A Hybrid Workflow for Auricular Epithesis: Proof of Concept Integrating Mold Design and the Virtual Patient
Abstract
1. Introduction
- Minimally invasive data acquisition using structured-light scanning and processing;
- Virtual patient creation and mirroring of the contralateral ear;
- Precise adaptation and positioning of the mirrored ear to the defect area;
- Virtual visualization and incorporation of patient approval and feedback before final fabrication;
- Digital mold design using open-source or commercial engineering CAD, allowing for a balanced approach between cost and functionality.
- Conventional prosthesis manufacturing with medical-grade silicone, followed by manual extrinsic color customization for a better resemblance to a natural ear, which leads to great comfort for the patient.
2. Materials and Methods
2.1. Clinical Scenario
2.2. Digital Workflow Overview
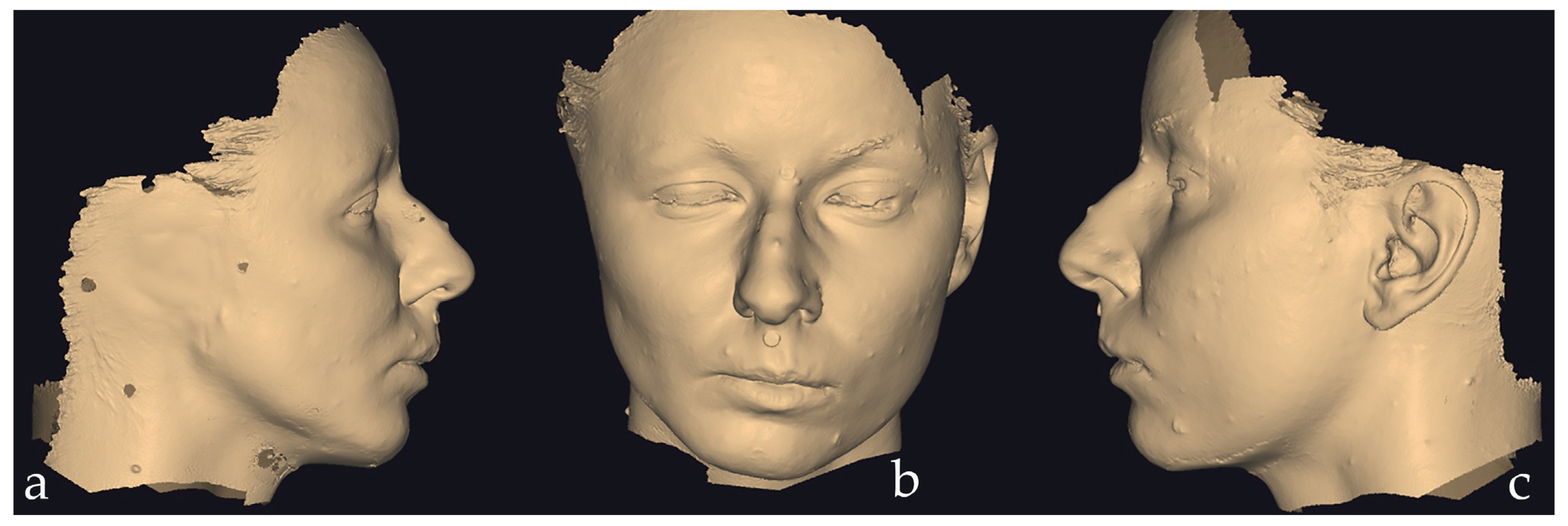
2.2.1. Data Acquisition
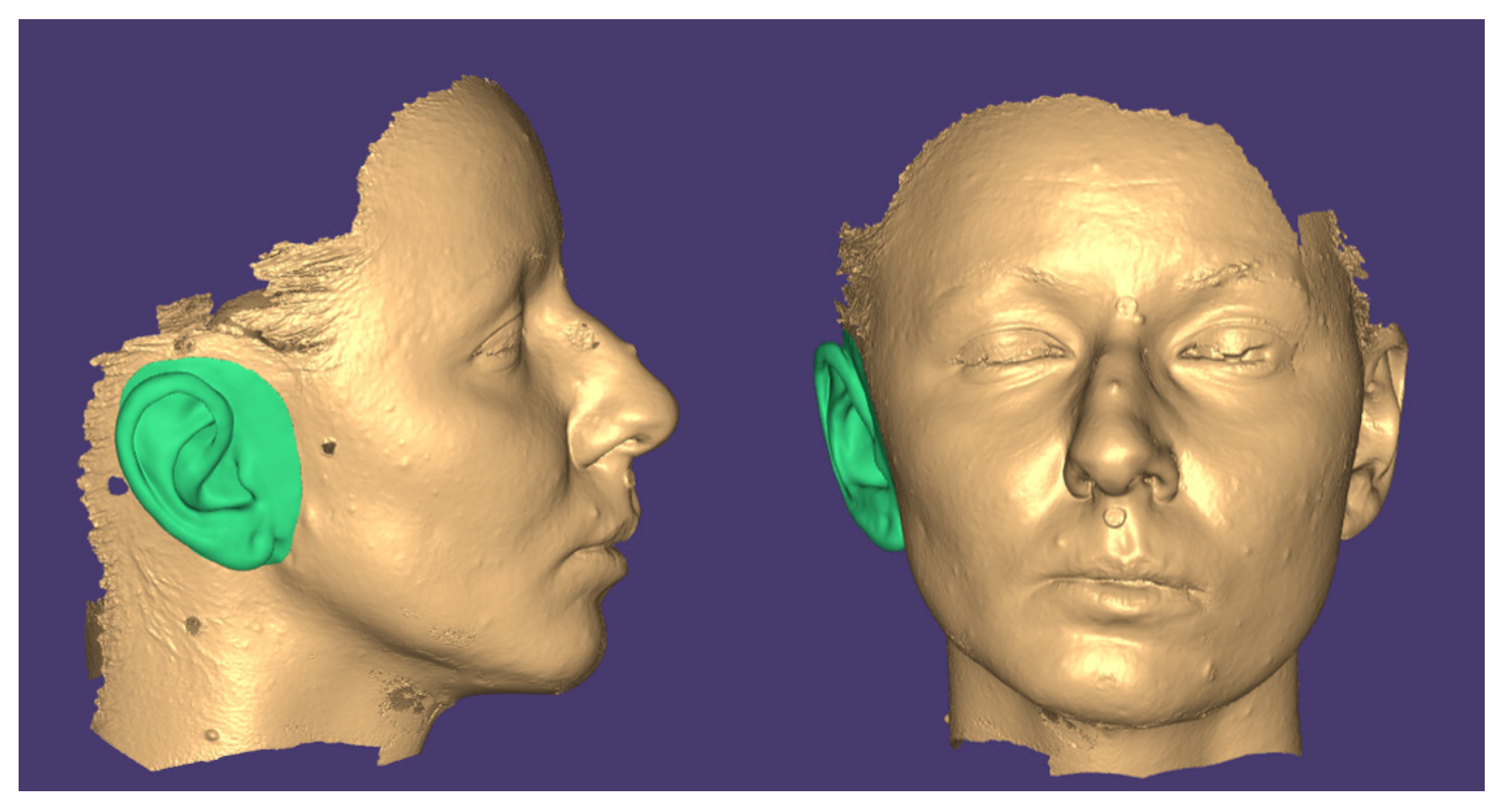
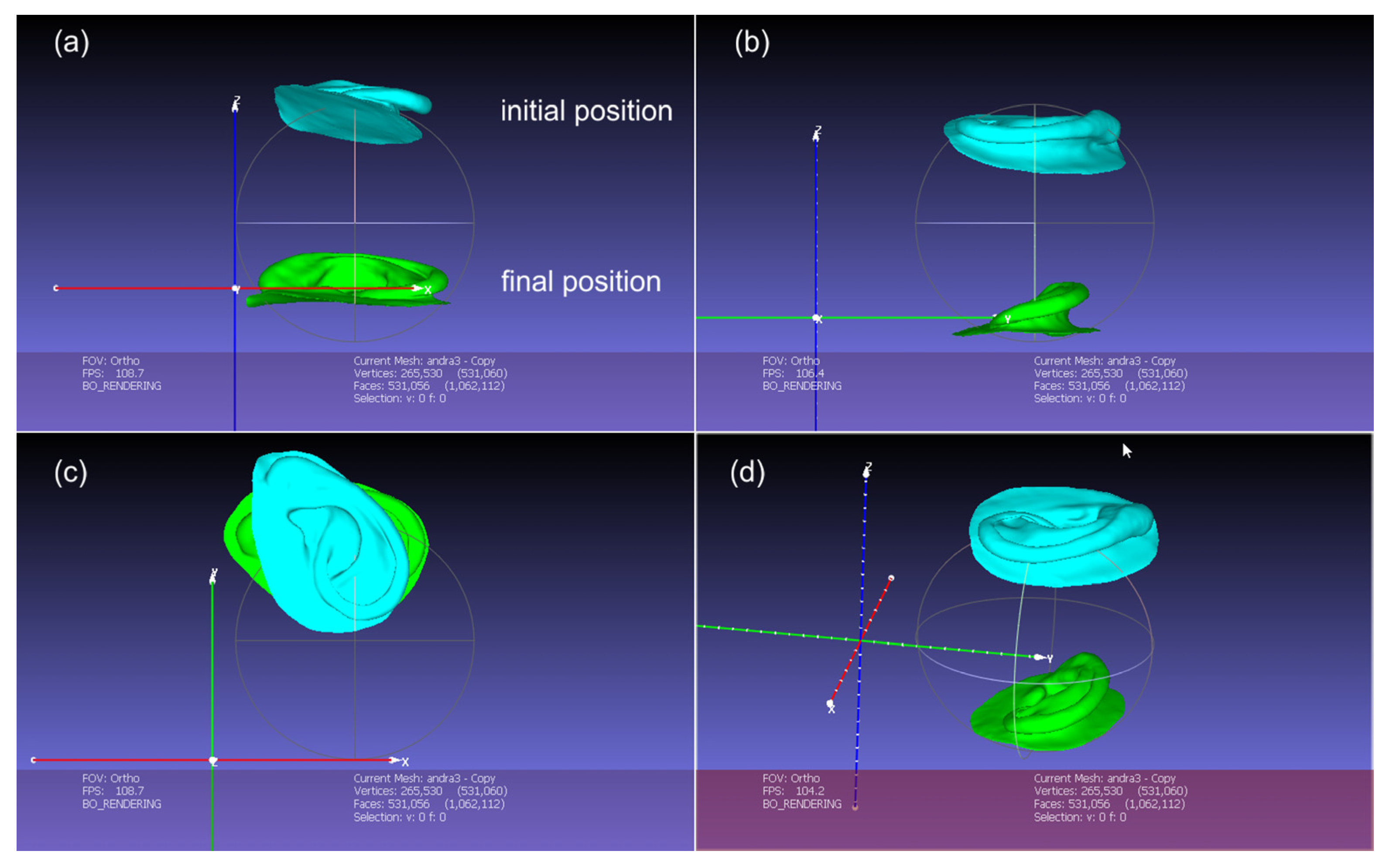
2.2.2. Digital Design of the Auricular Epithesis
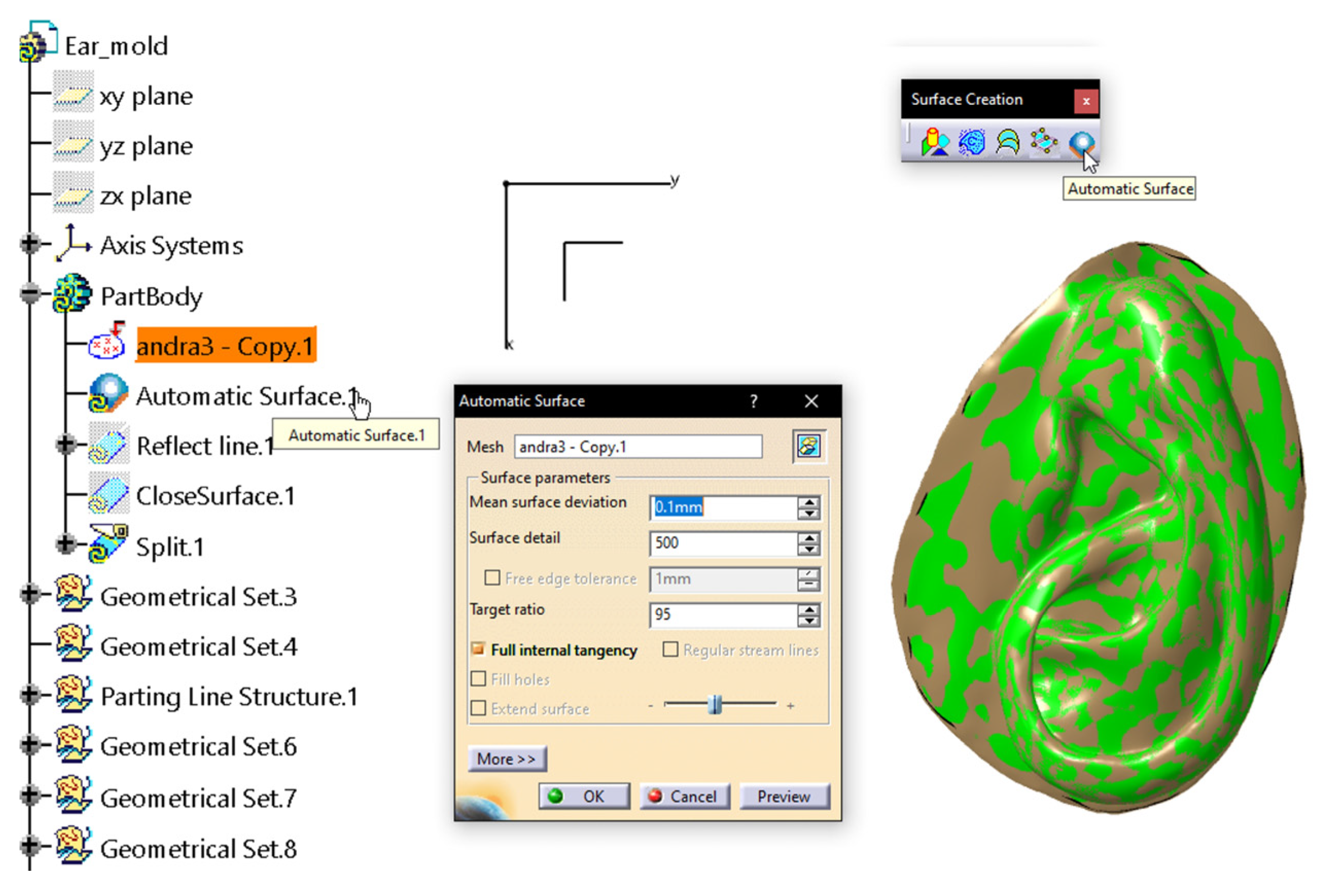
2.2.3. Digital Mold Design Using Meshmixer and CATIA V5R21
2.2.4. Digital Design of a Three-Part Mold in Blender
2.2.5. 3D Printing of the Molds and Ear Prosthesis Manufacturing
2.2.6. Objective and Subjective Evaluation of the Final Prostheses (Prior to Extrinsic Pigmentation)
Objective Evaluation
Subjective Evaluation
Statistical Analysis
3. Results
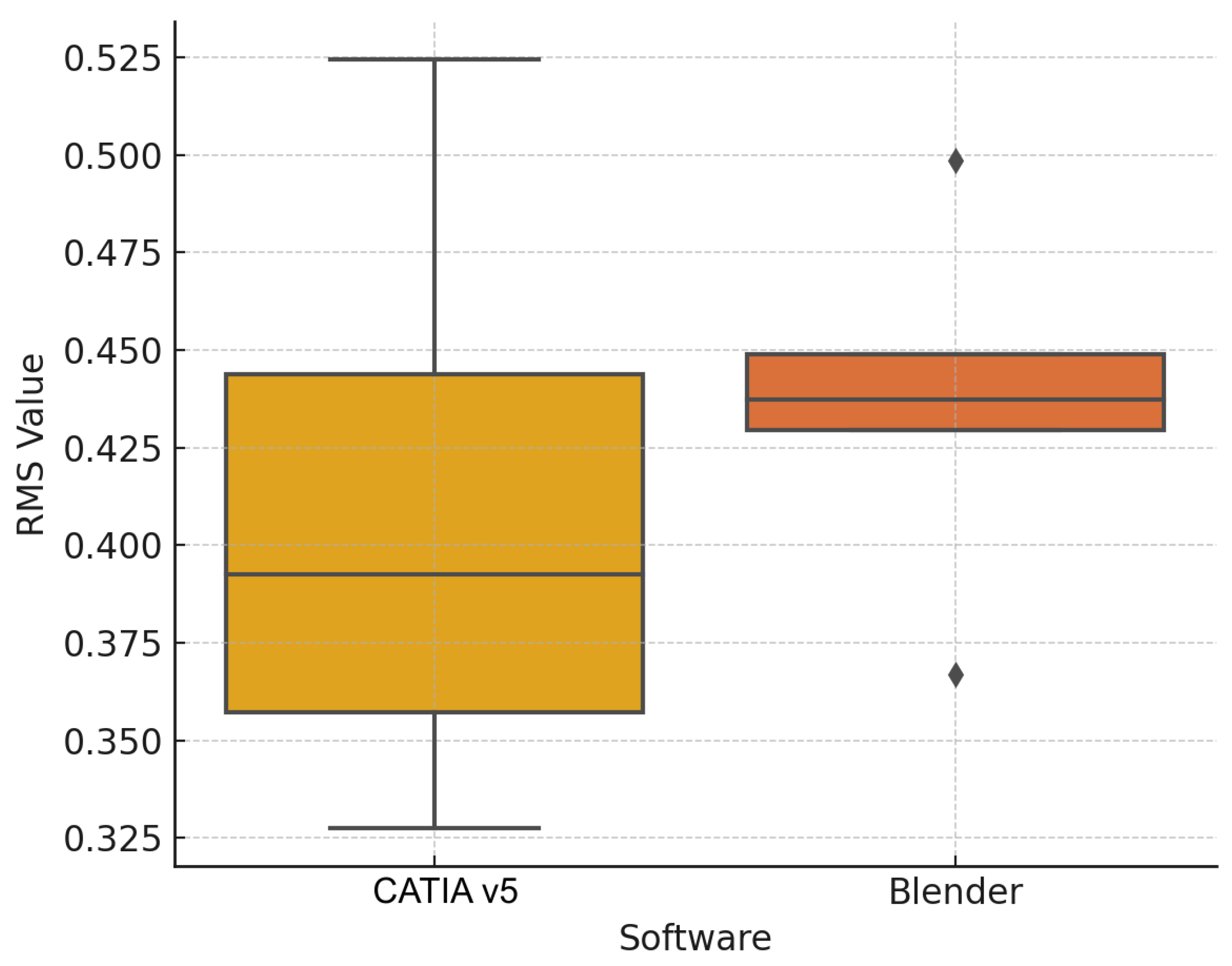
