1. Introduction
Ceramics were initially used with metal substructures to reinforce the more brittle ceramic materials. These combinations, known as porcelain-fused-to-metal (PFM) restorations, have long been considered the gold standard for fixed partial restorations due to their ability to fulfill both functional and esthetic demands. However, several disadvantages have been associated with PFMs, including reduced light transmission, gingival discoloration around abutment teeth, allergic reactions, and the release of metallic ions into gingival tissues. As a result, the growing demand for highly esthetic restorations has led to the development of metal-free all-ceramic restorations, using different resin-based adhesive systems, which provide superior esthetic outcomes [
1,
2].
Dental ceramics are commonly classified based on their composition into glass-based ceramics, resin-matrix ceramics, and polycrystalline ceramics [
3,
4]. Glass ceramics, such as lithium disilicate and leucite-reinforced ceramics, consist primarily of a silica-based matrix that provides excellent polish ability and esthetic characteristics. The crystalline phase enhances mechanical strength and fracture resistance [
3,
5]. Resin-matrix ceramics are composed of a ceramic network infiltrated with a polymer resin; combining the esthetics and wear resistance of ceramics with the resilience, ease of milling, and repairability of composite resins [
6]. In contrast, polycrystalline ceramics are composed entirely of densely packed crystals with no glassy phase. Among them, zirconia is notable for its superior mechanical properties, and compared with metal frameworks, it provides satisfactory esthetic outcomes [
3,
7].
Despite ongoing advances in ceramic materials, including efforts to improve the translucency of monolithic zirconia, this material remains relatively opaque. Therefore, zirconia is often used as a core structure, veneered with feldspathic porcelain or lithium disilicate powder, to achieve optimal esthetic outcomes [
8,
9].
All commercially available ceramic materials used for indirect restorations are prone to fracture during clinical service. Such failures may arise from fatigue, traumatic events, or parafunctional habits, as well as laboratory-related factors such as inadequate slow cooling, improper framework-to-veneer ratios, and inadequate firing procedures, etc. [
1,
10,
11].
Due to the inherent characteristics of ceramic processing, new porcelain cannot be added to an existing restoration intraorally. Consequently, intraoral repair represents a practical and often emergency approach for managing localized fractures. Direct repairs performed with resin-based composites in a single appointment offer several advantages, including reduced treatment time and lower costs for the patient. Moreover, this approach minimizes the need for further interventions or additional tooth preparation, which could compromise pulp vitality and weaken tooth structure, thereby enabling a more conservative and efficient restorative treatment [
3,
10,
12].
The repair of fractured ceramic restorations serves as a conservative method to restore both function and esthetics through the implementation of intraoral repair system components. This process needs mechanical surface preparation and chemical bonding agents to achieve strong composite resin adhesion with the ceramic surfaces [
2,
13,
14].
Researchers have developed multiple repair protocols over the past years to enhance the bond strength, durability, and esthetic results of ceramic restorations. The most effective repair system remains unclear because various elements determine the results. The success of ceramic repair depends on multiple factors, which include ceramic material selection, surface preparation methods, environmental exposure conditions, and the choice of repair materials [
15,
16].
Surface treatment is crucial in repairing fractured ceramic surfaces. It involves mechanically increasing the surface area, reducing tension, and creating a fine surface roughness. Chemically, it selectively dissolves the glassy matrix, promoting resin adhesion. Surface treatment alters the roughness of dental materials’ surfaces, which is essential for indirect restorations. Enhancing surface roughness improves mechanical interlocking with adhesive materials, ensuring long-lasting and stable restorations in challenging oral environments [
17,
18].
This in vitro study aims to assess the effect of mechanical surface treatments, along with two different intra-oral repair kits, on the shear bond strength between composite resin and two types of indirect restorative materials: metal (nickel–chromium alloy) and zirconia. The null hypotheses were as follows: (1) that applying different surface treatment methods to the indirect restorative materials would not result in a statistically significant difference in shear bond strength; (2) that there would be no significant difference in shear bond strength among the different types of restorative materials, regardless of the repair protocol used.
2. Materials and Methods
2.1. Study Design
A schematic representation of the study design and experimental workflow of this in vitro study is shown in
Figure 1.
2.2. Sample Preparation
The sample size was calculated using G*Power software (version 3.1.9.2; University of Kiel, Kiel, Germany) to ensure adequate statistical power for detecting significant differences among groups. The calculation was performed with 80% power, an alpha level of 0.05, and an effect size of 0.80. A total of 144 samples was deemed sufficient to achieve statistical significance. For each material, 72 square-shaped specimens (12 × 12 × 2 mm) were fabricated by a single operator following the manufacturer’s instructions.
Nickel–Chromium Alloy: Nickel–chromium samples (Realoy-N+, German Special Alloys GmbH, Carl-Friedrich-Benz-Str. 1b, 47877 Willich, Germany) were fabricated using selective laser melting technology following the alloy’s fabrication guidelines.
