Abstract
Background: The use of lower limb prosthesis can impact all aspects of daily life, activities and participation. Various studies have compared the microprocessor-controlled knee (MPK) to the non-microprocessor-controlled knee (NMPK) using a variety of different outcome measures, but results are inconsistent and raise the question of which type of knee is most effective. Therefore, we aimed to assess the effect of MPKs compared to NMPKs across all classified ICF domains in adult prosthesis users. Methods: Participants performed baseline measurements with the NMPK (T0). One week later, they started a four-to-six-week trial period with the MPK. Afterward, measurements were repeated with the MPK (T1). Functional tests (6MWT, TUG-test and activity monitor) and questionnaires (ABC, SQUASH, USER-P and PEQ) were used. For statistical analyses, paired t-tests, Wilcoxon signed-rank tests and Chi2 test were applied. The Benjamini–Hochberg procedure was applied to correct for multiple testing. Results: Twenty-five participants were included. Using an MPK compared to an NMPK significantly resulted in improvements in balance and walking confidence, safety, walking distance and self-reported walking ability, as well as a decrease in number of stumbles and falls. Additionally, participants using an MPK were significantly more satisfied with their participation, experienced fewer restrictions, reported greater satisfaction with the appearance and utility of the MPK, experienced less social burden and reported better well-being, compared to using an NMPK. Conclusions: Using an MPK instead of an NMPK can lead to significant improvements in all classified ICF domains, such as improved walking ability, confidence and satisfaction and reduced fall risk.
Keywords:
lower limb; prosthesis; amputation; ICF; participation; activities; falling; environmental factors 1. Introduction
Persons with a lower limb amputation (LLA) through or above the knee can use a prosthesis with a prosthetic knee unit to regain mobility. Prosthetic knees are generally divided into two categories: non-microprocessor-controlled knees (NMPKs), also known as mechanical knees, and microprocessor-controlled knees (MPKs) (Figure 1). NMPKs can be single axis or polycentric, use pneumatic or hydraulic systems for swing phase control and have various types of stance control. However, one characteristic that nearly all NMPKs have in common is their inability to adapt to different walking surfaces or walking speeds. In contrast, MPKs can automatically adjust to the user’s intent during the swing and stance phase due to built-in sensors and a microprocessor.

Figure 1.
Left: non-microprocessor-controlled knee (Össur Total Knee 2100); right: microprocessor-controlled knee (Ottobock C-leg 4). Reproduced with permission from Össur, Reykjavic, Iceland and Ottobock, Duderstadt, Germany, for use in this publication only.
Multiple studies have compared the effectiveness of MPKs to NMPKs based on different aspects and outcome measures, but results vary between studies and raise the question of which type of knee is most effective. The majority of studies [1,2,3,4,5,6,7] tended to focus on one aspect of prosthesis use, which is walking ability. Regaining and improving walking ability are the main goals of lower limb prosthesis (LLP) use. However, undergoing an amputation and having to use a prosthesis can change a person’s life in nearly all aspects of daily functioning, health, activities and participation [8].
Previous studies have demonstrated that the International Classification of Functioning, Disability and Health model (ICF model) can be used to classify outcome measures for persons with an LLA [9,10,11]. It was concluded that the existing literature primarily focused on laboratory-based measures of gait, while daily activities and participation were relatively underexplored [10,11,12]. Furthermore, it was recommended that future research should investigate the influence of MPKs and NMPKs on activities and participation in day-to-day living conditions [10,11]. Therefore, we aimed to explore a wider array of outcome measures by comparing the MPK to the NMPK using the classified domains of the ICF model [13]. The ICF model is used to characterize a person’s health and functioning based on body functions and structures, activities, participation and environmental factors. Due to the large societal and cultural variance, personal factors are currently not classified in the ICF model [14] and are therefore not included in this study.
1.1. Body Functions and Body Structures
“Body functions are the physiological aspects of body systems, while structures are the anatomical support” [14], meaning that the body itself is considered the structure, and the movements it makes are the functions.
Undergoing an amputation alters a person’s body structure, which can affect multiple areas of the body. For example, painful and non-painful sensations can occur in the unaffected limb, residual limb, phantom limb and back. Both phantom limb pain and residual limb pain are important determinants of a lower quality of life [15]. Other issues that can negatively affect quality of life are sweating in the prosthesis socket and the prevalence of skin irritation [16]. Four studies investigated the effect of different prosthetic knees on body structures, and all found a significant improvement in residual limb health and a decrease in pain in favor of the MPK compared to the NMPK [7,17,18,19].
An LLA can also have a negative impact on body functions, most importantly on one’s balance [20]. It has been demonstrated that balance is negatively affected after a lower limb amputation and that better balance is related to better walking ability [21]. Having an affected sense of balance can lead to lower balance confidence and a higher prevalence of falls and stumbles [22]. Ultimately, this can have a negative influence on the prosthesis user’s activities and participation. Previous studies have demonstrated that over 50% of LLP users reported they had fallen at least once in the previous year and 49% reported they experienced a fear of falling. From this group, 76% said they avoided certain activities due to the fear of falling [23,24]. In another study, LLP users ranked safety as the most important criterion for prosthesis use [25]. Several studies have compared the prevalence of falls and stumbles between the different types of prosthetic knees and reported a significant decrease in stumbles and falls in MPK users compared to NMPK users [3,4,17,26,27,28,29]. Furthermore, one study reported a decrease in falls for MPK users compared to NMPK users of 18.5%, which was conservative compared to reported reductions between 20.3% and 79% [30]. However, one study monitored the number of falls during their measurement period and did not find differences between the MPK users and NMPK users [25]. A different study that compared the use of the MPK compared to the NMPK in first-time prosthesis users also did not report significant differences in fall frequency between groups [31]. Lastly, balance confidence improved significantly in MPK users compared to NMPK users [1,28,31].
1.2. Activities
An individual’s actions and tasks are defined as activities [14]. Being able to perform physical activities can positively influence an individual’s quality of life [32]. Persons with an LLA tend to be less active than the general population, due to physical limitations or fear of falling [24]. Several studies have looked at the physical activities of LLP users. Most studies investigating the differences between MPKs and NMPKs have focused on various walking abilities. Walking ability can be expressed in terms of walking distance, walking speed, walking on even/uneven terrain, walking on an obstacle course, ability to multitask and ability to walk up and down stairs. The majority of the studies showed results in favor of the MPK compared to the NMPK [1,2,3,4,17,25,27,33,34]. However, two studies did not find significant differences between MPKs and NMPKs in walking speed [5,6]. Significant improvements in favor of the MPK over the NMPK were also found in self-reported walking ability scores [1,3,7,35,36].
1.3. Participation
Participation is a broad term to describe someone’s activities and involvement in life situations [14], such as work, study, hobbies and household. The use of an LLP can have a negative impact on an individual’s participation in society with respect to work, sports and leisure activities [12,37,38], with more than 50% of LLP users experiencing restrictions in these areas [12]. Consequently, a decrease in participation can lower the quality of life of LLP users [32]. While limited research has explored the effects of LLP use on participation, to the best of the authors’ knowledge, no studies have studied the difference in participation between MPK users and NMPK users so far.
1.4. Environmental Factors
Environmental factors can have a significant effect on an individual’s functioning [14]. One of the most relevant environmental factors for persons with an LLA is their prosthesis. Using a prosthesis can have an impact on various aspects of daily living, such as activities and participation [12,37], quality of life and satisfaction [16,32]. Previous studies have shown that using an MPK can have a positive influence on the user’s overall quality of life and satisfaction [4,7,17,18,25,27,35,39]. Furthermore, compared to NMPK users, MPK users reported a higher prosthesis-related quality of life while evaluating their prosthesis on different factors such as appearance and utility [4,7,35]. However, one study did not report a difference between MPK and NMPK usage [19].
Besides the prosthesis, LLP users can also make use of various walking aids such as crutches, canes or walkers. While the use of walking aids can help improve a person’s balance, it can also prevent them from participating in activities that require the use of both hands [40]. A previous study has reported that the use of walking aids in persons with an LLA had a negative impact on quality of life [8]. Furthermore, the use of walking aids can significantly increase the energy expenditure for LLP users [41].
Since the existing literature about the effectiveness of MPKs compared to NMPKs shows inconsistencies in the classified domains of the ICF model and knowledge on participation is lacking, it is difficult for rehabilitation teams to come to an evidence-based decision about the prosthesis choice for their patients. Considering that the acquisition costs of an MPK are higher than those of an NMPK [39,42], health insurance companies often require (scientific) proof that the MPK is more suitable for the user [39]. By incorporating all classified domains of the ICF model in one study, we aim to fill the evidence gap and clear up inconsistencies in the literature comparing MPKs and NMPKs.
The goal of this study was to investigate the effectiveness of MPKs compared to NMPKs in persons with an LLA in the standard healthcare system in the Netherlands, in terms of (1) walking ability as expressed in terms of walking distance and self-reported walking ability and (2) all classified ICF domains using a combination of functional tests and questionnaires. Based on the aforementioned literature, we expect to find superiority of the MPK over the NMPK in the classified domains of the ICF model.
2. Methods
The study protocol, including participant information and consent forms, was assessed by the Medical Ethics Committee of the University Medical Center Groningen (METc 2019/419) and was deemed as non-clinical research with human subjects as defined in the Medical Research Involving Human Subjects Act. A waiver for further formal approval was provided by the Medical Ethics Committee. Research was conducted according to the Declaration of Helsinki and its amendments. All participants provided written informed consent. This study was pre-registered on Clinicaltrials.gov: NCT06031922.
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement was utilized to present the results [43].
