Effects of Water Temperature and Structural Habitat Complexity on the Routine Swimming Speed and Escape Response of Post-Settlement Stage White Seabream
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Routine Swimming and Escape Response
2.3. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almihoub, A.A.A.; Mula, J.M.; Rahman, M.M. Marginal Abatement Cost Curves (MACCs): Important Approaches to Obtain (Firm and Sector) Greenhouse Gases (GHGs) Reduction. Int. J. Econ. Financ. 2013, 5, 35–54. [Google Scholar] [CrossRef]
- Fawzy, S.; Osman, A.I.; Doran, J.; Rooney, D.W. Strategies for mitigation of climate change: A review. Environ. Chem. Lett. 2020, 18, 2069–2094. [Google Scholar] [CrossRef]
- Jewett, L.; Romanou, A. Ocean acidification and other ocean changes. In Climate Science Special Report: Fourth National Climate Assessment; Wuebbles, D.J., Fahey, D.W., Hibbard, K.A., Dokken, D.J., Stewart, B.C., Maycock, T.K., Eds.; U.S. Global Change Research Program: Washington, DC, USA, 2017; Volume 1, pp. 364–392. [Google Scholar] [CrossRef]
- IPCC. Climate change 2021 the physical science basis summary for policymakers working group I contribution to the sixth assessment report of the intergovernmental panel on climate change. In Climate Change 2021: The Physical Science Basis; IPCC: Geneva, Switzerland, 2021. [Google Scholar]
- Thrush, S.F.; Dayton, P.K. Disturbance to marine benthic habitats by trawling and dredging: Implications for marine biodiversity. Annu. Rev. Ecol. Syst. 2002, 33, 449–473. [Google Scholar] [CrossRef]
- He, Q.; Silliman, B.R. Climate Change, Human Impacts, and Coastal Ecosystems in the Anthropocene. Curr. Biol. 2019, 29, R1021–R1035. [Google Scholar] [CrossRef] [PubMed]
- Seitz, R.D.; Wennhage, H.; Bergström, U.; Lipcius, R.N.; Ysebaert, T. Ecological value of coastal habitats for commercially and ecologically important species. ICES J. Mar. Sci. 2014, 71, 648–665. [Google Scholar] [CrossRef]
- Airoldi, L.; Balata, D.; Beck, M.W. The Gray Zone: Relationships between habitat loss and marine diversity and their applications in conservation. J. Exp. Mar. Biol. Ecol. 2008, 366, 8–15. [Google Scholar] [CrossRef]
- Duarte, C.M. The future of seagrass meadows. Environ. Conserv. 2002, 29, 192–206. [Google Scholar] [CrossRef]
- Lotze, H.K.; Lenihan, H.S.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.G.; Kay, M.C.; Kidwell, S.M.; Kirby, M.X.; Peterson, C.H.; Jackson, J.B.C. Depletion, Degradation, and Recovery Potential of Estuaries and Coastal Seas. Science 2006, 312, 1806–1809. [Google Scholar] [CrossRef]
