Comparative Analysis of the Physicochemical Properties of 3D-Printed and Conventional Resins for Temporary Dental Restorations
Abstract
1. Introduction
2. Materials and Methods
2.1. Roughness
2.2. Contact Angle Determination
2.3. Determination of Flexural Strength
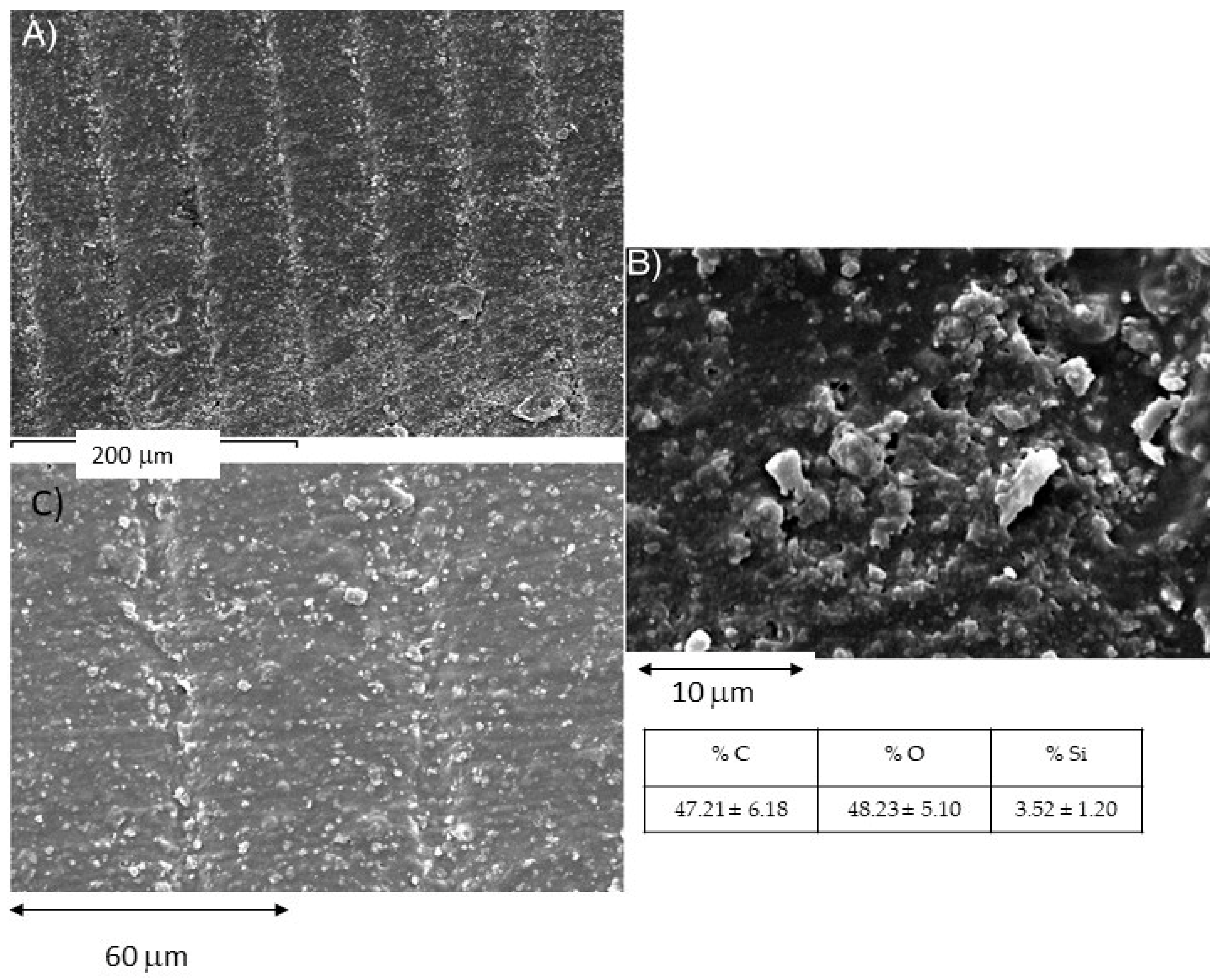
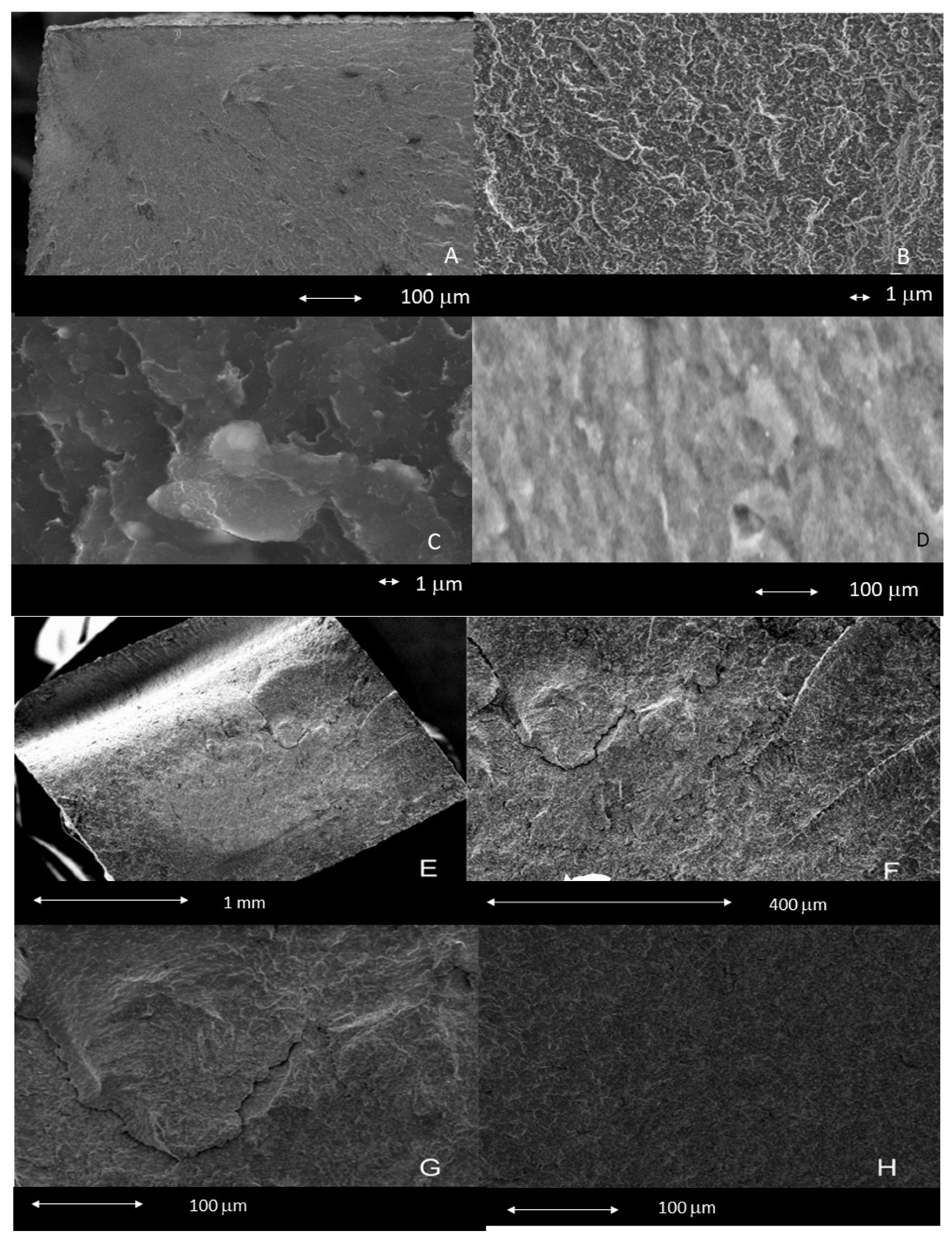
2.4. Fracture Surface Analysis Using Scanning Electron Microscopy
2.5. Density Determination
2.6. Microhardness Test
2.7. Water Absorption
2.8. Wear Resistance Study
2.9. Statistical Analysis
3. Results
3.1. Roughness and Wettability
3.2. Flexural Strength
3.3. Surface Study
3.4. Density
3.5. Water Absorption (SW)
3.6. Microhardness Determination
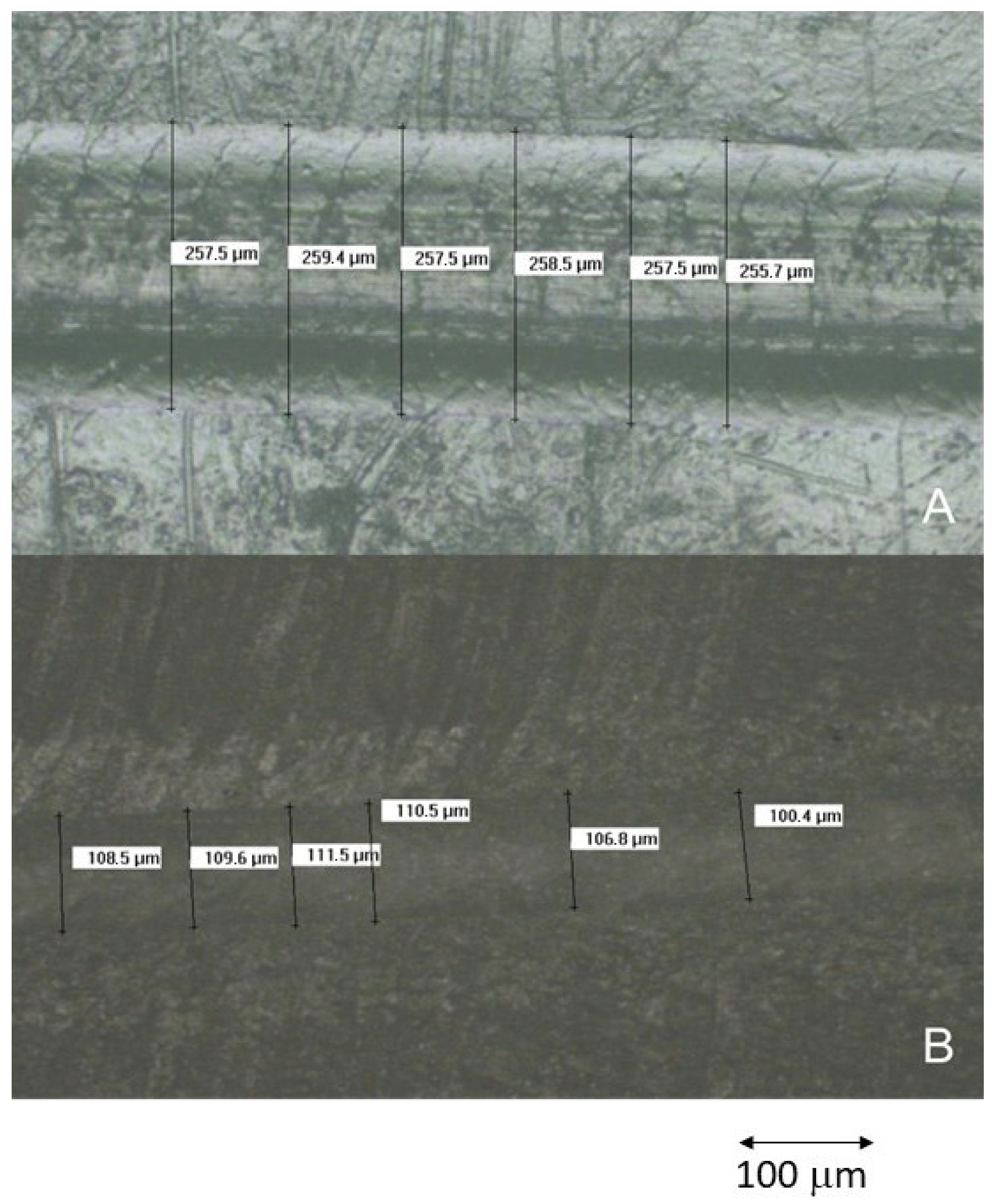
3.7. Wear Resistance Study
3.8. Scratch Resistance Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brånemark, P.I.; Adell, R.; Breine, U.; Hansson, B.O.; Lindström, J.; Ohlsson, A. Intra-osseous anchorage of dental prostheses: I. Experimental studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef]
- Luthardt, R.G.; Stössel, M.; Hinz, M.; Vollandt, R. Clinical performance and periodontal outcome of temporary crowns and fixed partial dentures: A randomized clinical trial. J. Prosthet. Dent. 2000, 83, 32–39. [Google Scholar] [CrossRef]
- Vahidi, F. The Provisional Restoration. Dent. Clin. N. Am. 1987, 31, 363–381. [Google Scholar] [CrossRef]
- Lowe, R.A. The art and science of provisionalization. Int. J. Period Rest. Dent. 1987, 7, 64–73. [Google Scholar]
- Baldissara, P.; Comin, G.; Martone, F.; Scotti, R. Comparative study of the marginal microleakage of six cements in fixed provisional crowns. J. Prosthet. Dent. 1998, 80, 417–422. [Google Scholar] [CrossRef]
- Zinner, I.D.; Trachtenberg, D.I.; Miller, R.D. Provisional Restorations in Fixed Partial Prosthodontics. Dent. Clin. N. Am. 1989, 33, 355–377. [Google Scholar] [CrossRef] [PubMed]
- Hull, C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent. US4575330A, 19 December 1989. [Google Scholar]
- Alshamrani, A.; Alhotan, A.; Owais, A.; Ellakwa, A. The Clinical Potential of 3D-Printed Crowns Reinforced with Zirconia and Glass Silica Microfillers. J. Funct. Biomater. 2023, 14, 267. [Google Scholar] [CrossRef]