Zirconia: Zirconia blocks (CaroZir
®, Carol Zircolite Pvt. Ltd., Bad Säckingen, Germany) were milled from pre-sintered blanks using a CAD/CAM system and sintered at 1530 °C for 10 h, as per the manufacturer’s instructions. The materials used in this study are presented in
Table 1.
All samples were embedded in cold-cured acrylic resin (Veracril, New Stetics, Colombia), leaving only one surface exposed for subsequent treatments (
Figure 2). The exposed surfaces were sequentially polished with 600-, 800-, 1000-, and 1200-grit silicon carbide abrasive papers under water cooling. This was followed by ultrasonic cleaning in distilled water for 5 min and drying with oil-free compressed air.
2.3. Sample Grouping and Surface Treatments
Specimens were numbered, thoroughly mixed, and then randomly allocated to the experimental groups in equal numbers using a simple random allocation method to minimize potential selection bias. Each material group (n = 72) was then randomly divided into three surface treatment subgroups (n = 24 each):
Group 1: (Control) Received no surface treatment.
Group 2: (Airborne Particle Abrasion) Performed using an air abrasion unit (Foshan Asin Dental Equipment Co., Foshan, China) mounted on a dental surveyor for standardization. Surfaces were treated with 50 µm aluminum oxide particles at a pressure of 2.5 bar through a nozzle with a 0.5 mm internal diameter, positioned perpendicular (90°) to the specimen surface, and maintained at a constant distance of 10 mm in a circular motion for 10 s. The samples were then rinsed and air-dried [
19].