- Cheminée, A.; Sala, E.; Pastor, J.; Bodilis, P.; Thiriet, P.; Mangialajo, L.; Cottalorda, J.M.; Francour, P. Nursery value of Cystoseira forests for Mediterranean rocky reef fishes. J. Exp. Mar. Biol. Ecol. 2013, 442, 70–79. [Google Scholar] [CrossRef]
- Alfonso, S.; Gesto, M.; Sadoul, B. Temperature increase and its effects on fish stress physiology in the context of global warming. J. Fish Biol. 2021, 98, 1496–1508. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Allan, B.J.M.; Booth, D.J.; Donelson, J.M.; Edgar, G.J.; Ravasi, T.; Rummer, J.L.; Vergés, A.; Mellin, C. The effects of climate change on the ecology of fishes. PLoS Clim. 2023, 2, 8. [Google Scholar] [CrossRef]
- Fuiman, L.A.; Smith, M.E.; Malley, V.N. Ontogeny of routine swimming speed and startle responses in red drum, with a comparison of responses to acoustic and visual stimuli. J. Fish Biol. 1999, 55, 215–226. [Google Scholar] [CrossRef]
- McCormick, M.I.; Fakan, E.; Allan, B.J.M. Behavioural measures determine survivorship within the hierarchy of whole-organism phenotypic traits. Funct. Ecol. 2018, 32, 958–969. [Google Scholar] [CrossRef]
- Roberts, C.M.; O’Leary, B.C.; Mccauley, D.J.; Cury, P.M.; Duarte, C.M.; Lubchenco, J.; Pauly, D.; Sáenz-Arroyo, A.; Sumaila, U.R.; Wilson, R.W.; et al. Marine reserves can mitigate and promote adaptation to climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 6167–6175. [Google Scholar] [CrossRef] [PubMed]
- Vigliola, L. Control and regulation of sparid recruitment (Teleostei) from the Mediterranean sea: Importance of pre- and post-settlement processes. Cybium 1999, 23, 413–414. [Google Scholar]
- Giacalone, V.M.; Pipitone, C.; Abecasis, D.; Badalamenti, F.; D’Anna, G. Movement ecology of the white seabream Diplodus sargus across its life cycle: A review. Environ. Biol. Fish 2022, 105, 1809–1823. [Google Scholar] [CrossRef]
- Harmelin-Vivien, M.L.; Harmelin, J.G.; Leboulleux, V. Microhabitat requirements for settlement of juvenile sparid fishes on Mediterranean rocky shores. Hydrobiologia 1995, 300–301, 309–320. [Google Scholar] [CrossRef]
- Macpherson, E. Ontogenetic shifts in habitat use and aggregation in juvenile sparid fishes. J. Exp. Mar. Biol. Ecol. 1998, 220, 127–150. [Google Scholar] [CrossRef]