- Zoabi, A.; Redenski, I.; Oren, D.; Kasem, A.; Zigron, A.; Daoud, S.; Moskovich, L.; Kablan, F.; Srouji, S. 3D Printing and Virtual Surgical Planning in Oral and Maxillofacial Surgery. J. Clin. Med. 2022, 11, 2385. [Google Scholar] [CrossRef] [PubMed]
- Vasiliadis, A.V.; Koukoulias, N.; Katakalos, K. From Three-Dimensional (3D)- to 6D-Printing Technology in Orthopedics: Science Fiction or Scientific Reality? J. Funct. Biomater. 2022, 13, 101. [Google Scholar] [CrossRef]
- Keßler, A.; Hickel, R.; Ilie, N. In vitro investigation of the influence of printing direction on the flexural strength, flexural modulus and fractographic analysis of 3D-printed temporary materials. Dent. Mater. J. 2021, 40, 641–649. [Google Scholar] [CrossRef]
- Park, S.-M.; Park, J.-M.; Kim, S.-K.; Heo, S.-J.; Koak, J.-Y. Flexural Strength of 3D-Printing Resin Materials for Provisional Fixed Dental Prostheses. Materials 2020, 13, 3970. [Google Scholar] [CrossRef]
- Piedra-Cascón, W.; Krishnamurthy, V.R.; Att, W.; Revilla-León, M. 3D printing parameters, supporting structures, slicing, and post-processing procedures of vat-polymerization additive manufacturing technologies: A narrative review. J. Dent. 2021, 109, 103630. [Google Scholar] [CrossRef]
- Finck, N.S.; Fraga, M.A.A.; Correr, A.B.; Dalmaschio, C.J.; Rodrigues, C.S.; Moraes, R.R. Effects of solvent type and UV post-cure time on 3D-printed restorative polymers. Dent. Mater. 2024, 40, 451–457. [Google Scholar] [CrossRef]
- Soto-Montero, J.; de Castro, E.F.; Romano Bde, C.; Nima, G.; Shimokawa, C.A.K.; Giannini, M. Color alterations, flexural strength, and microhardness of 3D printed resins for fixed provisional restoration using different post-curing times. Dent. Mater. 2022, 38, 1271–1282. [Google Scholar] [CrossRef]
- Anseth, K.S.; Newman, S.M.; Bowman, C.N. Polymeric dental composite: Properties and reaction behavior of multimethacrylate dental restorations. In Biopolymers II; Peppas, N.A., Langer, R.S., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 177–217. [Google Scholar]
- Schmalz, G.; Arenholt-Bindslev, D. Biocompatibility of Dental Materials: With 82 Tables; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Mousavinasab, S.M. Biocompatibility of composite resins. Dent. Res. J. 2011, 8, S21–S29. [Google Scholar]