3.1. Objective Evaluation
3.2. Weights of the Fabricated Ears
3.3. Subjective Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAD/CAM | Computer-aided design/manufacturing |
| CAE | Computer-aided engineering |
| PLY | Polygon File |
| HTML | HyperText Markup Language |
| STL | Stereolithography |
| PLA | Polylactic Acid |
| FDM | Fused Deposition Modeling |
| VAS | Visual Analogue Scale |
| NURBS | Non-Uniform Rational B-Splines |
| STEP | Standard for the Exchange of Product model data |
| IGES | Initial Graphics Exchange Specification |
| OBJ | Object File |
| RMS | Root mean square |
| DLP | Digital light processing |
References
- Tarba, C.I.; Cristache, M.A.; Baciu, I.M.; Cristache, C.M.; Burlacu Vatamanu, O.E.; Oancea, L. Advancements in Digital Workflows for 3D-Printed Maxillofacial Soft Prostheses: Exploring Design and Materials in Direct Additive Manufacturing: A Scoping Review. Appl. Sci. 2025, 15, 1701. [Google Scholar] [CrossRef]
- Domingue, D.; Glenn, N.C.; Vest, A.; White, J.R. Osseointegrated implant-retained auricular prosthesis constructed using cone-beam computed tomography and a prosthetically driven digital workflow: A case report. Clin. Case Rep. 2020, 9, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Unkovskiy, A.; Spintzyk, S.; Axmann, D.; Engel, E.M.; Weber, H.; Huettig, F. Additive Manufacturing: A Comparative Analysis of Dimensional Accuracy and Skin Texture Reproduction of Auricular Prostheses Replicas. J. Prosthodont. 