2.1. Participants
We included participants with a unilateral transfemoral amputation or knee disarticulation due to different etiology, who were using a prosthesis with an NMPK and were found eligible for a trial period on an MPK by their rehabilitation team. The inclusion criteria were (1) at least 18 years old; (2) at least one year post-amputation; and (3) ability to read and write in Dutch. Exclusion criteria were (1) a bilateral amputation; (2) osseointegration; and (3) previous experience using an MPK. Participants were recruited from 11 rehabilitation centers throughout the Netherlands. When rehabilitation physicians identified a potential participant among their patients, they provided the patient with an information letter about the study. If the patient expressed interest in enrolling in the study, the rehabilitation physician or one of the researchers (CB) contacted the potential participant to answer their questions and to ensure informed consent was obtained.
2.2. Study Design
In order to assess the effectiveness of the MPK and NMPK in standard day-to-day living conditions, we applied a multicenter pragmatic clinical trial design, in which participants were tested in a within-subject design in the control situation first (using their NMPK) and subsequently in the experimental situation (using the MPK). Opposed to explanatory trials, which evaluate the efficacy of a treatment in lab settings or under ideal conditions, pragmatic trials evaluate the effectiveness of a treatment in routine clinical practice [44].
2.3. Protocol
For this study, the standard care in the Netherlands was followed as closely as possible. To evaluate whether a person would benefit from an MPK compared to an NMPK, Dutch rehabilitation teams can apply for a trial period with an MPK of a maximum of six weeks, which is reimbursed by the health insurance company. In practice, the duration of this trial period ranges from four to six weeks, depending on the protocol of each rehabilitation center. During this period, the prosthesis user has to partake in several physical tests with both the MPK and the NMPK in order to evaluate the differences. Furthermore, they receive physical therapy during the trial period to help them adjust to the MPK. The trial period and the tests that should be performed are all described in a national protocol for provision of an LLP [45]. If, after the trial period, the prosthesis user and the rehabilitation team agree that an MPK could have a positive outcome on the user’s life, an application with all the test results is sent to the health insurance company.
In total, the participants were monitored for a year and performed four measurements. The first two measurements of this study were integrated into the standard care process and are described in this paper. Two follow-up measurements after the trial period will be published separately. A baseline measurement (T0) was performed while the participants were using their own NMPK (Figure 2). One week later, they received an MPK to start the trial period. During this trial period, they received 30 min of physical therapy twice a week. In the last week of the trial period, all measurements were repeated while using the MPK (T1). After the trial period, participants switched back to their NMPK, and after four weeks of getting used to that, the six-minute walking test (6MWT) and timed up and go test (TUG-test) were repeated (T2). In the case that the application for an MPK was approved by the health insurance company and the participant started using the MPK, the measurements were repeated again (T3), nine months after T2. A timeline of the measurements is displayed in Figure 2.
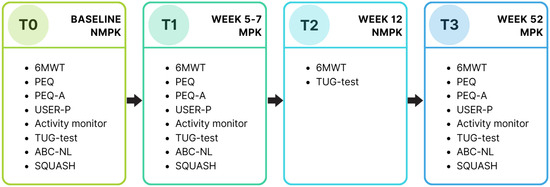
Figure 2.
Timeline of all measurements. Current study describes results of T0 and T1. T0: baseline measurement; T1: measurement 1; T2: measurement 2; T3: measurement 3; NMPK: non-microprocessor-controlled knee; MPK: microprocessor-controlled knee; PEQ: prosthesis evaluation questionnaire; USER-P: Utrechtse Schaal voor Evaluatie van Revalidatie-Participatie (Utrecht Scale for Evaluation of Rehabilitation-Participation); TUG-test: timed up and go test; 6MWT: six-minute walking test; ABC-NL: activities-specific balance confidence scale, Netherlands version (Dutch language version); SQUASH: short questionnaire to assess health-enhancing physical activity.
All measurements were performed by licensed physical therapists working at the participating rehabilitation center, who had experience in working with LLP users. All physical therapists received training from one of the researchers (CB) on the use of the activity monitor prior to the start of the study. Additionally, they were provided with verbal and written instructions for the physical tests.
Since the aim of this study was to follow standard care as closely as possible, and given that each rehabilitation center and prosthetist has their own preferences and makes individualized decisions regarding the type of MPK used, data on the specific models of MPK used for participants was recorded. However, we did not perform comparisons between different types of MPK, as our primary focus was on comparing the NMPK with the MPK, rather than comparing different types of MPK.
2.4. Sample Size
For the sample size calculation, we used the 6MWT, as this outcome measures the participants’ functional walking ability. To demonstrate a clinically relevant difference of 45 m [46] between the MPK and the NMPK, with an expected intra-patient variation of 63.6 [46], an α = 0.05 and power of 0.8, 18 participants were needed to demonstrate a significant difference. Assuming a 25% drop-out, we aimed to include 23 participants.
2.5. Outcome Measures
In order to obtain information across the classified domains of the ICF model, we used a combination of physical tests and questionnaires as outcome measures. All outcome measures were linked to level two and three categories of the ICF classification [9,47] (Appendix A—Table A1).
Walking ability is one of the main aims of LLP use and can be studied utilizing various outcome measures. To accommodate both functional outcomes and patient-reported outcomes, our primary outcome measures were the 6MWT and the ambulation scale of the prosthesis evaluation questionnaire (PEQ) [48].
The 6MWT measures functional capacity and is a very reliable outcome measure to use with persons with an LLA [46]. It is designed to measure walking ability based on walking distance in a set time [49].
The PEQ is a reliable and validated self-report questionnaire to evaluate several aspects of the prosthesis, prosthesis use and prosthesis-related quality of life [46,48]. The recall period for this questionnaire is four weeks. Questions are divided into nine scales (ambulation, appearance, frustration, perceived response, residual limb health, social burden, sounds, utility and well-being) and several themes with separate questions (satisfaction, pain, transfers, prosthetic care, self-efficacy and importance). The scales have also been validated separately and deemed to have a fairly to good reliability [46]. Most questions are scored on a visual analogue scale (VAS) from 0 to 100. Some questions about the frequency of certain problems (e.g., pain in residual limb, phantom limb or back) are scored on a seven-point Likert scale. A higher score on this questionnaire is linked to a more positive outcome. The minimal detectable change with a 90% confidence interval (MDC90) for all nine scales was established for LLP users [46]. Since this questionnaire covers a lot of aspects of prosthesis use, different scales were used to analyze the various domains of the ICF model (Appendix A—Table A2).
Secondary outcome measures were the TUG-test [50], the activities-specific balance confidence scale (ABC) [51], the Utrecht Scale for Evaluation of Rehabilitation-Participation (USER-P) [52], the short questionnaire to assess health-enhancing physical activity (SQUASH) [53], the prosthesis evaluation questionnaire addendum (PEQ-A) [4] and lastly, an activity monitor (Activ8 professional) [54].
2.5.1. Body Functions and Structures
To describe body function, specifically the participants’ confidence in their balance, we utilized the Dutch language version of the activities-specific balance confidence scale (ABC-NL). To describe body structures, we used the residual limb health scale and pain questions from the PEQ. Furthermore, we included the PEQ-A to assess the number of stumbles and falls the participants experienced and what impact this had on their confidence and sense of safety.
Balance confidence: ABC-NL
The ABC Scale is a self-report questionnaire consisting of 16 items [51]. The questionnaire has been validated and is considered to be a reliable outcome measure for persons with an LLA [55]. For each item, the participant has to indicate how much confidence they have in not falling or losing balance during various indoor and outdoor activities. Each question is scored on an 11-point scale, ranging from 0% to 100% confidence (steps of 10%). A higher score corresponds to a higher level of confidence in performing the activity. The MDC90 for this scale is 4.9, which was established for persons with an LLA [56].
Residual limb health and pain: PEQ
The residual limb health scale of the PEQ contains questions about the residual limb that are scored on a VAS (0–100). The pain questions of the PEQ are related to the phantom limb, residual limb, unaffected limb and back (Appendix A—Table A2). Participants are asked to score how often they experienced non-painful sensations and painful sensations (7-point Likert scale from ‘never’ to ‘all the time or almost all the time’) and how long this lasted (7-point Likert scale from ‘I have none’ to ‘more than two days’). Furthermore, participants are asked to rate how intense and bothersome these sensations were on a VAS (0–100).
Number of stumbles and falls: PEQ-A
The PEQ-A contains 14 questions related to the number of stumbles and falls participants experienced and what impact these had on their walking confidence (frustration, embarrassment and fear) [4]. Furthermore, they are asked about the concentration needed to walk with the prosthesis and their sense of safety. Eleven questions are scored on a VAS (0–100), and for the remaining three questions, participants have to fill out the number of falls and stumbles they experienced in the past four weeks. The outcome of this questionnaire is scored on three scales: confidence, concentration and safety. The scores of these scales are based on the 11 questions that were scored on a VAS. We combined the remaining three questions related to the number of falls and stumbles to form a new scale: ‘falls and stumbles’.
2.5.2. Activities
To acquire quantitative data on walking ability in terms of distance, intensity and mobility, participants were instructed to perform two physical tests under the supervision of a licensed physical therapist: the 6MWT and the TUG-test. Furthermore, participants received an activity monitor to collect data on the time spent standing and walking, as well as walking intensity. Since we were also interested in the participants’ perspectives and perceived differences between the MPK and NMPK on their activities, they were also asked to fill out the PEQ ambulation scale and separate questions related to transfers.
Walking distance: 6MWT
The maximum distance a participant can walk in six minutes is measured. The course can be 10, 30 or 50 m. The participant can use their walking aid if needed. The MDC90 was established for LLP users at a distance of 45 m [46].
Mobility and fall risk: TUG-test
The TUG-test assesses lower limb function, mobility and risk of falling [50]. It measures the time it takes to get up from a chair, walk three m comfortably, turn around, walk back and sit down. Participants are allowed to use their walking aids, but no physical assistance or encouragement should be given. This test is a very reliable outcome measure in persons with an LLA and has an MDC90 of 3.6 s [46].