Group 3: (Diamond Bur grinding) Surfaces were roughened with a medium-grit (approx. 100 µm) diamond bur (Jota, Rüthi, Switzerland) mounted in a dental surveyor. The bur was applied in a standardized back-and-forth motion (10 passes) at 200,000 RPM under water cooling; burs were replaced every five specimens [
20].
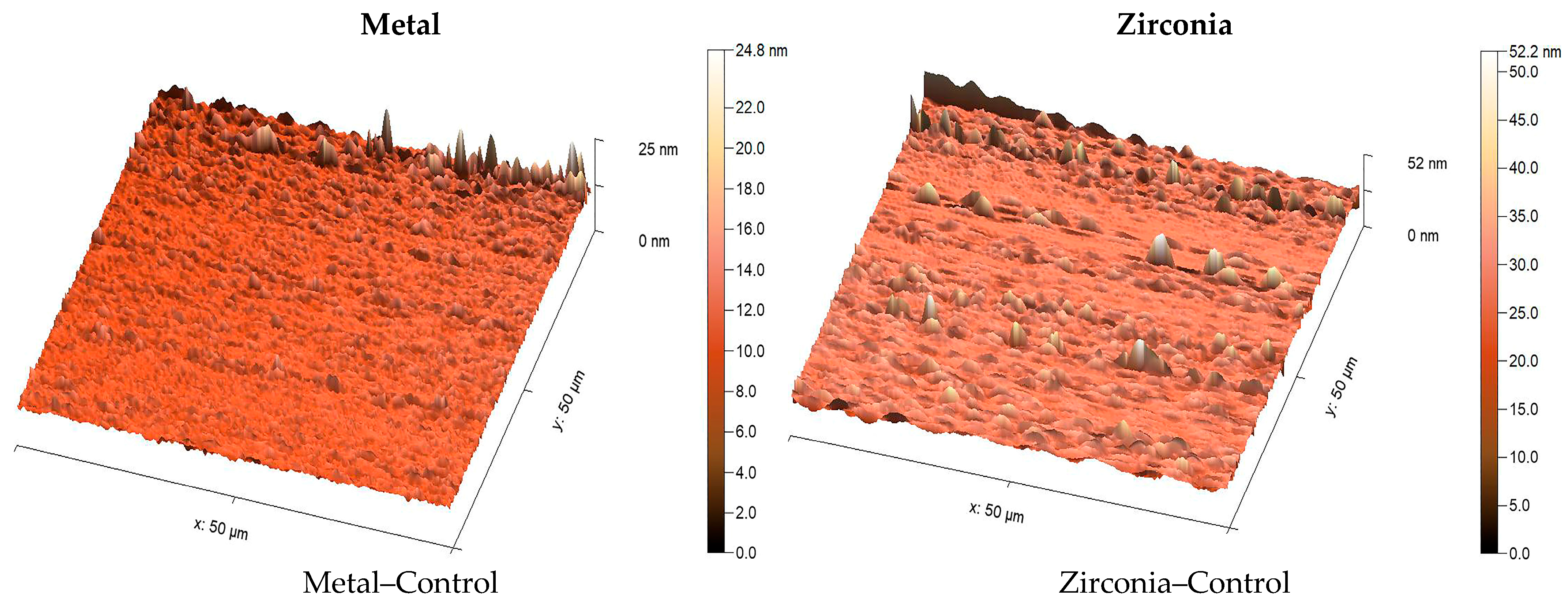
2.4. Surface Roughness Measurement
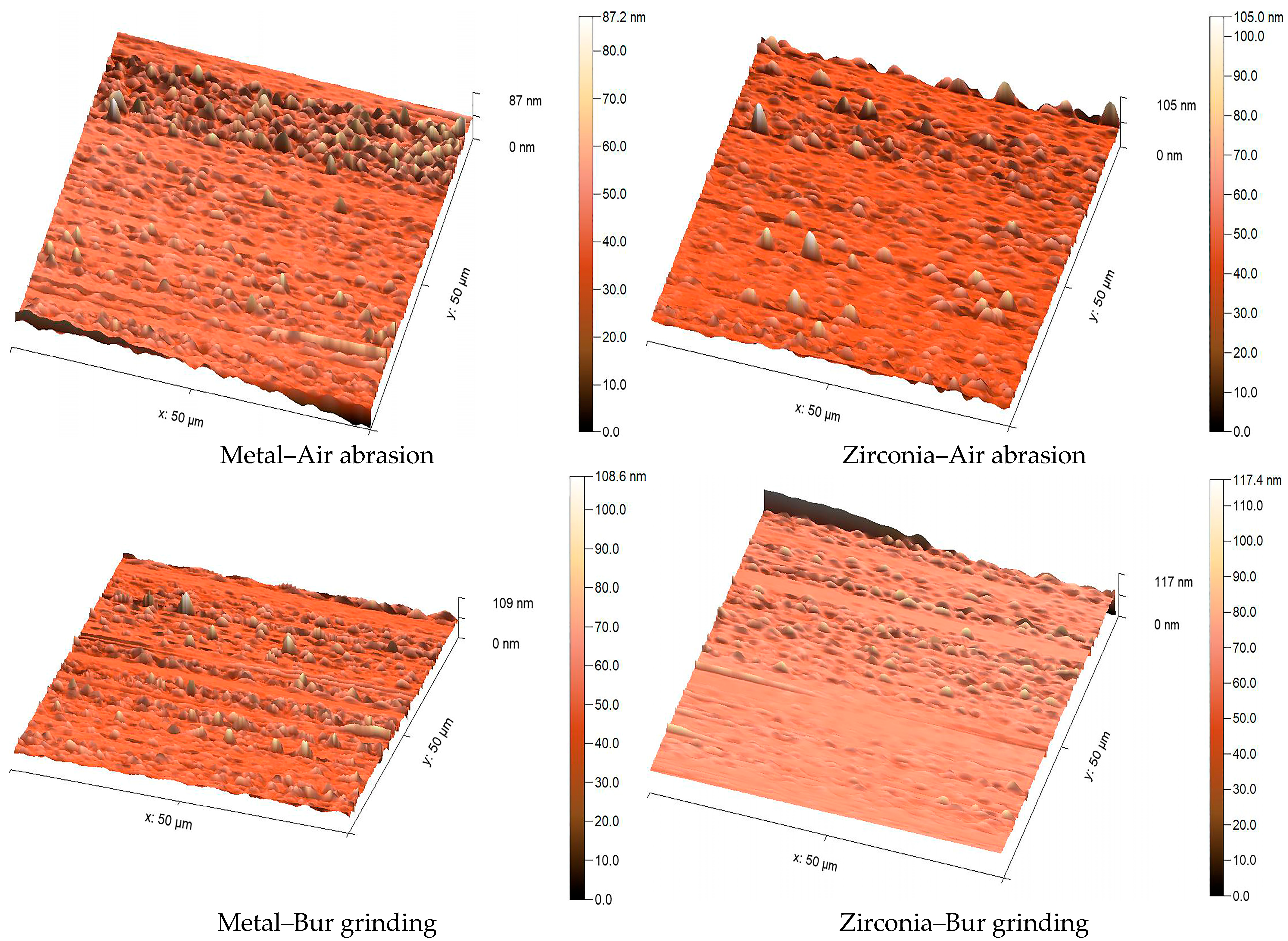
To assess surface roughness and topography of the specimens after surface treatment, two additional specimens were fabricated in each group, as explained earlier. They were analyzed using an atomic force microscope (AFM Workshop Model TT-2, Hilton Head Island, SC, USA). A silicon nitride tip with a 50 nm radius and 45° apex angle was used. Surfaces were scanned at 80 µm/s across a 50 × 50 µm area at 312 × 271-pixel resolution. AFM scans were taken from three randomly selected central areas on each sample, avoiding the edges to prevent measurement bias. The arithmetic mean height (Sa) value was calculated from these three readings to obtain a representative value for each surface. High-resolution 3D AFM images were used to illustrate surface morphology more clearly. Images were analyzed using MountainsSPIP
® software (Expert version 8.2.10392) [
21].
2.5. Bonding Procedures and Composite Resin Application
Each surface-treated group was further subdivided into two subgroups (n = 12) based on the adhesive system used. Adhesives were applied according to the manufacturer’s instructions. To ensure uniform adhesive thickness, all applications were performed by the same operator using a new microbrush every five specimens. The adhesive was gently air-dried for 5 s at a constant air pressure and 10 cm distance before light curing, resulting in a thin and consistent adhesive layer.