- Pascual, B.; Gurruchaga, M.; Ginebra, M.P.; Gil, F.J.; Planell, J.A.; Goñi, I. Influence of the modification of P/L ratio on a new formulation of acrylic bone cement. Biomaterials 1999, 20, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, B.; Ginebra, M.P.; Gil, F.J.; Planell, J.A.; López Bravo, A.; San Román, J. Radiopaque acrylic cements prepared with a new acrylic derivative of iodo-quinoline. Biomaterials 1999, 20, 2047–2053. [Google Scholar] [CrossRef]
- Manzano, M.; Arcos, D.; Delgado, M.R.; Ruiz, E.; Gil, F.J.; Vallet-Regí, M. Bioactive stargels. Chem. Mater. 2006, 18, 5696–5703. [Google Scholar] [CrossRef]
- Aparicio, C.; Manero, J.M.; Conde, F.; Pegueroles, M.; Planell, J.A.; Vallet-Regí, M.; Gil, F.J. Acceleration of apatite nucleation on microrough bioactive titanium for bone-replacing implants. J. Biomed. Mater. Res. A 2007, 82, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Buxadera-Palomero, J.; Calvo, C.; Torrent-Camarero, S.; Gil, F.J.; Mas-Moruno, C.; Canal, C.; Rodríguez, D. Biofunctional polyethylene glycol coatings on titanium: An in vitro-based comparison of functionalization methods. Colloids Surf. B Biointerfaces 2017, 152, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Mas-Moruno, C.; Garrido, B.; Rodriguez, D.; Ruperez, E.; Gil, F.J. Biofunctionalization strategies on tantalum-based materials for osseointegrative applications. J. Mater. Sci. Mater. Med. 2015, 26, 109. [Google Scholar] [CrossRef]
- Brizuela-Velasco, A.; Álvarez-Arenal, Á.; Gil-Mur, F.J.; Gil-Mur, F.J.; Herrero-Climent, M.; Chávarri-Prado, D.; Chento-Valiente, Y.; Dieguez-Pereira, M. Relationship Between Insertion Torque and Resonance Frequency Measurements, Performed by Resonance Frequency Analysis, in Micromobility of Dental Implants: An In Vitro Study. Implant. Dent. 2015, 24, 607–611. [Google Scholar] [CrossRef]
- Attaran, M. The rise of 3-D printing: The advantages of additive manufacturing over traditional manufacturing. Bus. Horiz. 2017, 60, 677–688. [Google Scholar] [CrossRef]
- Saratti, C.M.; Rocca, G.T.; Krejci, I. The potential of three-dimensional printing technologies to unlock the development of new ‘bio-inspired’ dental materials: An overview and research roadmap. J. Prosthodont. Res. 2019, 63, 131–139. [Google Scholar] [CrossRef]
