Abstract
Background: Culture-negative periprosthetic joint infections (PJIs) are dramatically increasing in prevalence. The success rate of implant-saving procedures in acute PJI cases is closely correlated with prompt diagnosis, rapid isolation/identification of the microorganism, and timely surgical intervention. Methods: A 70-year-old female patient with an acutely infected left total hip arthroplasty (THA) following a routine screening colonoscopy was rapidly treated with debridement, antibiotic pearls, and retention of the implant (DAPRI) after rapid identification of the microorganism using a molecular diagnostics-based algorithm. Results: Molecular diagnostics enabled the identification of Escherichia coli as the causative agent of the transient bacteremia and subsequent seeding of the left hip within less than an hour. Conclusions: This case suggests that endoscopic procedures may increase the risk to joint replacement patients. In acute PJI, the use of molecular diagnostics, which facilitates prompt identification of microorganisms, may increase the success rate of implant-saving surgical procedures.
1. Introduction
Joint replacement (JR) is the most common surgical procedure performed in the United States, with an estimated 3.48 million hip and knee replacements projected annually by 2030 [1]. Periprosthetic joint infection (PJI) is one of the most dramatic complications of JR: the overall one-year risk has been reported as 0.74% [2], while the overall incidence can reach 2.6% [3]. Most PJIs have an intraoperative etiopathogenesis and may manifest acutely, though there is no consensus on whether this occurs at 4, 6, or 12 weeks postoperatively [4]. PJIs may also manifest chronically, up to two years after the index procedure [5]. Late PJIs are often hematogenous and are associated with a transient bacteremia that causes septic arthritis, with an incidence rate of approximately 0.07% per prosthesis-year [6].
Hematogenous PJIs may arise from multiple sources, including the oral cavity, cutaneous tissue, the urinary tract, and soft tissues in general. Among those, the gut microbiota has recently been investigated as a source of microorganisms responsible for surgical site infections and PJIs [7]. Colonoscopy, a fundamental screening test for colon cancer, has historically been associated with a risk of transient bacteriemia and is therefore a well-recognized risk factor for the insurgence of a PJI [8]. Interestingly, the American Academy of Orthopaedic Surgeons (AAOS), the American Society for Gastrointestinal Endoscopy (ASGE), the Endoscopy Committee for the British Society of Gastroenterology (BSG), and the Second International Consensus Meeting on Musculoskeletal Infections do not recommend administering prophylactic antibiotics before colonoscopy in JR patients [9,10]. Unfortunately, there is limited literature examining the association between prior colonoscopies and an increased risk of PJI.
Historically, hematogenous PJIs occur suddenly after a long asymptomatic period and are caused by a highly virulent microorganism. If the patient has been symptomatic for less than 6 weeks and seeks medical care within that timeframe, they are eligible for an implant-saving procedure [5,11,12,13,14]. It has been established that the success rate of implant-saving procedures, especially Debridement Antibiotics Implant Retention (DAIR) [12], is highly correlated with the isolation/identification of the infecting microorganism [15]. Unfortunately, the PJI culture-negativity rate is rising worldwide, raising significant concerns within the adult reconstruction community [16]. To address this issue, new molecular diagnostic techniques and protocols have been developed in conjunction with traditional microbial culture methods to rapidly identify the infecting organism in PJI cases [17,18].
Here, we describe a case of a 70-year-old female patient who developed an acute PJI in her left hip caused by Escherichia coli, arising from acute bacteremia and subsequent acute seeding of her left total hip arthroplasty (THA) following a routine screening colonoscopy. The microorganism was quickly identified (<1 h) using an internally developed, molecular diagnostics-based algorithm. This case highlights the value of molecular diagnostics for rapid microorganism identification in acute hematogenous PJIs and raises the question of whether additional PJI risk stratification could help identify patients who might benefit from antibiotic prophylaxis. A narrative review of the literature on the use of molecular diagnostics in gastrointestinal-related septic arthritis was also performed.