Activity monitor
The Activ8 professional activity monitor (2M Engineering Valkenswaard, The Netherlands) [54] was used to register the participants’ time spent lying/sitting, standing, walking, running and cycling during one full week. The activity monitor was placed by the physical therapist on the ventral side of the thigh, 10 cm distal from the groin area, on the non-affected limb of the participant. The monitor was attached using 3M Tegaderm transparent film. During the week, the participant was asked to fill out a short diary with the time they got up and went to bed; whether they had worn the monitor for the full 24 h; whether they had performed any physical activity that was not in their usual day-to-day routine; and if so, during what time that activity was performed. In order to compare the MPK to the NMPK, we analyzed two outcome measures: (1) the time (percentage per day) participants spent standing, walking or cycling and (2) the walking intensity (expressed in walking counts per minute).
Ambulation and transfers: PEQ
The ambulation scale contains questions regarding a person’s walking abilities in different scenarios (Appendix A—Table A2). The transfer section consists of questions in which the participant is asked to rate their ability to make transfers in various places. All questions are rated on a VAS (0–100).
2.5.3. Participation
To gain insight into multiple areas of participation, participants were asked to fill out two questionnaires: the Utrecht Scale for Evaluation of Rehabilitation-Participation (USER-P) and the short questionnaire to assess health-enhancing physical activity (SQUASH).
Activities, restrictions and satisfaction: USER-P
The USER-P is a valid questionnaire [52] that is used to rate objective and subjective participation. It is composed of 31 items, divided over three scales: (1) frequency: 1a. how much time the participant spends on work, study and household per week, rated on a scale from 0 (not at all) up to 5 (36 h or more), and 1b. how often the participant partakes in certain activities per week, scored from 0 (not at all) up to 5 (19 times or more); (2) restrictions: whether the participant experiences limitations in daily life and whether they could perform certain activities with or without help, scored from 0 (not possible at all) to 3 (independent without difficulty); and (3) satisfaction: how satisfied the participant is with different aspects of their daily life, ranging from 0 (very dissatisfied) to 4 (very satisfied). For both the restrictions and satisfaction sections, it is also possible to choose a ‘not applicable’ option.
Physical activity and participation: SQUASH
The SQUASH is a questionnaire to measure physical activity related to participation in traveling, work, household and leisure time [53]. The list is based on the Dutch Standard for Healthy Exercise [57]. This questionnaire contains 11 items to be completed by the participant. The SQUASH measures the frequency, duration and intensity of four different physical activities, namely physical activity to and from work; household activities; activities at work; and physical activities performed in leisure time. Time spent on each activity is added together, resulting in the total minutes per week spent on physical activities. The higher the score, the more time is spent on physical activities. The SQUASH is a fairly reliable questionnaire for the adult population [53].
2.5.4. Environmental Factors
To evaluate specific features of the prosthesis, the remaining scales and questions of the PEQ were used (Appendix A—Table A2). The scales are appearance, frustration, perceived response, social burden, sounds, utility and well-being. The separate questions cover satisfaction, prosthetic care, self-efficacy and importance. All questions are rated on a VAS (0–100).
Furthermore, the use of walking aids was registered during the functional tests.
2.6. Statistical Analysis
Study data were managed using REDCap electronic data capture tools [58,59]. Statistical analyses were conducted using IBM SPSS Statistics software, version 28 (IBM Corp., Armonk, NY, USA). The dependent variables were functional capacity and all measures of the classified ICF domains. All dependent variables were checked for normal distribution based on histograms, kurtosis and skewness. For tests with normally distributed data, we also checked if the MDC90 value was achieved, as this cannot be performed for non-normally distributed data, which are expressed in medians. Normally distributed variables were compared with a paired t-test, whereas non-normally distributed variables were compared using a Wilcoxon signed-rank test. Categorical variables were compared with a Chi2 test. The independent variable in this study was the time of measurement, which corresponds with NMPK (T0) and MPK (T1) usage, respectively. To prevent a type-I error due to multiple testing, the Benjamini–Hochberg procedure was used to calculate the adjusted p-values [60]. A 95% confidence interval (CI) was used for all statistical tests to assess the precision of the estimated effects. In order to assess the practical significance of the results, we analyzed the effect size of each test. Cohen’s d was used for normally distributed data (0.2 = small effect; 0.5 = medium effect; 0.8 = large effect), and the Wilcoxon effect size (r) was calculated for the not normally distributed data (0.1 = small effect; 0.3 = medium effect; 0.5 = large effect) [61]. Lastly, Cramer’s V was used for categorical data (0.1 = small effect; 0.3 = medium effect; 0.5 = large effect) [61].
3. Results
The inclusion period spanned from January 2021 to August 2024. All consecutive eligible patients (n = 38) were informed about the study. Thirteen potential participants declined to participate in this study. In total, 25 participants (6 women and 19 men) with a median age of 57 years (range 19–75) were included and completed the measurements at T0 (Table 1). The most common cause of amputation was vascular disease (44%). One participant (male; 48 years) dropped out between T0 and T1 due to psychological issues. Another participant (female; 40 years) did complete the physical tests at T1, but refused to fill in the questionnaires and dropped out afterward due to medical issues. Table 2 presents the descriptive statistics and test statistics of all outcome measures at T0 and T1. The descriptive statistics and test statistics of the separate PEQ questions are presented in Appendix A Table A3. The effect sizes of all statistically significant results were medium to large.

Table 1.
Demographics of the participants.

Table 2.
Results.
3.1. Body Functions and Structures
Participants demonstrated significant improvements on the ABC-NL, reflecting an increase in balance confidence while using the MPK compared to the NMPK. Since data was not normally distributed, it is not possible to determine whether the MDC90 threshold was reached. Furthermore, a significant increase in walking confidence, concentration and safety was shown in the results of the PEQ-A with the use of the MPK compared to the NMPK. There was also a significant decrease in the number of stumbles and falls with the use of the MPK compared to the NMPK. No significant changes were found in the residual limb health scale of the PEQ.
Additionally, participants demonstrated no significant differences on nearly all PEQ questions related to pain. However, participants did report a significant decrease in the occurrence of back pain with the use of an MPK compared to the NMPK (Appendix A—Table A3).
3.2. Activities
Within this domain, participants significantly improved their walking distance on the 6MWT with the use of the MPK compared to the NMPK. Although the mean difference between the use of the MPK and NMPK was statistically significant (37.8 ± 38.8), it did not reach the MDC90 threshold of 45 m established for persons with an LLA [46]. The time needed to complete the TUG-test decreased significantly with the use of an MPK compared to an NMPK. However, since data was not normally distributed, it was not possible to determine whether the MDC90 for this scale was reached [46]. Participants also showed a significant improvement in self-reported walking ability with the MPK, which was demonstrated with significantly higher scores on the PEQ ambulation scale. Significant improvements with the use of the MPK compared to the NMPK were also found in all five PEQ questions related to transfers (Appendix A—Table A3).
Both the active time per day and walking intensity measured with the activity monitor did not demonstrate a significant difference between the MPK and NMPK.
3.3. Participation
Participants showed significantly greater satisfaction with the use of the MPK and also reported experiencing significantly less restrictions on the scales of the USER-P compared to the NMPK. No significant difference was demonstrated in the frequency scale of the USER-P or the SQUASH.
3.4. Environmental Factors
Participants reported significant improvement in the appearance and utility of the MPK compared to the NMPK. The improvements in score surpassed the MDC90 thresholds of 14 and 12, respectively. Furthermore, participants experienced significantly less social burden and reported better well-being. No significant differences were shown in the scales perceived response, frustration and sounds. The MDC90 thresholds for the frustration and well-being scales were not reached. Whether or not the MDC90 thresholds for the remaining scales were passed could not be assessed, since the data was non-normally distributed.
In the separate PEQ questions related to satisfaction, participants demonstrated significantly higher satisfaction with their prosthesis and their walking ability with the MPK compared to the NMPK. No significant differences were found on the questions related to prosthetic care. For two of the three questions related to self-efficacy, participants reported significant improvements with the MPK compared to the NMPK. Of the questions related to importance, one resulted in a significant difference (Appendix A—Table A3).
Of the twelve participants who used walking aids with the NMPK, three participants were able to walk without walking aids with the MPK, and one participant made use of one crutch instead of two.
4. Discussion
In this pragmatic multicenter study, we investigated the effectiveness of MPKs compared to NMPKs in terms of (1) walking ability and (2) all classified ICF domains in LLP users following the standard healthcare in the Netherlands. Since regaining walking ability is one of the main goals for LLP users, we assessed walking ability expressed in terms of walking distance and self-reported walking ability. Participants demonstrated significant improvements in walking distance measured with the 6MWT, as well as self-reported walking ability measured with the PEQ with the use of an MPK compared to an NMPK. Furthermore, they were significantly more satisfied with their walking ability while using the MPK compared to the NMPK. Previous studies demonstrated that the existing body of literature mainly focused on laboratory-based gait measurements and recommended that future research should investigate the effects of MPKs and NMPKs on activities and participation in day-to-day living conditions [10,11]. In this study, we compared the MPK to the NMPK across all classified ICF domains and found significant improvements in all domains with the use of an MPK compared to an NMPK. By adopting a pragmatic approach, the results are based on prosthesis use in daily living situations, rather than optimal research conditions, which were often applied in previous studies [2,3,4,6,17,19,25,34].
All statistically significant findings had medium–-to-large effect sizes, suggesting these findings can be used in a practical setting.