- Alharbi, N.; Osman, R.; Wismeijer, D. Factors Influencing the Dimensional Accuracy of 3D-Printed Full-Coverage Dental Restorations Using Stereolithography Technology. Int. J. Prosthodont. 2016, 29, 503–510. [Google Scholar] [CrossRef]
- Unkovskiy, A.; Bui, P.H.-B.; Schille, C.; Geis-Gerstorfer, J.; Huettig, F.; Spintzyk, S. Objects build orientation, positioning, and curing influence dimensional accuracy and flexural properties of stereolithographically printed resin. Dent. Mater. 2018, 34, e324–e333. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhu, W.; Liu, F.; He, J. Preparation of a Bis-GMA-Free Dental Resin System with Synthesized Fluorinated Dimethacrylate Monomers. Int. J. Mol. Sci. 2016, 17, 2014. [Google Scholar] [CrossRef]
- Polydorou, O.; König, A.; Hellwig, E.; Kümmerer, K. Urethane dimethacrylate: A molecule that may cause confusion in dental research. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Khatri, C.A.; Stansbury, J.W.; Schultheisz, C.R.; Antonucci, J.M. Synthesis, characterization and evaluation of urethane derivatives of Bis-GMA. Dent. Mater. 2003, 19, 584–588. [Google Scholar] [CrossRef]
- Hild, G. Model networks based on ‘endlinking’ processes: Synthesis, structure and properties. Prog. Polym. Sci. 1998, 23, 1019–1149. [Google Scholar] [CrossRef]
- Flory, P.J. Molecular Theory of Rubber Elasticity. Polym. J. 1985, 17, 1–12. [Google Scholar] [CrossRef]
- Dickens, S.H.; Stansbury, J.W.; Choi, K.M.; Floyd, D. Photopolymerization Kinetics of Methacrylate Dental Resins. Macromolecules 2003, 36, 6043–6053. [Google Scholar] [CrossRef]
- Lee, D.-W.; Kim, H.-N.; Lee, D.-S. Introduction of Reversible Urethane Bonds Based on Vanillyl Alcohol for Efficient Self-Healing of Polyurethane Elastomers. Molecules 2019, 24, 2201. [Google Scholar] [CrossRef]
- Lemon, M.T.; Jones, M.S.; Stansbury, J.W. Hydrogen bonding interactions in methacrylate monomers and polymers. J. Biomed. Mater. Res. A 2007, 83, 734–746. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I.M. A Guide through the Dental Dimethacrylate Polymer Network Structural Characterization and Interpretation of Physico-Mechanical Properties. Materials 2019, 12, 4057. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I.M. Characterization of urethane-dimethacrylate derivatives as alternative monomers for the restorative composite matrix. Dent. Mater. 2014, 30, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Barszczewska-Rybarek, I.M. Structure-property relationships in dimethacrylate networks based on Bis-GMA, UDMA and TEGDMA. Dent. Mater. 2009, 25, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins. Biomaterials 2002, 23, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, V.E.S.; Pfeifer, C.S.; Fróes-Salgado, N.R.G. Monomers used in resin composite: Degree of conversion, mechanical properties and water sorption/solubility. Braz. Dent. J. 2012, 23, 508–514. [Google Scholar] [CrossRef]
- Stansbury, J.W. Dimethacrylate network formation and polymer property evolution as determined by the selection of monomers and curing conditions. Dent. Mater. 2012, 28, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Senawongse, P.; Pongprueksa, P. Surface Roughness of Nanofill and Nanohybrid Resin Composite after Polishing and Brushing. J. Esth. Rest. Dent. 2007, 19, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L.; Pfeifer, C.S.; Hilton, T.J. Microstructural Features of Current Resin Composite Materials. Curr. Oral Health Rep. 2014, 1, 205–212. [Google Scholar] [CrossRef]
- ISO 4049:2019; Dentistry—Polymer-Based Restorative Materials. International Standard Organization: Geneva, Switzerland, 2019.
- ISO 10477:2020; Dentistry—Polymer-Based Crown and Veneering Materials. International Standard Organization: Geneva, Switzerland, 2020.
- Vöros, J.; Wieland, M.; Ruiz-Taylor, L.; Textor, M.; Brunette, D.M. Characterization of titanium surfaces. In Titanium in Medicine; Brunette, D.M., Tengvall, P., Textor, M., Thomsen, P., Eds.; Springer: Berlin, Germany, 2001; pp. 87–144. [Google Scholar]
- Keller, J.C.; Stanford, C.M.; Wightman, J.P.; Draughn, R.A.; Zaharias, R. Characterizations of titanium implant Surfaces. III. J. Biomed. Mater. Res. 1994, 28, 939–946. [Google Scholar] [CrossRef]