2. Materials and Methods
In June 2024, a 70-year-old female patient with a past medical history of hypertension, gastrointestinal (GI) reflux disease, melanoma under immunotherapy, depression, class III obesity (body mass index 40.5), and a prior successful left THA performed in 2016 was admitted to the GI unit of our Internal Medicine department for a scheduled, outpatient diagnostic colonoscopy. The procedure was intended to monitor the evolution of a 1.2 cm adenomatous polyp identified in 2021. The patient had an American Society of Anesthesiologists Score of 3 at the time of the diagnostic colonoscopy. Following the AAOS-ASGE-BSG recommendations [9], the patient underwent a diagnostic colonoscopy under general anesthesia without prophylactic antibiotics. The colonoscopy was performed using a standard, flexible, reusable, handheld endoscope (Olympus Corporation, Hamburg, Germany) equipped with a high-definition camera at the instrument tip and accessory channels for insufflation, irrigation, suction, and the insertion of additional equipment. During her colonoscopy, a biopsy of a polyp was performed using standard, single-use forceps (Olympus Corporation, Hamburg, Germany); the surgeon did not report any complications (i.e., bleeding or compromise of mucosal integrity) during the procedure. The patient was discharged as per routine a few hours following the procedure.
Two days after the colonoscopy, the patient presented to the Emergency Department (ED) at our Institution with a fever of 38.8 °C, diaphoresis, and acute left hip pain. Before visiting the ED, the patient had been placed on oral prophylactic amoxicillin (500 mg every 8 h) (Aurobindo, Saronno, Italy) by her primary care physician. The orthopedic surgery resident on call, in consultation with the attending, followed an institutional septic arthritis/PJI stepwise diagnostic protocol [17]. This included serologic and synovial fluid marker testing, followed by advanced molecular testing of the synovial fluid (Figure 1), as recently recommended by multiple experts [19].
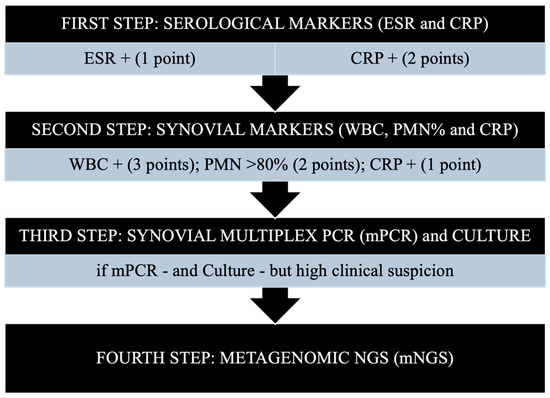
Figure 1.
A four-step diagnostic algorithm for periprosthetic joint infections (PJI). CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; mNGS, metagenomic next-generation sequencing; mPCR, multiplex PCR; PCR, polymerase chain reaction; PMN, polymorphonuclear leukocyte percentage; WBC, white blood cell count. The first two steps are based on the evaluation of standard serological/synovial markers; the third and fourth steps involve performing molecular diagnostics. From: Indelli PF, Totlis T, Lovreković B, Engl M, Violante B, Skowronek P et al. Molecular diagnostics for perioperative microbial identification in periprosthetic joint infection: A scoping review and proposal of a diagnostic flow chart. J Exp Orthop. 2025 Apr 5;12(2) [17]. Permission to publish granted by the authors.
Initial serological analysis showed 15.03 × 1000 µL (normal 3.60–10.50) serological WBC, an erythrocyte sedimentation rate (ESR) of 79 mm/h (normal < 35), and a C-reactive protein of 12.1 mg/L (normal < 0.50). Due to abnormal serological test results and worsening left hip pain that rendered the patient unable to bear weight on the affected limb, radiographs of the left hip were obtained, showing no signs of complication. A fluoroscopy-guided aspiration of the left hip was then promptly performed. Following the protocol (Figure 1), the synovial fluid was sent for measurement of synovial markers: a WBC count and differential were performed first, leaving 1 cc of fluid reserved for possible molecular diagnostic.
Because the aspiration yielded 51.10 × 1000 µL WBCs with 88% PMNs, meeting the algorithm scoring criteria for PJI (score ≥ 6), the synovial fluid was submitted for molecular diagnostics analysis and traditional culture to identify the causative microorganism.
A commercially available multiplex PCR assay (BioFire JI Panel, bioMérieux, Marcy-l’Étoile, France) was used. The Biofire JI Panel features automated nucleic acid extraction, reverse transcription, and nucleic acid amplification [17,18]. This system enables rapid (<1 h) identification of 31 microorganisms and up to 8 antimicrobial resistance (AMR) genes. The microorganism identified was E. coli, and no AMR genes were detected (Supplement S1).
These findings prompted surgical intervention, and the patient underwent Debridement, Antibiotic Pearls, and Retention of the Implant (DAPRI), following a previously described DAIR-like surgical technique [13,15]. The procedure aimed to remove the intra-articular biofilm and to increase and sustain local antibiotic levels by using calcium sulfate antibiotic-loaded beads (Stimulan, Biocomposites, Keele, UK) in cases of acute PJI with pathogen identification (Figure 2).
