Innovations in Amputee Care in the United States: Access, Ethics, and Equity
Abstract
1. Introduction
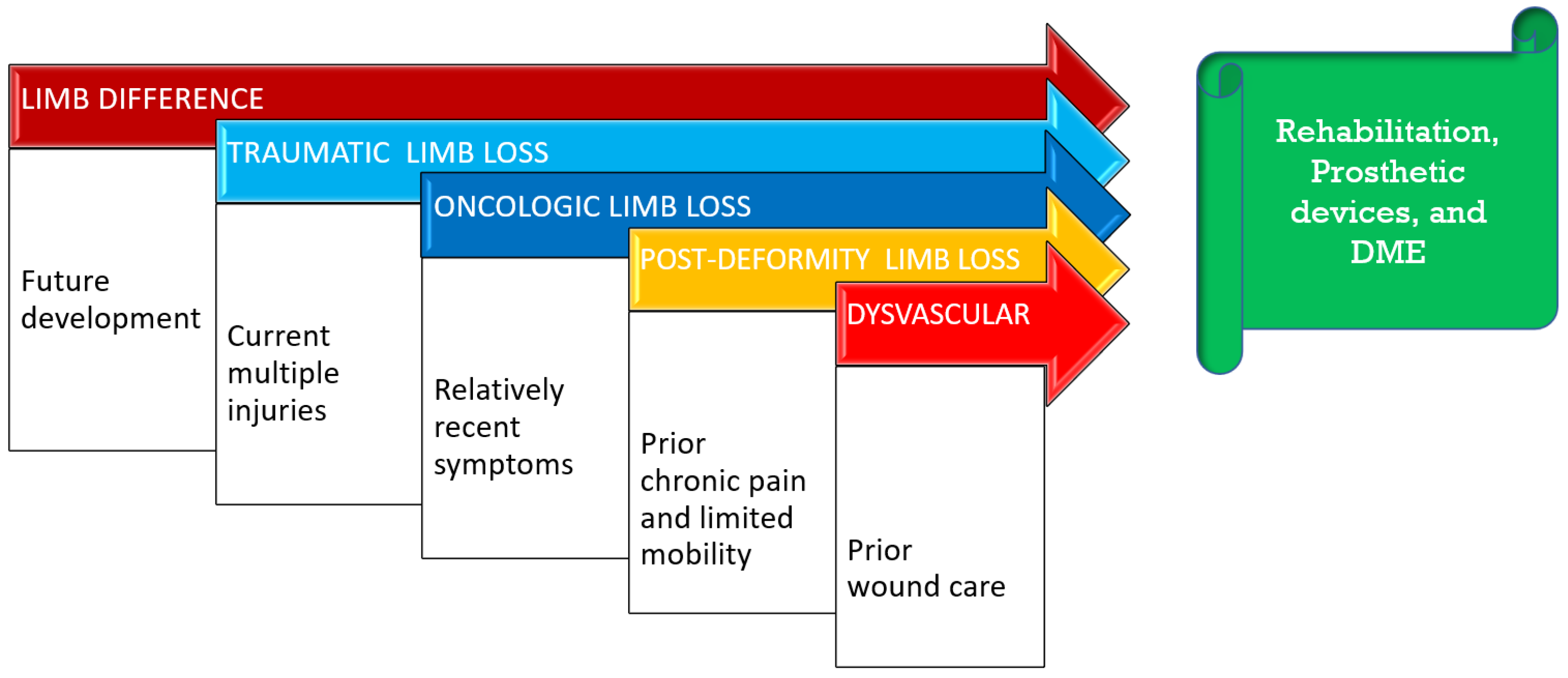
2. Background and Patient Forecast
3. Innovations in Prosthetic Technology
4. Advances in Amputation Surgery
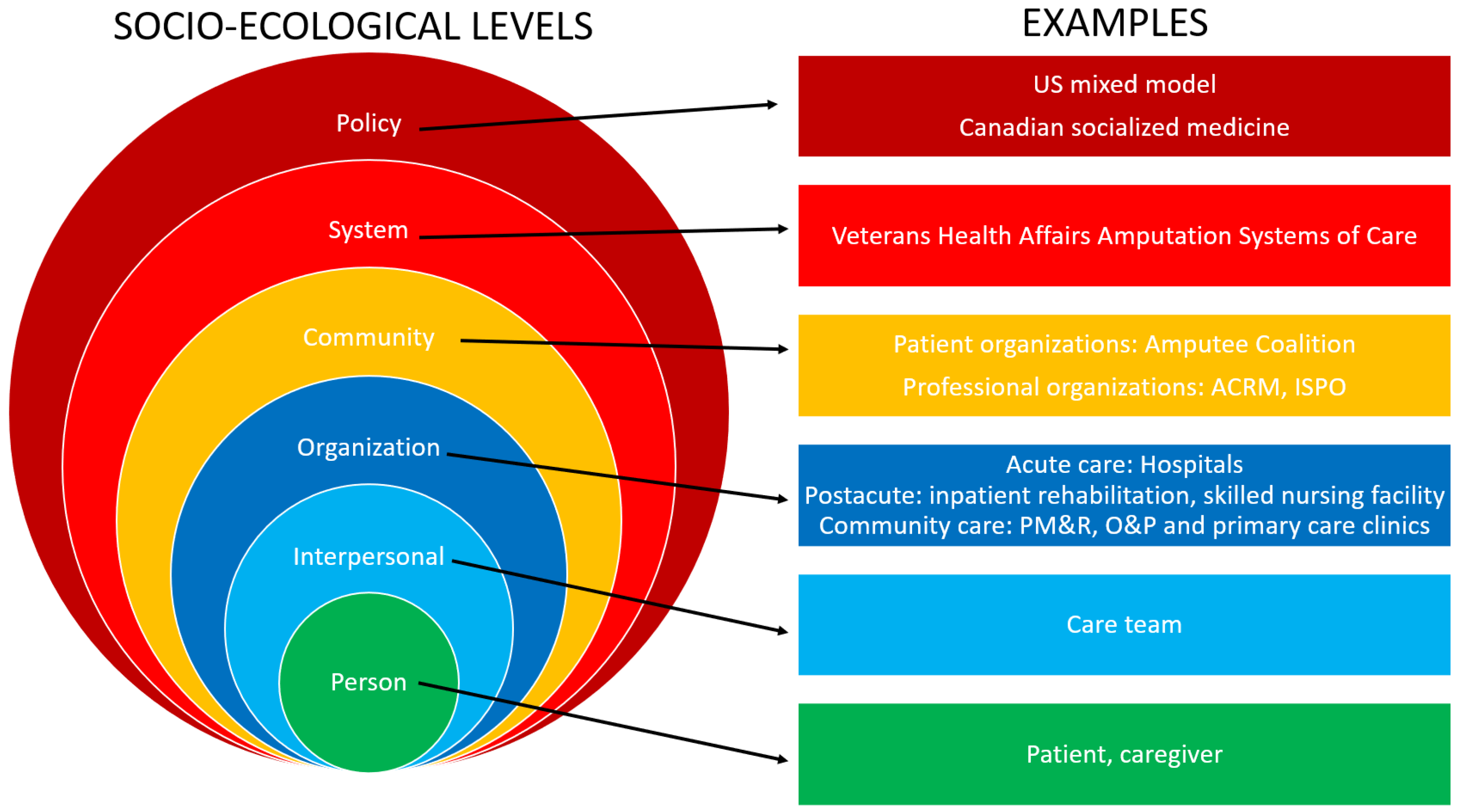
5. Limb Care Systems and Limb Loss Rehabilitation Continuum
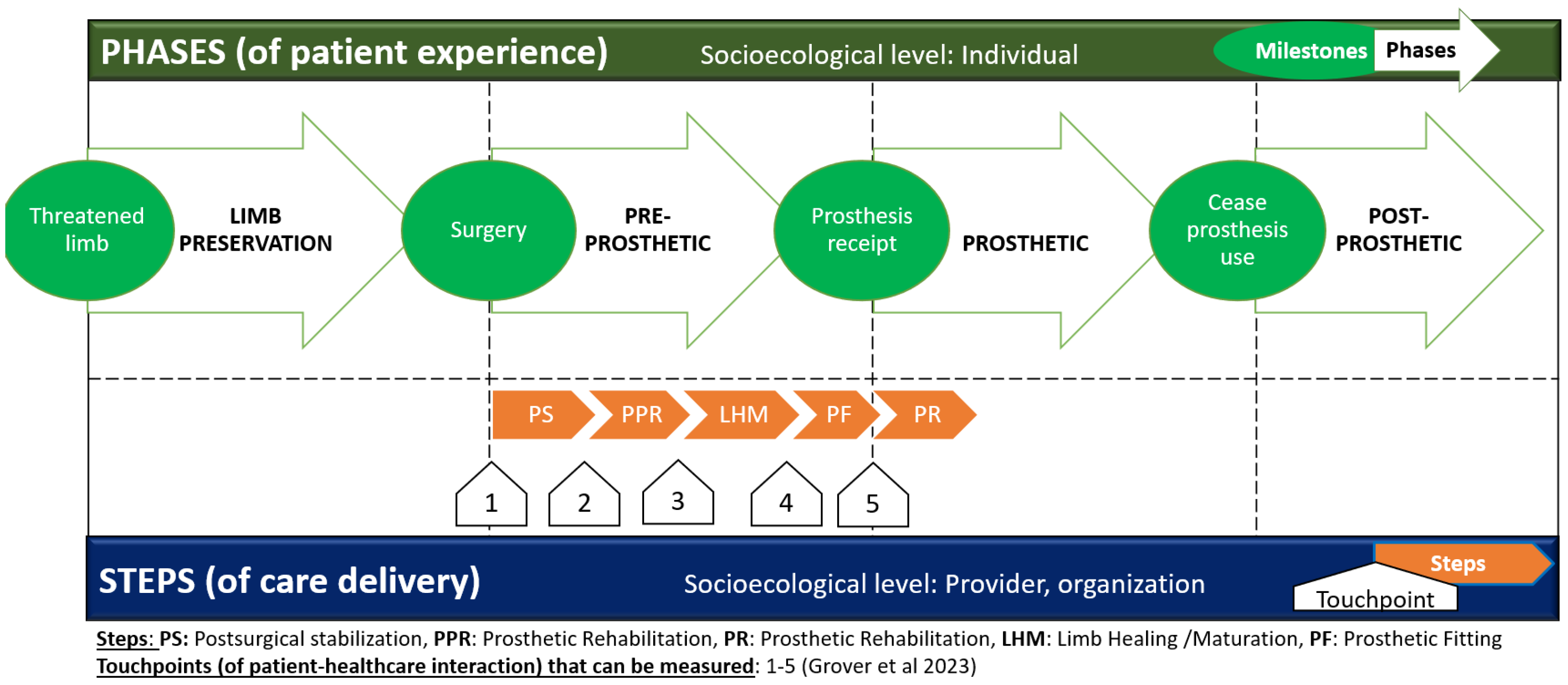
5.1. The Patient Experience: Care Delivery Model of the Patient Journey with Limb Loss
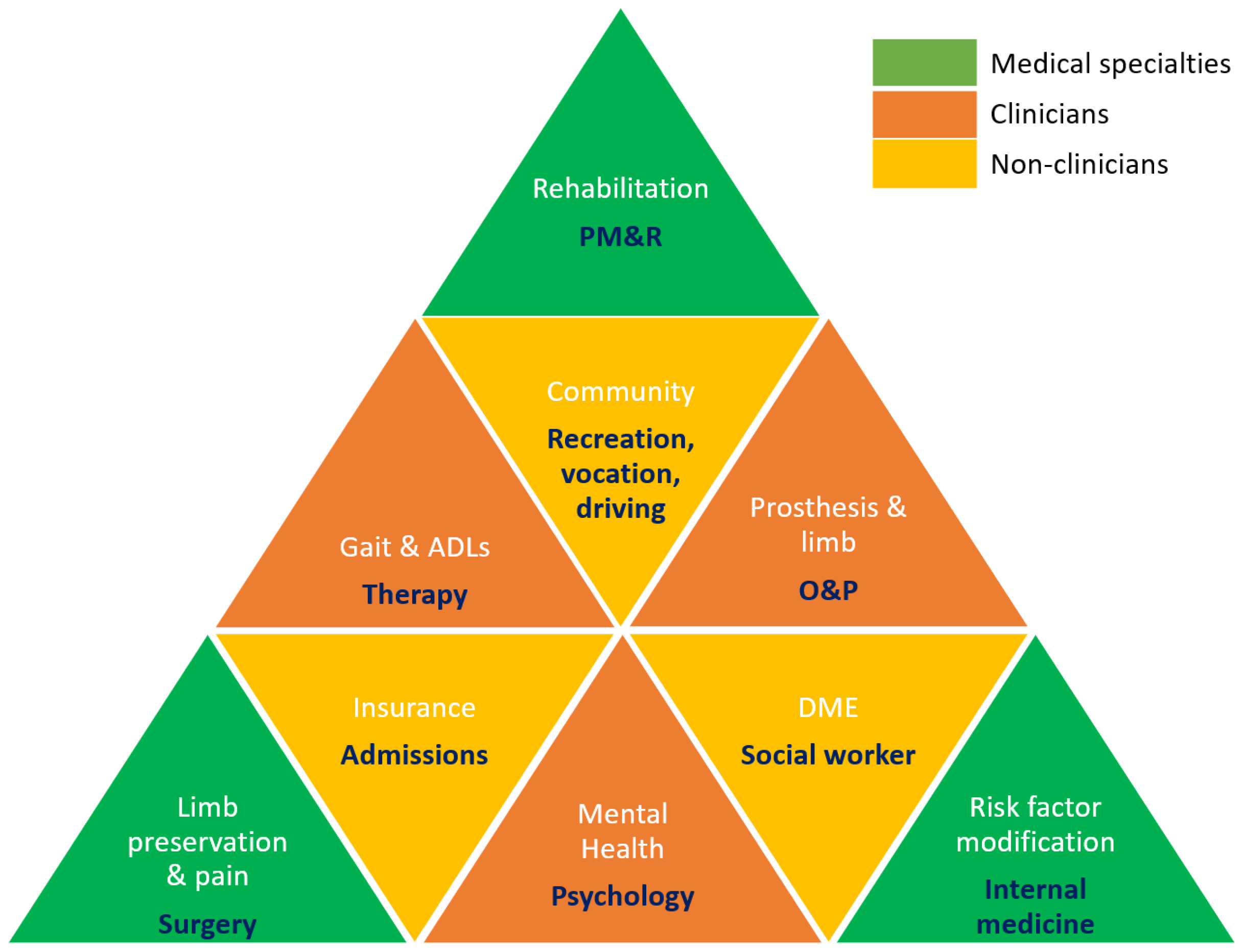
5.2. Stakeholders in Limb Care
5.3. Understanding Contexual Factors for Limb Care Continuum Program Development
5.4. Summarizing Steps to Effective, Efficient, and Equitable System Design for Limb Care Continuum Programs
6. Insurance Coverage and Reimbursement Challenges
7. Ethical Considerations of Advanced Treatment Modalities
7.1. Informed Decision Making and Plan of Care
7.2. Setting Personal, Physical, and Emotional Goals
7.3. Receiving Support, Funding, and Follow-Up Care
7.4. Sharing of Information Between Healthcare Providers
8. Health Equity Considerations
9. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hooper, R.C.; Kelly, B.; Cederna, P.S.; Siegel, G. Amputation Surgery. Phys. Med. Rehabil. Clin. N. Am. 2024, 35, 725–737. [Google Scholar] [CrossRef]
- Mendez, V.; Iberite, F.; Shokur, S.; Micera, S. Current Solutions and Future Trends for Robotic Prosthetic Hands. Annu. Rev. Control. Robot. Auton. Syst. 2021, 4, 595–627. [Google Scholar] [CrossRef]
- Asif, M.; Tiwana, M.I.; Khan, U.S.; Qureshi, W.S.; Iqbal, J.; Rashid, N.; Naseer, N. Advancements, Trends and Future Prospects of Lower Limb Prosthesis. IEEE Access 2021, 9, 85956–85977. [Google Scholar] [CrossRef]
- Donaghy, A.; Miranda, A. Amputation Rehabilitation, an Issue of Physical Medicine and Rehabilitation Clinics of North America; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Ziegler-Graham, K.; MacKenzie, E.J.; Ephraim, P.L.; Travison, T.G.; Brookmeyer, R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch. Phys. Med. Rehabil. 2008, 89, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Caruso, M.; Harrington, S. Prevalence of limb loss and limb difference in the United States: Implications for public policy. In White Paper; Avalere and Amputation Coalition: Manassas, VA, USA, 2024. [Google Scholar]
- McDonald, C.L.; Westcott-McCoy, S.; Weaver, M.R.; Haagsma, J.; Kartin, D. Global prevalence of traumatic non-fatal limb amputation. Prosthet. Orthot. Int. 2021, 45, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Kejlaa, G. The social and economic outcome after upper limb amputation. Prosthet. Orthot. Int. 1992, 16, 25–31. [Google Scholar] [CrossRef]
- Tintle, L.S.M.; Keeling, C.J.J.; Shawen, L.S.B.; Forsberg, L.J.A.; Potter, M.B.K. Traumatic and trauma-related amputations: Part I: General principles and lower-extremity amputations. J. Bone Jt. Surg. 2010, 92, 2852–2868. [Google Scholar] [CrossRef]
- Postema, S.G.; Bongers, R.M.; Brouwers, M.A.; Burger, H.; Norling-Hermansson, L.M.; Reneman, M.F.; Dijkstra, P.U.; Van der Sluis, C.K. Upper limb absence: Predictors of work participation and work productivity. Arch. Phys. Med. Rehabil. 2016, 97, 892–899. [Google Scholar] [CrossRef]
- Pierce, R.O.; Kernek, C.B.; Ambrose, T.A. The plight of the traumatic amputee. Orthopedics 1993, 16, 793–797. [Google Scholar] [CrossRef]
