Surgeon Learning Curve for Minimally Invasive Hemiarthroplasty Using the Direct Anterior Approach for Treatment of Femoral Neck Fractures in Elderly Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Database Constitution
2.2. Ethical Considerations
2.3. Inclusion and Exclusion Criteria
2.4. Surgeon Experience Classification
2.5. Surgical Technique
2.6. Methodology for Determination of Learning Curve
2.7. Statistical Analysis
3. Results
3.1. Study Population
3.2. Surgeon Groups
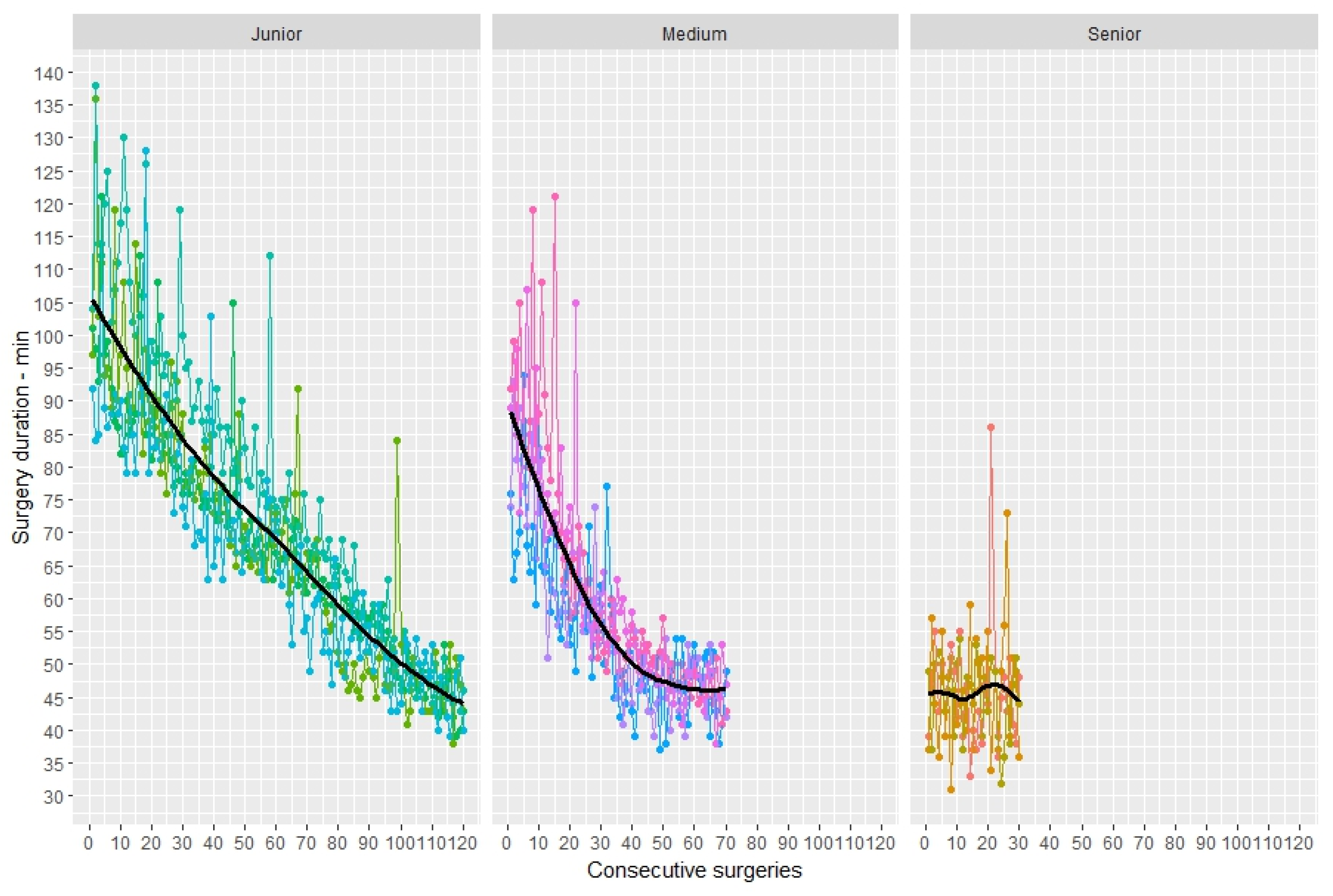
3.3. Duration of Surgery
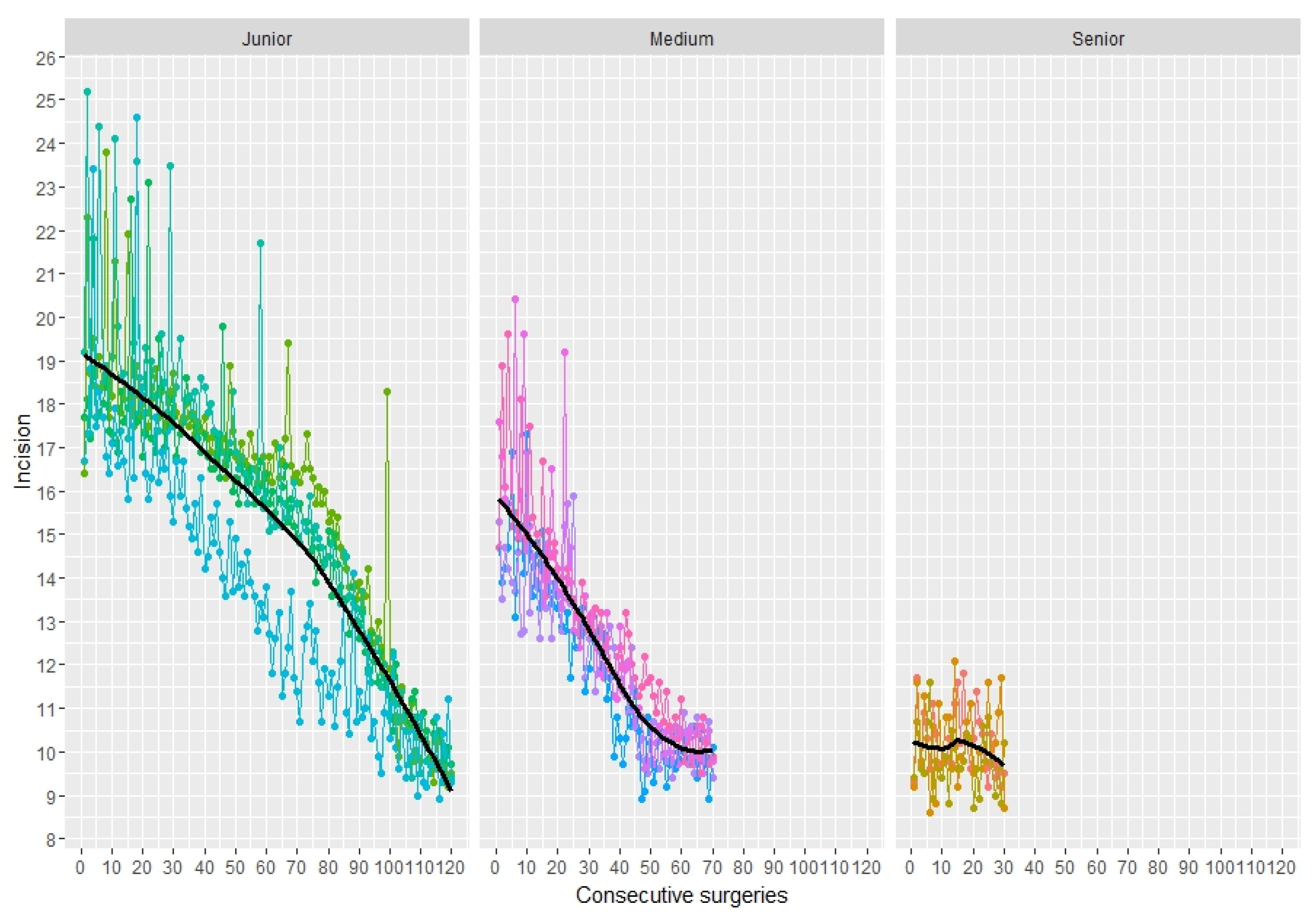
3.4. Skin Incision Length
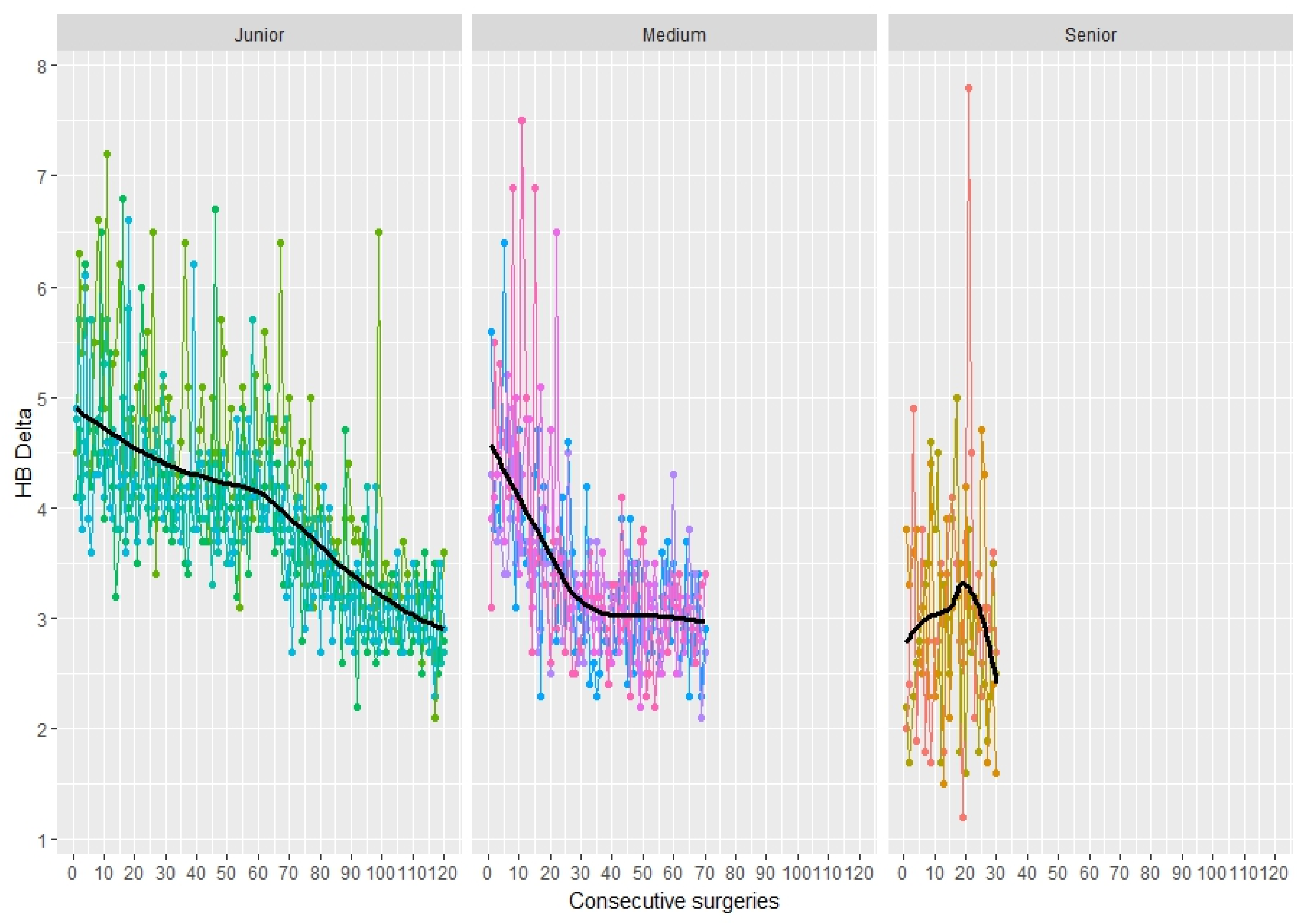
3.5. Blood Loss
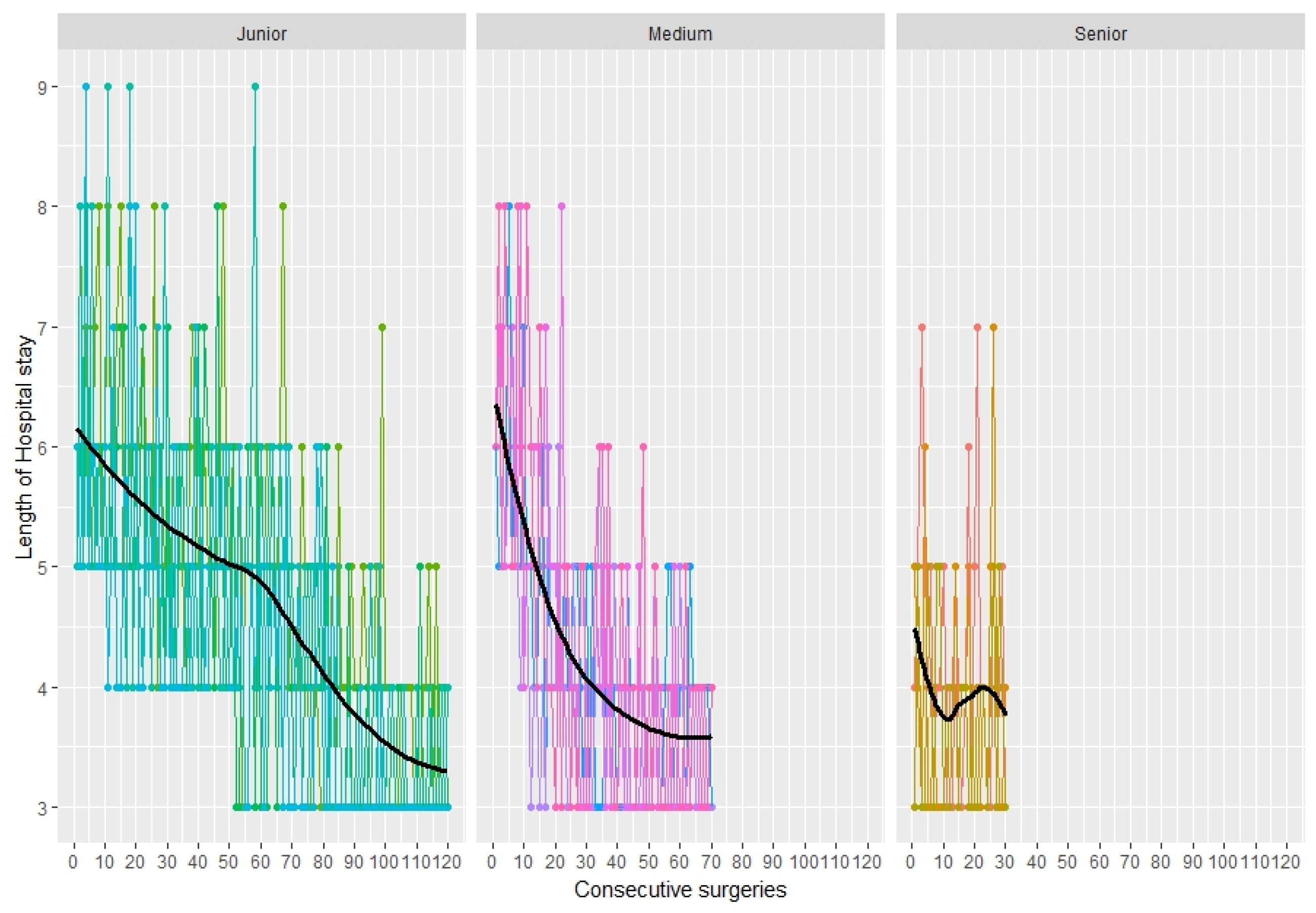
3.6. Length of Stay in Hospital
3.7. Complications
3.8. Harris Hip Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FNFs | Femoral Neck Fractures |
| HA | Hemiarthroplasty |
| DAA | Direct Anterior Approach |
| AMIS | Anterior Minimally Invasive Surgery |
| THA | Total Hip Arthroplasty |
| TFL | Tensor Fascia Lata |
| LoS | Length of Stay in Hospital |
| HHS | Harris Hip Score |
| ΔHb | Decrease in Hemoglobin Level |
| SD | Standard Deviation |
| ASA | American Society of Anesthesiologists |
| BMI | Body Mass Index |
References
- Spina, M.; Luppi, V.; Chiappi, J.; Bagnis, F.; Balsano, M. Direct anterior approach versus direct lateral approach in total hip arthroplasty and bipolar hemiarthroplasty for femoral neck fractures: A retrospective comparative study. Aging Clin. Exp. Res. 2021, 33, 1635–1644. [Google Scholar] [CrossRef]