Intraoral Repair Kit Group:
Z-Prime plus: One layer of Z-prime plus (Bisco, USA) was applied to each treated sample using a microbrush and dried with an air spray for 5 s. Porcelain Bonding Resin (Bisco, USA): A thin layer of porcelain bonding resin was applied to the treated samples, and the solvent was evaporated using air-drying followed by light curing for 10 s.
GC Repair Kit Group:
G-Multi Primer (GC Corporation, Tokyo, Japan): One layer of G-Multi Primer was applied onto the all-treated sample, then it was air-dried. G-Premio Bond Universal Adhesive (GC Corporation, Japan): A single layer was applied, followed by a 10 s wait. The adhesive was then dried for 5 s under a strong stream of air and finally light-cured for 10 s.
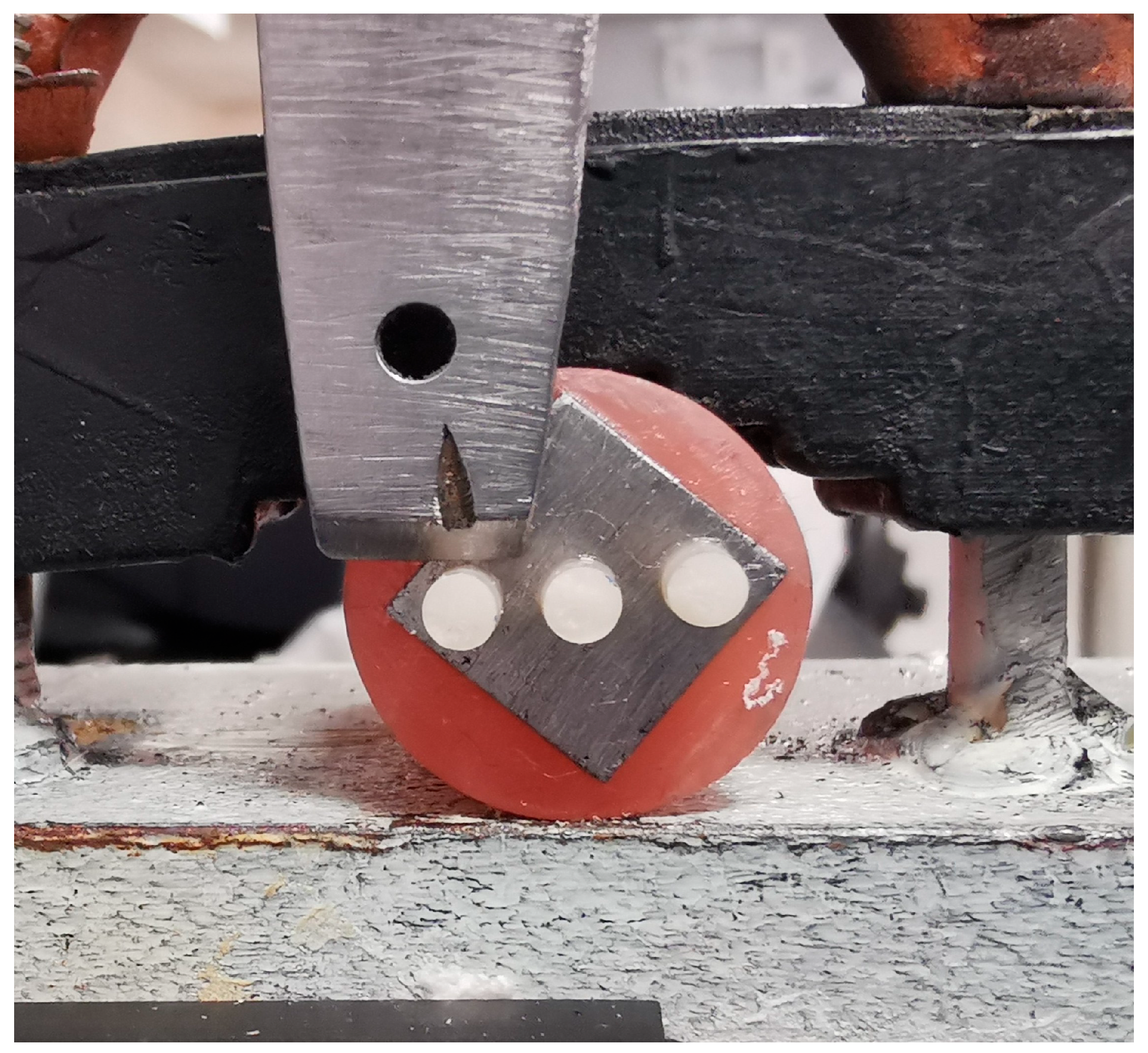
Three polyethylene microtubules (2 mm internal diameter, 3 mm height) were placed over each surface and filled with Tokuyama Palfique LX5 composite resin (A2 shade, Japan) in three increments. Each increment was light-cured for 20 s using a curing unit (Foshan Coxo Medical Instrument Co., Ltd.) at an intensity of 1200 mW/cm
2. Subsequently, after the removal of the microtubule, the entire restoration received an additional 20 s of light curing [
22].