2019, 28, e460–e468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, J.; Li, J.; Zhou, X.; Xie, J.; Wang, A. Selective Laser Melting Molding of Individualized Femur Implant: Design, Process, Optimization. J. Bionic Eng. 2021, 18, 128–137. [Google Scholar] [CrossRef]
- Bi, Y.; Zhou, M.; Wei, H. Digital workflow for auricular prosthesis fabrication with a negative mold. J. Prosthet. Dent. 2024, 131, 1254–1258. [Google Scholar] [CrossRef]
- Pickrell, B.B.; Hughes, C.D.; Maricevich, R.S. Partial Ear Defects. Semin. Plast. Surg. 2017, 31, 134–140. [Google Scholar] [CrossRef]
- Cristache, C.M.; Tudor, I.; Moraru, L.; Cristache, G.; Lanza, A.; Burlibasa, M. Digital Workflow in Maxillofacial Prosthodontics—An Update on Defect Data Acquisition, Editing and Design Using Open-Source and Commercial Available Software. Appl. Sci. 2021, 11, 973. [Google Scholar] [CrossRef]
- Tanaka, E. Biomechanical and tribological properties of the temporomandibular joint. Front. Oral Maxillofac. Med. 2021, 3, 15. [Google Scholar] [CrossRef]
- Cruz, R.L.J.; Ross, M.T.; Skewes, J.; Allenby, M.C.; Powell, S.K.; Woodruff, M.A. An advanced prosthetic manufacturing framework for economic personalised ear prostheses. Sci. Rep. 2020, 10, 11453. [Google Scholar] [CrossRef]
- Ciocca, L.; Gastaldi, G. Restoration of Facial Defects with Digital Technology; Elsevier: London, UK, 2022; ISBN 9780323902953. [Google Scholar]
- Unkovskiy, A. Digital Workflow of Facial Prostheses Manufacturing; Freie Universität: Berlin, Germany, 2022. [Google Scholar]
