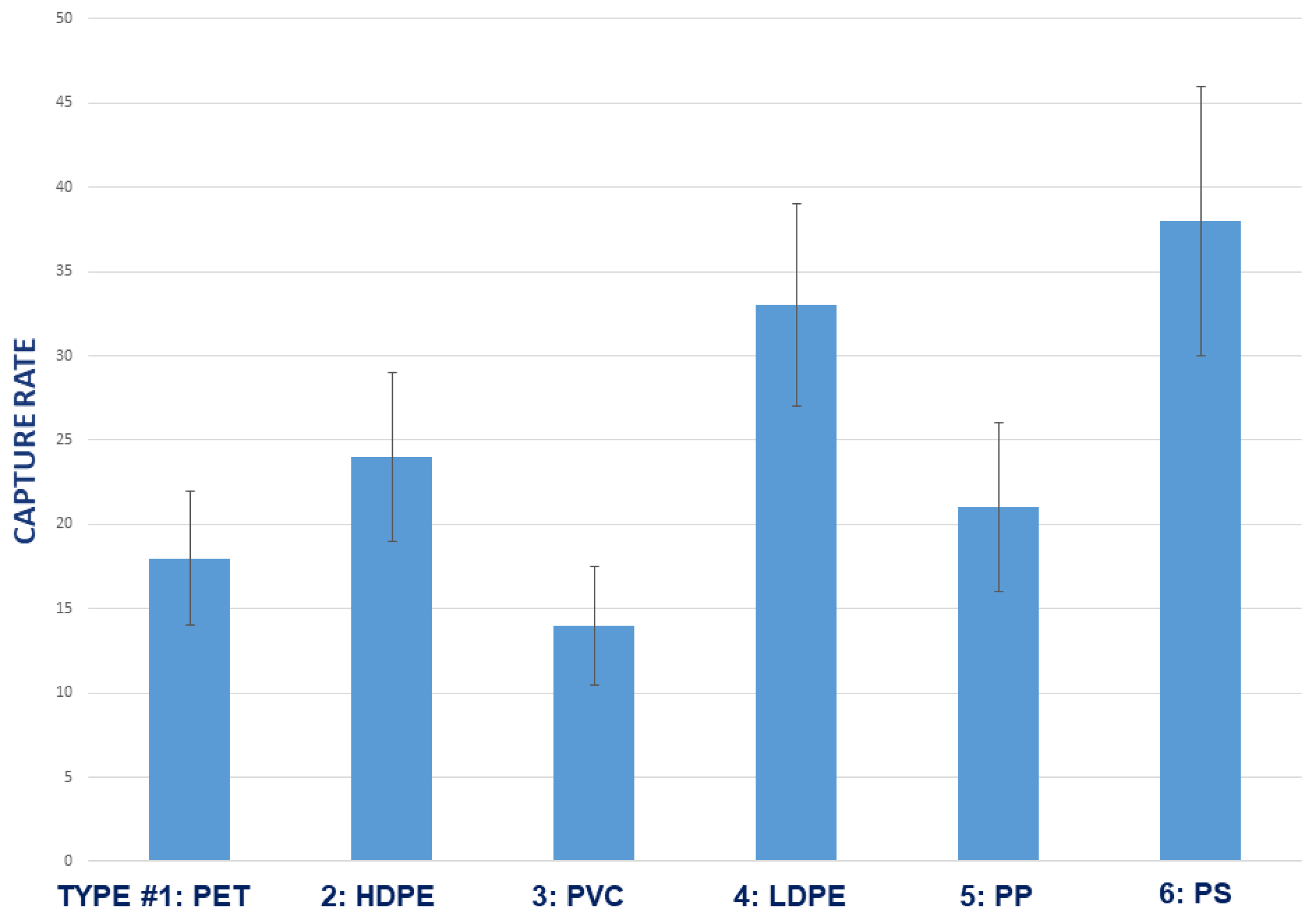
3.5. Effect of Plastic Polymer Type
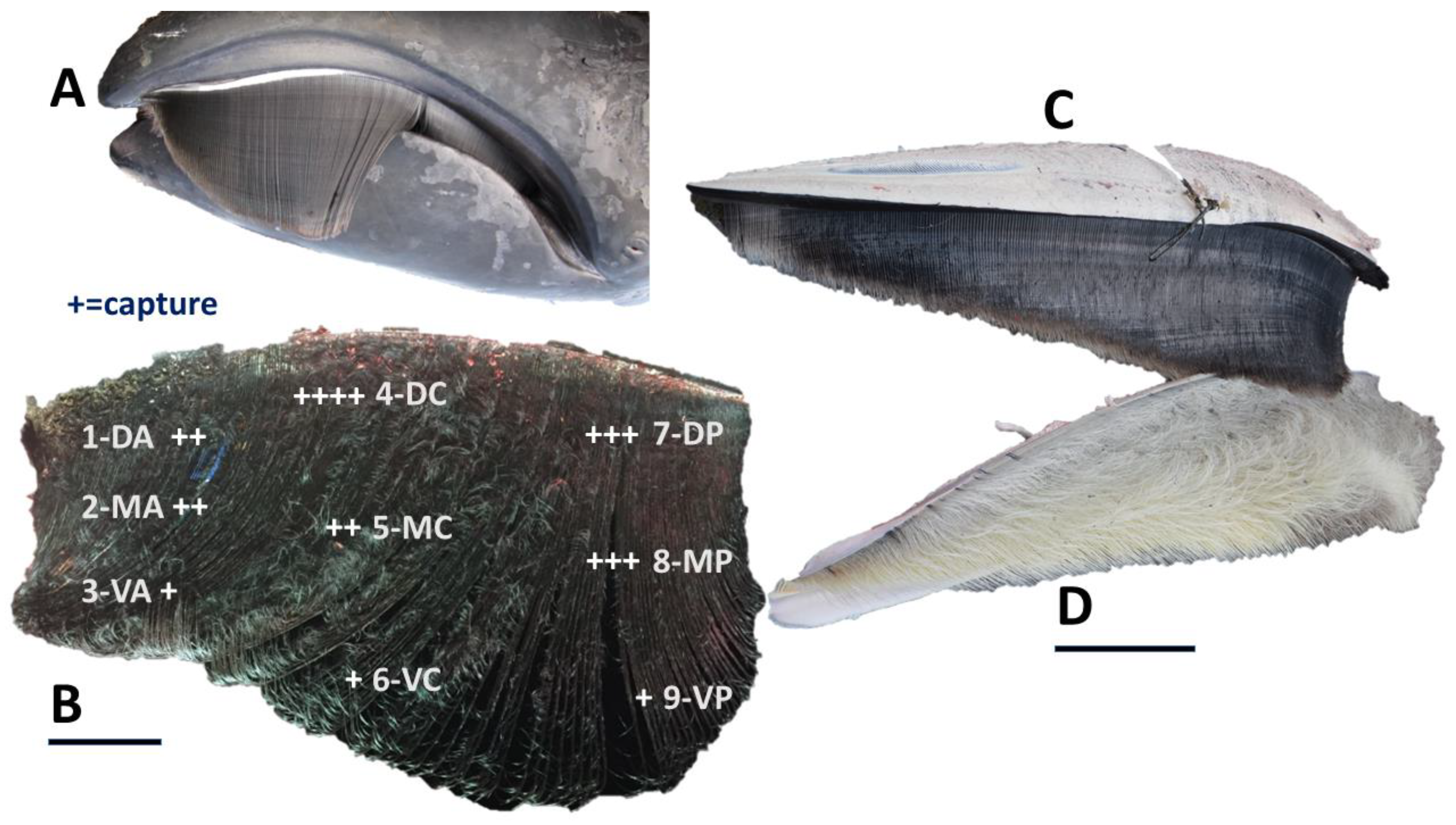
3.6. Differential Capture within Regions of Baleen Rack
3.7. Capture of Plastic Residues
3.8. Filter Bypass and Ingestion Rate Estimation
4. Discussion
4.1. Comparative Species Results
4.2. Analysis of Diverse Plastic Pollutants
4.3. Risk Factors of Plastics for Various Cetacean Species
4.4. Reducing and Mitigating Threats to Baleen-Filtering Whales from Plastic Pollution
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gallo, F.; Fossi, C.; Weber, R.; Santillo, D.; Sousa, J.; Ingram, I.; Nadal, A.; Romano, D. Marine litter plastics and microplastics and their toxic chemicals components: The need for urgent preventive measures. Environ. Sci. Eur. 2018, 30, 13. [Google Scholar] [CrossRef]
- Brown, E.; MacDonald, A.; Allen, S.; Allen, D. The potential for a plastic recycling facility to release microplastic pollution and possible filtration remediation effectiveness. J. Hazard. Mater. Adv. 2023, 10, 100309. [Google Scholar] [CrossRef]
- GESAMP (IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UNEP/UNDP/ISA Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection). Guidelines for the monitoring and assessment of plastic litter and microplastics in the ocean. In GESAMP Reports & Studies Series; Kershaw, P.J., Turra, A., Galgani, F., Eds.; GESAMP: London, UK, 2019; Volume 99, 130p, ISSN 1020-4873. [Google Scholar]
- Bucci, K.; Tulio, M.; Rochman, C.M. What is known and unknown about the effects of plastic pollution: A meta-analysis and systematic review. Ecol. Appl. 2020, 30, e02044. [Google Scholar] [CrossRef]
- Gregorietti, M.; Atzori, F.; Carosso, L.; Frau, F.; Pellegrino, G.; Sarà, G.; Arcangeli, A. Cetacean presence and distribution in the central Mediterranean Sea and potential risks deriving from plastic pollution. Mar. Pollut. Bull. 2021, 173, 112943. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Redondo-Hasselerharm, P.E.; Nor, N.H.M.; de Ruijter, V.M.; Mintenig, S.M.; Kooi, M. Risk assessment of microplastic particles. Nat. Rev. Mater. 2022, 7, 138–152. [Google Scholar] [CrossRef]
- Law, K.L. Plastics in the marine environment. Annu. Rev. Mar. Sci. 2017, 9, 205–229. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, M.; Arp, H.P.H.; Tekman, M.B.; Jahnke, A. The global threat from plastic pollution. Science 2021, 373, 61–65. [Google Scholar] [CrossRef]
- Panti, C.; Baini, M.; Lusher, A.; Hernandez-Milan, G.; Bravo Rebolledo, E.L.; Unger, B.; Syberg, K.; Simmonds, M.K.; Fossi, M.C. Marine litter: One of the major threats for marine mammals. Environ. Pollut. 2019, 247, 72–79. [Google Scholar] [CrossRef]
- Santos, R.G.; Machovsky-Capuska, G.E.; Andrades, R. Plastic ingestion as an evolutionary trap: Toward a holistic understanding. Science 2021, 373, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Fossi, M.C.; Baini, M.; Simmonds, M.P. Cetaceans as ocean health indicators of marine litter impact at global scale. Front. Environ. Sci. 2020, 8, 586627. [Google Scholar] [CrossRef]
- Scacco, U.; Mancini, E.; Marcucci, F.; Tiralongo, F. Microplastics in the deep: Comparing dietary and plastic ingestion data between two Mediterranean bathyal opportunistic feeder species, Galeus melastomus, Rafinesque 1810 and Coelorinchus caelorhincus, Risso 1810, through stomach content analysis. J. Mar. Sci. Eng. 2022, 10, 624. [Google Scholar] [CrossRef]