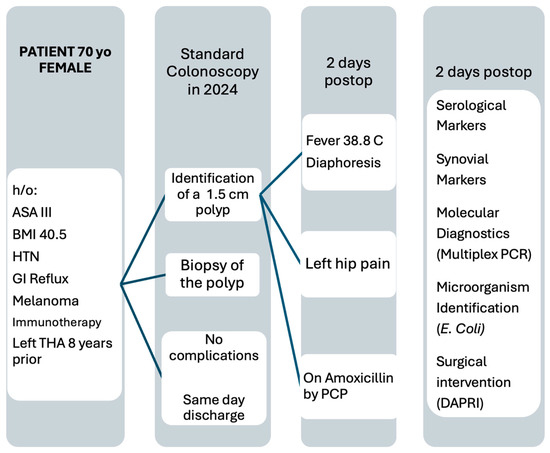
Figure 2.
Flow diagram showing the evolution of the patient’s symptoms and the steps from symptom onset to surgical intervention. ASA: American Society of Anesthesiologists Score; HTN: Hypertension; THA: total hip arthroplasty; PCP: primary care physician; PCR: polymerase chain reaction; DAPRI: Debridement, Antibiotic Pearls, Retention of the Implant.
After sending the synovial fluid for standard culture, three sequential surgical steps were performed: (1) A tumor-like synovectomy (mechanical debridement guided by the injection of 50 cc of dilute methylene blue—ProvayBlue®, Provepharm Life Solutions, Marseille, France—used as a disclosing agent) (Figure 3); (2) Chemical debridement [intra-articular use of 10% dilute povidone iodine [20] (Viatris, Canonsburg, PA, USA), irrigation with one liter of saline, intra-articular application of an acetic acid-based irrigation (BactisureTM, Zimmer Biomet, Warsaw, IN, USA) [21], an additional irrigation with one liter of saline, and extra-articular use of a polyhexanide–poloxamer antiseptic solution (Preventia, Hartmann, Heidenheim, Baden-Württemberg, Germany) [22], aimed at removing the bacterial biofilm from the implant without removing the original hardware except for the polyethylene insert and the femoral head; (3) Local delivery of antibiotics [23]. In this case, guided by the Multiplex PCR report, the authors used 10 cc of calcium sulfate beads (Stimulan, Biocomposites, Keele, UK) mixed with 1 g of Meropenem and 240 mg of Gentamycin. To complete the surgical procedure, all mobile parts—the ceramic femoral head and polyethylene insert—were replaced before the capsule and wound were closed (Figure 2).
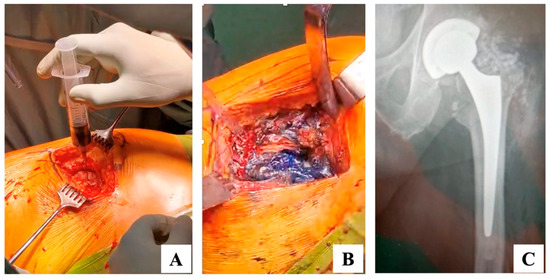
Figure 3.
(A,B) Intraoperative surgical steps. (C) Left hip postoperative radiogram. (A) After skin incision and before capsulotomy, 50 cc of dilute methylene blue (40 cc saline and 10 cc of 0.5% methylene blue solution) were injected into the joint to serve as a disclosing agent [13]; (B) After capsulotomy, the intra-articular space is dyed by the disclosing agent; at this point a tumor-like synovectomy is performed to remove the dyed tissue; (C) Left hip postoperative antero-posterior non-weightbearing radiogram showing the intra-articular positioning of antibiotic added, calcium sulfate beads. Postoperatively, the patient was started on a two-week course of IV antibiotics (Cefotaxime) followed by 10 weeks of oral antibiotics (Cefixime, 200 mg twice daily), in accordance with the European Bone and Joint Infection Society (EBJIS) recommendations [20,21].
Written informed consent has been obtained from the patient to be included in this case report.
A narrative review was performed using PubMed and Scopus databases. The search terms were “colonoscopy” and “periprosthetic joint infections”: 10 articles were selected and ultimately included in the current narrative review.
3. Results
During the postoperative period, all serological markers (ESR, C-reactive protein, WBCs) consistently decreased throughout the patient’s hospital stay. Final intraoperative synovial fluid cultures identified E. coli as the infecting microorganism: the antibiogram confirmed that the microorganism was sensitive to the originally selected Cefotaxime, and the postoperative course of antibiotic prophylaxis was not changed.