- Kunkel, S.T.; Sabatino, M.J.; Kang, R.; Jevsevar, D.S.; Moschetti, W.E. A systematic review and meta-analysis of the direct anterior approach for hemiarthroplasty for femoral neck fracture. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 217–232. [Google Scholar] [CrossRef]
- Mallon, Z.O.; Prentice, H.A.; Schlauch, A.M.; Fasig, B.H.; Paxton, E.W.; Sadeghi, C.; Okike, K. Femoral Neck System Compared with 3 Cannulated Screws in the Treatment of Femoral Neck Fracture in Patients Aged 60 and Older: A Multicenter Registry-Based Study. J. Bone Jt. Surg. Am. 2025, 107, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.; Audigé, L.; Kuehnel, S.P.; Helmy, N. The direct anterior approach in hemiarthroplasty for displaced femoral neck fractures. Int. Orthop. 2012, 36, 1773–1781. [Google Scholar] [CrossRef]
- Lewis, D.P.; Wæver, D.; Thorninger, R.; Donnelly, W.J. Erratum to ‘Hemiarthroplasty Versus Total Hip Arthroplasty for the Management of Displaced Neck of Femur Fractures: A Systematic Review and Meta-Analysis’. J. Arthroplast. 2019, 34, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, H.; Zhang, Z.; Ma, C.; Feng, X. Comparison of bipolar hemiarthroplasty and total hip arthroplasty for displaced femoral neck fractures in the healthy elderly: A meta-analysis. BMC Musculoskelet Disord. 2015, 16, 229. [Google Scholar] [CrossRef] [PubMed]