4.1. Body Functions and Structures
Participants reported a significant decrease in the number of stumbles and falls with the use of the MPK compared to the NMPK, which confirms earlier findings [4,17,27,62]. Furthermore, participants reported an increased sense of balance confidence and walking confidence and safety when using the MPK compared to the NMPK, which could be related to the decrease in number of stumbles and falls. A previous study has demonstrated that a decrease in number of falls had a positive influence on the balance confidence of LLP users [22]. As safety was noted as the most important factor with prosthesis use [25], the improved confidence may have contributed to an increased sense of safety.
Similar to previous studies [28,31], participants reported a significant improvement in balance confidence based on the ABC. However, while one study reported a significant decrease in falls and stumbles, similar to our findings [28], another study did not report any significant changes in fall frequency [31]. Therefore, we recommend that future research should further investigate the relationship between balance confidence and fall frequency.
No significant difference was found in residual limb health, which contrasts with the findings of three previous studies [7,16,19]. The questions in the residual limb health scale of the PEQ pertain to the amount of sweating in the prosthesis socket, the smell of the prosthesis, swelling of the residual limb and the presence of rashes, ingrown hair and pimples on the residual limb. Sweating and smell of the prosthesis are mostly related to the socket and liner that are used, as opposed to the prosthetic knee that is attached to it, which could explain the minimal difference between the MPK and the NMPK. The other factors are directly tied to the status of the residual limb and the presence of skin issues. One study demonstrated that over 40% of persons with an LLA experienced at least one skin problem and that a higher activity level can increase the chance of developing skin issues [63]. However, these issues may take more time to develop or to be resolved than the relatively short time frame of the trial period.
4.2. Activities
Participants demonstrated significant improvements in walking ability in terms of distance and mobility based on the functional tests within this domain, which is in line with previous studies that found significant functional improvements with the MPK compared to the NMPK [1,25]. These findings were substantiated by the significant improvements in self-reported walking ability, which also complement earlier findings [3,18,35,62]. One study that compared the MPK to the NMPK in first-time prosthesis users did not report significant differences in walking distance on the 6MWT [31], contrasting our findings as well as other studies [1,25]. This disparity could be attributed to the limited walking experience of first-time prosthesis users, regardless of the type of prosthetic knee used.
The combination of functional tests and self-reported outcomes in this domain reveals that the use of an MPK can significantly improve walking abilities in multiple circumstances compared to the NMPK.
Although the measurements with the activity monitor showed a small increase in active time and walking intensity with the use of an MPK compared to the NMPK, these findings were not significant. These findings support earlier findings that demonstrated that the use of an MPK compared to an NMPK had no significant effect on the level or duration of daily activities [64], but contradict another study that found significant improvements in the active time per day with the use of the MPK [62]. Compared to the aforementioned study [62], our participants were younger (mean age 57 vs. 69 years) and had higher active time per day with both types of knee (27% vs. 16% and 30% vs. 20% for the NMPK and MPK, respectively). This could have contributed to the difference in findings.
Walking with an LLP requires significantly more energy than walking with two intact legs. Compared to healthy individuals, the energy cost for persons with a non-vascular or vascular transfemoral amputation was 41% and 102% higher, respectively [65]. While one study did not find statistically significant differences in energy expenditure between the MPK and NMPK [18], another study demonstrated that the use of an MPK compared to an NMPK could result in significantly lower energy expenditure and stated that based on this lower energy expenditure, the use of the MPK could increase walking ability [33]. While our study did not measure the energy expenditure, this could have been a factor that contributed to the increased walking ability we found.
4.3. Participation
Previous research has demonstrated that the literature exploring participation in LLP users is limited, and it was recommended to include this domain in future research [10,12]. To the authors’ knowledge, no previous studies have investigated the influence that the usage of the MPK and NMPK can have on participation. Participants in this study reported a significantly greater satisfaction with their participation and a significant decrease in experienced participation restrictions. However, no significant difference was demonstrated in the self-reported frequency and time spent on physical activities. Changing one’s daily routine of activities and participation might take longer than the observed trial period. One study demonstrated it can take roughly three months to make a small change in exercise behavior [66]. This would comply with the findings of a different study that compared MPKs to NMPKs. They found a 10% increase in the use of a specific mode on the MPK (dedicated sports) six months after prescription of the MPK compared to the end of the trial period [27], which suggested an increase in activity over time. It is advised in future research to evaluate the usage of MPKs after a longer duration of wearing time than the 4–6 weeks trial period to obtain insight into durable shifts in participation and physical activity.
4.4. Environmental Factors
Within this ICF domain, participants reported significantly higher satisfaction with the utility of the MPK; they experienced significantly less social burden and reported significantly better well-being with the MPK compared to the NMPK, which supports the findings of earlier studies [7,18,35,62]. Similar to previous studies [18,35,62], our participants also reported a significantly higher score on the appearance of the MPK compared to the NMPK, which was unexpected since the prosthesis generally does not receive a cosmetic finish during the trial period.
No significant differences were found in the frustration scale. Of all previous studies that utilized the frustration scale to compare the MPK to the NMPK, one reported a significant change [18], while others also did not report any significant differences [3,19,35,62]. The use of an MPK had little effect on the perceived responses that the participants received from their partners, friends and family, and how these responses affected their relationship. Participants scored very high on this scale with both the MPK and NMPK. This suggests that the responses related to their prosthesis use that the participants received from their close relationships were not or only slightly related to the type of prosthetic knee they used. With regard to the sounds scale of the PEQ, we did not find significant differences between the MPK and the NMPK, which supports earlier findings [3,4,19,35].
Three participants who had used walking aids while walking with the NMPK were able to walk without walking aids when they used the MPK. In addition, one participant used one crutch with the MPK instead of the two crutches he utilized while walking with the NMPK. Due to the fact that these participants were now able to engage in activities requiring the use of both hands, it might have resulted in a reduction in perceived participation restrictions. Additionally, walking without walking aids has the advantage of requiring less energy compared to walking with walking aids. However, it is not possible for every user to walk without walking aids, and the higher energy expenditure might be worth it if it results in greater balance and safety [41].
4.5. Strengths and Limitations
The use of a multicenter pragmatic trial design, combined with a substantial number of participants from diverse demographic backgrounds, provided a strong representation of our target population. Even though the male/female ratio in this study (76% male) was slightly higher compared to other studies (61–69%) [67,68], we believe our study sample was representative of the real-world clinical setting. The medium-to-large effect sizes for the significant results further highlight the validity and generalizability of our findings. Additionally, the combination of functional tests and patient-reported outcome measures demonstrates that the observed improvements with the use of an MPK compared to an NMPK across the classified ICF domains were not only measurable but also perceived by participants.
Participants who were included in this study were all in some way unsatisfied with the use of their NMPK, which is why the process of applying for a trial period with an MPK was started. This could have resulted in lower baseline scores on the questionnaires for the NMPK if compared to satisfied NMPK users. However, this presumably would not translate to lower scores on the functional tests.
The pragmatic study protocol is a strength of this study since it was designed to follow the standard care in the Netherlands as closely as possible. While a double-blinded randomized controlled trial could offer a stronger internal validity, it can also limit external validity and generalizability. Our design aimed to balance a strong methodology with clinical relevance, providing insights that are directly applicable to everyday clinical settings.
We aimed to equalize any differences in physical therapy or gait training between the rehabilitation centers. However, there was most likely a slight variation in the exact form of physical therapy each participant received, due to the different physical therapists and each individual’s abilities.
The current analyses focused on the initial phase of the study, presenting data from measurements T0 and T1. Although additional follow-up measurements (T2 and T3) were conducted, these results will be addressed in a follow-up publication. As such, the findings presented in this paper primarily reflect short-term outcomes and should be interpreted within that temporal context.
The classified domains of the ICF model provided a structured framework to evaluate the effectiveness of the MPK compared to the NMPK. However, individual differences (such as demographic or psychosocial characteristics) that are captured in the non-classified domain ‘personal factors’ may have influenced the observed findings. Future research should explore these dimensions to better understand the variability in patient responses.
The PEQ is widely used and has been translated and validated in numerous languages [69,70,71,72,73,74]. However, the PEQ and PEQ-A had not yet been translated and validated for use in Dutch. Before the start of this study, we translated and validated the PEQ and PEQ-A according to the guidelines for the process of cross-cultural adaptation of self-report measures [75]. The results of our validation study demonstrated that the Dutch language version is a valid and reliable questionnaire and will be published at a later stage.
The Dutch national protocol for the prescription of LLPs contains a specific protocol for the evaluation of the MPK trial period. At the baseline and at the end of the trial period, six functional tests are performed to compare the MPK to the NMPK. These tests are (1) 6MWT; (2) TUG-test; (3) obstacle course/outside training area/slope; (4) Berg Balance Scale; (5) multitasking; and (6) walking on stairs. Some of these tests are not standardized, and facilities to perform these tests vary between different rehabilitation centers. Furthermore, with regard to factors such as fear of falling, balance confidence, dynamic use and participation, no pre- and post-tests are required. The rehabilitation team is asked to provide feedback on the relevance of potential MPK use related to these factors, instead of reporting the results of reliable tests. This study demonstrated that these factors can be measured and could improve significantly with the use of an MPK. We would recommend including validated and reliable patient-reported outcome measures such as the ABC or PEQ in these evaluations as well to further support the findings of the rehabilitation team and the LLP user.
Furthermore, we found that 19 out of the 25 included participants had undergone their amputation in the last two years. The total costs per year associated with the prescriptions of LLPs in the Netherlands have increased in the last decade [76], and although this increase cannot fully be attributed to the prescription of MPKs, we would recommend that research should be conducted to identify potential indicators in first-time prosthesis users to reveal if an MPK would be more beneficial. By starting with an MPK instead of trying the NMPK first, this could save time, effort and frustration for the end-user and the rehabilitation team, as well as lower the healthcare cost. Although these recommendations pertain specifically to the Dutch healthcare system, they are also strongly advised for the evaluation of LLP use worldwide.