- Heydenrych, A.; van der Walt, J.G.; van den Heever, H.J. Auricular prosthesis positioning using virtual planning in combination with additive manufacturing. J. Stomatol. Oral Maxillofac. Surg. 2023, 124, 101258. [Google Scholar] [CrossRef]
- Abdelaziz, M.S. Digital Designing of a 3D-Printed Stackable Ear Mold for Auricular Rehabilitation: A Technical Note. Int. J. Prosthodont. 2023, 36, 637–641. [Google Scholar] [CrossRef]
- Bannink, T.; Bouman, S.; Wolterink, R.; van Veen, R.; van Alphen, M. Implementation of 3D technologies in the workflow of auricular prosthetics: A method using optical scanning and stereolithography 3D printing. J. Prosthet. Dent. 2021, 125, 708–713. [Google Scholar] [CrossRef]
- Tanveer, W.; Ridwan-Pramana, A.; Molinero-Mourelle, P.; Forouzanfar, T. Applications of CAD/CAM Technology for Craniofacial Implants Placement and Manufacturing of Auricular Prostheses—Systematic Review. J. Clin. Med. 2023, 12, 5950. [Google Scholar] [CrossRef] [PubMed]
- Ballo, A.M.; Nguyen, C.T.; Lee, V.S.K. Digital Workflow of Auricular Rehabilitation: A Technical Report Using an Intraoral Scanner. J. Prosthodont. 2019, 28, 596–600. [Google Scholar] [CrossRef]
- McHutchion, L.; Aalto, D. Simulation of tissue-prosthesis margin interface by using surface scanning and digital design for auricular prostheses. J. Prosthet. Dent. 2021, 125, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.L.J.; Ross, M.T.; Nightingale, R.; Pickering, E.; Allenby, M.C.; Woodruff, M.A.; Powell, S.K. An automated parametric ear model to improve frugal 3D scanning methods for the advanced manufacturing of high-quality prosthetic ears. Comput. Biol. Med. 2023, 162, 107033. [Google Scholar] [CrossRef] [PubMed]