- Madeira, D.; Narciso, L.; Cabral, H.N.; Vinagre, C. Thermal tolerance and potential impacts of climate change on coastal and estuarine organisms. J. Sea Res. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Chang, C.H.; Mayer, M.; Rivera-Ingraham, G.; Blondeau-Bidet, E.; Wu, W.Y.; Lorin-Nebel, C.; Lee, T.H. Effects of temperature and salinity on antioxidant responses in livers of temperate (Dicentrarchus labrax) and tropical (Chanos Chanos) marine euryhaline fish. J. Therm. Biol. 2021, 99, 103016. [Google Scholar] [CrossRef]
- Domenici, P.; Allan, B.J.M.; Lefrançois, C.; McCormick, M.I. The effect of climate change on the escape kinematics and performance of fishes: Implications for future predator-prey interactions. Conserv. Physiol. 2019, 7, coz078. [Google Scholar] [CrossRef] [PubMed]
- Gregor, C.A.; Anderson, T.W. Relative importance of habitat attributes to predation risk in a temperate reef fish. Environ. Biol. Fishes 2016, 99, 539–556. [Google Scholar] [CrossRef]
- Fakan, E.P.; Allan, B.J.M.; Illing, B.; Hoey, A.S.; McCormick, M.I. Habitat complexity and predator odours impact on the stress response and antipredation behaviour in coral reef fish. PLoS ONE 2023, 18, e0286570. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.; Lopes, A.R.; Ribeiro, L.; Castanho, S.; Candeias-Mendes, A.; Pousão-Ferreira, P.; Faria, A.M. Effects of exposure to elevated temperature and different food levels on the escape response and metabolism of early life stages of white seabream, Diplodus sargus. Conserv. Physiol. 2022, 10, coac023. [Google Scholar] [CrossRef] [PubMed]
- Warren, D.T.; Donelson, J.M.; McCormick, M.I. Extended exposure to elevated temperature affects escape response behaviour in coral reef fishes. PeerJ 2017, 5, e3652. [Google Scholar] [CrossRef]
- Walker, J.A.; Ghalambor, C.K.; Griset, O.L.; McKenney, D.; Reznick, D.N. Do faster starts increase the probability of evading predators? Funct. Ecol. 2005, 19, 808–815. [Google Scholar] [CrossRef]
- Bleicher, S.S. The landscape of fear conceptual framework: Definition and review of current applications and misuses. PeerJ 2017, 5, e3772. [Google Scholar] [CrossRef]
- von Krogh, K.; Sørensen, C.; Nilsson, G.E.; Øverli, Ø. Forebrain cell proliferation, behavior, and physiology of zebrafish, Danio rerio, kept in enriched or barren environments. Physiol. Behav. 2010, 101, 32–39. [Google Scholar] [CrossRef]
- Enefalk, Å.; Bergman, E. Effect of fine wood on juvenile brown trout behaviour in experimental stream channels. Ecol. Freshw. Fish 2016, 25, 664–673. [Google Scholar] [CrossRef]