- Carrasco, B.; Brizuela, A.; Romero-Ruiz, M.M.; Gil-Mur, J. Investigation of the influence of roughness and dental implant design on primary stability via analysis of insertion torque and implant stability quotient: An in vitro study. J. Clin. Med. 2023, 12, 4190. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, L.; Milan, A.; Del Lupo, V.; Maglione, M.; Dolzani, L. Biofilms developed on dental implant titanium surfaces with different roughness: Comparison between in vitro and in vivo studies. Curr. Microbiol. 2018, 75, 766–772. [Google Scholar] [CrossRef]
- Pfeifer, C.S.; Shelton, Z.R.; Braga, R.R.; Windmoller, D.; Machado, J.C.; Stansbury, J.W. Characterization of dimethacrylate polymeric networks: A study of the crosslinked structure formed by monomers used in dental composite. Eur. Polym. J. 2011, 47, 162–170. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Bociong, K.; Szczesio, A.; Sokolowski, K.; Domarecka, M.; Sokolowski, J.; Krasowski, M.; Lukomska-Szymanska, M. The Influence of Water Sorption of Dental Light-Cured Composite on Shrinkage Stress. Materials 2017, 10, 1142. [Google Scholar] [CrossRef]
- Vila, M.M.; Ginebra, M.P.; Gil, F.J.; Planell, J.A. Effect of porosity and environment on the mechanical behavior of acrylic bone cement modified with acrylonitrile-butadiene- styrene particles: I. Fracture toughness. J. Biomed. Mater. Res. 1999, 48, 121–127. [Google Scholar] [CrossRef]
- Gil, F.J.; Solano, E.; Peña, J.; Engel, E.; Mendoza, A.; Planell, J.A. Microstructural, mechanical and citotoxicity evaluation of different NiTi and NiTiCu shape memory alloys. J. Mater. Sci. Mater. Med. 2004, 15, 1181–1185. [Google Scholar] [CrossRef]
- Andrzejewska, E. Photopolymerization kinetics of multifunctional monomers. Prog. Polimer. Sci. 2001, 26, 605–665. [Google Scholar] [CrossRef]
- Drummond, J.L. Degradation, fatigue, and failure of resin dental composite materials. J. Dent. Res. 2008, 87, 710–719. [Google Scholar] [CrossRef]
- Lovell, L.G.; Berchtold, K.A.; Elliott, J.E.; Stansbury, J.W.; Bowman, C.N. Understanding the kinetics and network formation of dimethacrylate dental resins. Dent. Mater. 2001, 12, 335–345. [Google Scholar] [CrossRef]
- Gil, F.X.; Rodríguez, D.; Planell, J.A. Grain growth kinetics of pure titanium. Scr. Metall. Mater. 1995, 33, 1361–1366. [Google Scholar] [CrossRef]