- Dobson, A.; El-Gamil, A.; Shimer, M.; DaVonzo, J.E. Retrospective Cohort Study of the Economic Value of Orthotic and Prosthetic Services Among Medicare Beneficiaries; Dobson DaVanzo & Associates, LLC: Vienna, VA, USA, 2013; pp. 11–114. [Google Scholar]
- Stevens, P.M.; Wurdeman, S.R. Prosthetic Knee Selection for Individuals with Unilateral Transfemoral Amputation: A Clinical Practice Guideline. JPO J. Prosthet. Orthot. 2019, 31, 2–8. [Google Scholar] [CrossRef]
- Wurdeman, S.R.; Miller, T.; Stevens, P.M.; Campbell, J.H. Microprocessor Knee Technology Reduces Odds of Incurring an Injurious Fall for Diabetic/Dysvascular Amputees. JPO J. Prosthet. Orthot. 2020, 32, 64. [Google Scholar]
- Igual, C.; Pardo, L.A.; Hahne, J.M.; Igual, J. Myoelectric Control for Upper Limb Prostheses. Electronics 2019, 8, 1244. [Google Scholar] [CrossRef]
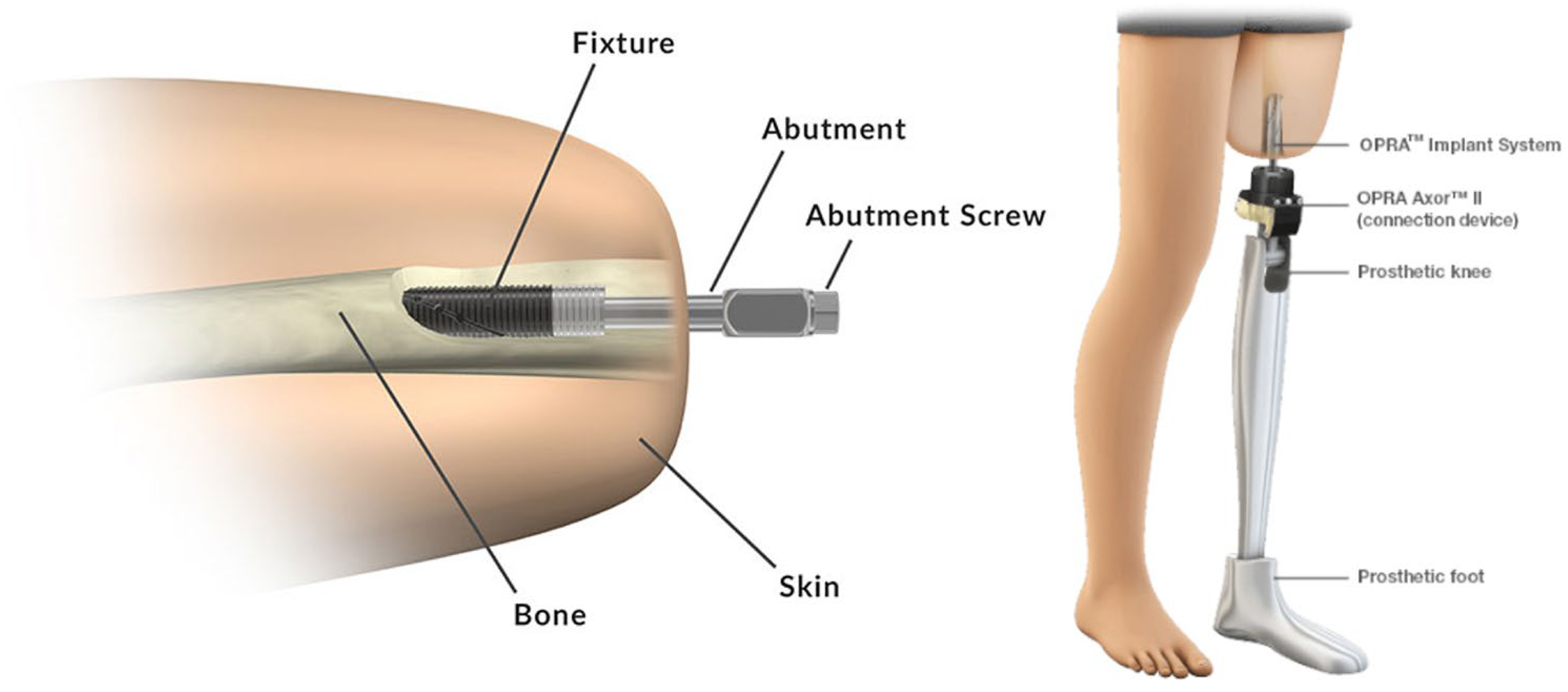
- Zaid, M.B.; O’Donnell, R.J.; Potter, B.K.; Forsberg, J.A. Orthopaedic Osseointegration: State of the Art. J. Am. Acad. Orthop. Surg. 2019, 27, e977–e985. [Google Scholar] [CrossRef]
- Hoellwarth, J.S.; Tetsworth, K.; Rozbruch, S.R.; Handal, M.B.; Coughlan, A.; Al Muderis, M. Osseointegration for Amputees. CJBJS Rev. 2020, 8, e0043. [Google Scholar] [CrossRef]
- Fisher, L.E.; Gaunt, R.A.; Huang, H. Sensory restoration for improved motor control of prostheses. Curr. Opin. Biomed. Eng. 2023, 28, 100498. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.; Stafford, N.; Huang, S.; Hu, X.; Ferris, D.P.; Huang, H. Myoelectric control of robotic lower limb prostheses: A review of electromyography interfaces, control paradigms, challenges and future directions. J. Neural Eng. 2021, 18, 041004. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo-Yánez, A.; Benalcázar, M.E.; Mena-Maldonado, E. Real-Time Hand Gesture Recognition Using Surface Electromyography and Machine Learning: A Systematic Literature Review. Sensors 2020, 20, 2467. [Google Scholar] [CrossRef]
- Simon, A.M.; Turner, K.L.; Miller, L.A.; Potter, B.K.; Beachler, M.D.; Dumanian, G.A.; Hargrove, L.J.; Kuiken, T.A. User Performance With a Transradial Multi-Articulating Hand Prosthesis During Pattern Recognition and Direct Control Home Use. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 271–281. [Google Scholar] [CrossRef]
- Amsüss, S.; Paredes, L.P.; Rudigkeit, N.; Graimann, B.; Herrmann, M.J.; Farina, D. Long term stability of surface EMG pattern classification for prosthetic control. In Proceedings of the 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; Volume 2013, pp. 3622–3625. [Google Scholar]
- Hahne, J.M.; Farina, D.; Jiang, N.; Liebetanz, D. A novel percutaneous electrode implant for improving robustness in advanced myoelectric control. Front. Neurosci. 2016, 10, 114. [Google Scholar] [CrossRef]
- Earley, E.J.; Kristoffersen, M.B.; Ortiz-Catalan, M. Comparing implantable epimysial and intramuscular electrodes for prosthetic control. Front. Neurosci. 2025, 19, 1568212. [Google Scholar] [CrossRef]
- Kristoffersen, M.B.; Munoz-Novoa, M.; Möller, N.; Ortiz-Catalan, M. In MyoCognition, a rehabilitation platform using serious games controlled with myoelectric pattern recognition. In Proceedings of the International Conference for Virtual Rehabilitation, Rotterdam, The Netherlands, 26–28 July 2022; pp. 23–24. [Google Scholar]