- Jacob, H.; Besson, M.; Swarzenski, P.W.; Lecchini, D.; Metian, M. Effects of virgin micro- and nano-plastics on fish: Trends, meta-analysis and perspectives. Environ. Sci. Technol. 2020, 54, 4733–4745. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Allen, S.; Krumpen, T.; Allen, D. High levels of microplastics in the Arctic sea ice alga Melosira arctica, a vector to ice-associated and benthic food webs. Environ. Sci. Technol. 2023, 57, 6799–6807. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, P.M.; Twayana, K.S.; Ravanan, P.; Thomas, J.; Mukherjee, A.; Jenkins, D.F.; Chandrasekharan, N. Prospects on the nano-plastic particles internalization and induction of cellular response in human keratinocytes. Part. Fibre Toxicol. 2021, 18, 35. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Cowger, W.; Erdle, L.M.; Coffin, S.; Villarrubia-Gómez, P.; Moore, C.J.; Carpenter, E.J.; Day, R.H.; Thiel, M.; Wilcox, C. A growing plastic smog, now estimated to be over 170 trillion plastic particles afloat in the world’s oceans—Urgent solutions required. PLoS ONE 2023, 18, e0281596. [Google Scholar] [CrossRef] [PubMed]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “plastisphere: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef] [PubMed]
- Kaandorp, M.L.A.; Lobelle, D.; Kehl, C.; Dijkstra, H.A.; van Sebille, E. Global mass of buoyant marine plastics dominated by large long-lived debris. Nat. Geosci. 2023, 16, 689–694. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Lopes, C.; Oliveira, P.; Bessa, F.; Otero, V.; Henriques, B.; Raimundo, J.; Caetano, M.; Vale, C.; Guilhermino, L. Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci. Total Environ. 2020, 717, 134625. [Google Scholar] [CrossRef]
- Ramsperger, A.F.R.M.; Narayana, V.K.B.; Gross, W.; Mohanraj, J.; Thalakkat, M.; Greiner, A.; Schmalz, H.; Kress, H.; Laforsch, C. Environmental exposure enhances the internalization of microplastic particles into cells. Sci. Adv. 2020, 6, eabd1211. [Google Scholar] [CrossRef]
- Lear, L.; Padfield, D.; Dowsett, T.; Jones, M.; Kay, S.; Hayward, A.; Vos, M. Bacterial colonisation dynamics of household plastics in a coastal environment. Sci. Total Environ. 2022, 838, 156199. [Google Scholar] [CrossRef]
- Focardi, A.; Moore, L.R.; Raina, J.B.; Seymour, J.R.; Paulsen, I.T.; Tetu, S.G. Plastic leachates impair picophytoplankton and dramatically reshape the marine microbiome. Microbiome 2022, 10, 179. [Google Scholar] [CrossRef]
- Fackelmann, G.; Pham, C.K.; Rodríguez, Y.; Mallory, M.L.; Provencher, J.F.; Baak, J.E.; Sommer, S. Current levels of microplastic pollution impact wild seabird gut microbiomes. Nat. Ecol. Evol. 2023, 7, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.G. Ingestion of plastics by marine organisms. In Hazardous Chemicals Associated with Plastics in the Marine Environment, The Handbook of Environmental Chemistry; Takada, H., Karapanagioti, H., Eds.; Springer: Cham, Switzerland, 2016; Volume 78, pp. 235–266. [Google Scholar] [CrossRef]
- Gove, J.M.; Whitney, J.L.; McManus, M.A.; Williams, G.J. Prey-size plastics are invading larval fish nurseries. Proc. Natl. Acad. Sci. USA 2019, 116, 24143–24149. [Google Scholar] [CrossRef] [PubMed]
- Zantis, L.J.; Bosker, T.; Lawler, F.; Nelms, S.E.; O’Rorke, R.; Constantine, R.; Sewell, M.; Carroll, E.L. Assessing microplastic exposure of large marine filter feeders. Sci. Total Environ. 2022, 818, 121815. [Google Scholar] [CrossRef] [PubMed]