- Church, K.D.W.; Grant, J.W.A. Does increasing habitat complexity favour particular personality types of juvenile Atlantic salmon, Salmo salar? Anim. Behav. 2018, 135, 139–146. [Google Scholar] [CrossRef]
- De Pasquale, C.; Neuberger, T.; Hirrlinger, A.M.; Braithwaite, V.A. The influence of complex and threatening environments in early life on brain size and behaviour. Proc. R. Soc. B Biol. Sci. 2016, 283. [Google Scholar] [CrossRef]
- Marcon, M.; Mocelin, R.; Benvenutti, R.; Costa, T.; Herrmann, A.P.; De Oliveira, D.L.; Koakoski, G.; Barcellos, L.J.G.; Piato, A. Environmental enrichment modulates the response to chronic stress in zebrafish. J. Exp. Biol. 2018, 221, jeb176735. [Google Scholar] [CrossRef] [PubMed]
- Marras, S.; Killen, S.S.; Claireaux, G.; Domenici, P.; McKenzie, D.J. Behavioural and kinematic components of the fast-start escape response in fish: Individual variation and temporal repeatability. J. Exp. Biol. 2011, 214, 3102–3110. [Google Scholar] [CrossRef] [PubMed]
- Batzina, A.; Karakatsouli, N. The presence of substrate as a means of environmental enrichment in intensively reared gilthead seabream Sparus aurata: Growth and behavioral effects. Aquaculture 2012, 370–371, 54–60. [Google Scholar] [CrossRef]
- Rosengren, M.; Kvingedal, E.; Naslund, J.; Johnsson, J.I.; Sundell, K. Born to be wild: Effects of rearing density and environmental enrichment on stress, welfare and smolt migration in hatchery reared Atlantic salmon. Can. J. Fish. Aquat. Sci. 2017, 74, 396–405. [Google Scholar] [CrossRef]
- Zhang, Z.; Bai, Q.; Xu, X.; Guo, H.; Zhang, X. Effects of environmental enrichment on the welfare of juvenile black rockfish Sebastes schlegelii: Growth, behavior and physiology. Aquaculture 2020, 518, 734782. [Google Scholar] [CrossRef]
- Ojelade, O.C.; Durosaro, S.O.; Akinde, A.O.; Abdulraheem, I.; Oladepo, M.B.; Sopein, C.A.; Bhadmus, A.S.; Olateju, M. Environmental enrichment improves the growth rate, behavioral and physiological response of juveniles of Clarias gariepinus under laboratory conditions. Front. Vet. Sci. 2022, 9, 1566. [Google Scholar] [CrossRef]
- von Herbing, I.H. Effects of temperature on larval fish swimming performance: The importance of physics to physiology. J. Fish Biol. 2002, 61, 865–876. [Google Scholar] [CrossRef]