- Earley, E.J.; Zbinden, J.; Munoz-Novoa, M.; Mastinu, E.; Smiles, A.; Ortiz-Catalan, M. Competitive motivation increased home use and improved prosthesis self-perception after Cybathlon 2020 for neuromusculoskeletal prosthesis user. J. Neuroeng. Rehabil. 2022, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Resnik, L.; Acluche, F.; Borgia, M.; Latlief, G.; Phillips, S. EMG Pattern Recognition Control of the DEKA Arm: Impact on User Ratings of Satisfaction and Usability. IEEE J. Transl. Eng. Health Med. 2018, 7, 2100113. [Google Scholar] [CrossRef]
- Tarantino, S.; Clemente, F.; Barone, D.; Controzzi, M.; Cipriani, C. The myokinetic control interface: Tracking implanted magnets as a means for prosthetic control. Sci. Rep. 2017, 7, 17149. [Google Scholar] [CrossRef]
- Gherardini, M.; Masiero, F.; Ianniciello, V.; Cipriani, C. The myokinetic interface: Implanting permanent magnets to restore the sensory-motor control loop in amputees. Curr. Opin. Biomed. Eng. 2023, 27, 100460. [Google Scholar] [CrossRef]
- Yin, Z.; Chen, H.; Yang, X.; Liu, Y.; Zhang, N.; Meng, J.; Liu, H. A Wearable Ultrasound Interface for Prosthetic Hand Control. IEEE J. Biomed. Health Inform. 2022, 26, 5384–5393. [Google Scholar] [CrossRef]
- Mendez, J.; Murray, R.; Gabert, L.; Fey, N.P.; Liu, H.; Lenzi, T. A-Mode Ultrasound-Based Prediction of Transfemoral Amputee Prosthesis Walking Kinematics via an Artificial Neural Network. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 1511–1520. [Google Scholar] [CrossRef]
- Raspopovic, S.; Valle, G.; Petrini, F.M. Sensory feedback for limb prostheses in amputees. Nat. Mater. 2021, 20, 925–939. [Google Scholar] [CrossRef]
- Jabban, L.; Dupan, S.; Zhang, D.; Ainsworth, B.; Nazarpour, K.; Metcalfe, B.W. Sensory Feedback for Upper-Limb Prostheses: Opportunities and Barriers. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 738–747. [Google Scholar] [CrossRef]
- Wolpert, D.M.; Ghahramani, Z.; Flanagan, J.R. Perspectives and problems in motor learning. Trends Cogn. Sci. 2001, 5, 487–494. [Google Scholar] [CrossRef]
- Rusaw, D.; Hagberg, K.; Nolan, L.; Ramstrand, N. Can vibratory feedback be used to improve postural stability in persons with transtibial limb loss? J. Rehabil. Res. Dev. 2012, 49, 1239–1254. [Google Scholar] [CrossRef]
- Crea, S.; Edin, B.B.; Knaepen, K.; Meeusen, R.; Vitiello, N. Time-Discrete Vibrotactile Feedback Contributes to Improved Gait Symmetry in Patients with Lower Limb Amputations: Case Series. Phys. Ther. 2017, 97, 198–207. [Google Scholar] [CrossRef]
- De Nunzio, A.M.; Dosen, S.; Lemling, S.; Markovic, M.; Schweisfurth, M.A.; Ge, N.; Graimann, B.; Falla, D.; Farina, D. Tactile feedback is an effective instrument for the training of grasping with a prosthesis at low- and medium-force levels. Exp. Brain Res. 2017, 235, 2547–2559. [Google Scholar] [CrossRef] [PubMed]
- Markovic, M.; Schweisfurth, M.A.; Engels, L.F.; Farina, D.; Dosen, S. Myocontrol is closed-loop control: Incidental feedback is sufficient for scaling the prosthesis force in routine grasping. J. Neuroeng. Rehabil. 2018, 15, 81. [Google Scholar] [CrossRef] [PubMed]
- Witteveen, H.J.; Rietman, H.S.; Veltink, P.H. Vibrotactile grasping force and hand aperture feedback for myoelectric forearm prosthesis users. Prosthet. Orthot. Int. 2015, 39, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Earley, E.J.; Johnson, R.E.; Sensinger, J.W.; Hargrove, L.J. Wrist speed feedback improves elbow compensation and reaching accuracy for myoelectric transradial prosthesis users in hybrid virtual reaching task. J. Neuroeng. Rehabil. 2023, 20, 9. [Google Scholar] [CrossRef]