- Torres, L.G.; Brander, S.M.; Parker, J.I.; Bloom, E.M.; Norman, R.; Van Brocklin, J.E.; Lasdin, K.S.; Hildebrand, L. Zoop to poop: Assessment of microparticle loads in gray whale zooplankton prey and fecal matter reveal high daily consumption rates. Front. Mar. Sci. 2023, 10, 1201078. [Google Scholar] [CrossRef]
- Allen, A.S.; Seymour, A.C.; Rittschof, D. Chemoreception drives plastic consumption in a hard coral. Mar. Pollut. Bull. 2017, 124, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Savoca, M.S.; Tyson, C.W.; McGill, M.; Slager, C.J. Odours from marine plastic debris induce food search behaviours in a forage fish. Proc. Roy. Soc. B 2017, 284, 20171000. [Google Scholar] [CrossRef] [PubMed]
- Germanov, E.S.; Marshall, A.D.; Bejder, L.; Fossi, M.C.; Lonergan, N.R. Microplastics: No small problem for filter-feeding megafauna. Trends Ecol. Evol. 2018, 33, 227–232. [Google Scholar] [CrossRef]
- Axworthy, J.B.; Padilla-Gamiño, J.L. Microplastics ingestion and heterotrophy in thermally stressed corals. Sci. Rep. 2019, 9, 18193. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, J.B.; Goforth, K.M.; Gil, M.A.; Savoca, M.S.; Lohmann, K.J. Odors from marine plastic debris elicit foraging behavior in sea turtles. Curr. Biol. 2020, 30, R213–R214. [Google Scholar] [CrossRef] [PubMed]
- Procter, J.; Hopkins, F.E.; Fileman, E.S.; Lindeque, P.K. Smells good enough to eat: Dimethyl sulfide (DMS) enhances copepod ingestion of microplastics. Mar. Pollut. Bull. 2019, 138, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Savoca, M.S.; Wihlfeil, M.E.; Ebeler, S.E.; Nevitt, G.A. Marine plastic debris emits a keystone info chemical for olfactory foraging seabirds. Sci. Adv. 2016, 2, e1600395. [Google Scholar] [CrossRef] [PubMed]
- Savoca, M.S.; Nevitt, G. Evidence that dimethyl sulfide facilitates a tritrophic mutualism between marine primary producers and top predators. Proc. Natl. Acad. Sci. USA 2014, 111, 4157–4161. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, B.; Barnagaud, J.-Y.; Poupard, M.; Glotin, H.; Gauffier, P.; Torres Ortiz, S.; Lisney, T.J.; Campagna, S.; Rasmussen, M.; Célérier, A. Behavioural responses of humpback whales to food-related chemical stimuli. PLoS ONE 2019, 14, e0212515. [Google Scholar] [CrossRef]
- Burkhardt-Holm, P.; N’Guyen, A. Ingestion of microplastics by fish and other prey organisms of cetaceans, exemplified for two large baleen whale species. Mar. Pollut. Bull. 2019, 144, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Alava, J.J. Modeling the bioaccumulation and biomagnification potential of microplastics in a cetacean foodweb of the Northeastern Pacific: A prospective tool to assess the risk exposure to plastic particles. Front. Mar. Sci. 2020, 7, 566101. [Google Scholar] [CrossRef]
- Kahane-Rapport, S.R.; Czapanskiy, M.F.; Fahlbusch, J.A.; Friedlaender, A.S.; Calambokidis, J.; Hazen, E.L.; Goldbogen, J.A.; Savoca, M.S. Field measurements reveal exposure risk to microplastic ingestion by filter-feeding megafauna. Nat. Commun. 2022, 13, 6327. [Google Scholar] [CrossRef]
- Zantis, L.J.; Carroll, E.L.; Nelms, S.E.; Bosker, T. Marine mammals and microplastics: A systematic review and call for standardization. Environ. Pollut. 2021, 269, 116142. [Google Scholar] [CrossRef]
- Eisfeld-Pierantonio, S.M.; Pierantonio, N.; Simmonds, M.P. The impact of marine debris on cetaceans with consideration of plastics generated by the COVID-19 pandemic. Environ. Pollut. 2022, 300, 118967. [Google Scholar] [CrossRef]