5. Conclusions
The effectiveness of MPKs compared to NMPKs across all classified domains of the ICF model was investigated. As was hypothesized based on the existing literature, we found significant improvements with the MPK compared to the NMPK in all classified domains of the ICF model. Participants using an MPK significantly improved their walking ability in terms of distance, speed and self-reported walking ability, compared to using an NMPK. Furthermore, we found a significant increase in balance and walking confidence and sense of safety, which was also reflected in a significant decrease in number of stumbles and falls with the use of an MPK compared to an NMPK. Lastly, participants using an MPK were significantly more satisfied with their participation, their prosthesis and their walking ability, compared to using an NMPK. We therefore recommend considering all classified ICF domains when selecting or evaluating a new prosthetic knee, rather than focusing solely on regaining or improving walking ability.
Author Contributions
Conceptualization, C.E.B., B.L.S., J.H.B.G., A.H.V. and C.K.v.d.S.; methodology, C.E.B., J.H.B.G., A.H.V. and C.K.v.d.S.; formal analysis, C.E.B. and B.L.S.; investigation, C.E.B., B.F., I.E.N. and M.A.P.; data curation, C.E.B.; writing—original draft preparation, C.E.B.; writing—review and editing, B.L.S., J.H.B.G., B.F., I.E.N., M.A.P., A.H.V. and C.K.v.d.S.; visualization, C.E.B.; supervision, B.L.S., J.H.B.G., A.H.V. and C.K.v.d.S.; project administration, C.E.B.; funding acquisition, J.H.B.G., A.H.V. and C.K.v.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by ZonMW as part of a larger research project entitled ‘Effectiveness and cost-effectiveness of lower limb prostheses’ (project number: 853001109; URL: https://projecten.zonmw.nl/nl/project/doelmatige-zorg-van-beenprothesen).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and its amendments. The Medical Ethics Committee of the University Medical Center Groningen assessed the study protocol and deemed it as non-clinical research with human subjects as defined in the Medical Research Involving Human Subjects Act. A waiver for further formal approval was provided (METc 2019/419 approval date: 19 April 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
Data will be available through DataverseNL, upon reasonable request.
Acknowledgments
The authors would like to express their sincere gratitude to all the rehabilitation physicians and physical therapists from the participating rehabilitation centers listed below for their assistance in recruiting participants and conducting measurements. Adelante Rehabilitation; Heliomare Rehabilitation Center; Hoogstraat Rehabilitation; Libra Rehabilitation and Audiology; Revant Rehabilitation Center; Rijndam Rehabilitation Center; Roessingh Center for Rehabilitation; Tolbrug Rehabilitation; University Medical Center Groningen, Center for Rehabilitation; and Vogellanden, Center for Rehabilitation.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| MPK | Microprocessor-controlled knee |
| NMPK | Non-microprocessor-controlled knee |
| LLA | Lower limb amputation |
| LLP | Lower limb prosthesis |
| ICF | International Classification of Functioning, Disability and Health |
| STROBE | The Strengthening the Reporting of Observational Studies in Epidemiology |
| 6MWT | Six-minute walking test |
| TUG-test | Timed up and go test |
| PEQ | Prosthesis evaluation questionnaire |
| VAS | Visual analogue scale |
| ABC | Activities-specific balance confidence |
| USER-P | Utrecht Scale for Evaluation of Rehabilitation-Participation |
| SQUASH | Short questionnaire to assess health-enhancing physical activity |
| PEQ-A | Prosthesis evaluation questionnaire—addendum |
| MDC90 | Minimal detectable change with 90% confidence interval |
Appendix A

Table A1.
Outcome measures linked to ICF categories.
Table A1.
Outcome measures linked to ICF categories.
| Outcome Measure | Measured Construct | ICF Category * | Explanation [47] |
|---|---|---|---|
| Body functions and structures | |||
| ABC-NL | Balance confidence | b755 | Functions of involuntary contractions of large muscles or the whole body induced by body position, balance and threatening stimuli. |
| b1266 | Mental functions that produce a personal disposition that is self-assured, bold and assertive, in contrast to being timid, insecure and self-effacing. | ||
| PEQ-RL | Residual limb health | s750 | Structure of lower extremity. |
| s8104 | Skin of lower extremity. | ||
| PEQ-A | Walking confidence, concentration, safety, stumbles and falls | b1266 | Mental functions that produce a personal disposition that is self-assured, bold and assertive, in contrast to being timid, insecure and self-effacing. |
| b147 | Specific mental functions of control both motor and psychological events at the body level. | ||
| Activities | |||
| 6MWT | Walking distance | d4500 | Walking for less than a kilometer, such as walking around rooms or hallways, within a building or for short distances outside. |
| TUG | Mobility and fall risk | d499 | Mobility, unspecified. |
| PEQ-AM | Self-reported walking ability | d450 | Moving along a surface on foot, step by step, so that one foot is always on the ground, such as when strolling, sauntering, walking forwards, backwards or sideways. |
| Activity monitor | Active time and walking intensity | d920 | Engaging in any form of play, recreational or leisure pursuit, such as informal or organized play and sports, programs of physical fitness, relaxation, amusement or diversion, going to art galleries, museums, cinemas or theatres; engaging in crafts or hobbies, reading or singing for enjoyment, playing musical instruments; sightseeing, tourism and traveling for pleasure. |
| Participation | |||
| USER-P | Activities, restrictions and satisfaction | d859 | Work and employment. |
| Squash | Physical activity and participation | d920 | Recreation and leisure. |
| Environmental factors | |||
| PEQ | Prosthesis features | e1151 | Adapted or specially designed equipment, products and technologies that assist people in daily living, such as prosthetic and orthotic devices, neural prostheses (e.g., functional stimulation devices that control bowels, bladder, breathing and heart rate) and environmental control units aimed at facilitating individuals’ control over their indoor setting (scanners, remote control systems, voice-controlled systems, timer switches). |
| Walking aid | Walking aid use | e1201 | Adapted or specially designed equipment, products and technologies that assist people to move inside and outside buildings, such as walking devices (such as canes or crutches), special cars and vans, adaptations to vehicles, wheelchairs, scooters and transfer devices. |
* All domains are divided into various categories, which are coded. The letters b, s, d and e represent the domains, followed by a numeric code. This code starts with a single-digit chapter number, followed by two digits for the second level and additional digits for the third and fourth levels. E.g., the category d450 walking is subdivided into d4500 walking short distances, d4501 walking long distances, etc. [14]. ABC-NL: activities-specific balance confidence scale—Dutch language version; PEQ-A: prosthesis evaluation questionnaire addendum; PEQ: prosthesis evaluation questionnaire; 6MWT: six-minute walking test; TUG-test: timed up and go test; A8: Activ8 activity monitor; USER-P: Utrecht Scale for Evaluation of Rehabilitation-Participation; SQUASH: short questionnaire to assess health-enhancing physical activity.

Table A2.
PEQ scales and questions divided into ICF categories.
Table A2.
PEQ scales and questions divided into ICF categories.
| Theme (Scale or Questions) | Number of Questions | Measured Constructs | MDC90 |
|---|---|---|---|
| Body functions and structures | |||
| Residual limb health (scale) | 6 |
| 8 |
| Pain (questions) | 16 | Pain and (non-)painful sensations in
| N/A |
| Activities | |||
| Ambulation (scale) | 8 |
| 11 |
| Transfer (questions) | 5 |
| N/A |
| Environmental factors | |||
| Appearance (scale) | 5 |
| 14 |
| Frustration (scale) | 2 |
| 16 |
| Perceived response (scale) | 5 |
| 9 |
| Social burden (scale) | 3 |
| 14 |
| Sounds (scale) | 2 |
| 17 |
| Utility (scale) | 8 |
| 12 |
| Well-being (scale) | 2 |
| 14 |
| Satisfaction (questions) | 3 |
| N/A |
| Prosthetic care (questions) | 3 |
| N/A |
| Self-efficacy (questions) | 3 |
| N/A |
| Importance (questions) | 10 |
| N/A |

Table A3.
Results of separate questions from PEQ.
Table A3.
Results of separate questions from PEQ.