- Clemente, F.; D’Alonzo, M.; Controzzi, M.; Edin, B.B.; Cipriani, C. Non-Invasive, Temporally Discrete Feedback of Object Contact and Release Improves Grasp Control of Closed-Loop Myoelectric Transradial Prostheses. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 1314–1322. [Google Scholar] [CrossRef]
- Smiles, A.; Earley, E.J.; Jiang, N.; Ortiz-Catalan, M. Sensory Feedback of Grasp Security by Direct Neural Stimulation Improves Amputee Prediction of Object Slip. Prosthesis 2024, 7, 3. [Google Scholar] [CrossRef]
- Nag, S.; Thakor, N.V. Implantable neurotechnologies: Electrical stimulation and applications. Med. Biol. Eng. Comput. 2016, 54, 63–76. [Google Scholar] [CrossRef]
- Valle, G.; Mazzoni, A.; Iberite, F.; D’Anna, E.; Strauss, I.; Granata, G.; Controzzi, M.; Clemente, F.; Rognini, G.; Cipriani, C.; et al. Biomimetic Intraneural Sensory Feedback Enhances Sensation Naturalness, Tactile Sensitivity, and Manual Dexterity in a Bidirectional Prosthesis. Neuron 2018, 100, 37–45.e37. [Google Scholar] [CrossRef]
- George, J.A.; Kluger, D.T.; Davis, T.S.; Wendelken, S.M.; Okorokova, E.V.; He, Q.; Duncan, C.C.; Hutchinson, D.T.; Thumser, Z.C.; Beckler, D.T.; et al. Biomimetic sensory feedback through peripheral nerve stimulation improves dexterous use of a bionic hand. Sci. Robot. 2019, 4, eaax2352. [Google Scholar] [CrossRef]
- Graczyk, E.L.; Christie, B.P.; He, Q.; Tyler, D.J.; Bensmaia, S.J. Frequency Shapes the Quality of Tactile Percepts Evoked through Electrical Stimulation of the Nerves. J. Neurosci. 2022, 42, 2052–2064. [Google Scholar] [CrossRef]
- Collu, R.; Earley, E.J.; Barbaro, M.; Ortiz-Catalan, M. Non-rectangular neurostimulation waveforms elicit varied sensation quality and perceptive fields on the hand. Sci. Rep. 2023, 13, 1588. [Google Scholar] [CrossRef] [PubMed]
- Petrini, F.M.; Bumbasirevic, M.; Valle, G.; Ilic, V.; Mijović, P.; Čvančara, P.; Barberi, F.; Katic, N.; Bortolotti, D.; Andreu, D.; et al. Sensory feedback restoration in leg amputees improves walking speed, metabolic cost and phantom pain. Nat. Med. 2019, 25, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Christie, B.P.; Freeberg, M.; Memberg, W.D.; Pinault, G.J.C.; Hoyen, H.A.; Tyler, D.J.; Triolo, R.J. Long-term stability of stimulating spiral nerve cuff electrodes on human peripheral nerves. J. Neuroeng. Rehabil. 2017, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Roche, A.D.; Bailey, Z.K.; Gonzalez, M.; Vu, P.P.; Chestek, C.A.; Gates, D.H.; Kemp, S.W.P.; Cederna, P.S.; Ortiz-Catalan, M.; Aszmann, O.C. Upper limb prostheses: Bridging the sensory gap. J. Hand Surg. 2023, 48, 182–190. [Google Scholar] [CrossRef]
- Wendelken, S.; Page, D.M.; Davis, T.; Wark, H.A.; Kluger, D.T.; Duncan, C.; Warren, D.J.; Hutchinson, D.T.; Clark, G.A. Restoration of motor control and proprioceptive and cutaneous sensation in humans with prior upper-limb amputation via multiple Utah Slanted Electrode Arrays (USEAs) implanted in residual peripheral arm nerves. J. Neuroeng. Rehabil. 2017, 14, 121. [Google Scholar] [CrossRef]
- Sponheim, C.; Papadourakis, V.; Collinger, J.L.; Downey, J.; Weiss, J.; Pentousi, L.; Elliott, K.; Hatsopoulos, N.G. Longevity and reliability of chronic unit recordings using the Utah, intracortical multi-electrode arrays. J. Neural Eng. 2021, 18, 066044. [Google Scholar] [CrossRef]
- Günter, C.; Delbeke, J.; Ortiz-Catalan, M. Safety of long-term electrical peripheral nerve stimulation: Review of the state of the art. J. Neuroeng. Rehabil. 2019, 16, 13. [Google Scholar] [CrossRef]