- Kruse, K.; Knickmeier, K.; Brennecke, D.; Unger, B.; Siebert, U. Plastic Debris and Its Impacts on Marine Mammals. In Marine Mammals; Brennecke, D., Knickmeier, K., Pawliczka, I., Siebert, U., Wahlberg, M., Eds.; Springer: Cham, Switzerland, 2023; pp. 49–62. [Google Scholar] [CrossRef]
- Merrill, G.B.; Hermabessiere, L.; Rochman, C.M.; Nowacek, D.P. Microplastics in marine mammal blubber, melon, & other tissues: Evidence of translocation. Environ. Pollut. 2023, 335, 122252. [Google Scholar] [CrossRef]
- Tarpley, R.J.; Marwitz, S. Plastic ingestion by cetaceans along the Texas coast: Two case reports. Aquat. Mamm. 1993, 19, 93–98. [Google Scholar]
- de Stephanis, R.; Giménez, J.; Carpinelli, E.; Gutierrez-Exposito, C.; Cañadas, A. As main meal for sperm whales: Plastics debris. Mar. Pollut. Bull. 2013, 69, 206–214. [Google Scholar] [CrossRef]
- Brentano, R.; Petry, M.V. Marine debris ingestion and human impacts on the pygmy sperm whale (Kogia breviceps) in southern Brazil. Mar. Pollut. Bull. 2020, 150, e110595. [Google Scholar] [CrossRef] [PubMed]
- Tonay, A.M.; Öztürk, A.A.; Salman, A.; Dede, A.; Danyer, I.A.; Danyer, E.; Öztürk, B. Stranding records of sperm whale (Physeter macrocephalus) on the Turkish coast in 2019-2020 with a note on the opportunistic sampling of stomach content. J. Black Sea/Mediterr. Environ. 2021, 27, 281–293. [Google Scholar]
- Moore, R.C.; Loseto, L.; Noel, M.; Etemadifar, A.; Brewster, J.D.; MacPhee, S.; Bendell, L.; Ross, P.S. Microplastics in beluga whales (Delphinapterus leucas) from the Eastern Beaufort Sea. Mar. Pollut. Bull. 2020, 150, 110723. [Google Scholar] [CrossRef] [PubMed]
- Secchi, E.R.; Zarzur, S. Plastic debris ingested by a Blainville’s beaked whale, Mesoplodon densirostris, washed ashore in Brazil. Aquat. Mamm. 1999, 25, 21–24. [Google Scholar]
- Lusher, A.L.; Hernandez-Milian, G.; O’Brien, J.; Berrow, S.; O’Connor, I.; Officer, R. Microplastic and microplastic ingestion by a deep diving, oceanic cetacean: The True’s beaked whale Mesoplodon mirus. Environ. Pollut. 2015, 199, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Abreo, N.A.S.; Macusi, E.D.; Blatchley, D.D.; Cuenca-Ocay, G.C. First evidence of plastic ingestion by the rare Deraniyagala’s beaked whale. Int. J. Ecol. Cons. 2016, 19, 16–36. [Google Scholar]
- Kelly, N.E.; Feyre, L.; Gavel, H.; Trela, O.; Ledwell, W.; Breeze, H.; Marotte, E.C.; McConney, L.; Whitehead, H. Long term trends in floating plastic pollution within a marine protected area identifies threats for endangered northern bottlenose whales. Environ. Res. 2023, 227, 115686. [Google Scholar] [CrossRef]
- Alexiadou, P.; Foskolos, I.; Frantzis, A. Ingestion of macroplastics by odontocetes of the Greek Seas, Eastern Mediterranean: Often deadly! Mar. Pollut. Bull. 2019, 146, 67–75. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, S.; Giménez-Luque, E.; Molina-Pardo, J.L.; Manzano-Medina, S.; Arribas-Arias, H.; Gavara, R.; Morales-Caselles, C.; Rivas, M.L. Plastic ingestion by two cetacean groups: Ziphiidae and Delphinidae. Environ. Pollut. 2023, 333, 121932. [Google Scholar] [CrossRef]
- Sá, S.; Torres-Pereira, A.; Ferreira, M.; Monteiro, S.S.; Fradoca, R.; Sequeira, M.; Vingada, J.; Eira, C. Microplastics in Cetaceans Stranded on the Portuguese Coast. Animals 2023, 13, 3263. [Google Scholar] [CrossRef]
- Alzugaray, L.; Di Martino, M.; Beltramino, L.; Rowntree, V.J.; Sironi, M.; Uhart, M.M. Anthropogenic debris in the digestive tract of a southern right whale (Eubalaena australis) stranded in Golfo Nuevo, Argentina. Mar. Pollut. Bull. 2020, 161A, 111738. [Google Scholar] [CrossRef]