- Peck, M.A.; Buckley, L.J.; Bengtson, D.A. Effects of temperature and body size on the swimming speed of larval and juvenile Atlantic cod (Gadus morhua): Implications for individual-based modelling. Environ. Biol. Fishes 2006, 75, 419–429. [Google Scholar] [CrossRef]
- Bignami, S.; Sponaugle, S.; Hauff, M.; Cowen, R.K. Combined effects of elevated pCO2, temperature, and starvation stress on larvae of a large tropical marine fish. ICES J. Mar. Sci. 2017, 74, 1220–1229. [Google Scholar] [CrossRef]
- Madeira, D.; Madeira, C.; Costa, P.M.; Vinagre, C.; Pörtner, H.-O.; Diniz, M. Different sensitivity to heatwaves across the life cycle of fish reflects phenotypic adaptation to environmental niche. Mar. Environ. Res. 2020, 162, 105192. [Google Scholar] [CrossRef] [PubMed]
- Green, B.S.; Fisher, R. Temperature influences swimming speed, growth and larval duration in coral reef fish larvae. J. Exp. Mar. Biol. Ecol. 2004, 299, 115–132. [Google Scholar] [CrossRef]
- Fielder, D.S.; Bardsley, W.J.; Allan, G.L.; Pankhurst, P.M. The effects of salinity and temperature on growth and survival of Australian snapper, Pagrus auratus larvae. Aquaculture 2005, 250, 201–214. [Google Scholar] [CrossRef]
- Salvanes, A.G.V.; Moberg, O.; Ebbesson, L.O.E.; Nilsen, T.O.; Jensen, K.H.; Braithwaite, V.A. Environmental enrichment promotes neural plasticity and cognitive ability in fish. Proc. R. Soc. B Biol. Sci. 2013, 280, 1–7. [Google Scholar] [CrossRef]
- Arechavala-Lopez, P.; Caballero-Froilán, J.C.; Jiménez-García, M.; Capó, X.; Tejada, S.; Saraiva, J.L.; Sureda, A.; Moranta, D. Enriched environments enhance cognition, exploratory behaviour and brain physiological functions of Sparus aurata. Sci. Rep. 2020, 10, 11252. [Google Scholar] [CrossRef] [PubMed]
- Arechavala-Lopez, P.; Nuñez-Velazquez, S.; Diaz-Gil, C.; Follana-Berná, G.; Saraiva, J.L. Suspended Structures Reduce Variability of Group Risk-Taking Responses of Dicentrarchus labrax Juvenile Reared in Tanks. Fishes 2022, 7, 126. [Google Scholar] [CrossRef]
- Lönnstedt, O.M.; Mccormick, M.I.; Chivers, D.P.; Ferrari, M.C.O. Habitat degradation is threatening reef replenishment by making fish fearless. J. Anim. Ecol. 2014, 83, 1178–1185. [Google Scholar] [CrossRef]
- McCormick, M.I.; Lönnstedt, O.M. Disrupted learning: Habitat degradation impairs crucial antipredator responses in naive prey. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160441. [Google Scholar] [CrossRef]