- Segil, J.L.; Roldan, L.M.; Graczyk, E.L. Measuring embodiment: A review of methods for prosthetic devices. Front. Neurorobotics 2022, 16, 902162. [Google Scholar] [CrossRef]
- Bekrater-Bodmann, R. Factors Associated with Prosthesis Embodiment and Its Importance for Prosthetic Satisfaction in Lower Limb Amputees. Front. Neurorobotics 2021, 14, 604376. [Google Scholar] [CrossRef]
- Maimon-Mor, R.O.; Makin, T.R. Is an artificial limb embodied as a hand? Brain decoding in prosthetic limb users. PLoS Biol. 2020, 18, e3000729. [Google Scholar] [CrossRef] [PubMed]
- Engdahl, S.M.; Meehan, S.K.; Gates, D.H. Differential experiences of embodiment between body-powered and myoelectric prosthesis users. Sci. Rep. 2020, 10, 15471. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Belsi, A.; McGregor, A.H. Issues Faced by Prosthetists and Physiotherapists During Lower-Limb Prosthetic Rehabilitation: A Thematic Analysis. Front. Rehabil. Sci. 2022, 2, 795021. [Google Scholar] [CrossRef] [PubMed]
- Earley, E.J.; Zbinden, J.; Munoz-Novoa, M.; Just, F.; Vasan, C.; Holtz, A.S.; Emadeldin, M.; Kolankowska, J.; Davidsson, B.; Thesleff, A.; et al. Cutting Edge Bionics in Highly Impaired Individuals: A Case of Challenges and Opportunities. IEEE Trans. Neural Syst. Rehabil. Eng. 2024, 32, 1013–1022. [Google Scholar] [CrossRef]
- Brånemark, R.; Brånemark, P.I.; Rydevik, B.; Myers, R.R. Osseointegration in skeletal reconstruction and rehabilitation: A review. J. Rehabil. Res. Dev. 2001, 38, 175–181. [Google Scholar]
- Carvalho, A. Osseointegration may improve function for people with poor outcomes in a traditional transfemoral socket. Am. Acad. Orthotists Prosthetists Crit. Apprais. Top. 2020. [Google Scholar]
- Awad, M.E.; Lev, G.; Melton, D.H.; Shaw, K.G.; Thomsen-Freitas, P.B.; Gaffney, B.M.M.; Christiansen, C.L.; Stoneback, J.W. Colorado Limb Donning-Timed Up and Go (COLD-TUG) Test in Lower-Extremity Amputation. J. Bone Jt. Surg. 2025, 107, 1611–1619. [Google Scholar] [CrossRef]
- Davis-Wilson, H.C.; Christiansen, C.L.; Gaffney, B.M.M.; Lev, G.; Enabulele, E.; Stoneback, J.W. Improvements in disability and function in people with lower-limb amputation one year after prosthesis osseointegration. Prosthet. Orthot. Int. 2023, 47, 343–349. [Google Scholar] [CrossRef]
- Diaz Balzani, L.; Ciuffreda, M.; Vadalà, G.; Di Pino, G.; Papalia, R.; Denaro, V. Osseointegration for lower and upper-limb amputation a systematic review of clinical outcomes and complications. J. Biol. Regul. Homeost. Agents 2020, 34, 315–326. [Google Scholar]
- Earley, E.J.; Milius, D.W.; Awad, M.E.; Melton, D.H.; Ahmed, K.; Leijendekkers, R.A.; Potter, B.K.; Stevens, P.M.; Gaffney, B.M.M.; Christiansen, C.L.; et al. Establishing Consensus for Prescription of Prosthetic Components for Transfemoral Bone-Anchored Limbs: An International Delphi Method Study. Arch. Phys. Med. Rehabil. 2025, 106, 1565–1574. [Google Scholar] [CrossRef]
- Kuiken, T.A.; Dumanian, G.A.; Lipschutz, R.D.; Miller, L.A.; Stubblefield, K.A. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthet. Orthot. Int. 2004, 28, 245–253. [Google Scholar] [CrossRef]
- Chappell, A.G.; Ramsey, M.D.; Park, S.; Dumanian, G.A.; Ko, J.H. Targeted Muscle Reinnervation—An Up-to-Date Review: Evidence, Indications, and Technique. Arch. Plast. Surg. 2025, 52, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Roche, A.D.; Rehbaum, H.; Farina, D.; Aszmann, O.C. Prosthetic Myoelectric Control Strategies: A Clinical Perspective. Curr. Surg. Rep. 2014, 2, 44. [Google Scholar] [CrossRef]