| Week 1 (NMPK) | Week 7 (MPK) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | M ± SD; Median (Range) | N | M ± SD; Median (Range) | Mean Difference ± SD (Confidence Interval); Z-Value | DoF | p-Value | Adjusted p-Value | Cohen’s d; [r] | |
| Satisfaction | |||||||||
| Over the past four weeks, rate how happy you have been with your current prosthesis. | 25 | 56 (2–87) | 23 | 92 (27–100) | −3.3 | 22 | <0.001 ‡ | 0.006 ‡ | [0.38] |
| Over the past four weeks, rate how satisfied you have been with your prosthesis. | 23 | 63 (22–100) | 22 | 95 (65–100) | −3.4 | 19 | <0.001 ‡ | 0.005 † | [0.41] |
| Over the past four weeks, rate how satisfied you have been with how you are walking. | 24 | 53.5 (21–78) | 22 | 88.5 (22–100) | −3.9 | 20 | <0.001 ‡ | 0.004 ‡ | [0.43] |
| Pain | |||||||||
| Over the past four weeks, rate how often you have been aware of non-painful sensations in your phantom limb. | 18 | 3 (0–6) | 21 | 2 (0–6) | −0.2 | 13 | 0.852 | 0.894 | [0.12] |
| If you had non-painful sensations in your phantom limb during the past month, rate how intense they were on average. | 15 | 62.2 ± 27.2 | 17 | 69.8 ± 25.8 | 5.5 ± 16.3 (−5.5–16.4) | 10 | 0.292 | 0.486 | 0.34 |
| Over the past four weeks, how bothersome were these sensations in your phantom limb? | 18 | 64.9 ± 29.6 | 18 | 66.9 ± 29.5 | −1.9 ± 36.7 (−23.1–19.3) | 13 | 0.853 | 0.894 | −0.05 |
| Over the past four weeks, rate how often you had pain in your phantom limb. | 23 | 1 (0–6) | 22 | 1 (0–6) | −1 | 19 | 0.310 | 0.496 | [0.23] |
| How long does your phantom limb pain usually last? | 22 | 1 (0–6) | 22 | 1 (0–6) | −0.3 | 18 | 0.782 | 0.894 | [0.12] |
| If you had any pain in your phantom limb this past month, rate how intense it was on average. | 14 | 47.3 ± 23.9 | 16 | 58 ± 27 | 4.0 ± 18.9 (−10.5–18.5) | 8 | 0.544 | 0.725 | 0.21 |
| In the past four weeks, how bothersome was the pain in your phantom limb? | 14 | 66.5 (3–96) | 15 | 71 (9–96) | −0.6 | 7 | 0.574 | 0.741 | [0.26] |
| Over the past four weeks, rate how often you had pain in your residual limb. | 22 | 1 (0–4) | 22 | 1 (0–5) | −0.2 | 18 | 0.822 | 0.894 | [0.11] |
| If you had any pain in your residual limb over the past four weeks, rate how intense it was on average. | 12 | 61 ± 26.1 | 15 | 65.1 ± 31.6 | 2.0 ± 6.0 (−3.0–7.0) | 7 | 0.375 | 0.578 | 0.34 |
| Over the past four weeks, how bothersome was the pain in your residual limb? | 13 | 65 ± 23.2 | 14 | 66 ± 28.1 | −2.8 ± 18.9 (−18.5–13.0) | 7 | 0.692 | 0.865 | −0.15 |
| Over the past four weeks, rate how often you had pain in your other leg or foot. | 22 | 1 (0–6) | 23 | 0 (0–5) | −1.3 | 20 | 0.203 | 0.386 | [0.25] |
| If you had any pain in your other leg or foot over the past four weeks, rate how intense it was on average. | 14 | 55.9 ± 27.9 | 13 | 63 ± 31.7 | 0.4 ± 21.0 (−14.6–15.4) | 9 | 0.953 | 0.953 | 0.02 |
| Over the past four weeks, how bothersome was the pain in your other leg or foot? | 14 | 55.1 ± 31.3 | 13 | 64.9 ± 33.4 | 2.1 ± 23.7 (−14.9–19.1) | 9 | 0.786 | 0.894 | 0.09 |
| Over the past four weeks, rate how often you experienced back pain. | 23 | 1 (0–6) | 23 | 0 (0–5) | −2.5 | 21 | 0.012 † | 0.044 † | [0.34] |
| If you had any back pain over the past four weeks, rate how intense it was on average. | 17 | 66.1 ± 24.3 | 11 | 71.9 ± 29.1 | 5.3 ± 11.2 (−2.7–13.3) | 9 | 0.169 | 0.356 | 0.47 |
| Over the past four weeks, how bothersome was the back pain? | 17 | 67 (11–100) | 11 | 78 (20–100) | −1.2 | 9 | 0.233 | 0.424 | [0.35] |
| Transfer | |||||||||
| Over the past four weeks, rate your ability to get in and out of a car when using your prosthesis. | 23 | 80 (32–100) | 22 | 95 (73–100) | −3.4 | 20 | <0.001 ‡ | 0.005 ‡ | [0.4] |
| Over the past four weeks, rate your ability to sit down and get up from a chair with a high seat (e.g., a dining chair, a kitchen chair, an office chair). | 24 | 78.5 (31–100) | 22 | 96 (14–100) | −2.7 | 20 | 0.008 ‡ | 0.031 † | [0.36] |
| Over the past four weeks, rate your ability to sit down and get up from a low or soft chair (e.g., an easy chair or deep sofa). | 24 | 57 (8–85) | 22 | 87.5 (24–100) | −3.6 | 20 | <0.001 ‡ | 0.004 ‡ | [0.41] |
| Over the past four weeks, rate your ability to sit down and get up from the toilet. | 24 | 78 (2–100) | 22 | 95.5 (2–100) | −3.6 | 20 | <0.001 ‡ | 0.004 ‡ | [0.41] |
| Over the past four weeks, rate your ability to shower or bathe safely. | 24 | 83 (3–100) | 22 | 95 (7–100) | −2.7 | 20 | 0.006 ‡ | 0.027 † | [0.36] |
| Prosthetic care | |||||||||
| How satisfied are you with the person who fits your current prosthesis? | 24 | 92.5 (28–100) | 22 | 93.5 (17–100) | −1.4 | 20 | 0.150 | 0.343 | [0.26] |
| How satisfied are you with the training you have received on using your current prosthesis? | 21 | 88 (54–100) | 22 | 94 (31–100) | −1.4 | 17 | 0.154 | 0.343 | [0.28] |
| Overall, how satisfied are you with the gait and prosthetic training you have received since your amputation? | 22 | 78.5 (15–98) | 22 | 90.5 (25–100) | −2.1 | 18 | 0.035 † | 0.108 | [0.33] |
| Self-efficacy | |||||||||
| When the fit of my prosthesis is poor, I will get… [VAS: nothing done–everything done] | 23 | 33.6 ± 23.5 | 20 | 51.8 ± 21.8 | 20.8 ± 28.1 (7.3–34.4) | 18 | 0.005 ‡ | 0.023 † | 0.74 |
| When the comfort of my prosthesis is poor, I will get… [VAS: nothing done–everything done] | 23 | 38.2 ± 24.7 | 19 | 52.8 ± 19.8 | 18.7 ± 23.0 (7.3–30.2) | 17 | 0.003 ‡ | 0.017 † | 0.81 |
| Without my prosthesis, I will get… [VAS: nothing done–everything done] | 23 | 31.7 ± 28.4 | 21 | 37.4 ± 29.7 | 3.6 ± 21.2 (−6.4–13.5) | 19 | 0.464 | 0.669 | 0.17 |
| Importance | |||||||||
| How important is it that the weight of your prosthesis feel right? | 24 | 90 (3–100) | 21 | 93 (65–100) | −1.9 | 19 | 0.061 | 0.163 | [0.31] |
| How important is the ease of putting on (donning) your prosthesis? | 24 | 93.5 (49–100) | 21 | 95 (64–100) | −1.3 | 19 | 0.198 | 0.386 | [0.25] |
| How important is the appearance of your prosthesis (how it looks)? | 24 | 60.8 ± 31 | 21 | 60.8 ± 35 | 3.8 ± 25.8 (−8.3–15.9) | 19 | 0.519 | 0.715 | 0.15 |
| How important is it to you to be able to wear different kinds of shoes (heights or styles)? | 24 | 87.5 (3–100) | 21 | 79 (9–100) | −0.7 | 19 | 0.469 | 0.669 | [0.19] |
| How important is it that your prosthesis’ covering is durable (cannot be torn, dented, easily scratched or discolored)? | 23 | 63 ± 38.8 | 20 | 69.9 ± 31.3 | 8.6 ± 18.2 (−0.3–17.4) | 18 | 0.056 | 0.159 | 0.47 |
| How bothersome is it when you sweat a lot inside your prosthesis (in the sock, liner, socket)? | 24 | 20 (2–100) | 23 | 50 (0–100) | −1.8 | 21 | 0.073 | 0.183 | [0.29] |
| How bothersome to you is swelling in your residual limb (stump)? | 24 | 14 (0–100) | 22 | 32 (0–100) | −2.5 | 20 | 0.013 † | 0.044 † | [0.34] |
| How important is it to avoid having any ingrown hairs (pimples) on your residual limb (stump)? | 24 | 92 (6–100) | 23 | 92 (0–100) | −0.3 | 21 | 0.777 | 0.894 | [0.11] |
| How bothersome is it to see people looking at you and your prosthesis? | 24 | 93 (3–100) | 23 | 94 (0–100) | −0.2 | 21 | 0.872 | 0.894 | [0.09] |
| How important is being able to walk up a steep hill? | 24 | 90.5 (30–100) | 23 | 80 (0–100) | −1.1 | 21 | 0.291 | 0.486 | [0.22] |
† Significant at p ≤ 0.05; ‡ Significant at p ≤ 0.01. Cohen’s d: 0.2 = small effect; 0.5 = medium effect; 0.8 = large effect Wilcoxon effect size (r): 0.1 = small effect; 0.3 = medium effect; 0.5 = large effect.