The infectious disease team at our institution hypothesized that the patient’s left hip PJI resulted from hematogenous seeding of microorganisms from the intestinal microbiome during the biopsy phase of her colonoscopy. The patient completed a 12-week course of antibiotic prophylaxis without adverse effects.
Periodic orthopedic surgery and infectious disease consults confirmed that the patient remained afebrile and completely asymptomatic, with resolution of her left hip pain and no clinical or radiological concerns. The patient underwent PJI-related serological markers screening (ESR, C-reactive protein, WBCs) at 3 weeks, 6 weeks, 12 weeks, 6 months, and 12 months postoperatively. At the most recent follow-up, the patient was able to walk up to 5 blocks without assistive devices.
4. Discussion
This case demonstrates that a screening colonoscopy with biopsy of a suspicious polyp led to transient bacteremia and subsequent development of an acute left hip PJI. To the authors’ knowledge, this report is the first to describe the implementation of a previously published [17] molecular diagnostics-based algorithm to quickly identify the infecting microorganism in an acute PJI following standard colonoscopy. The use of the algorithm [17] in this particular setting guided an implant-saving surgical treatment. In this case, prompt surgical intervention targeting biofilm formation resulted in complete symptom resolution.
In our literature review, we identified several studies assessing the link between gastrointestinal endoscopy and the development of PJI. Forlenza et al. [22] demonstrated that gastrointestinal endoscopy significantly increased PJI rates when performed within a year of JR. They strongly recommended avoiding endoscopy within two months after TKA and one month after THA (21). Coelho-Prabhu et al. [23] also quantified the increased risk of PJI following esophagogastroduodenoscopy with biopsy (OR 3, 95% CI 1.1–7). Shin et al. [24], in a nationwide propensity score-matched study, confirmed that colonoscopy was associated with a higher risk of PJI in TKA patients, regardless of any concurrent invasive procedures. In the same study [24], the one-year cumulative incidence of PJI after colonoscopy was 0.07%, with a hazard ratio of 1.83 compared to patients who did not undergo colonoscopy. Several case reports also describe PJIs occurring after GI endoscopies, involving bacteria such as Group B streptococcus, Citrobacter freundii, Streptococcus milleri, and other enteric and Gram-negative organisms [25,26].
Interestingly, three retrospective cohort studies using national databases [27,28,29] did not find an association between colonoscopy and the development of PJI. Chiu et al. [27] demonstrated that diagnostic colonoscopy was not a significant risk factor for revision following unicompartmental knee arthroplasty (UKA), TKA, or THA. However, their study did not include patients treated with DAIR or DAIR-like procedures, such as the one described in the present report. Wang et al. [28], analyzing an extensive North American database, found that GI endoscopies performed after a recent JR were not associated with an increased risk of 60-day PJI compared to patients who did not undergo endoscopy. The main limitation of this study was that the database excluded outpatient endoscopic procedures, which constitute the vast majority of such cases. Anderson et al. [29], in a retrospective cohort study within the military population, showed that the timing of colonoscopy relative to the JR event was not associated with increased PJI risk 1 year after surgery. However, they also noted that several comorbidities—such as cerebrovascular disease, cardiovascular disease, diabetes, kidney disease, COPD, and other pulmonary diseases—should be preoperatively managed, and JR patients should be optimized before perioperative colonoscopy. Recently, a national database analysis showed that a colonoscopy performed 6 months before TKA is not associated with an increased risk of PJI [28].
In recent years, the role of gut microbiota and epithelial integrity in the development of PJIs has been extensively studied [30]; this case report confirms that mucosal tissue trauma during endoscopy can lead to the translocation of endogenous microorganisms into the bloodstream, resulting in transient bacteremia. Recent evidence supports the theory (“Trojan Horse” hypothesis) that immune cells, such as neutrophils and macrophages, are responsible for transporting microorganisms to the surgical site and contribute to early biofilm formation [30]. Considering this emerging evidence, there is growing support for investigating the risk of PJI in JR patients undergoing gastrointestinal endoscopy [24,31]. This stands in contrast to the historical lack of consensus among orthopedic surgeons, infectious disease specialists, and gastroenterologists regarding appropriate risk stratification. Several authors have already recommended using PJI risk algorithms that evaluate the presence of gastrointestinal pathologies in relation to JR surgery [32,33].