- Manju, V.; Babu, A.S.; Krishnapriya, V.N.; Chandrashekar, J. Rapid Prototyping Technology for Silicone Auricular Prosthesis Fabrication: A Pilot Study. J. Head Neck Physicians Surg. 2021, 9, 35–40. [Google Scholar] [CrossRef]
- Mukhtarkhanov, M.; Perveen, A.; Talamona, D. Application of Stereolithography Based 3D Printing Technology in Investment Casting. Micromachines 2020, 11, 946. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Q.; Wang, S.; Tao, J.; Gou, M. Digital Light Processing Based Three-dimensional Printing for Medical Applications. Int. J. Bioprinting 2020, 6, 12–27. [Google Scholar] [CrossRef]
- Totu, E.E.; Cristache, C.M. Could the old poly(methylmethacrylate) face arrising challanges of new advanced technologies for dental prosthesis manufacturing? Rev. Chim. 2017, 68, 2102–2107. [Google Scholar] [CrossRef]
- Guide to Inhibiting Cure of Addition-Cure Silicone in 3D Printed Molds. Available online: https://www.siliconeab.com/solutions/3Dprinted-molds-inhibiting-silicone.html (accessed on 17 July 2025).
- Salazar-Gamarra, R.; Cárdenas-Bocanegra, A.; Masch, U.; Da Costa Moraes, C.A.; Seelaus, R.; Lopes Da Silva, J.V.; Lauria Dib, L. Color translation from monoscopic photogrammetry +ID Methodology into a Polyjet final 3D printed facial prosthesis. F1000Research 2022, 11, 582. [Google Scholar] [CrossRef] [PubMed]