- Kuiken, T.A. Targeted Muscle Reinnervation for Real-time Myoelectric Control of Multifunction Artificial Arms. JAMA 2009, 301, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Spanias, J.A.; Simon, A.M.; Ingraham, K.A.; Hargrove, L.J. Effect of additional mechanical sensor data on an EMG-based pattern recognition system for a powered leg prosthesis. In Proceedings of the 2015 7th International IEEE/EMBS Conference on Neural Engineering (NER), Montpellier, France, 22–24 April 2015. [Google Scholar]
- Dumanian, G.A.; Potter, B.K.; Mioton, L.M.; Ko, J.H.; Cheesborough, J.E.; Souza, J.M.; Ertl, W.J.; Tintle, S.M.; Nanos, G.P.; Valerio, I.L.; et al. Targeted Muscle Reinnervation Treats Neuroma and Phantom Pain in Major Limb Amputees. Ann. Surg. 2019, 270, 238–246. [Google Scholar] [CrossRef]
- Elabd, R.; Dow, T.; Jabori, S.; Alhalabi, B.; Lin, S.J.; Dowlatshahi, S. Pain and Functional Outcomes following Targeted Muscle Reinnervation: A Systematic Review. Plast. Reconstr. Surg. 2024, 153, 494–508. [Google Scholar] [CrossRef]
- Kuiken, T.A.; Marasco, P.D.; Lock, B.A.; Harden, R.N.; Dewald, J.P.A. Redirection of cutaneous sensation from the hand to the chest skin of human amputees with targeted reinnervation. Proc. Natl. Acad. Sci. USA 2007, 104, 20061–20066. [Google Scholar] [CrossRef]
- Clites, T.R.; Carty, M.J.; Ullauri, J.B.; Carney, M.E.; Mooney, L.M.; Duval, J.-F.; Srinivasan, S.S.; Herr, H.M. Proprioception from a neurally controlled lower-extremity prosthesis. Sci. Transl. Med. 2018, 10, eaap8373. [Google Scholar] [CrossRef]
- Kung, T.A.; Langhals, N.B.; Martin, D.C.; Johnson, P.J.; Cederna, P.S.; Urbanchek, M.G. Regenerative peripheral nerve interface viability and signal transduction with an implanted electrode. Plast. Reconstr. Surg. 2014, 133, 1380–1394. [Google Scholar] [CrossRef]
- Vu, P.P.; Vaskov, A.K.; Lee, C.; Jillala, R.R.; Wallace, D.M.; Davis, A.J.; Kung, T.A.; Kemp, S.W.P.; Gates, D.H.; Chestek, C.A.; et al. Long-term upper-extremity prosthetic control using regenerative peripheral nerve interfaces and implanted EMG electrodes. J. Neural Eng. 2023, 20, 026039. [Google Scholar] [CrossRef]
- Zbinden, J.; Sassu, P.; Mastinu, E.; Earley, E.J.; Munoz-Novoa, M.; Brånemark, R.; Ortiz-Catalan, M. Improved control of a prosthetic limb by surgically creating electro-neuromuscular constructs with implanted electrodes. Sci. Transl. Med. 2023, 15, eabq3665. [Google Scholar] [CrossRef]
- Gardetto, A.; Baur, E.-M.; Prahm, C.; Smekal, V.; Jeschke, J.; Peternell, G.; Pedrini, M.T.; Kolbenschlag, J. Reduction of Phantom Limb Pain and Improved Proprioception through a TSR-Based Surgical Technique: A Case Series of Four Patients with Lower Limb Amputation. J. Clin. Med. 2021, 10, 4029. [Google Scholar] [CrossRef]
- Sullivan, C.; Shu, T.; Berger, L.; Chiao, R.; Clites, K.; Ferrone, M.; O’Donnell, R.; Brånemark, R.; Herr, H.; Carty, M. Transfemoral Amputation Incorporating eOPRA and Agonist-Antagonist Myoneural Interface (AMI) Design: Surgical Technique and Perioperative Care. Plast. Reconstr. Surg. Glob. Open 2023, 11, 133–134. [Google Scholar] [CrossRef]
- Schober, T.-L.; Abrahamsen, C. Patient perspectives on major lower limb amputation—A qualitative systematic review. Int. J. Orthop. Trauma Nurs. 2022, 46, 100958. [Google Scholar] [CrossRef] [PubMed]