- Saxer, F.; Studer, P.; Jakob, M.; Suhm, N.; Rosenthal, R.; Dell-Kuster, S.; Vach, W.; Bless, N. Minimally invasive anterior muscle-sparing versus a transgluteal approach for hemiarthroplasty in femoral neck fractures-a prospective randomised controlled trial including 190 elderly patients. BMC Geriatr. 2018, 18, 222. [Google Scholar] [CrossRef]
- Maccagnano, G.; Maruccia, F.; Rauseo, M.; Noia, G.; Coviello, M.; Laneve, A.; Quitadamo, A.P.; Trivellin, G.; Malavolta, M.; Pesce, V. Direct Anterior versus Lateral Approach for Femoral Neck Fracture: Role in COVID-19 Disease. J. Clin. Med. 2022, 11, 4785. [Google Scholar] [CrossRef]
- Petis, S.; Howard, J.L.; Lanting, B.L.; Vasarhelyi, E.M. Surgical approach in primary total hip arthroplasty: Anatomy, technique and clinical outcomes. Can. J. Surg. 2015, 58, 128–139. [Google Scholar] [CrossRef]
- Ait Mokhtar, M. Postero-posterolateral approach in total hip arthroplasty. Int. Orthop. 2020, 44, 2577–2585. [Google Scholar] [CrossRef]
- Hardinge, K. The Direct Lateral Approach To The Hip. J. Bone Jt. Surg. Br. Vol. 1982, 64, 17–19. [Google Scholar] [CrossRef]
- Solarino, G.; Moretti, L.; Vicenti, G.; Bizzoca, D.; Piazzolla, A.; Moretti, B. Hip hemiarthroplasty with modular neck: Is it useful in residents’ learning curve? A prospective clinical trial. HIP Int. 2020, 30, 30–36. [Google Scholar] [CrossRef]
- Graf, R.; Azizbaig Mohajer, M. The Stolzalpe technique: A modified Watson-Jones approach. Int. Orthop. 2007, 31, 21–24. [Google Scholar] [CrossRef]