- Nuseir, A.; Hatamleh, M.M.d.; Alnazzawi, A.; Al-Rabab’ah, M.; Kamel, B.; Jaradat, E. Direct 3D Printing of Flexible Nasal Prosthesis: Optimized Digital Workflow from Scan to Fit. J. Prosthodont. 2019, 28, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Vennam, S.; Vijayasankar, K.N.; Pati, F. 3D printed personalized assistive devices: A material, technique, and medical condition perspective. Appl. Mater. Today 2024, 40, 102403. [Google Scholar] [CrossRef]
- Totu, E.E.; Voicila, E.; Pistritu, V.; Nechifor, G.; Cristache, C.M. Evaluation of Electrical Characteristics for PMMA-TiO2 Nanocomposites Used in Dentistry. Rev. Chim. 2018, 69, 155–159. [Google Scholar] [CrossRef]
- Culmone, C.; Smit, G.; Breedveld, P. Additive manufacturing of medical instruments: A state-of-the-art review. Addit. Manuf. 2019, 27, 461–473. [Google Scholar] [CrossRef]
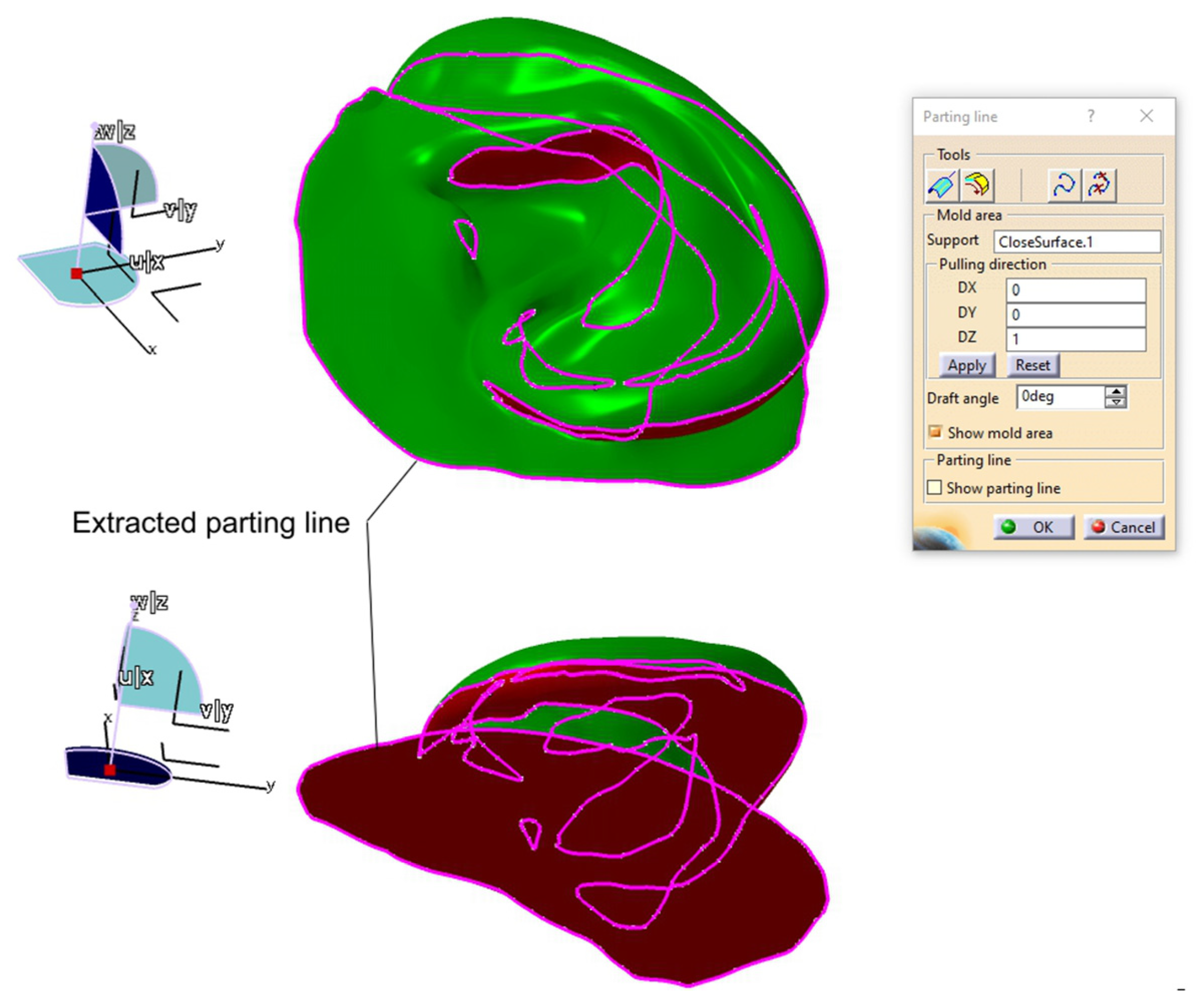

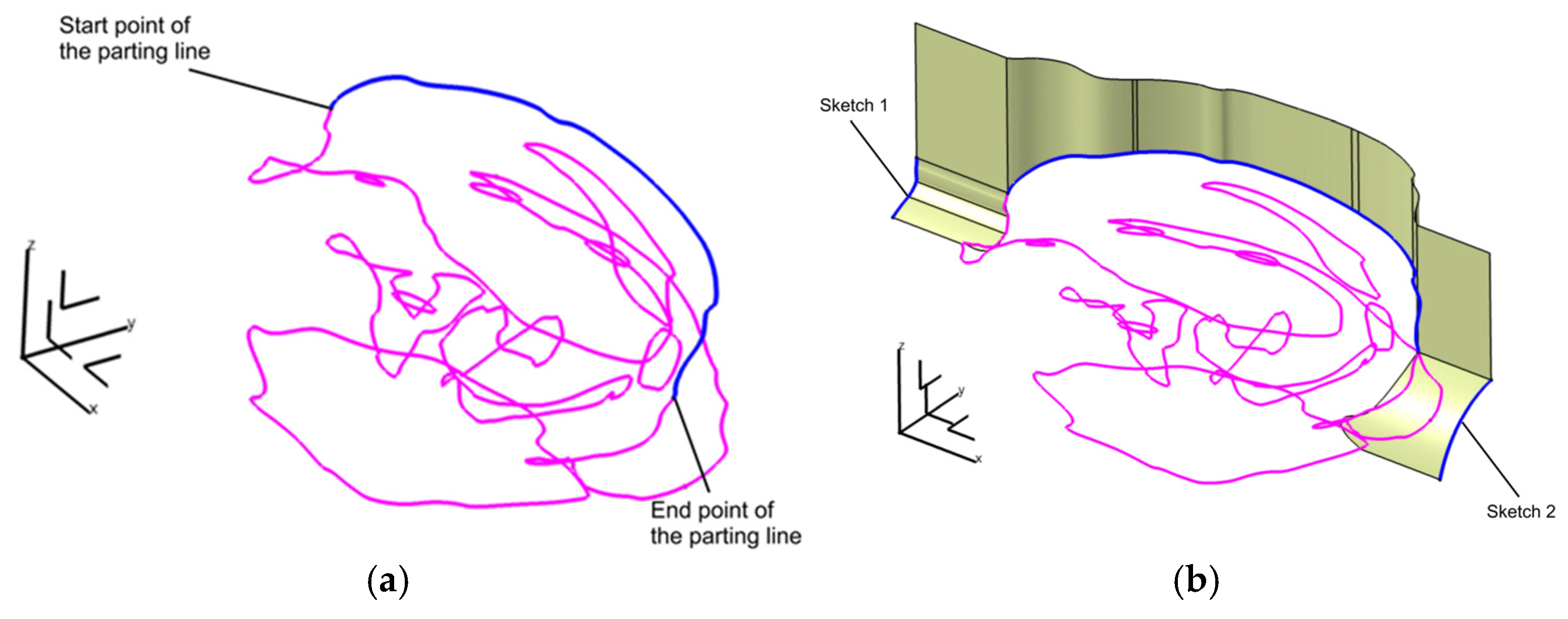
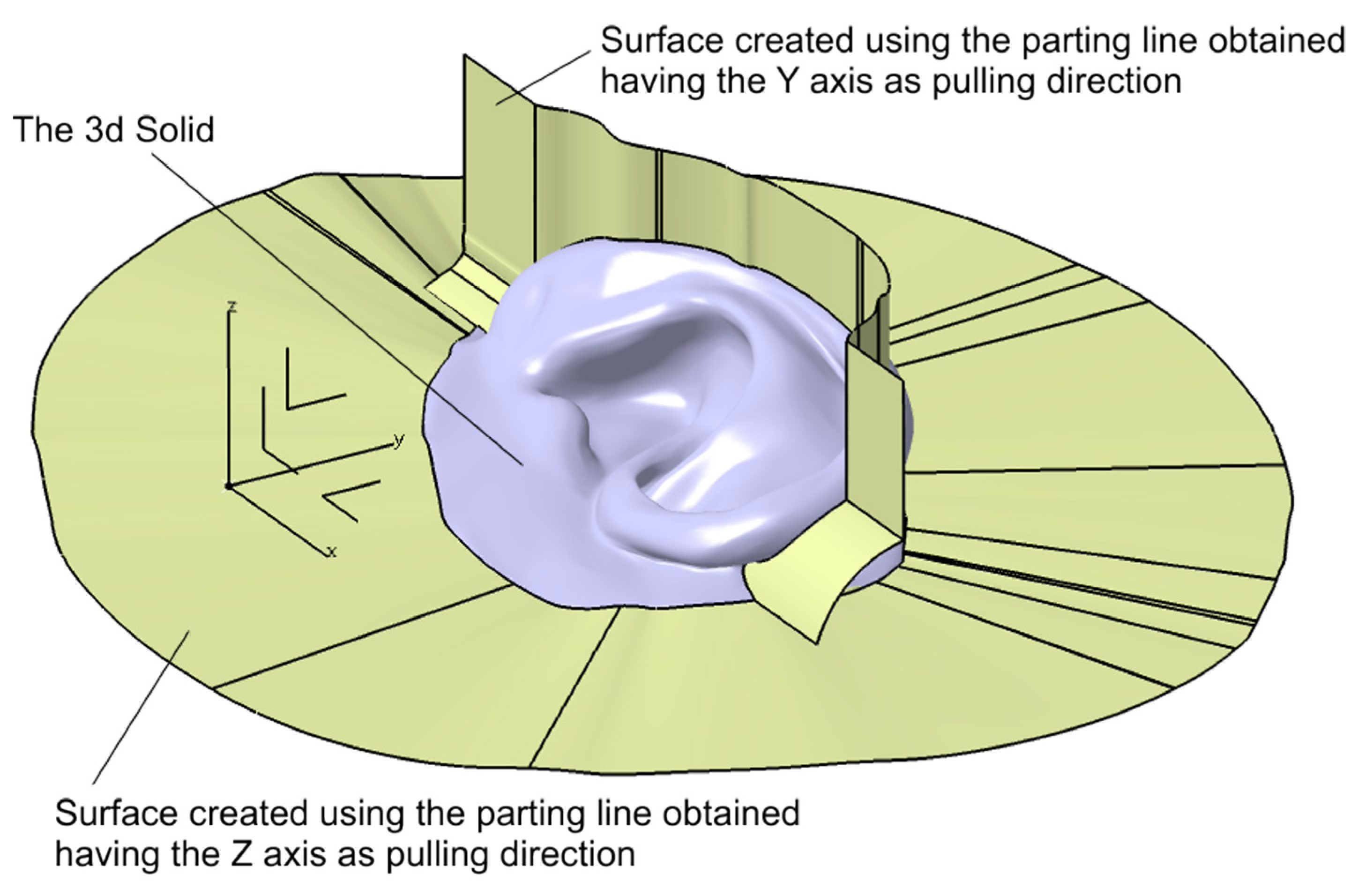
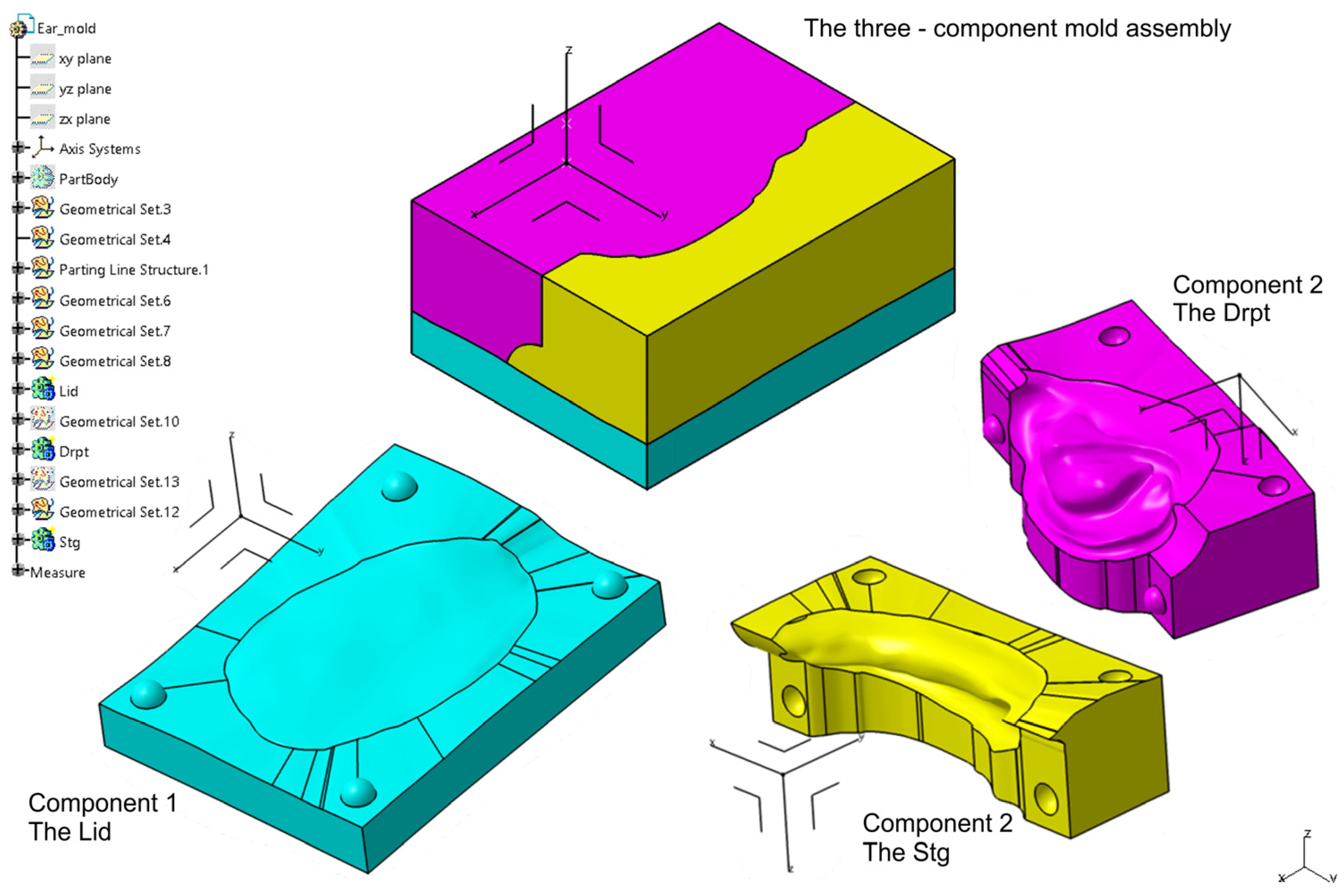
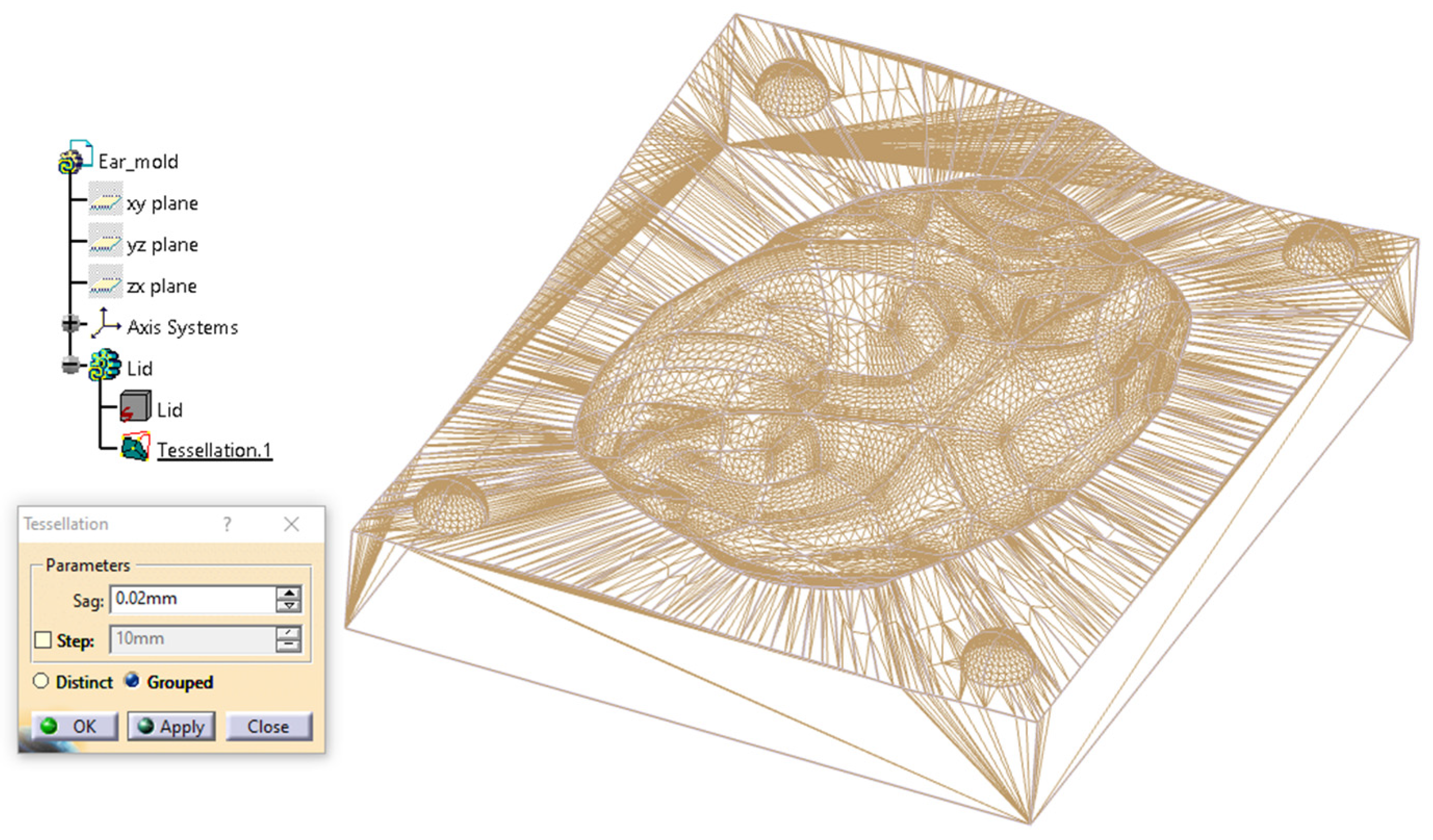
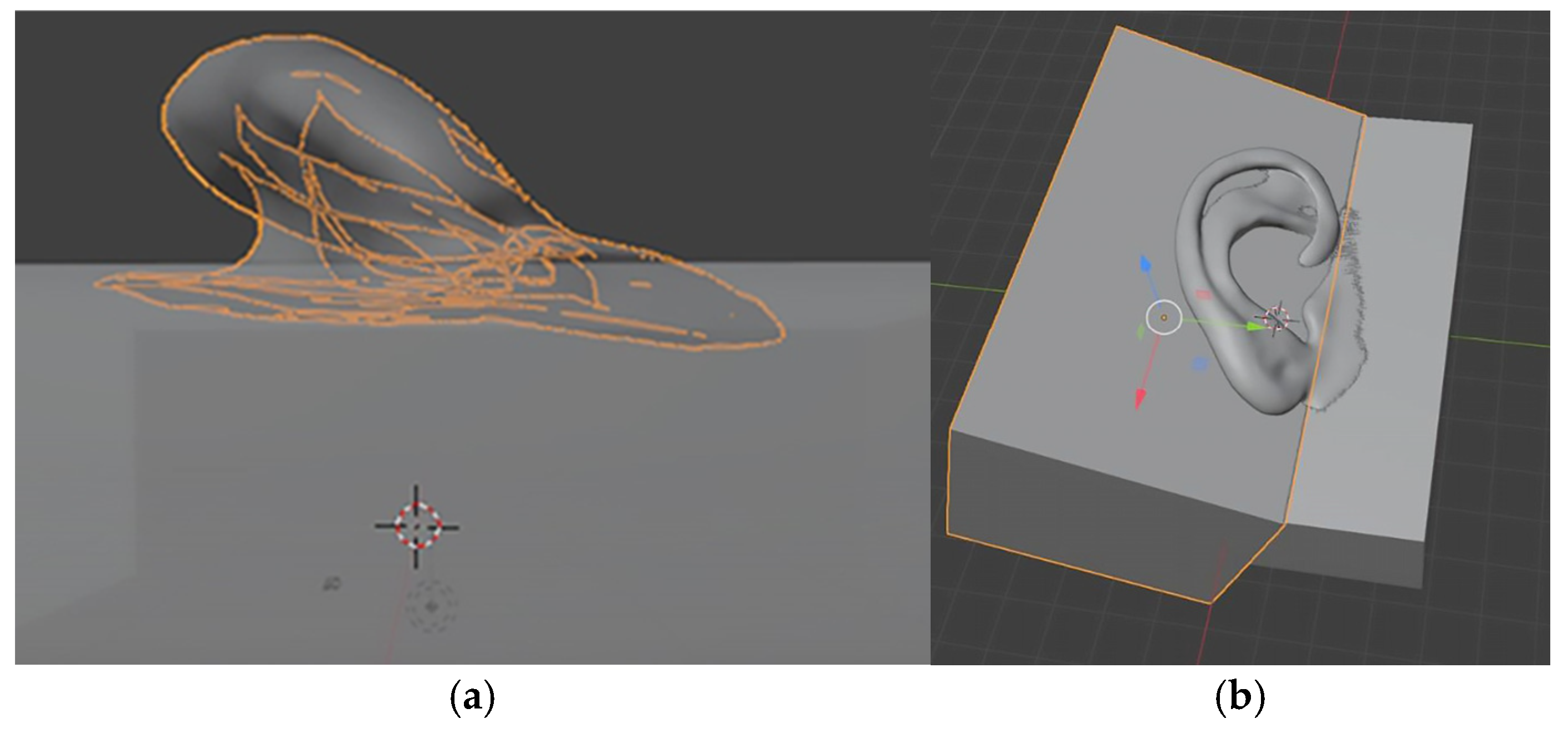
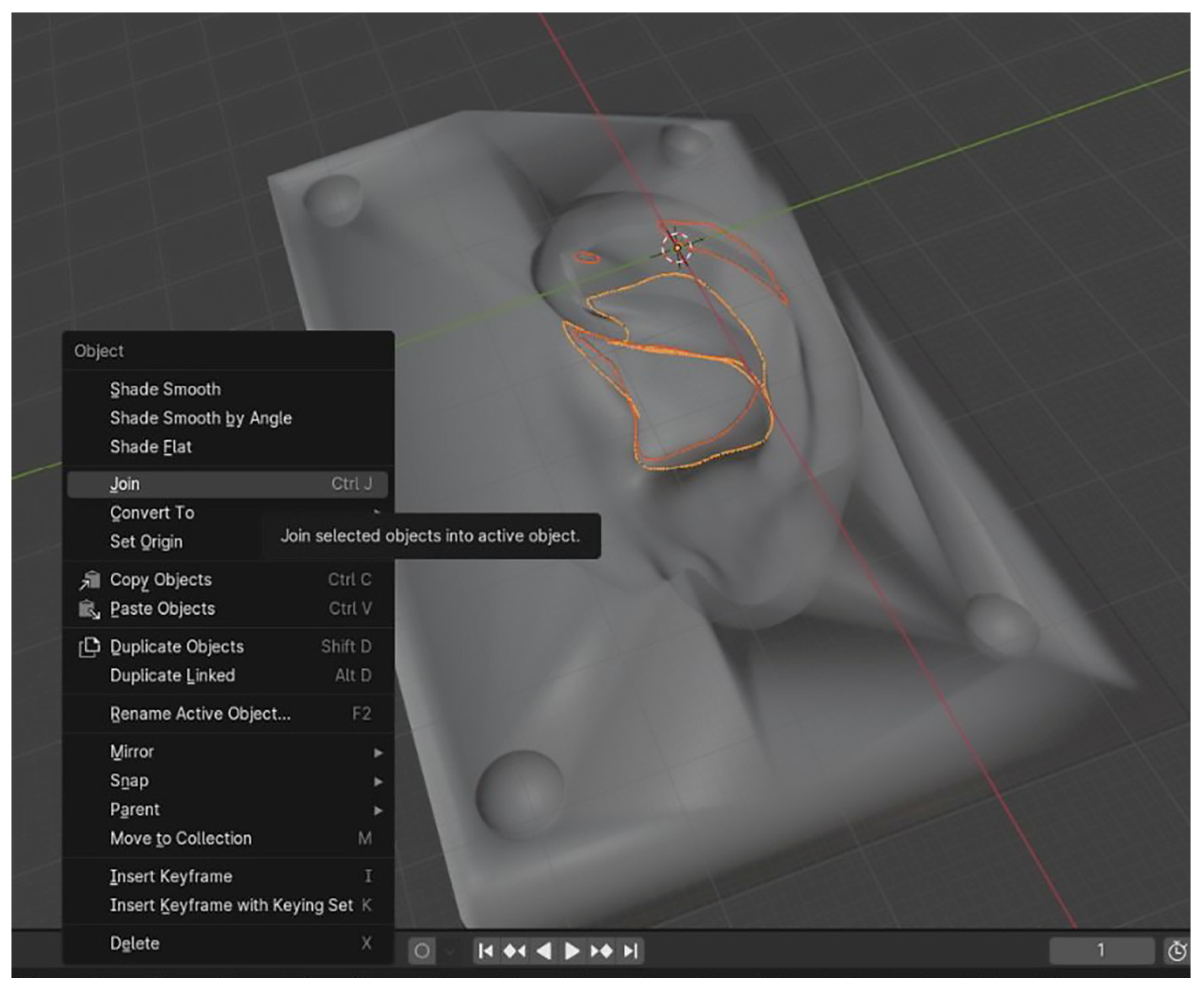

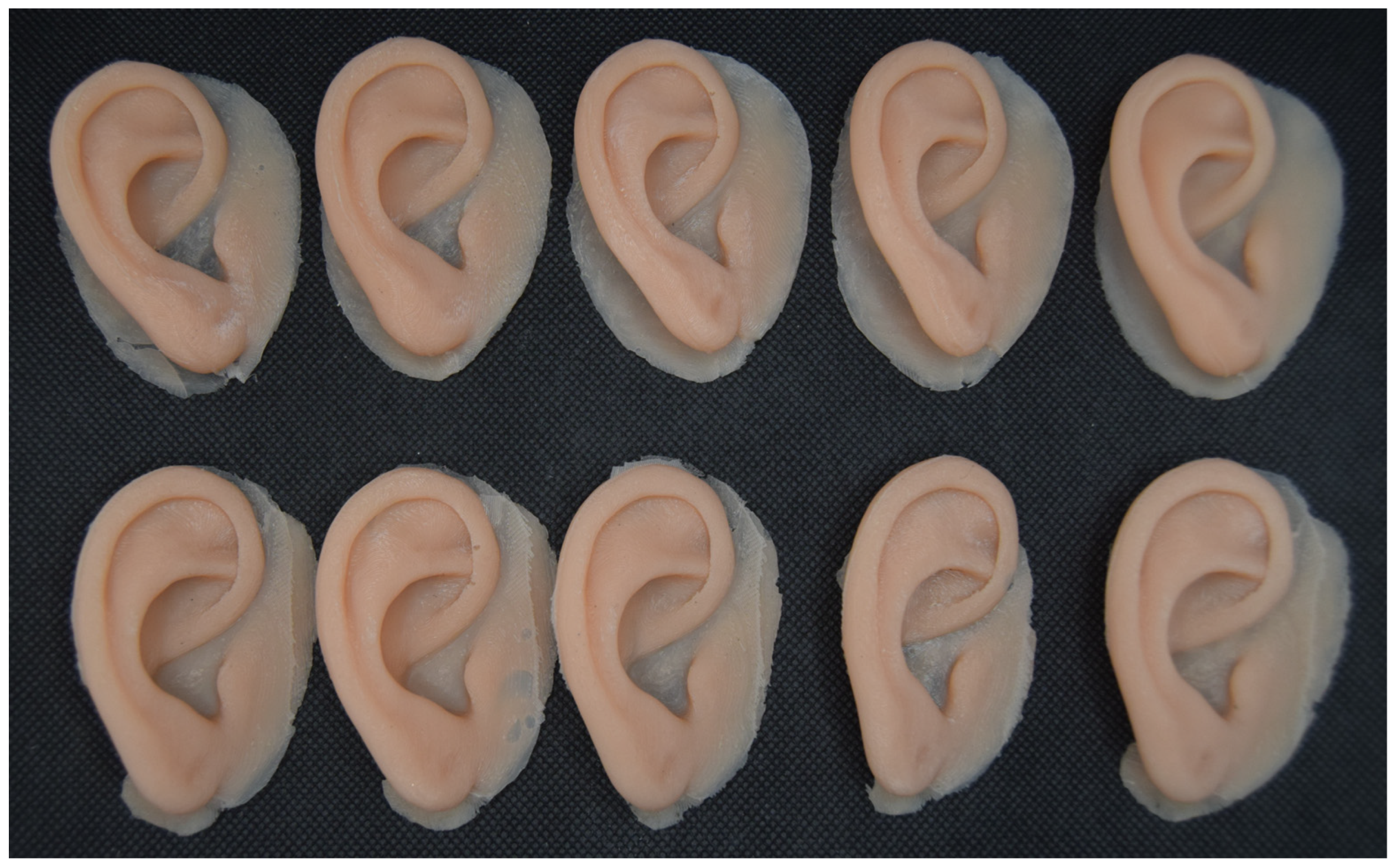



| Category | CATIA V5R21 (Proprietary CAD) | Blender (Open-Source) |
|---|---|---|
| Cost | High licensing fees | Free and open-source |
| User Interface | Engineering-oriented; steeper learning curve | Intuitive for artists/designers; community-supported |