- Issa, Y.; Watts, D.C.; Brunton, P.A.; Waters, C.M.; Duxbury, A.J. Resin composite monomers alter MTT and LDH activity of human gingival fibroblasts in vitro. Dent. Mater. 2004, 20, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Reichl, F.-X.; Simon, S.; Esters, M.; Seiss, M.; Kehe, K.; Kleinsasser, N.; Hickel, R. Cytotoxicity of dental composite (co)monomers and the amalgam component Hg(2+) in human gingival fibroblasts. Arch. Toxicol. 2006, 80, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Manuelli, M.; Marcolina, M.; Nardi, N.; Bertossi, D.; De Santis, D.; Ricciardi, G.; Luciano, U.; Nocini, R.; Mainardi, A.; Lissoni, A.; et al. Oral mucosal complications in orthodontic treatment. Minerva Stomatol. 2019, 68, 84–88. [Google Scholar] [CrossRef]


| Resin | Self-Curing Dimethacrylate Composite | Photopolymerized 3D-Printed Composite |
|---|---|---|
| Manufacturer | VOCO GmbH Cuxhaven Germany | Vertex-Dental B.V. AV Soesterberg The Netherlands |
| Brand | Structur 3 | NextDent C&B MFH |
| Batch | SC-34523657-AB | PPDC-675000001238-B |
| Resin type | Self-polymerizing | Photopolymerizing |
| Composition | Bis-GMA: 5–10% UDMA: 10–25% Inorganic Filler Nanofiller 32% (size 50nm) Amines Terpenes Benzoyl peroxide butylhydroxyteluene | UDMA: 50–75% HEMA:0–25% Bis-EMA:0–10% EGDMA:0–10% Silicon dioxide: 1–5% (TPO): 1–5% Mequinol: 0–0.1 Titanium dioxide: 0–0.1 |
| Residual monomer | 2.6% | 3.3% |
| Material | Sa (mm) | Sy (mm) | Sm (mm) | Pc (1/mm) | Index Area | CA (°) |
|---|---|---|---|---|---|---|
| Self-curing dimethacrylate composite | 0.53 ± 0.09 | 0.87 ± 0.13 | 12.25 ± 3.12 | 23.30 ± 10.42 | 1.07 ± 0.01 | 70.17 ± 5.65 |
| Photopolymer, 3D-printed composite | 0.63 ± 0.04 | 0.65 ± 0.06 | 13.00 ± 0.78 | 25.37 ± 12.63 | 1.05 ± 0.03 | 85.85 ± 1.84 * |
| Resin | Flexural Strength (MPa) | Modulus of Elasticity (GPa) | Toughness (mJ) | Displacement at Break (mm) |
|---|---|---|---|---|
| Self-curing dimethacrylate composite | 122 ± 12 | 1.94 ± 0.10 | 38.52 ± 9.00 | 2.49 ± 0.48 |
| Photopolymerized 3D-printed composite | 138 ± 10 | 2.50 ± 0.11 | 66.51 ± 9.16 | 3.20 ± 0.53 |
| Student’s t-test (p < 0.05) | 0.049 | 0.000 | 0.000 | 0.000 |
| Material | Self-Curing Dimethacrylate Composite (g/cm3) | Photopolymerized 3D-Printed Composite (g/cm3) |
|---|---|---|
| 1.328 ± 0.005 | 1.260 ± 0.008 |