All bonding and testing procedures were carried out in a controlled laboratory environment at 23 ± 2 °C and 50 ± 10% relative humidity, consistent with the ISO guideline for dental material testing.
2.6. Storage and Thermocycling
Specimens were stored in distilled water at 37 °C for 15 h. Half of the specimens were thermocycled for 5000 cycles between 5 °C and 55 °C with a 30 s dwell time. Afterward, they were again stored in distilled water at 37 °C for 15 h before testing [
23].
2.7. Micro-Shear Bond Strength (μSBS) Testing
Specimens were mounted in a universal testing machine (MultiTest 1 d, Mecmesin, Slinfold, UK) equipped with an AFG 500 N digital force gauge. The machine was calibrated before testing using a certified reference load cell, following the manufacturer’s standard procedure to ensure measurement accuracy and consistency. A shear force was applied to the bonded composite interface at a crosshead speed of 1.0 mm/min using a knife-edge blade until failure occurred (
Figure 3). The maximum load at failure (N) was recorded for each sample. Bond strength values, expressed in megapascals (MPa), were calculated using the formula: Bond Strength (MPa) = Failure Load (N)/Bonding Area (mm
2) [
9].
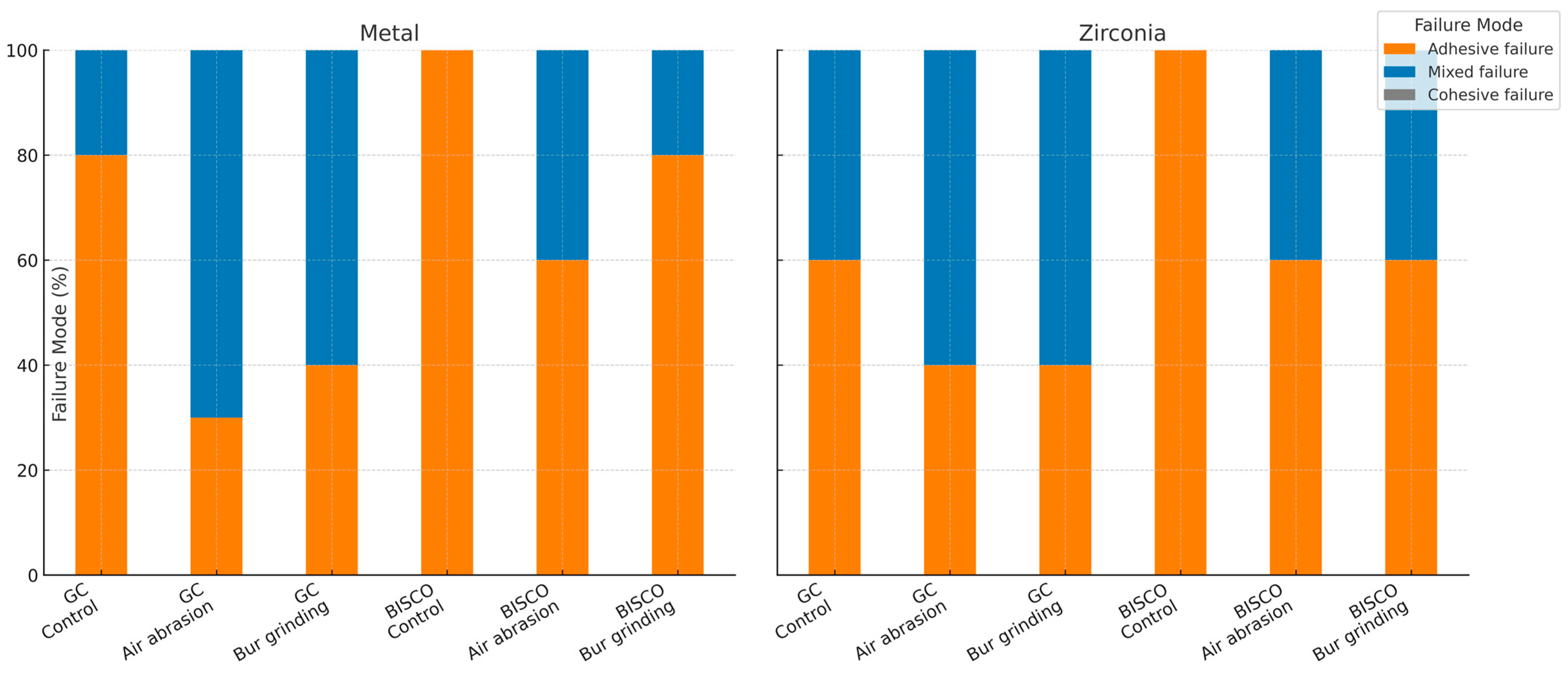
2.8. Failure Mode Analysis
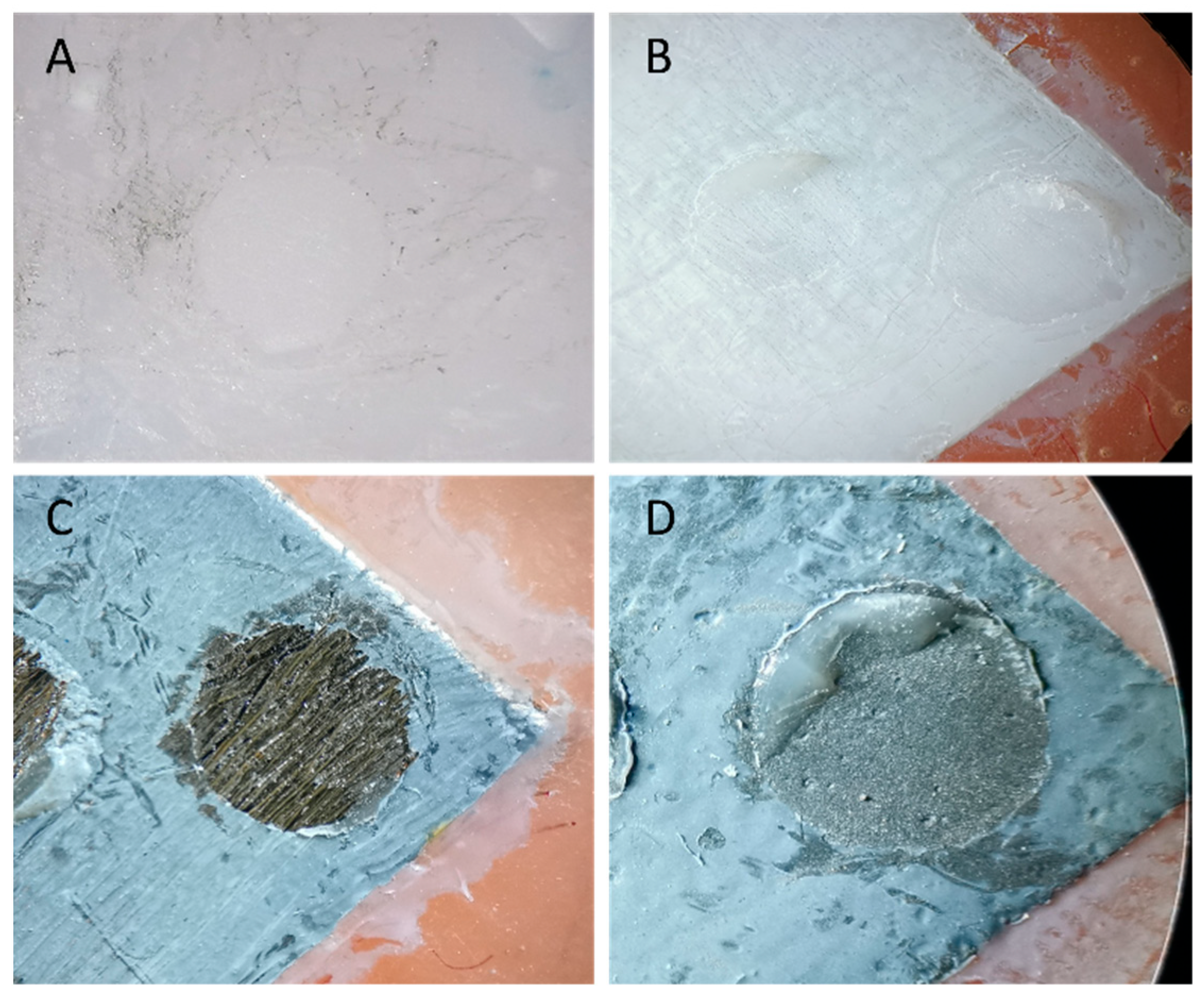
Failure modes were examined visually under a stereomicroscope (AmScope, Irvine, CA, USA) at ×20 magnification. Single calibrated examiners categorized based on predefined criteria as:
Adhesive failure (A): at the adhesive–substrate interface.
Cohesive failure (C): within the composite resin.
Mixed failure (M): a combination of adhesive and cohesive failures.
The percentage of each failure mode was calculated from the number of samples per category relative to the total in each group. No software was used. Representative failure modes were photographed for illustration.
2.9. Statistical Analysis
Data were analyzed with IBM SPSS Statistics software (Version 27). The Shapiro–Wilk test confirmed that all data points followed a normal distribution pattern. One-way ANOVA was used for surface roughness analysis. Three-way ANOVA was applied to assess the main effects and interactions of surface treatment, adhesive system, and aging on μSBS. Tukey HSD tests were conducted for pairwise comparisons, with a p-value threshold of 0.05 to determine statistical significance.