- Fossi, M.C.; Panti, C.; Guerranti, C.; Copola, D.; Gianetti, M.; Marsili, L.; Minutoli, R. Are baleen whales exposed to the threat of microplastics? A case study of the Mediterranean fin whale (Balaenoptera physalus). Mar. Pollut. Bull. 2012, 64, 2374–2379. [Google Scholar] [CrossRef] [PubMed]
- Fossi, M.C.; Marsili, L.; Baini, M.; Gianetti, M.; Coppola, D.; Guerranti, C.; Caliani, I.; Minutoli, R.; Lauriano, G.; Finoia, M.G.; et al. Fin whales and microplastics: The Mediterranean Sea and the Sea of Cortez scenarios. Environ. Pollut. 2016, 209, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Fossi, M.C.; Baini, M.; Panti, C.; Baulch, S. Impacts of marine litter on cetaceans: A focus on plastic pollution. In Marine Mammal Ecotoxicology; Fossi, M.C., Panti, C., Eds.; Academic Press: San Diego, CA, USA, 2018; pp. 147–184. [Google Scholar] [CrossRef]
- Im, J.; Joo, S.; Lee, Y.; Kim, B.Y.; Kim, T. First record of plastic debris ingestion by a fin whale (Balaenoptera physalus) in the sea off East Asia. Mar. Pollut. Bull. 2020, 159, 111514. [Google Scholar] [CrossRef]
- Garcia-Garin, O.; Sahyoun, W.; Net, S.; Vighi, M.; Aguila, A.; Ouddane, B.; Vikingsoon, G.A.; Chosson, V.; Borrell, A. Intrapopulation and temporal differences of phthalate concentrations in North Atlantic fin whales (Balaenoptera physalus). Chemosphere 2022, 300, e134453. [Google Scholar] [CrossRef]
- Jauniaux, T.; Haelters, J.; Degraer, S.; Coignoul, F. Fatal plastic impaction in a minke whale (Balaenoptera acutorostrata). In Proceedings of the 28th Annual Conference of European Cetacean Society: Marine Mammals as Sentinels of Changing Environment, Liege, Belgique, 5–9 April 2014. poster 168. [Google Scholar]
- Besseling, E.; Foekema, E.M.; Van Franeker, J.A.; Leopold, M.F.; Kühn, S.; Bravo Rebolledo, E.L.; Heße, E.; Mielke, L.; Ijzer, J.; Kamminga, P.; et al. Microplastic in a macro filter feeder: Humpback whale Megaptera novaeangliae. Mar. Pollut. Bull. 2015, 95, 248–252. [Google Scholar] [CrossRef]
- Gibbs, S.E.; Kent, S.; Slat, B.; Morales, D.; Fouda, L.; Reisser, J. Cetacean sightings within the Great Pacific Garbage Patch. Mar. Biodivers. 2019, 49, 2021–2027. [Google Scholar] [CrossRef]
- Guerrini, F.; Lorenzo, M.; Renato, C. Modeling plastics exposure for the marine biota: Risk maps for fin whales in the Pelagos Sanctuary (North-Western Mediterranean). Front. Mar. Sci. 2019, 6, 299. Available online: https://www.frontiersin.org/articles/10.3389/fmars.2019.00299 (accessed on 24 May 2023). [CrossRef]
- Werth, A.J. Flow-dependent porosity of baleen from the bowhead whale (Balaena mysticetus). J. Exp. Biol. 2013, 216, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Werth, A.J.; Blakeney, S.M.; Cothren, A.I. Oil adsorption does not structurally or functionally alter whale baleen. Roy. Soc. Open Sci. 2019, 6, e182194. [Google Scholar] [CrossRef] [PubMed]
- Charlton-Howard, H.S.; Bond, A.L.; Rivers-Auty, J.; Lavers, J.L. ‘Plasticosis’: Characterising macro- and microplastic-associated fibrosis in seabird tissues. J. Hazard. Mater. 2023, 450, 131090. [Google Scholar] [CrossRef] [PubMed]
- Rivers-Auty, J.; Bond, A.L.; Grant, M.L.; Lavers, J.L. The one-two punch of plastic exposure: Macro- and micro-plastics induce multi-organ damage in seabirds. J. Hazard. Mater. 2023, 442, 130117. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Ye, T.; Zhang, Y.; Xu, R.; Lei, Y.; Chen, Z.; Yang, Z.; Zhang, Y.; Song, J.; Yue, X.; et al. Global estimates of daily ambient fine particulate matter concentrations and unequal spatiotemporal distribution of population exposure: A machine learning modelling study. Lancet Planet. Health 2023, 7, e209–e218. [Google Scholar] [CrossRef] [PubMed]