| Design Precision | Very high, parametric, and feature-based modeling, making it suitable for any complex solid and surface design challenge | Moderate to high; mesh-based modeling |
| Workflow Structure | Modular (Generative Shape Design, Core & Cavity, etc.) | Non-parametric, flexible, but more manual |
| Types of curves for 3D modeling | NURBS—default curves, but also supports Bézier Curves (less common) | Bézier Curves—default curve type, but also supports NURBS |
| Mold Alignment | Easier and precise to define planes and alignment geometrically | Manual alignment using basic primitives and transforms |
| Export Compatibility | STL, STEP, IGES—excellent integration with industry tools | STL and OBJ—widely used, especially for 3D printing |
| Time to Final Mold | Slightly longer due to the initial setup of the mold components, but repeatable and consistent | Faster for experienced users; requires manual steps |
| Learning Curve | Steep, especially for non-engineers, but after mastering its tools, it may become the best solution, no matter the complexity of the project | Moderate; easier for creative users |
| Mesh Handling | Native support for solids and surfaces | Native support for meshes and sculpting |
| Automation Potential | Higher for industrial workflows (via macros/scripts) | Moderate; can be scripted in Python but lacks built-in AI |
| Mean (SD) | CATIA | Blender |
|---|---|---|
| Patient evaluation | ||
| Esthetic | 9 (±0.71) | 6.6 (±0.55) |
| Comfort and Adaptation | 8.2 (±1.30) | 7.2 (±0.84) |
| Overall Preference | 8.6 (±0.89) | 6.6 (±0.55) |