References
- Burnfield, J.M.; Eberly, V.J.; Gronely, J.K.; Perry, J.; Yule, W.J.; Mulroy, S.J. Impact of stance phase microprocessor-controlled knee prosthesis on ramp negotiation and community walking function in K2 level transfemoral amputees. Prosthet. Orthot. Int. 2012, 36, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Eberly, V.J.; Mulroy, S.J.; Gronley, J.K.; Perry, J.; Yule, W.J.; Burnfield, J.M. Impact of a stance phase microprocessor-controlled knee prosthesis on level walking in lower functioning individuals with a transfemoral amputation. Prosthet. Orthot. Int. 2013, 38, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Hafner, B.J.; Smith, D.G. Differences in function and safety between Medicare Functional Classification Level-2 and -3 transfemoral amputees and influence of prosthetic knee joint control. J. Rehabil. Res. Dev. 2009, 46, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Hafner, B.J.; Willingham, L.L.; Buell, N.C.; Allyn, K.J.; Smith, D.G. Evaluation of Function, Performance, and Preference as Transfemoral Amputees Transition from Mechanical to Microprocessor Control of the Prosthetic Knee. Arch. Phys. Med. Rehabil. 2007, 88, 207–217. [Google Scholar] [CrossRef]
- Cao, W.; Yu, H.; Zhao, W.; Meng, Q.; Chen, W. The comparison of transfemoral amputees using mechanical and microprocessor- controlled prosthetic knee under different walking speeds: A randomized cross-over trial. Technol. Health Care 2018, 26, 581–592. [Google Scholar] [CrossRef]
- Williams, R.M.; Turner, A.P.; Orendurff, M.; Segal, A.D.; Klute, G.K.; Pecoraro, J.; Czerniecki, J. Does having a computerized prosthetic knee influence cognitive performance during amputee walking? Arch. Phys. Med. Rehabil. 2006, 87, 989–994. [Google Scholar] [CrossRef]
- Theeven, P.; Hemmen, B.; Geers, R.; Smeets, R.; Brink, P.; Seelen, H. Influence of advanced prosthetic knee joints on perceived performance and everyday life activity level of low-functional persons with a transfemoral amputation or knee disarticulation. J. Rehabil. Med. 2012, 44, 454–461. [Google Scholar] [CrossRef]
- Sinha, R.; van den Heuvel, W.J.; Arokiasamy, P. Factors affecting quality of life in lower limb amputees. Prosthet. Orthot. Int. 2011, 35, 90–96. [Google Scholar] [CrossRef]
- Xu, J.; Kohler, F.; Dickson, H. Systematic review of concepts measured in individuals with lower limb amputation using the International Classification of Functioning, Disability and Health as a reference. Prosthet. Orthot. Int. 2011, 35, 262–268. [Google Scholar] [CrossRef]
- Clarke, L.; Ridgewell, E.; Dillon, M.P. Identifying and linking prosthetic outcomes to the ICF framework: A step to inform the benefits measured in prosthetic health economic evaluations. Disabil. Rehabil. 2022, 45, 1103–1113. [Google Scholar] [CrossRef]
- Theeven, P.J.; Hemmen, B.; Brink, P.R.; Smeets, R.J.; Seelen, H.A. Measures and procedures utilized to determine the added value of microprocessor-controlled prosthetic knee joints: A systematic review. BMC Musculoskelet. Disord. 2013, 14, 333. [Google Scholar] [CrossRef]
- Gallagher, P.; O’dOnovan, M.-A.; Doyle, A.; Desmond, D. Environmental barriers, activity limitations and participation restrictions experienced by people with major limb amputation. Prosthet. Orthot. Int. 2011, 35, 278–284. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Classification of Functioning, Disability and Health: ICF. 2001. Available online: https://apps.who.int/iris/handle/10665/42407 (accessed on 7 September 2023).
- World Health Organization. How to Use the ICF: A Practical Manual for Using the International Classification of Functioning, Disability and Health (ICF). Exposure Draft for Comment. October 2013. Available online: https://cdn.who.int/media/docs/default-source/classification/icf/drafticfpracticalmanual2.pdf?sfvrsn=8a214b01_4&download=true (accessed on 7 September 2023).
- van der Schans, C.P.; Geertzen, J.H.; Schoppen, T.; Dijkstra, P.U. Phantom Pain and Health-Related Quality of Life in Lower Limb Amputees. J. Pain. Symptom Manag. 2002, 24, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, K.; Brånemark, R. Consequences of non-vascular trans-femoral amputation: A survey of quality of life, prosthetic use and problems. Prosthet. Orthot. Int. 2001, 25, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Kahle, J.T.; Highsmith, M.J.; Hubbard, S.L. Comparison of nonmicroprocessor knee mechanism versus C-Leg on Prosthesis Evaluation Questionnaire, stumbles, falls, walking tests, stair descent, and knee preference. J. Rehabil. Res. Dev. 2008, 45, 1–14. [Google Scholar] [CrossRef]
- Kaufman, K.R.; Levine, J.A.; Brey, R.H.; McCrady, S.K.; Padgett, D.J.; Joyner, M.J. Energy Expenditure and Activity of Transfemoral Amputees Using Mechanical and Microprocessor-Controlled Prosthetic Knees. Arch. Phys. Med. Rehabil. 2008, 89, 1380–1385. [Google Scholar] [CrossRef]
- Prinsen, E.C.; Nederhand, M.J.; Olsman, J.; Rietman, J.S. Influence of a user-adaptive prosthetic knee on quality of life, balance confidence, and measures of mobility: A randomised cross-over trial. Clin. Rehabil. 2014, 29, 581–591. [Google Scholar] [CrossRef]
- Buckley, J.G.; O’dRiscoll, D.; Bennett, S.J. Postural Sway and Active Balance Performance in Highly Active Lower-Limb Amputees. Am. J. Phys. Med. Rehabil. 2002, 81, 13–20. [Google Scholar] [CrossRef]
- van Velzen, J.M.; van Bennekom, C.A.; Polomski, W.; Slootman, J.R.; van der Woude, L.H.; Houdijk, H. Physical capacity and walking ability after lower limb amputation: A systematic review. Clin. Rehabil. 2006, 20, 999–1016. [Google Scholar] [CrossRef]
- Wong, C.K.; Rheinstein, J.; Stern, M.A. Benefits for Adults with Transfemoral Amputations and Peripheral Artery Disease Using Microprocessor Compared with Nonmicroprocessor Prosthetic Knees. Am. J. Phys. Med. Rehabil. 2015, 94, 804–810. [Google Scholar] [CrossRef]
- Miller, W.C.; Deathe, A.; Speechley, M.; Koval, J. The influence of falling, fear of falling, and balance confidence on prosthetic mobility and social activity among individuals with a lower extremity amputation. Arch. Phys. Med. Rehabil. 2001, 82, 1238–1244. [Google Scholar] [CrossRef]
- Miller, W.C.; Speechley, M.; Deathe, B. The prevalence and risk factors of falling and fear of falling among lower extremity amputees. Arch. Phys. Med. Rehabil. 2001, 82, 1031–1037. [Google Scholar] [CrossRef]
- Lansade, C.; Vicaut, E.; Paysant, J.; Ménager, D.; Cristina, M.-C.; Braatz, F.; Domayer, S.; Pérennou, D.; Chiesa, G. Mobility and satisfaction with a microprocessor-controlled knee in moderately active amputees: A multi-centric randomized crossover trial. Ann. Phys. Rehabil. Med. 2018, 61, 278–285. [Google Scholar] [CrossRef]
- Berry, D.C.; Olson, M.D.; Larntz, K. Perceived Stability, Function, and Satisfaction Among Transfemoral Amputees Using Microprocessor and Nonmicroprocessor Controlled Prosthetic Knees: A Multicenter Survey. JPO J. Prosthet. Orthot. 2009, 21, 32–42. [Google Scholar] [CrossRef]
- Lansade, C.; Chiesa, G.; Paysant, J.; Vicaut, E.; Cristina, M.-C.; Ménager, D. Impact of C-LEG on mobility, satisfaction and quality of life in a multicenter cohort of femoral amputees. Ann. Phys. Rehabil. Med. 2021, 64, 101386. [Google Scholar] [CrossRef]
- Davie-Smith, F.; Carse, B. Comparison of patient-reported and functional outcomes following transition from mechanical to microprocessor knee in the low-activity user with a unilateral transfemoral amputation. Prosthet. Orthot. Int. 2021, 45, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Wurdeman, S.R.; Miller, T.A.; Stevens, P.M.; Campbell, J.H. Stability and Falls Evaluations in AMPutees (SAFE-AMP 1): Microprocessor knee technology reduces odds of incurring an injurious fall for individuals with diabetic/dysvascular amputation. Assist. Technol. 2023, 35, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Dobson, A.; Beins, M.; DaVanzo, J.; Kim, S.; McMahon, P.; Haught, R.; Hasselbrink, R.; Gonzalez, S.; Kannenberg, A.; Seidinger, S. Retrospective cohort study of the economic value of providing microprocessor knees to the population of Medicare fee-for-service K2 beneficiaries with a knee disarticulation/above knee amputation. Prosthet. Orthot. Int. 2024. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.J.; Friedly, J.L.; Nelson, I.K.; Rosen, R.E.; Humbert, A.T.; Hafner, B.J. The effects of microprocessor prosthetic knee use in early rehabilitation: A pilot randomized controlled trial. PMR 2025, 17, 371–383. [Google Scholar] [CrossRef]
- Deans, S.A.; McFadyen, A.K.; Rowe, P.J. Physical activity and quality of life: A study of a lower-limb amputee population. Prosthet. Orthot. Int. 2008, 32, 186–200. [Google Scholar] [CrossRef]
- Seymour, R.; Engbretson, B.; Kott, K.; Ordway, N.; Brooks, G.; Crannell, J.; Hickernell, E.; Wheeler, K. Comparison between the C-leg microprocessor-controlled prosthetic knee and non-microprocessor control prosthetic knees: A preliminary study of energy expenditure, obstacle course performance, and quality of life survey. Prosthet. Orthot. Int. 2007, 31, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Segal, A.D.; Orendurff, M.S.; Klute, G.K.; McDowell, M.L.; Pecoraro, J.A.; Shofer, J.; Czerniecki, J.M. Kinematic and kinetic comparisons of transfemoral amputee gait using C-Leg and Mauch SNS prosthetic knees. J. Rehabil. Res. Dev. 2006, 43, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Alzeer, A.M.; Raj, N.B.; Shahine, E.M.; Nadiah, W.-A. Impacts of Microprocessor-Controlled Versus Non-microprocessor-Controlled Prosthetic Knee Joints Among Transfemoral Amputees on Functional Outcomes: A Comparative Study. Cureus 2022, 14, e24331. [Google Scholar] [CrossRef] [PubMed]
- Gerzeli, S.; Torbica, A.; Fattore, G. Cost utility analysis of knee prosthesis with complete microprocessor control (C-leg) compared with mechanical technology in trans-femoral amputees. Eur. J. Health Econ. 2008, 10, 47–55. [Google Scholar] [CrossRef]
- Couture, M.; Caron, C.D.; Desrosiers, J. Leisure activities following a lower limb amputation. Disabil. Rehabil. 2009, 32, 57–64. [Google Scholar] [CrossRef]
- Whyte, A.S.; Carroll, L.J. A preliminary examination of the relationship between employment, pain and disability in an amputee population. Disabil. Rehabil. 2002, 24, 462–470. [Google Scholar] [CrossRef]
- Seelen, H.; Hemmen, B.; Schmeets, A.; Ament, A.; Evers, S. Costs and consequences of a prosthesis with an electronically stance and swing phase controlled knee joint. Technol. Disabil. 2009, 21, 25–34. [Google Scholar] [CrossRef]
- Azuma, Y.; Chin, T.; Miura, Y. The relationship between balance ability and walking ability using the Berg Balance Scale in people with transfemoral amputation. Prosthet. Orthot. Int. 2019, 43, 396–401. [Google Scholar] [CrossRef]
- Vllasolli, T.; Zafirova, B.; Orovcanec, N.; Poposka, A.; Murtezani, A.; Krasniqi, B. Energy Expenditure and Walking Speed in Lower Limb Amputees: A Cross Sectional Study. Ortop. Traumatol. Rehabil. 2014, 16, 419–426. [Google Scholar] [CrossRef]
- Brodtkorb, T.-H.; Henriksson, M.; Johannesen-Munk, K.; Thidell, F. Cost-Effectiveness of C-Leg Compared with Non–Microprocessor-Controlled Knees: A Modeling Approach. Arch. Phys. Med. Rehabil. 2008, 89, 24–30. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Roland, M.; Torgerson, D.J. What are pragmatic trials? BMJ 1998, 316, 285. [Google Scholar] [CrossRef] [PubMed]
- Steering Committee Protocol and Pricingsystem Prosthetics (PPP)–Lower Limb. Protocol for the Provision Process of Lower Limb Prostheses. Available online: https://www.ispo.nl/protocol-verstrekkingsproces-beenprothesen (accessed on 22 August 2023).