- Chen, H.; Li, J.; Chang, Z.; Liang, X.; Tang, P. Treatment of femoral neck nonunion with a new fixation construct through the Watson-Jones approach. J. Orthop. Transl. 2019, 19, 126–132. [Google Scholar] [CrossRef]
- Meermans, G.; Konan, S.; Das, R.; Volpin, A.; Haddad, F.S. The direct anterior approach in total hip arthroplasty: A systematic review of the literature. Bone Jt. J. 2017, 99-B, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Nogler, M.; Randelli, F.; Macheras, G.A.; Thaler, M. Hemiarthroplasty of the hip using the direct anterior approach. Oper. Orthop. Traumatol. 2021, 33, 304–317. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, K.A.; Krez, A.N.; Anastasio, A.T. Direct anterior compared to posterior approach for hip hemiarthroplasty following femoral neck fractures. World J. Orthop. 2024, 15, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Filippini, M.; Bortoli, M.; Montanari, A.; Pace, A.; Di Prinzio, L.; Lonardo, G.; Parisi, S.C.; Persiani, V.; De Cristofaro, R.; Sambri, A.; et al. Does Surgical Approach Influence Complication Rate of Hip Hemiarthroplasty for Femoral Neck Fractures? A Literature Review and Meta-Analysis. Medicina 2023, 59, 1220. [Google Scholar] [CrossRef]