Within this evolving framework, molecular diagnostics played a critical role in this case by enabling rapid microorganism identification and informing surgical management without the delay associated with traditional culture results. The selection of an implant-saving procedure, such as DAIR and DAPRI, has traditionally depended on the timing of diagnosis: the success rate of DAIR has been associated with the interval between symptom onset and surgical treatment, with the best outcomes observed when the procedure is performed within a week of symptom onset and the causative microorganism has been identified [34]. The current authors previously reported satisfactory results with local antibiotic delivery [13] in an acute PJI setting. Recent recommendations from the 2025 International Consensus Meeting on PJI endorse using local antibiotic delivery whenever possible, alongside mechanical and chemical debridement, exchanging mobile parts, and a 12-week postoperative antibiotic course [35].
The use of molecular diagnostic techniques is transforming the management of PJIs, especially in acute cases [17]. In this case report, the infectious microorganism was identified in less than an hour after joint aspiration. This step allowed prompt surgical intervention and the selection of appropriate antibiotics, administered both locally (via antibiotic-added calcium sulfate beads) and systemically. Several studies have supported combining molecular diagnostics with standard culture to guide the surgical approach in PJI cases [36,37]. However, only a few diagnostic algorithms have been proposed, including the one used in the current study [17].
The current study also raises concerns about the application of existing guidelines for prophylactic antibiotics in JR patients undergoing endoscopic procedures. The American Academy of Orthopaedic Surgeons (AAOS) and The Knee Society currently recommend against antibiotic prophylaxis in JR patients during colonoscopy [9,34]. The issue of antibiotic stewardship has been raised when deciding on prophylactic antibiotics in JR patients, given that 20 million GI endoscopies are performed annually in the United States [36]. The present case, along with other studies [29], suggests that antibiotic prophylaxis should be considered for patients undergoing tissue-disrupting procedures such as biopsies, particularly in the presence of preoperative and postoperative comorbidities that are already independent risk factors for PJI. Interestingly, a dedicated study group from the 2025 International Consensus Meeting on PJI recently recommended against routine antimicrobial prophylaxis for patients who have undergone joint arthroplasty and are scheduled to undergo dental, urologic, or gastrointestinal procedures [37].
The current case report has several limitations. First, as a case report, there is an inherent inability to generalize the findings to a broader population and a potential difficulty in replicating the reported outcome in different clinical scenarios. Second, the syndromic multiplex PCR test used here (Biofire JI panel, bioMérieux, Marcy-l’Étoile, France) can identify only 31 microorganisms and 8 AMR genes, making this test ideal as a screening test (detecting test in the current case report) but represents an intrinsic limitation for its wide use if the infecting microorganism is not detectable within the panel. Third, this type of molecular diagnostic does not allow the preparation of a traditional antibiogram; the choice of an appropriate antibiotic is based on the presence of an AMR gene (when detected) and the indication of an infectious disease specialist. Finally, the patient in this case review presented multiple comorbidities, which could have significantly contributed to the development of a PJI. PJI risk algorithms have been published to quantify the relative risk of PJI in patients undergoing surgical procedures [38].
5. Conclusions
This case illustrates the successful use of a molecular diagnostic approach in managing an acute PJI caused by bacterial translocation of E. coli following a diagnostic colonoscopy.
The combination of timely diagnosis, an implant-preserving procedure to remove the newly formed biofilm, and the initiation of targeted local and systemic antibiotic therapy enabled the avoidance of a two-stage revision, a surgical procedure associated with high morbidity. Although rare, this case supports PJI risk stratification and the occasional use of antibiotic prophylaxis in a specific group of high-risk patients undergoing GI endoscopic procedures. The authors recognize that routine antibiotic prophylaxis is not recommended for patients undergoing gastrointestinal procedures after total joint arthroplasty. In conclusion, this case illustrates a potential risk scenario and the usefulness of rapid diagnostics [38], but it does not provide evidence to change practice.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/prosthesis7060152/s1, Molecular diagnostics report identifying the infecting microorganism (E. coli) (Supplement S1).