- Resnik, L.; Borgia, M. Reliability of Outcome Measures for People with Lower-Limb Amputations: Distinguishing True Change from Statistical Error. Phys. Ther. 2011, 91, 555–565. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Modernized ICF Online Browser. 2025. Available online: https://icd.who.int/browse/2025-01/icf/en (accessed on 24 August 2023).
- Legro, M.W.; Reiber, G.D.; Smith, D.G.; del Aguila, M.; Larsen, J.; Boone, D. Prosthesis evaluation questionnaire for persons with lower limb amputations: Assessing prosthesis-related quality of life. Arch. Phys. Med. Rehabil. 1998, 79, 931–938. [Google Scholar] [CrossRef]
- Cox, P.D.; Frengopoulos, C.A.; Hunter, S.W.; Sealy, C.M.; Deathe, A.B.; Payne, M.W. Impact of Course Configuration on 6-Minute Walk Test Performance of People with Lower Extremity Amputations. Physiother. Can. 2017, 69, 197–203. [Google Scholar] [CrossRef]
- Herman, T.; Giladi, N.; Hausdorff, J.M. Properties of the ‘timed up and go’ test: More than meets the eye. Gerontology 2011, 57, 203–210. [Google Scholar] [CrossRef]
- Powell, L.E.; Myers, A.M. The Activities-specific Balance Confidence (ABC) Scale. J. Gerontol.. A. Biol. Sci. Med. Sci. 1995, 50A, M28–M34. [Google Scholar] [CrossRef]
- Post, M.W.M.; van der Zee, C.H.; Hennink, J.; Schafrat, C.G.; Visser-Meily, J.M.A.; van Berlekom, S.B. Validity of the Utrecht Scale for Evaluation of Rehabilitation-Participation. Disabil. Rehabil. 2012, 34, 478–485. [Google Scholar] [CrossRef]
- Wendel-Vos, G.C.; Schuit, A.J.; Saris, W.H.; Kromhout, D. Reproducibility and relative validity of the short questionnaire to assess health-enhancing physical activity. J. Clin. Epidemiol. 2003, 56, 1163–1169. [Google Scholar] [CrossRef]
- 2M Engineering. Activ8 Professional GEN1. 2023. Available online: https://activ8all.com/wearable-activity-tracker-portfolio/activ8-professional-gen1/ (accessed on 25 August 2023).
- Miller, W.C.; Deathe, A.B.; Speechley, M. Psychometric properties of the Activities-specific Balance Confidence Scale among individuals with a lower-limb amputation. Arch. Phys. Med. Rehabil. 2003, 84, 656–661. [Google Scholar] [CrossRef]
- Hafner, B.J.; Morgan, S.J.; Askew, R.L.; Salem, R. Psychometric evaluation of self-report outcome measures for prosthetic applications. J. Rehabil. Res. Dev. 2016, 53, 797–812. [Google Scholar] [CrossRef] [PubMed]
- Health Council of the Netherlands. Physical Activity Guidelines 2017. Available online: https://www.gezondheidsraad.nl/documenten/adviezen/2017/08/22/beweegrichtlijnen-2017 (accessed on 22 August 2023).
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Kaufman, K.R.; Bernhardt, K.A.; Symms, K. Functional assessment and satisfaction of transfemoral amputees with low mobility (FASTK2): A clinical trial of microprocessor-controlled vs. non-microprocessor-controlled knees. Clin. Biomech. 2018, 58, 116–122. [Google Scholar] [CrossRef]
- Dudek, N.L.; Marks, M.B.; Marshall, S.C.; Chardon, J.P. Dermatologic conditions associated with use of a lower-extremity prosthesis. Arch. Phys. Med. Rehabil. 2005, 86, 659–663. [Google Scholar] [CrossRef]
- Klute, G.K.; Berge, J.S.; Orendurff, M.S.; Williams, R.M.; Czerniecki, J.M. Prosthetic Intervention Effects on Activity of Lower-Extremity Amputees. Arch. Phys. Med. Rehabil. 2006, 87, 717–722. [Google Scholar] [CrossRef]
- Ettema, S.; Kal, E.; Houdijk, H. General estimates of the energy cost of walking in people with different levels and causes of lower-limb amputation: A systematic review and meta-analysis. Prosthet. Orthot. Int. 2021, 45, 417–427. [Google Scholar] [CrossRef]
- Lally, P.; van Jaarsveld, C.H.M.; Potts, H.W.W.; Wardle, J. How are habits formed: Modelling habit formation in the real world. Eur. J. Soc. Psychol. 2010, 40, 998–1009. [Google Scholar] [CrossRef]
- Kaser, S.; Radlinger, B.; Blasinger, J.; Koellenberger, N.; Streitberger, V.; Kopp, L.; Bifano, E.; Aziz, F.; Sourij, H.; Goebel, G.; et al. Non-Traumatic Lower-Limb Amputations: Outcome, Sex-Differences, Comorbidity Patterns and Temporal Trends from 2006 to 2022. J. Clin. Med. 2025, 14, 4030. [Google Scholar] [CrossRef]
- Eidmann, A.; Kamawal, Y.; Luedemann, M.; Raab, P.; Rudert, M.; Stratos, I. Demographics and Etiology for Lower Extremity Amputations—Experiences of an University Orthopaedic Center in Germany. Medicina 2023, 59, 200. [Google Scholar] [CrossRef]
- Benavent, J.V.; Igual, C.; Mora, E.; Antonio, R.; Tenias, J.M. Cross-cultural validation of the Prosthesis Evaluation Questionnaire in vascular amputees fitted with prostheses in Spain. Prosthet. Orthot. Int. 2015, 40, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.; Doherty, P.; Bjorner, J.B.; Langberg, H. Reliability and construct validity of a new Danish translation of the Prosthesis Evaluation Questionnaire in a population of Danish amputees. Prosthet. Orthot. Int. 2017, 41, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Ferriero, G.; Dughi, D.; Orlandini, D.; Moscato, T.; Nicita, D.; Franchignoni, F. Measuring long-term outcome in people with lower limb amputation: Cross-validation of the Italian versions of the Prosthetic Profile of the Amputee and Prosthesis Evaluation Questionnaire. Eura Medicophys 2005, 41, 1–6. [Google Scholar]
- Safer, V.B.; Yavuzer, G.; Demir, S.O.; Yanikoglu, I.; Guneri, F.D. The prosthesis evaluation questionnaire: Reliability and cross-validation of the Turkish version. J. Phys. Ther. Sci. 2015, 27, 1677–1680. [Google Scholar] [CrossRef] [PubMed]
- Day, S.J.; Buis, A. Cross cultural equivalence testing of the Prosthetic Evaluation Questionnaire (PEQ) for an Arabic speaking population. Prosthet. Orthot. Int. 2012, 36, 173–180. [Google Scholar] [CrossRef]
- Repo, J.P.; Piitulainen, K.; Häkkinen, A.; Roine, R.P.; Kautiainen, H.; Becker, P.; Tukiainen, E.J. Reliability and validity of the Finnish version of the prosthesis evaluation questionnaire. Disabil. Rehabil. 2017, 40, 2081–2087. [Google Scholar] [CrossRef]
- Beaton, D.E.; Bombardier, C.; Guillemin, F.; Ferraz, M.B. Guidelines for the Process of Cross-Cultural Adaptation of Self-Report Measures. Spine 2000, 25, 3186–3191. [Google Scholar] [CrossRef]
- National Health Care Institute. GIP Databank Multi-Year Table for Assistive Devices 2023. Available online: https://www.gipdatabank.nl/servicepagina/open-data (accessed on 24 August 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).