| Material | Self-Curing Dimethacrylate Composite (µg/mm3) | Photopolymerized 3D-Printed Composite (µg/mm3) |
|---|---|---|
| 16 ± 2 | 57 ± 3 |
| Material | Self-Curing Dimethacrylate Composite (HV) | Photopolymerized 3D-Printed Composite (HV) |
|---|---|---|
| 8.3 ± 0.5 | 19.5 ± 1.2 | |
| Mann–Whitney U Test (p > 0.05) | 6.70 × 10−8 | |
| Property | Self-Curing Dimethacrylate Composite | Photopolymerized 3D-Printed Composite |
|---|---|---|
| Wear channel area/(mm2) | 0.0444 (0.0302–0.0644) | 0.0310 (0.0128–0.0499) |
| Wear Rate/(m3/Nm) × 10−13 | 2.379 (1.5550–3.14598) | 1.489 (0.5023–2.8976) |
| Material | Scratch Channel Width (µm) | Friction Force (N) | Dynamic Friction Coef. | Penetration Depth (µm) |
|---|---|---|---|---|
| self-curing dimethacrylate composite | 252.39 ± 4.85 | 1.878 ± 0.386 | 0.255 ± 0.010 | 389.77 ± 1.867 |
| photopolymerized 3D-printed composite | 93.87 ± 12.78 | 1.99 ± 0.557 | 0.19 ± 0.01 | 263.367 ± 6.643 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valencia-Blanco, O.J.; Pérez-Pevida, E.; Robles-Cantero, D.; Montalvillo, E.; Gil, J.; Brizuela-Velasco, A. Comparative Analysis of the Physicochemical Properties of 3D-Printed and Conventional Resins for Temporary Dental Restorations. Prosthesis 2025, 7, 129. https://doi.org/10.3390/prosthesis7050129
Valencia-Blanco OJ, Pérez-Pevida E, Robles-Cantero D, Montalvillo E, Gil J, Brizuela-Velasco A. Comparative Analysis of the Physicochemical Properties of 3D-Printed and Conventional Resins for Temporary Dental Restorations. Prosthesis. 2025; 7(5):129. https://doi.org/10.3390/prosthesis7050129
Chicago/Turabian StyleValencia-Blanco, Oscar Javier, Esteban Pérez-Pevida, Daniel Robles-Cantero, Enrique Montalvillo, Javier Gil, and Aritza Brizuela-Velasco. 2025. "Comparative Analysis of the Physicochemical Properties of 3D-Printed and Conventional Resins for Temporary Dental Restorations" Prosthesis 7, no. 5: 129. https://doi.org/10.3390/prosthesis7050129
APA StyleValencia-Blanco, O. J., Pérez-Pevida, E., Robles-Cantero, D., Montalvillo, E., Gil, J., & Brizuela-Velasco, A. (2025). Comparative Analysis of the Physicochemical Properties of 3D-Printed and Conventional Resins for Temporary Dental Restorations. Prosthesis, 7(5), 129. https://doi.org/10.3390/prosthesis7050129