Author Contributions
Conceptualization, P.V. and P.F.I.; methodology, A.G.S.; software, M.P.F.C.; validation, P.F.I., F.Q. and A.G.S.; formal analysis, A.G.S.; investigation, P.F.I.; resources, S.S.; data curation, P.V., A.G.S. and M.P.F.C.; writing—original draft preparation, P.F.I.; writing—review and editing, P.F.I., F.Q. and J.K.; visualization, F.Q.; supervision, P.F.I.; project administration, P.F.I.; funding acquisition, P.F.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study received approval from the ethics committee “Comitato Etico dell’Azienda Sanitaria dell’Alto Adige” (SABES 103-2023, 15 November 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The data will be available upon written request to the senior author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| JR | Joint Replacement |
| PJI | Periprosthetic Joint Infection |
| PCR | Polymerase Chain Reaction |
| DAIR | Debridement, Antibiotics, Implant Retention |
| DAPRI | Debridement, Antibiotic Pearls, Retention of the Implant |
| THA | Total Hip Arthroplasty |
| TKA | Total Knee Arthroplasty |
| GI | Gastrointestinal |
References
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of Primary and Revision Hip and Knee Arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Lau, E.C.; Son, M.S.; Chang, E.T.; Zimmerli, W.; Parvizi, J. Are We Winning or Losing the Battle with Periprosthetic Joint Infection: Trends in Periprosthetic Joint Infection and Mortality Risk for the Medicare Population. J. Arthroplast. 2018, 33, 3238–3245. [Google Scholar] [CrossRef]
- Kamath, A.F.; Ong, K.L.; Lau, E.; Chan, V.; Vail, T.P.; Rubash, H.E.; Berry, D.J.; Bozic, K.J. Quantifying the Burden of Revision Total Joint Arthroplasty for Periprosthetic Infection. J. Arthroplast. 2015, 30, 1492–1497. [Google Scholar] [CrossRef]
- Piuzzi, N.S. Rethinking How We Define and Treat Periprosthetic Joint Infection. J. Bone Jt. Surg. 2025, 107, 664. [Google Scholar] [CrossRef]
- Wouthuyzen-Bakker, M.; Sebillotte, M.; Lomas, J.; Taylor, A.; Palomares, E.B.; Murillo, O.; Parvizi, J.; Shohat, N.; Reinoso, J.C.; Sánchez, R.E.; et al. Clinical outcome and risk factors for failure in late acute prosthetic joint infections treated with debridement and implant retention. J. Infect. 2019, 78, 40–47. [Google Scholar] [CrossRef]
- Huotari, K.; Peltola, M.; Jämsen, E. The incidence of late prosthetic joint infections. Acta Orthop. 2015, 86, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Krezalek, M.A.; Alverdy, J.C. The Role of the Gut Microbiome on the Development of Surgical Site Infections. Clin. Colon. Rectal Surg. 2023, 36, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Low, D.E.; Shoenut, J.P.; Kennedy, J.K.; Sharma, G.P.; Harding, G.K.; Den Boer, B.; Micflikier, A.B. Prospective assessment of risk of bacteremia with colonoscopy and polypectomy. Dig. Dis. Sci. 1987, 32, 1239–1243. [Google Scholar] [CrossRef]
- ASGE Standards of Practice Committee; Khashab, M.A.; Chithadi, K.V.; Acosta, R.D.; Bruining, D.H.; Chandrasekhara, V.; Eloubeidi, M.A.; Fanelli, R.D.; Faulx, A.L.; Fonkalsrud, L.; et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest. Endosc. 2015, 81, 81–89. [Google Scholar] [CrossRef]
- Bravo, T.; Budhiparama, N.; Flynn, S.; Gaol, I.L.; Hidayat, H.; Ifran, N.N.; O’Byrne, J.; Utomo, D.N. Hip and Knee Section, Prevention, Postoperative Issues: Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2019, 34, S321–S323. [Google Scholar] [CrossRef] [PubMed]
- Rakow, A.; Perka, C.; Trampuz, A.; Renz, N. Origin and characteristics of haematogenous periprosthetic joint infection. Clin. Microbiol. Infect. 2019, 25, 845–850. [Google Scholar] [CrossRef]
- Longo, U.G.; De Salvatore, S.; Bandini, B.; Lalli, A.; Barillà, B.; Budhiparama, N.C.; Lustig, S. Debridement, antibiotics, and implant retention (DAIR) for the early prosthetic joint infection of total knee and hip arthroplasties: A systematic review. J. ISAKOS 2024, 9, 62–70. [Google Scholar] [CrossRef]