- Hansen, B.J.; Hallows, R.K.; Kelley, S.S. The Rottinger approach for total hip arthroplasty: Technique and review of the literature. Curr. Rev. Musculoskelet Med. 2011, 4, 132–138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Delanois, R.E.; Sultan, A.A.; Albayar, A.A.; Khlopas, A.; Gwam, C.U.; Sodhi, N.; Lamaj, S.; Newman, J.M.; Mont, M.A. The Röttinger approach for total hip arthroplasty: Technique, comparison to the direct lateral approach and review of literature. Ann. Transl. Med. 2017, 5, S31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gusho, C.; Hoskins, W.; Ghanem, E. A Comparison of Surgical Approaches for Hip Hemiarthroplasty Performed for the Treatment of Femoral Neck Fracture: A Systematic Review and Network Meta-analysis of Randomized Controlled Trials. JBJS Rev. 2024, 12, e24.00067. [Google Scholar] [CrossRef]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D. The CARE guidelines: Consensus-based clinical case reporting guideline development. Headache 2013, 53, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Masionis, P.; Uvarovas, V.; Mazarevičius, G.; Popov, K.; Venckus, Š.; Baužys, K.; Porvaneckas, N. The reliability of a Garden, AO and simple II stage classifications for intracapsular hip fractures. Orthop. Traumatol. Surg. Res. 2019, 105, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Horvath, B.; Kloesel, B.; Todd, M.M.; Cole, D.J.; Prielipp, R.C. Review of the ASA Physical Status Classification. Anesthesiology 2022, 135, 866–867. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T. Surgeons’ Level of Expertise. J. Wrist Surg. 2020, 9, 185. [Google Scholar] [CrossRef]