4. Discussion
4.1. Surface Treatment Effects on Bond Strength
Mechanical surface treatments are fundamental to enhancing resin adhesion by increasing surface roughness and surface free energy, thereby facilitating micromechanical interlocking and chemical bonding [
24,
25]. In the current study, diamond bur roughening produced the maximum quantitative surface roughness (Sa) values for both metal and zirconia, yet this was not consistently associated with the strongest bond strength, particularly in metal substrates, where air abrasion yielded superior SBS results. This discrepancy can be attributed to the nature and quality of surface irregularities induced, as reported by Jain et al. (2013), diamond burs create deeper and more irregular grooves, which may introduce microcracks or stress concentration points detrimental to adhesive durability. This explains that excessive mechanical roughening can weaken substrate integrity and reduce long-term bond strength [
2]. Conversely, airborne particle abrasion with 50 µm aluminum oxide particles effectively increased surface roughness and energy by producing a uniformly frosted surface with shallow, interconnected furrows that facilitate capillary infiltration of primers and adhesives, resulting in pronounced improvements in SBS for metal substrates [
2,
26]. This observation aligns with multiple studies, SBS improvements following Al
2O
3 air abrasion on substrate surfaces, emphasizing its ability to clean and activate the surface by generating microporosities favorable for resin infiltration and retention [
27].
For zirconia, both mechanical treatments produced statistically similar SBS increases relative to control groups, confirming the substrate’s responsiveness to varied surface modifications due to its polycrystalline structure that exposes fresh zirconia grains upon abrasion. This aligns with the work of Yeğin (2018), who concluded that both grinding and sandblasting are equally effective chairside treatments for enhancing the bond strength of resin cement to Y-TZP ceramics [
28]. Valizadeh et al. (2020) and Dieckmann et al. (2020) found aluminum oxide sandblasting and bur abrasion to be equally effective for repairing composite restorations [
29,
30]. (Chatterjee and Ghosh 2022) corroborated these findings, noting comparable bond strength improvements with air abrasion and bur grinding methods on zirconia ceramics [
31]. These consistent findings across different materials underscore that the critical mechanism is the creation of surface roughness and mechanical retention, which can be achieved equally well by either abrasion method However, some studies (e.g., Fathpour et al., 2022) (Libecki et al. 2017) argue that the clinical superiority of air abrasion combined with chemical primers may ultimately yield more durable bonds due to synergistic mechanical and chemical effects [
8,
32]. Therefore, while surface roughness is necessary, it is insufficient alone, and careful selection of accompanying adhesive chemistry is paramount.
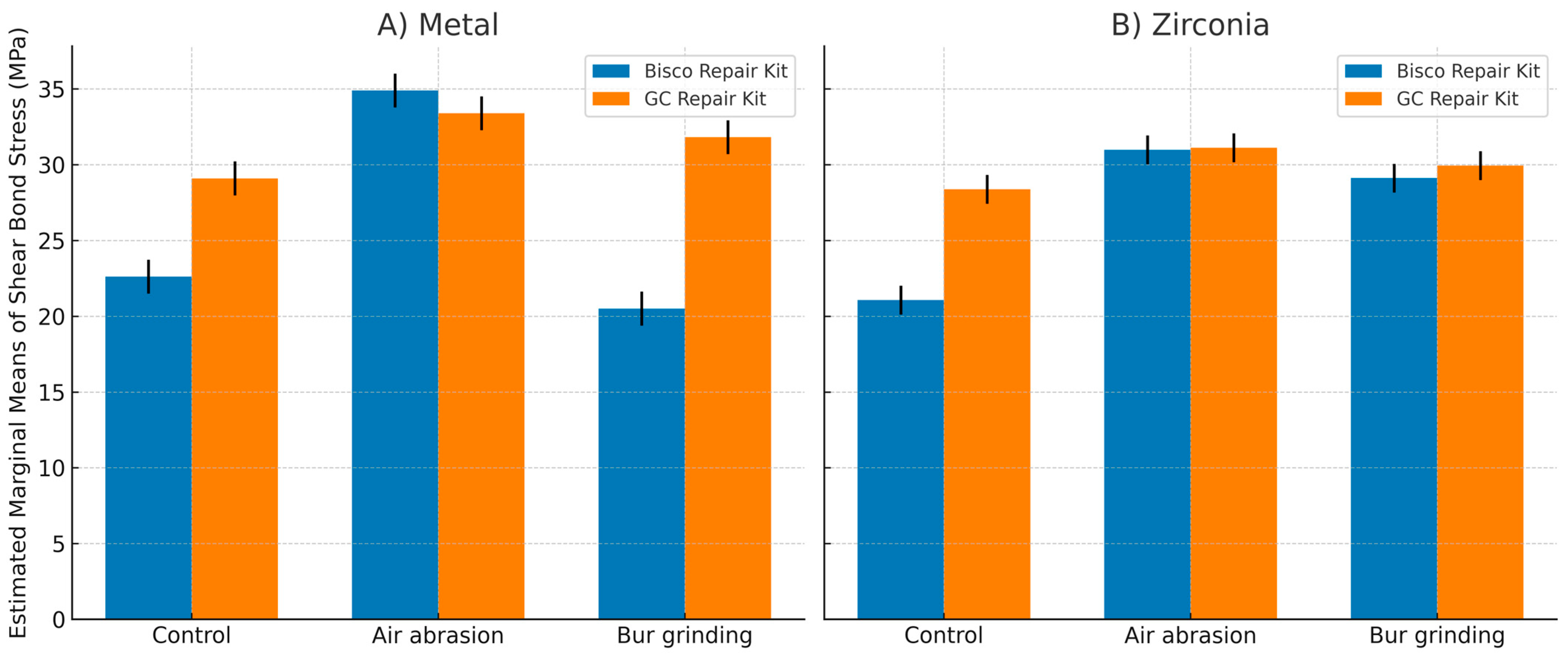
4.2. Impact of Adhesive Systems on Repair Performance
The adhesive repair system used also significantly affected bond strength and its longevity after thermocycling; a decrease in bond strength was potentially due to hydrolysis at the interface by water absorption [
33,
34]. The superior post-aging stability of GC repair systems can be attributed to the adhesive composition. Both G-Multi Primer and G-Primeo Bond contain Methacryloyloxydecyl dihydrogen phosphate (10-MDP), a hydrophobic functional monomer that creates ionic bonds with metal and zirconia oxide and covalently links to the resin composite, resulting in hydrolytically stable adhesion [
35]. Klaisiri et al. (2021) demonstrated that universal adhesives rich in phosphate monomers better preserve bond strength under long-term simulated oral aging than some traditional bonding systems [
36]. Furthermore, a silica filler in G-Primeo bond renders it less sensitive to water absorption, contributing to the adhesive’s resistance against hydrolytic breakdown [
34,
37].