- Indelli, P.; Ghirardelli, S.; Valpiana, P.; Bini, L.; Festini, M.; Iannotti, F. Debridement, Antibiotic Pearls, and Retention of the Implant (DAPRI) in the Treatment of Early Periprosthetic Joint Infections: A Consecutive Series. Pathogens 2023, 12, 605. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.B.; German, G.; Abdelbary, H.; Grammatopoulos, G.; Garceau, S. Timing from Admission to Debridement, Antibiotic, and Implant Retention (DAIR) Affects Treatment Success in Total Knee Arthroplasty Periprosthetic Joint Infection. J. Arthroplast. 2025; in press. [Google Scholar]
- Qasim, S.N.; Swann, A.; Ashford, R. The DAIR (debridement, antibiotics and implant retention) procedure for infected total knee replacement—A literature review. SICOT J. 2017, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.W.; Mu, W.; Parvizi, J. Culture-negative periprosthetic joint infections: Do we have an issue? J. Clin. Orthop. Trauma 2024, 52, 102430. [Google Scholar] [CrossRef] [PubMed]
- Ghirardelli, S.; Scaggiante, F.; Troi, C.; Valpiana, P.; Cristofolini, G.; Aloisi, G.; Violante, B.; Russo, A.; Schaller, S.; Indelli, P.F. Multiplex PCR in septic arthritis and periprosthetic joint infections microorganism identification: Results from the application of a new molecular testing diagnostic algorithm. J. Exp. Orthop. 2024, 11, e12097. [Google Scholar] [CrossRef]
- Indelli, P.F.; Totlis, T.; Lovreković, B.; Engl, M.; Violante, B.; Skowronek, P.; Demey, G.; Ghirardelli, S.; Maci, C.; Castagna, A.; et al. Molecular diagnostics for perioperative microbial identification in periprosthetic joint infection: A scoping review and proposal of a diagnostic flow chart. J. Exp. Orthop. 2025, 12, e70263. [Google Scholar] [CrossRef]
- Esteban, J.; Salar-Vidal, L.; Schmitt, B.H.; Waggoner, A.; Laurent, F.; Abad, L.; Bauer, T.W.; Mazariegos, I.; Balada-Llasat, J.-M.; Horn, J.; et al. Multicenter evaluation of the BIOFIRE Joint Infection Panel for the detection of bacteria, yeast, and AMR genes in synovial fluid samples. J. Clin. Microbiol. 2023, 61, e0035723. [Google Scholar] [CrossRef]
- Hanusrichter, Y.; Frieler, S.; Gessmann, J.; Schulte, M.; Krejczy, M.; Schildhauer, T.; Baecker, H. Does the Implementation of the PRO-IMPLANT Foundation Treatment Algorithm Improve the Outcome of Chronic Periprosthetic Knee Infections? Mid-Term Results of a Prospective Study. Z. Orthop. Unfall. 2023, 161, 260–270. [Google Scholar] [CrossRef]
- Saadana, J.; Abdeljelil, M.; Khemili, K.; Chaouch, F.; Saad, L.; Belgacem, H.; Jellali, M.; Fekih, A.; Toumi, A.; Abid, A. Strategies for periprosthetic joint infection management in resource-limited settings: The applicability of EBJIS criteria. Int. Orthop. 2025, 49, 1027–1035. [Google Scholar] [CrossRef]
- Forlenza, E.M.; Terhune, E.B.; Higgins, J.D.D.; Jones, C.; Geller, J.A.; Della Valle, C.J. Invasive Gastrointestinal Endoscopy Following Total Joint Arthroplasty Increases the Risk for Periprosthetic Joint Infection. J. Arthroplast. 2023, 38, S394–S398.e1. [Google Scholar] [CrossRef]
- Coelho-Prabhu, N.; Oxentenko, A.S.; Osmon, D.R.; Baron, T.H.; Hanssen, A.D.; Wilson, W.R.; Steckelberg, J.M.; Baddour, L.M.; Harmsen, W.S.; Mandrekar, J.; et al. Increased risk of prosthetic joint infection associated with esophago-gastro-duodenoscopy with biopsy. Acta Orthop. 2013, 84, 82–86. [Google Scholar] [CrossRef]
- Shin, K.H.; Han, S.B.; Song, J.E. Risk of Periprosthetic Joint Infection in Patients with Total Knee Arthroplasty Undergoing Colonoscopy: A Nationwide Propensity Score Matched Study. J. Arthroplast. 2022, 37, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Triesenberg, S.N.; Clark, N.M.; Kauffman, C.A. Group B Streptococcal Prosthetic Joint Infection Following Sigmoidoscopy. Clin. Infect. Dis. 1992, 15, 374–375. [Google Scholar] [CrossRef] [PubMed]