- Foissey, C.; Fauvernier, M.; Fary, C.; Servien, E.; Lustig, S.; Batailler, C. Total hip arthroplasty performed by direct anterior approach—Does experience influence the learning curve? SICOT-J 2020, 6, 15. [Google Scholar] [CrossRef]
- Burnham, R.R., Jr.; Kiernan, H.; Ortega, L.F.; Wesolowski, M.; Tauchen, A.; Russo, M.; Gerscovich, D.; Brown, N.M. Defining the Learning Curve of Anterior Total Hip Arthroplasty After Fellowship-specific Training. J. Am. Acad. Orthop. Surg. 2022, 30, e131–e138. [Google Scholar] [CrossRef]
- Wilson, M.D.; Dowsey, M.M.; Spelman, T.; Choong, P.F.M. Impact of surgical experience on outcomes in total joint arthroplasties. ANZ J. Surg. 2016, 86, 967–972. [Google Scholar] [CrossRef]
- York, P.J.; Logterman, S.L.; Hak, D.J.; Mavrogenis, A.; Mauffrey, C. Orthopaedic trauma surgeons and direct anterior total hip arthroplasty: Evaluation of learning curve at a level I academic institution. Eur. J. Orthop. Surg. Traumatol. 2017, 27, 421–424. [Google Scholar] [CrossRef]
- Carlet, A.; Buono, C.; Scaramuzzi, L.; Amendolagine, M.; Moretti, B.; Solarino, G. Efficacy of capsular repair in partial hip hemiarthroplasties: Outcome of 100 consecutive cases. J. Orthop. 2023, 15, 9–13. [Google Scholar]