- Jerbi, H.; Pérez, W.; Tellechea, J.S. Ingestion of floating net debris in sperm whales and larynx strangulation with gillnet parts in bottlenose dolphins on the coast of Tunisia. Aquat. Mamm. 2021, 47, 239–244. [Google Scholar] [CrossRef]
- Mghili, B.; Keznine, M.; Analla, M.; Aksissou, M. The impacts of abandoned, discarded and lost fishing gear on marine biodiversity in Morocco. Ocean. Coast. Manag. 2023, 239, e106593. [Google Scholar] [CrossRef]
- Werth, A.J. Marine Mammals. In Feeding: Form, Function, and Evolution in Tetrapod Vertebrates; Schwenk, K., Ed.; Academic Press: New York, NY, USA, 2000; pp. 475–514. [Google Scholar]
- Williamson, G.R. Counting and measuring baleen and ventral grooves of whales. Sci. Rep. Whales Res. Inst. 1973, 24, 279–292. [Google Scholar]
- Carroll, G.M.; George, J.C.; Lowry, L.F.; Coyle, K.O. Bowhead whale (Balaena mysticetus) feeding near Point Barrow, Alaska, during the 1985 spring migrations. Arctic 1987, 40, 105–110. [Google Scholar] [CrossRef]
- Nowacek, D.P.; Johnson, M.; Tyack, P.; Shorter, K.A.; McLellan, W.A.; Pabst, D.A. Buoyant balaenids: The ups and downs of buoyancy in right whales. Proc. R. Soc. Lond. 2001, 268, 1811–1816. [Google Scholar] [CrossRef]
- Goldbogen, J.A.; Calambokidis, J.; Croll, D.A.; Harvey, J.T.; Newton, K.M.; Oleson, E.M.; Schorr, G.; Shadwick, R.E. Foraging behavior of humpback whales: Kinematic and respiratory patterns suggest a high cost for a lunge. J. Exp. Biol. 2008, 211, 3712–3719. [Google Scholar] [CrossRef]
- Simon, M.J.; Johnson, M.; Tyack, P.; Madsen, P.T. Behaviour and kinematics of continuous ram filtration in bowhead whales (Balaena mysticetus). Proc. R. Soc. B 2009, 276, 3819–3828. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Johnson, M.; Madsen, P.T. Keeping momentum with a mouthful of water: Behavior and kinematics of humpback whale lunge feeding. J. Exp. Biol. 2012, 215, 3786–3798. [Google Scholar] [CrossRef] [PubMed]
- Werth, A.J.; Potvin, J. Baleen hydrodynamics and morphology of cross-flow filtration in balaenid whale suspension feeding. PLoS ONE 2016, 11, e0150106. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, D.; Yang, G. Theoretical analysis of the hydrodynamic filtering system in the balaenid whales suspension feeding. Bioinspiration Biomim. 2020, 16, e26006. [Google Scholar] [CrossRef]
- Vandenberg, M.L.; Cohen, K.E.; Rubin, R.D.; Goldbogen, J.A.; Summer, A.P.; Paig-tran, E.M.; Kahane-Rapport, S.R. Formation of a fringe: A look inside baleen morphology using a multimodal visual approach. J. Morphol. 2023, 284, e21574. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Saido, K.; Kodera, Y.; Kimukai, H.; Sato, H.; Okabe, A.; Koizumi, K.; Takatama, K.; Kwon, B.G.; Chung, S.Y.; Nishimura, M.; et al. Waste polystyrene degradation in the world oceans: Newly identified sources of contamination. Res. Sq. 2020; preprint v3. [Google Scholar] [CrossRef]
- Sridhar, A.; Kanna, D.; Kapoor, A.; Prabhakar, S. Extraction and detection methods of microplastics in food and marine systems: A critical review. Chemosphere 2022, 286, e131653. [Google Scholar] [CrossRef]
- Werth, A.J.; Rita Espada, D.; Lysiak, N.S.; George, J.C.; Sformo, T.L. Baleen turnover and gut transit in mysticete whales and its environmental implications. Polar Biol. 2020, 43, 707–723. [Google Scholar] [CrossRef]