- Weiler, P.J. Late infection of a bipolar prosthesis following endoscopy. A case report. J. Bone Jt. Surg. 1995, 77, 1129–1130. [Google Scholar] [CrossRef]
- Chiu, A.K.; Malyavko, A.; Das, A.; Agarwal, A.R.; Gu, A.; Zhao, A.; Thakkar, S.C.; Campbell, J. Diagnostic and Invasive Colonoscopy Are Not Risk Factors for Revision Surgery Due to Periprosthetic Joint Infection. J. Arthroplast. 2023, 38, 1591–1596.e3. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Chen, B.; Huang, X.; Li, S.; Huang, Y.; Bansal, P. Gastrointestinal Endoscopy and the Risk of Prosthetic Joint Infection: A Nationwide Database Analysis. Dig. Dis. Sci. 2022, 67, 5562–5570. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.B.; Slaven, S.E.; Watson, N.L.; Cody, J.P.; McGill, R.J.; Potter, B.K.; Nealeigh, M.D. Periprosthetic Joint Infection in Patients with Arthroplasty Undergoing Perioperative Colonoscopy. JAMA Netw. Open 2024, 7, e2410123. [Google Scholar] [CrossRef]
- Krezalek, M.A.; Hyoju, S.; Zaborin, A.; Okafor, E.; Chandrasekar, L.; Bindokas, V.; Guyton, K.; Montgomery, C.P.; Daum, R.S.; Zaborina, O.; et al. Can Methicillin-resistant Staphylococcus aureus Silently Travel from the Gut to the Wound and Cause Postoperative Infection? Modeling the “Trojan Horse Hypothesis”. Ann. Surg. 2018, 267, 749–758. [Google Scholar] [CrossRef]
- Elmenawi, K.; Khan, S.; Pasqualini, I.; Visperas, A.; Deren, M.; Krebs, V.; Molloy, R.; Piuzzi, N. Colonoscopy within 6 Months before TKA May Be Associated with Worse Postoperative Outcomes, but Not Infection: A National Database Analysis. In Proceedings of the 35th Annual Open Scientific Meeting Musculoskeletal Infection Society, Jersey City, NJ, USA, 1–2 August 2025. [Google Scholar]
- Tan, T.L.; Maltenfort, M.G.; Chen, A.F.; Shahi, A.; Higuera, C.A.; Siqueira, M.; Parvizi, J. Development and Evaluation of a Preoperative Risk Calculator for Periprosthetic Joint Infection Following Total Joint Arthroplasty. J. Bone Jt. Surg. 2018, 100, 777–785. [Google Scholar] [CrossRef]
- Longo, U.G.; Lalli, A.; Bandini, B.; Angeletti, S.; Lustig, S.; Budhiparama, N.C. The influence of gut microbiome on periprosthetic joint infections: State-of-the art. J. ISAKOS 2024, 9, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Bains, S.S.; Sax, O.C.; Chen, Z.; Gilson, G.A.; Nace, J.; Mont, M.A.; Delanois, R.E. Antibiotic Prophylaxis Is Often Unnecessary for Screening Colonoscopies Following Total Knee Arthroplasty. J. Arthroplast. 2023, 38, S331–S336. [Google Scholar] [CrossRef] [PubMed]
- Cashman, J.; Mortazavi, S.M.J.; Indelli, P.F.; Rele, S.; Haasper, C.; Yildiz, F.; Holland, C.T.; Lizcano, J.D.; Auñón-Rubio, Á.; Tai, D.B.G.; et al. 2025 ICM: Debridement, Antibiotics, and Implant Retention (DAIR). J. Arthroplast. 2025; epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Seeff, L.C.; Richards, T.B.; Shapiro, J.A.; Nadel, M.R.; Manninen, D.L.; Given, L.S.; Dong, F.B.; Winges, L.D.; McKenna, M.T. How many endoscopies are performed for colorectal cancer screening? Results from CDC’s survey of endoscopic capacity. Gastroenterology 2004, 127, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Coope, A.M.; Certain, L.; Buterin, A.; Cortés-Penfield, N.; Alkhawashki, H.M.; Suleman, L. Should antimicrobial prophylaxis be given to patients who have a joint arthroplasty in place and who are undergoing invasive procedures (e.g., dental, colonoscopy, cystoscopy, etc.)? In Proceedings of the 2025 International Consensus Meeting on Periprosthetic Joint Infections, Istanbul, Turkey, 8–10 May 2025. [Google Scholar]
- Martinazzi, B.J.; Indelli, P.F.; Azboy, I.; Babis, G.; Dikmen, G.; Flores, H.; Franceschini, M.; Goswami, K.; Han, H.S.; Huddleston, J.; et al. 2025 ICM: Diagnostic Techniques: Molecular Tests. J. Arthroplast. 2025; epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).