| Overall Score | 8.6 (±0.40) | 6.8 (±0.35) |
| Experts Evaluation | ||
| Surface Texture | 8.73 (±0.76) | 7.89 (±0.18) |
| Anatomic Details | 9.27 (±0.68) | 7.67 (±0.00) |
| Ease of Handling | 9.67 (±0.00) | 7.47 (±0.18) |
| Overall Score | 9.22 (±0.47) | 7.67 (±0.20) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarba, C.I.; Dragomir, I.; Baciu, I.M.; Burlacu Vatamanu, O.E.; Ghionea, I.G.; Cristache, C.M. A Hybrid Workflow for Auricular Epithesis: Proof of Concept Integrating Mold Design and the Virtual Patient. Prosthesis 2025, 7, 114. https://doi.org/10.3390/prosthesis7050114
Tarba CI, Dragomir I, Baciu IM, Burlacu Vatamanu OE, Ghionea IG, Cristache CM. A Hybrid Workflow for Auricular Epithesis: Proof of Concept Integrating Mold Design and the Virtual Patient. Prosthesis. 2025; 7(5):114. https://doi.org/10.3390/prosthesis7050114
Chicago/Turabian StyleTarba, Cristian Ioan, Ioana Dragomir, Ioana Medeea Baciu, Oana Elena Burlacu Vatamanu, Ionut Gabriel Ghionea, and Corina Marilena Cristache. 2025. "A Hybrid Workflow for Auricular Epithesis: Proof of Concept Integrating Mold Design and the Virtual Patient" Prosthesis 7, no. 5: 114. https://doi.org/10.3390/prosthesis7050114
APA StyleTarba, C. I., Dragomir, I., Baciu, I. M., Burlacu Vatamanu, O. E., Ghionea, I. G., & Cristache, C. M. (2025). A Hybrid Workflow for Auricular Epithesis: Proof of Concept Integrating Mold Design and the Virtual Patient. Prosthesis, 7(5), 114. https://doi.org/10.3390/prosthesis7050114