In contrast, Bisco’s Z-Prime Plus is formulated with Hydroxyethyl methacrylate (HEMA), a hydrophilic monomer prone to water absorption and hydrolysis, which compromises bond stability over time [
38]. The presence of Bisphenol A-glycidyl methacrylate (Bis-GMA) in both Z-Prime Plus and Porcelain Bonding Resin may further decrease durability because of the hydroxyl group, which facilitates water uptake and degradation of the adhesive interface [
39]. Additionally, Triethylene Glycol Dimethacrylate (TEGDMA) in the Porcelain Bonding Resin raises polymerization shrinkage and water absorption, further undermining bond integrity [
40]. These compositional differences probably explain why the GC kit shows better bond durability in this study, aligning with the previous reports that show MDP-based adhesives have better hydrolytic stability than those with higher levels of HEMA and Bis-GMA.
The interaction between the surface treatment and the adhesive system effectiveness highlights that optimal intra-oral repair requires both mechanical and chemical preparation. For metal substrates, GC outperformed Bisco in control and diamond bur groups, but both were similar after air abrasion, indicating that strong mechanical preparation reduces reliance on adhesive type. On zirconia, GC showed higher bond strength in controls, while both adhesives were comparable after mechanical treatment, emphasizing the key role of primer chemistry when roughening is limited.
4.3. Effect of Aging on Bond Durability
Simulated aging through thermocycling significantly diminished bond strength across all experimental groups, reflecting the deteriorative effects of fluctuating thermal and moisture stresses commonly endured intraorally [
41]. The most substantial SBS reductions were observed in control groups without mechanical surface treatment, especially when repaired with the Bisco adhesive, corroborating extensive literature demonstrating the vulnerability of unprepared surfaces and inadequate chemical adhesive systems to hydrolytic degradation and resin–substrate debonding.
These findings align with Mokeem et al. (2023), who noted that unprepared surfaces and inadequate primer (or adhesive) treatment are especially vulnerable to hydrolytic degradation, leading to adhesive failures in dental composites [
42]. Similarly, pronounced bond strength losses post-thermocycling were observed in zirconia repairs without mechanical roughening or robust primers. These collective findings highlight the necessity of surface modification and appropriate adhesive monomers to withstand the challenging oral environment [
43].
Importantly, sandblasted surfaces treated with the GC repair kit retained approximately 80% of their initial bond strength after aging, indicating the synergistic benefits of mechanical interlocking combined with chemical durability in maintaining restoration integrity [
44]. Klaisiri et al. (2022) demonstrated that composite repairs employing sandblasting plus 10-MDP-containing primers exhibited significantly enhanced resistance to thermal and mechanical fatigue compared to other protocols [
45,
46].
4.4. Clinical Implications
This resilience is essential clinically, as insufficiently durable repairs can lead to premature restoration failure, increased patient costs, and additional invasive procedures. Accordingly, our results advocate for repair approaches combining airborne particle abrasion with chemically stable universal adhesives to optimize repair longevity and clinical outcomes. The present findings support that airborne particle abrasion combined with the MDP-containing universal adhesive (GC repair kit) shows superior and durable intraoral repair performance for fractured metal and zirconia restorations, offering a conservative and cost-effective alternative to full replacement.
However, as an in vitro study, the findings are limited by the inability to fully replicate the complex oral environment, including masticatory fatigue, chemical degradation from pH variation, and biofilm colonization. The results are also specific to the two materials and repair systems tested.
Future works should incorporate extended aging, mechanical fatigue testing, and randomized clinical trials, as well as newer substrates and adhesives, to validate and broaden these results.