- Choy, C.A.; Robison, B.H.; Gagne, T.O.; Erwin, B.; Firl, E.; Halden, R.U.; Hamilton, J.A.; Katija, K.; Lisin, S.E.; Rolsky, C.; et al. The vertical distribution and biological transport of marine microplastics across the epipelagic and mesopelagic water column. Sci. Rep. 2019, 9, 7843. [Google Scholar] [CrossRef]
- Kashiwabara, L.M.; Kahane-Rapport, S.R.; King, C.; DeVogelaere, M.; Goldbogen, J.A.; Savoca, M.S. Microplastics and microfibers in surface waters of Monterey Bay National Marine Sanctuary, California. Mar. Pollut. Bull. 2021, 165, 112148. [Google Scholar] [CrossRef]
- Rochman, C.M.; Brookson, C.; Bikker, J.; Djuric, N.; Earn, A.; Bucci, K.; Athey, S.; Huntington, A.; McIlwraith, H.; Munno, K.; et al. Rethinking microplastics as a diverse contaminant suite. Environ. Toxicol. Chem. 2019, 38, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Werth, A.J. Models of hydrodynamic flow in the bowhead whale filter feeding apparatus. J. Exp. Biol. 2004, 207, 3569–3580. [Google Scholar] [CrossRef]
- van der Hoop, J.M.; Nousek-McGregor, A.E.; Nowacek, D.P.; Parks, S.E.; Tyack, P.; Madsen, P.T. Foraging rates of ram-filtering North Atlantic right whales. Funct. Ecol. 2019, 33, 1290–1306. [Google Scholar] [CrossRef]
- Rodríguez, Y.; Silva, M.A.; Pham, C.K.; Duncan, E.M. Cetaceans playing with single-use plastics (SUPs): A widespread interaction with likely severe impacts. Mar. Pollut. Bull. 2023, 194, 115428. [Google Scholar] [CrossRef]
- Cózar, A.; Echevarria, F.; González-Gordillo, I.; Duarte, C.M. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef]
- van Sebille, E.; Wilcox, C.; Lebreton, L.; Maximenko, N.; Hardesy, B.D.; van Franekere, J.A.; Eriksen, M.; Siegel, D.; Galgani, F.; Lavender Law, K. A global inventory of small floating plastic debris. Environ. Res. Lett. 2015, 10, 124006. [Google Scholar] [CrossRef]
- Cózar, A.; Sanz-Martin, M.; Marti, E.; González-Gordillo, I.; Ubeda, B.; Gálvez, J.A.; Irigoien, X.; Duarte, C.M. Plastic accumulation in the Mediterranean Sea. PLoS ONE 2015, 10, e121762. [Google Scholar] [CrossRef]
- Fossi, M.C.; Copploa, D.; Baini, M.; Giannetti, M.; Guerranti, C.; Marsli, L.; Panti, C.; de Sabata, E.; Clò, S. Large filter feeding marine organisms as indicators of microplastic in the pelagic environment: The case studies of the Mediterranean basking shark (Cetorhinus maximus) and fin whale (Balaenoptera physalus). Mar. Environ. Res. 2014, 100, 17–24. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Lavender, K. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- de Vos, A.; Brownell, R.L.; Tershy, B.; Croll, D. Anthropogenic threats and conservation needs of blue whales, Balaenoptera musculus indica, around Sri Lanka. J. Mar. Sci. 2016, 8420846, 8420846. [Google Scholar] [CrossRef]
- Connan, M.; Perold, V.; Dilley, B.J.; Barbraud, C.; Cherel, Y.; Ryan, P.G. The Indian Ocean ‘garbage patch’: Empirical evidence from floating macro-litter. Mar. Pollut. Bull. 2021, 169, 112559. [Google Scholar] [CrossRef]
- Pattiaratchi, C.; van der Mheen, M.; Schlundt, C.; Narayanaswamy, B.E.; Sura, A.; Hajbane, S.; White, R.; Kumar, N.; Fernandes, M.; Wijeratne, S. Plastics in the Indian Ocean: Sources, transport, distribution, and impacts. Ocean Sci. 2022, 18, 1–28. [Google Scholar] [CrossRef]
- Rosel, P.E.; Wilcox, L.A.; Yamada, T.K.; Mullin, K.D. A new species of baleen whale (Balaenoptera) from the Gulf of Mexico, with a review of its geographic distribution. Mar. Mammal Sci. 2021, 37, 577–610. [Google Scholar] [CrossRef]