- Keeves, J.; Hutchison, A.; D’Cruz, K.; Anderson, S. Social and community participation following traumatic lower limb amputation: An exploratory qualitative study. Disabil. Rehabil. 2023, 45, 4404–4412. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, A.; D’Cruz, K.; Ross, P.; Anderson, S. Exploring the barriers and facilitators to community reintegration for adults following traumatic upper limb amputation: A mixed methods systematic review. Disabil. Rehabil. 2024, 46, 1471–1484. [Google Scholar] [CrossRef]
- Khetarpaul, V.; Kirby, J.P.; Geraghty, P.; Felder, J.; Grover, P. Socioecological model-based design and implementation principles of lower limb preservation programs as partners for limb-loss rehabilitation programs—A mini-review. Front. Rehabil. Sci. 2022, 3, 983432. [Google Scholar] [CrossRef] [PubMed]
- Sansam, K.; O’Connor, R.J.; Neumann, V.; Bhakta, B. Clinicians’ perspectives on decision making in lower limb amputee rehabilitation. J. Rehabil. Med. 2014, 46, 447–453. [Google Scholar] [CrossRef]
- Spyrou, J.M.; Minns Lowe, C. An exploration of specialist clinicians’ experiences and beliefs about inpatient amputee rehabilitation as a pathway option for adult primary amputees. Disabil. Rehabil. 2022, 44, 6710–6721. [Google Scholar] [CrossRef]
- Allen, A.P.; Bolton, W.S.; Jalloh, M.B.; Halpin, S.J.; Jayne, D.G.; Scott, J.D. Barriers to accessing and providing rehabilitation after a lower limb amputation in Sierra Leone–a multidisciplinary patient and service provider perspective. Disabil. Rehabil. 2022, 44, 2392–2399. [Google Scholar] [CrossRef]
- Grover, P.; Karuppan, C.M. The lower limb-loss rehabilitation continuum (LLRC)—A framework for program design and implementation. Disabil. Rehabil. 2024, 46, 1652–1661. [Google Scholar] [CrossRef] [PubMed]
- Kassavin, D.; Mota, L.; Ostertag-Hill, C.A.; Kassavin, M.; Himmelstein, D.U.; Woolhandler, S.; Wang, S.X.; Liang, P.; Schermerhorn, M.L.; Vithiananthan, S.; et al. Amputation Rates and Associated Social Determinants of Health in the Most Populous US Counties. JAMA Surg. 2024, 159, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Minc, S.D.; Goodney, P.P.; Misra, R.; Thibault, D.; Smith, G.S.; Marone, L. The effect of rurality on the risk of primary amputation is amplified by race. J. Vasc. Surg. 2020, 72, 1011–1017. [Google Scholar] [CrossRef]
- Hughes, K.; Mota, L.; Nunez, M.; Sehgal, N.; Ortega, G. The effect of income and insurance on the likelihood of major leg amputation. J. Vasc. Surg. 2019, 70, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Limb Loss: Rehabilitation Services and Outcomes for Medicare Beneficiaries; U.S. Government Accountability Office: Washington, DC, USA, 2024.
- Resnik, L.J.; Borgia, M.; Clark, M.A.; Ni, P. Out-of-pocket costs and affordability of upper limb prostheses. Prosthet. Orthot. Int. 2024, 48, 108–114. [Google Scholar] [CrossRef]
- Roberts, D.J.; Murphy, C.; Strauss, S.A.; Brandys, T.; Corrales-Medina, V.; Zhang, J.; Lalonde, K.A.; Meulenkamp, B.; Jennings, A.; Forster, A.J. Structure, processes, and initial outcomes of The Ottawa Hospital Multi-Specialist Limb-Preservation Clinic and Programme: A unique-in-Canada quality improvement initiative. Int. Wound J. 2022, 19, 326–338. [Google Scholar] [CrossRef]
- Kannenberg, A.; Seidinger, S. Health Economics in the Field of Prosthetics and Orthotics: A Global Perspective. Can. Prosthet. Orthot. J. 2021, 4. [Google Scholar] [CrossRef]
- Gruber, J. Financing Health Care Delivery. Annu. Rev. Financ. Econ. 2022, 14, 209–229. [Google Scholar] [CrossRef]
- 2025 Mid-Year Health Insurance Industries Analysis Report; National Association of Insurance Commissioners: Washington, DC, USA, 2025.