- Gordon, T.A.C.; Harding, H.R.; Wong, K.E.; Merchant, N.D.; Meekan, M.G.; McCormick, M.I.; Radford, A.N.; Simpson, S.D. Habitat degradation negatively affects auditory settlement behavior of coral reef fishes. Proc. Natl. Acad. Sci. USA 2018, 115, 5193–5198. [Google Scholar] [CrossRef]
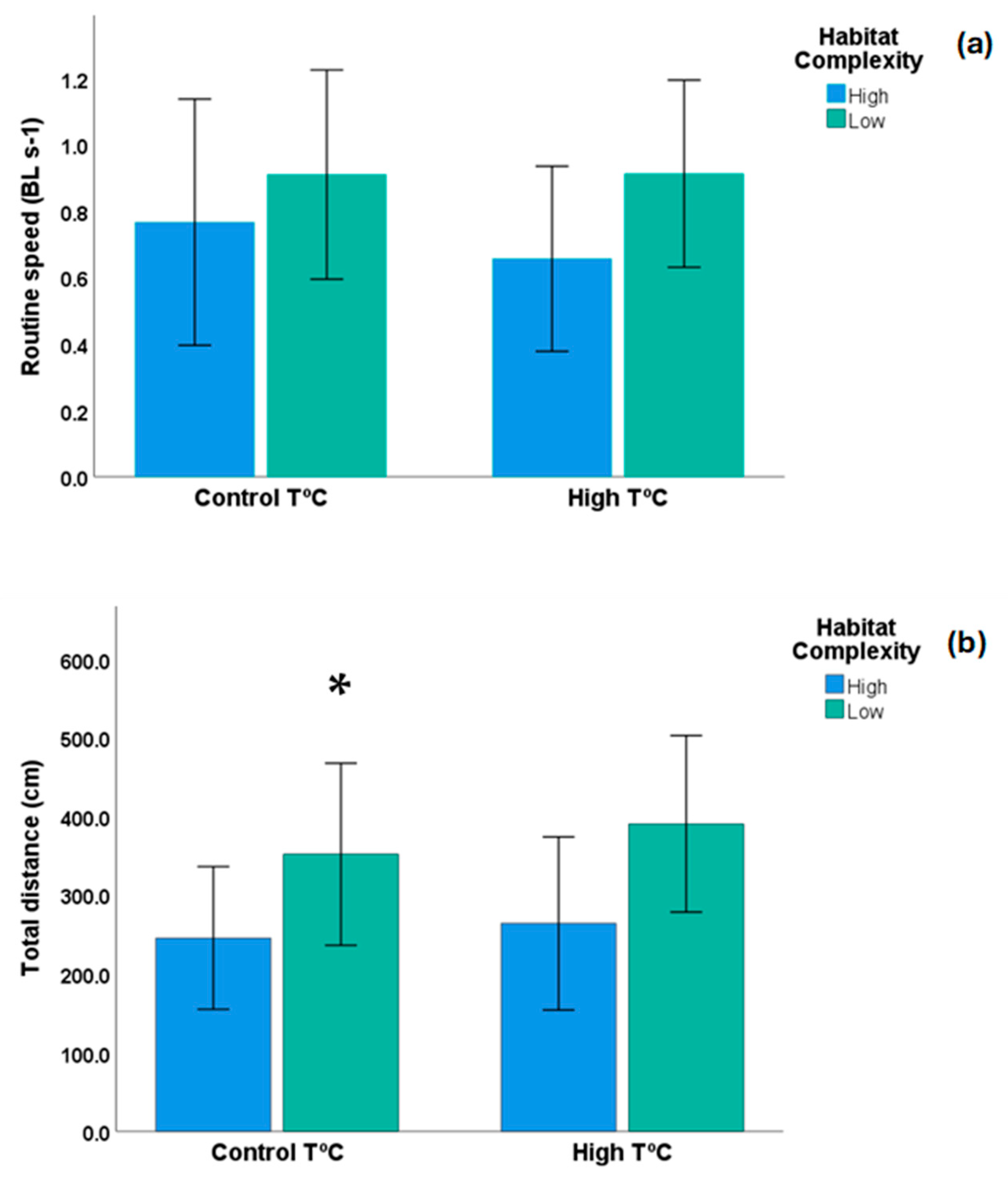
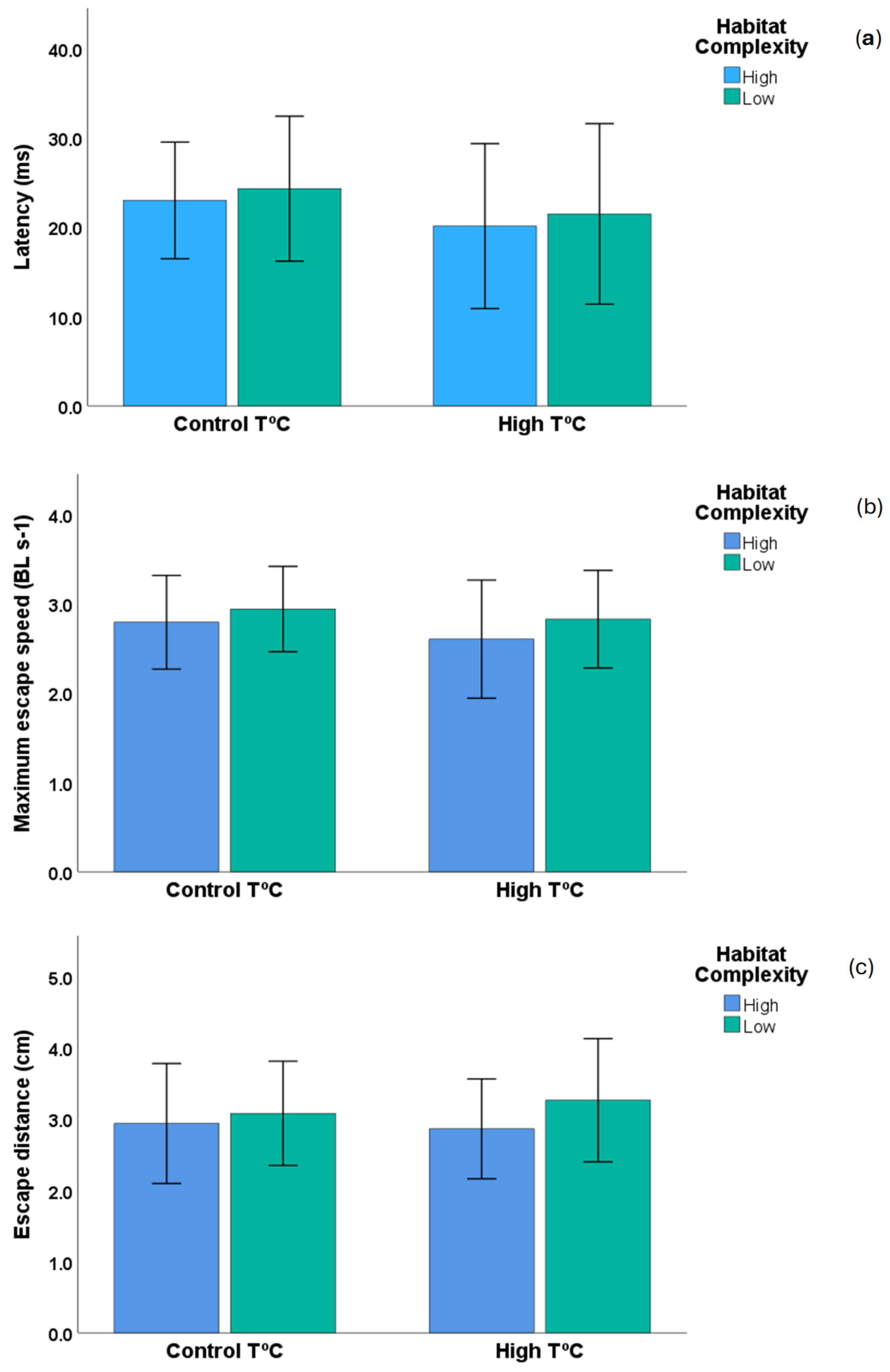
| Variables | Control T °C × High Complex | Control T °C × Low Complex | High T °C × Low Complex | High T °C × High Complex |
|---|---|---|---|---|
| Standard length (cm) | 3.22 ± 0.11 | 3.41 ± 0.09 | 3.62 ± 0.91 | 3.60 ± 0.12 |
| Aver routine speed (BL/s) | 0.85 ± 0.19 | 0.91 ± 0.16 | 0.91 ± 0.14 | 0.66 ± 0.15 |
| Routine distance (cm) | 271.96 ± 47.85 | 351.34 ± 75.84 | 389.93 ± 56.09 | 275.94 ± 62.13 |
| Responsiveness (%) | 89 | 100 | 100 | 86 |
| Directionality (%) | 88 | 96 | 95 | 94 |
| Latency (ms) | 41.84 ± 14.04 | 24.27 ± 4.05 | 21.45 ± 5.04 | 20.64 ± 4.84 |
| Escape distance (cm) | 2.89 ± 0.44 | 2.90 ± 0.42 | 3.01 ± 0.51 | 2.60 ± 0.37 |
| Max escape speed (BL/s) | 2.70 ± 0.24 | 2.94 ± 0.24 | 2.82 ± 0.27 | 2.69 ± 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicente, P.; Almeida, J.; Ribeiro, L.; Castanho, S.; Candeias-Mendes, A.; Pousão-Ferreira, P.; Faria, A.M. Effects of Water Temperature and Structural Habitat Complexity on the Routine Swimming Speed and Escape Response of Post-Settlement Stage White Seabream. Oceans 2024, 5, 38-47. https://doi.org/10.3390/oceans5010003
Vicente P, Almeida J, Ribeiro L, Castanho S, Candeias-Mendes A, Pousão-Ferreira P, Faria AM. Effects of Water Temperature and Structural Habitat Complexity on the Routine Swimming Speed and Escape Response of Post-Settlement Stage White Seabream. Oceans. 2024; 5(1):38-47. https://doi.org/10.3390/oceans5010003
Chicago/Turabian StyleVicente, Patrícia, João Almeida, Laura Ribeiro, Sara Castanho, Ana Candeias-Mendes, Pedro Pousão-Ferreira, and Ana Margarida Faria. 2024. "Effects of Water Temperature and Structural Habitat Complexity on the Routine Swimming Speed and Escape Response of Post-Settlement Stage White Seabream" Oceans 5, no. 1: 38-47. https://doi.org/10.3390/oceans5010003
APA StyleVicente, P., Almeida, J., Ribeiro, L., Castanho, S., Candeias-Mendes, A., Pousão-Ferreira, P., & Faria, A. M. (2024). Effects of Water Temperature and Structural Habitat Complexity on the Routine Swimming Speed and Escape Response of Post-Settlement Stage White Seabream. Oceans, 5(1), 38-47. https://doi.org/10.3390/oceans5010003