- Fu, J.; Liu, N.; Peng, Y.; Wang, G.; Wang, X.; Wang, Q.; Lv, M.; Chen, L. An ultra-light sustainable sponge for elimination of microplastics and nanoplastics. J. Hazard. Mater. 2023, 456, 131685. [Google Scholar] [CrossRef]
- Dubner, S. The first great American industry. Freakonomics 2023, 549, 1–10. Available online: https://freakonomics.com/podcast/the-first-great-american-industry/ (accessed on 24 May 2023).
- Stevenson, C.H. Whalebone: Its Production and Utilization; US Government Printing Office: Washington, DC, USA, 1907; Volume 626, pp. 1–12.
- Virginia Marine Debris Reduction Plan, 2021–2025. U.S. NOAA Office of Response and Restoration Marine Debris Program. 2021. Available online: https://marinedebris.noaa.gov/report/virginia-marine-debris-reduction-plan (accessed on 12 June 2023).
- Tan, Z.; Berry, A.; Charalambides, M.; Mijic, A.; Pearse, W.; Porter, A.; Ryan, M.P.; Shorten, R.N.; Stettler, M.E.J.; Tetley, T.D.; et al. Tyre Wear Particles Are Toxic for Us and the Environment; Imperial College London Report; Imperial College London: London, UK, 2023. [Google Scholar] [CrossRef]
- Borrelle, S.B.; Ringma, J.; Law, K.L.; Monnahan, C.C.; Lebreton, L.; McGivern, A.; Murphy, E.; Jambeck, J.; Leonard, G.H.; Hilleary, M.A.; et al. Predicted growth in plastic waste exceeds efforts to mitigate plastic production. Science 2020, 369, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
- Barnett, M.J.; Hancock, P.I.; Klotz, L.E.; Attari, S.Z. Recycling bias and reduction neglect. Nat. Sustain. 2023, 6, 1418–1425. [Google Scholar] [CrossRef]
- Fernández-d’Arlas, B. Tough and functional cross-linked bioplastics from sheep wool keratin. Sci. Rep. 2019, 9, 14810. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; van Putten, R.-J.; Gruter, G.J.M. Plastic pollution: The role of (bio)degradable plastics and other solutions. In Biodegradable Polymers in the Circular Plastics Economy; Dusselier, M., Lange, J.-P., Eds.; Wiley: Hoboken, NJ, USA, 2022; pp. 59–82. [Google Scholar]
- Perera, P.; Herath, H.; Paranagama, P.A.; Wijesinghe, P.; Attanayake, R.N. Wood decay fungi show enhanced biodeterioration of low density polyethylene in the absence of wood in culture media. PLoS ONE 2023, 18, e0288133. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werth, A.J.; Kahane-Rapport, S.R.; Potvin, J.; Goldbogen, J.A.; Savoca, M.S. Baleen–Plastic Interactions Reveal High Risk to All Filter-Feeding Whales from Clogging, Ingestion, and Entanglement. Oceans 2024, 5, 48-70. https://doi.org/10.3390/oceans5010004
Werth AJ, Kahane-Rapport SR, Potvin J, Goldbogen JA, Savoca MS. Baleen–Plastic Interactions Reveal High Risk to All Filter-Feeding Whales from Clogging, Ingestion, and Entanglement. Oceans. 2024; 5(1):48-70. https://doi.org/10.3390/oceans5010004
Chicago/Turabian StyleWerth, Alexander J., Shirel R. Kahane-Rapport, Jean Potvin, Jeremy A. Goldbogen, and Matthew S. Savoca. 2024. "Baleen–Plastic Interactions Reveal High Risk to All Filter-Feeding Whales from Clogging, Ingestion, and Entanglement" Oceans 5, no. 1: 48-70. https://doi.org/10.3390/oceans5010004
APA StyleWerth, A. J., Kahane-Rapport, S. R., Potvin, J., Goldbogen, J. A., & Savoca, M. S. (2024). Baleen–Plastic Interactions Reveal High Risk to All Filter-Feeding Whales from Clogging, Ingestion, and Entanglement. Oceans, 5(1), 48-70. https://doi.org/10.3390/oceans5010004