- Kehoe, S.; Cain, J.; Montgomery, A.; Mitsou, L. A Multi-State Analysis of the Fiscal and Social Impact of Commercial Insurance Coverage for Recreational Prostheses in the United States. Med. Res. Arch. 2023, 11. [Google Scholar] [CrossRef]
- Racine, J. Orthopedic medical devices: Ethical questions, implant recalls and responsibility. Rhode Isl. Med. J. 2013, 96, 16. [Google Scholar]
- Silver, J.K.; Flores, L.E.; Mondriguez González, A.; Frontera, W.R. An Analysis of the Inclusion of Women, Older Individuals, and Racial/Ethnic Minorities in Rehabilitation Clinical Trials. Am. J. Phys. Med. Rehabil. 2021, 100, 546–554. [Google Scholar] [CrossRef]
- Amputee Coalition of America. Amputee Bill of Rights. Available online: https://tarrant.tx.nocbeta.org/kids/library/article.aspx?id=2265 (accessed on 15 July 2025).
- World Medical Association. Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Participants. JAMA 2025, 333, 71. [Google Scholar] [CrossRef]
- Mercuri, J.J.; Bosco, J.A.; Iorio, R.; Schwarzkopf, R. The Ethics of Patient Cost-Sharing for Total Joint Arthroplasty Implants. J. Bone Jt. Surg. 2016, 98, e111. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, J.; Demiris, G.; Shevin, M.; Thadaney-Israni, S.; Carney, T.J.; Cupito, A. Health technology for all: An equity-based paradigm shift opportunity. NAM Perspect. 2022, 2022, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Health Inequities and Their Causes; World Health Organization: Geneva, Switzerland, 2018.
- Baciu, A.; Negussie, Y.; Geller, A.; Weinstein, J.N.; National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on Community-Based Solutions to Promote Health Equity in the United States. The root causes of health inequity. In Communities in Action: Pathways to Health Equity; National Academies Press: Washington, DC, USA, 2017. [Google Scholar]
- Li, Y.; Burrows, N.R.; Gregg, E.W.; Albright, A.; Geiss, L.S. Declining rates of hospitalization for nontraumatic lower-extremity amputation in the diabetic population aged 40 years or older: US, 1988–2008. Diabetes Care 2012, 35, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.S.; Goodman, D.C.; Chandra, A. Disparities in Health and Health Care Among Medicare Beneficiaries: A Brief Report of the Dartmouth Atlas Project; Dartmouth Institute for Health Policy and Clinical Practice: Lebanon, NH, USA, 2022. [Google Scholar]
- Bancks, M.P.; Kershaw, K.; Carson, A.P.; Gordon-Larsen, P.; Schreiner, P.J.; Carnethon, M.R. Association of modifiable risk factors in young adulthood with racial disparity in incident type 2 diabetes during middle adulthood. JAMA 2017, 318, 2457–2465. [Google Scholar] [CrossRef]
- Waldera, K.E.; Heckathorne, C.W.; Parker, M.; Fatone, S. Assessing the prosthetic needs of farmers and ranchers with amputations. Disabil. Rehabil. Assist. Technol. 2013, 8, 204–212. [Google Scholar] [CrossRef]
- Ikeda, A.J.; Grabowski, A.M.; Lindsley, A.; Sadeghi-Demneh, E.; Reisinger, K.D. A scoping literature review of the provision of orthoses and prostheses in resource-limited environments 2000–2010. Part one. Prosthet. Orthot. Int. 2014, 38, 269–286. [Google Scholar] [CrossRef]
- SwedeAmp Steering Committee. Annual Report 2024: SwedeAmp Amputation- and Prosthetics Registry for the Lower Extremity; Quality Registry Center South: Gothenburg, Sweden, 2024. [Google Scholar]
- Alimusaj, M.; Michel, K.; Block, J.; Daub, U.; Heitzmann, D.; Nguyen, T.-D.; Bisele, M.; Wolf, S.I.; Schneider, U. Update Amputationsregister Deutschland (AMP-Register). Die Unfallchirurgie 2025, 128, 240–247. [Google Scholar] [CrossRef]
- NIMHD Research Framework. Available online: https://www.nimhd.nih.gov/resources/nimhd-research-framework (accessed on 15 July 2025).

| You Have the Right to: |
| Receive clear, complete information about your surgery, medical care and therapy. |
| Take part in decisions affecting your health and well-being. |
| Be involved in developing your plan of care. |
| Set goals for what you want to achieve. |
| Set goals for your physical and emotional well-being. |
| Set goals for preventing other health conditions that may result from your amputation. |
| Receive support from a certified peer visitor. |
| Be informed about funding for healthcare. |
| Be informed about returning to work and opportunities for recreation. |
| Be informed about prosthetic services, healthcare products and new technology. |
| Select qualified healthcare providers. |
| Ask for help when you are unhappy with healthcare products or the care you receive. |
| You Have the Responsibility to: |
| Stay informed about healthcare products and services. |
| Learn about products and services that are appropriate, safe and effective for you. |
| Express concerns about quality of care, billing practices, and products or services. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cain, J.; Earley, E.J.; Potter, B.K.; Grover, P.; Thomas, P.; Stark, G.; White, A. Innovations in Amputee Care in the United States: Access, Ethics, and Equity. Prosthesis 2025, 7, 153. https://doi.org/10.3390/prosthesis7060153
Cain J, Earley EJ, Potter BK, Grover P, Thomas P, Stark G, White A. Innovations in Amputee Care in the United States: Access, Ethics, and Equity. Prosthesis. 2025; 7(6):153. https://doi.org/10.3390/prosthesis7060153
Chicago/Turabian StyleCain, Jeffrey, Eric J. Earley, Benjamin K. Potter, Prateek Grover, Peter Thomas, Gerald Stark, and Ashlie White. 2025. "Innovations in Amputee Care in the United States: Access, Ethics, and Equity" Prosthesis 7, no. 6: 153. https://doi.org/10.3390/prosthesis7060153
APA StyleCain, J., Earley, E. J., Potter, B. K., Grover, P., Thomas, P., Stark, G., & White, A. (2025). Innovations in Amputee Care in the United States: Access, Ethics, and Equity. Prosthesis, 7(6), 153. https://doi.org/10.3390/prosthesis7060153