- Bascom, N.; Levy, E.; Golden, T. Saddle to Incision Relationship: A Novel Approach to Localizing the Incision for Direct Anterior Hip Arthroplasty. Arthroplast. Today 2021, 10, 79–81. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, L.; Qin, J.; Hu, H.; Wei, Q. Direct anterior approach versus posterolateral approach for total hip arthroplasty in the treatment of femoral neck fractures in elderly patients: A meta-analysis and systematic review. Ann. Med. 2023, 55, 1378–1392. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bourget-Murray, J.; Horton, I.; Meniawy, S.E.; Papp, S.; Kim, P.R.; Grammatopoulos, G. The direct anterior approach is safe and shortens hospital length of stay following hemiarthroplasty for neck of femur fracture. Injury 2023, 54, 1186–1190. [Google Scholar] [CrossRef]
- Vasudevan, R.S.; Brzezinski, A.; Kaszuba, S.; Ani, L.; Essis, M.D.; Rubin, L.E. Direct anterior approach for femoral neck fractures: Why and how? J. Clin. Orthop. Trauma 2025, 67, 102995. [Google Scholar] [CrossRef]
| Characteristics | Group A | Group B | Group C | p-Value |
|---|---|---|---|---|
| Number (n) | 90 | 280 | 480 | |
| Age, years, mean (SD) | 81.6 (5.48) | 81.7 (5.83) | 81.9 (6.23) | 0.896 |
| Body mass index (kg/cm2) | 25.4 (3.90) | 25.4 (3.90) | 25.8 (4.36) | 0.706 |
| Women, n (%) | 56 (62.2%) | 158 (56.4%) | 273 (56.9%) | 0.772 |
| FNF on right side, n (%) | 36 (40.0%) | 147 (52.5%) | 255 (53.1%) | 0.068 |
| Characteristics | Group A | Group B | Group C | p-Value | A vs. B | A vs. C | B vs. C |
|---|---|---|---|---|---|---|---|
| Surgical Time, min (SD) | 45.8 (8.20) | 58.15 (15.50) | 70 (19.66) | 0.0002 | 0.0061 | <0.001 | 0.0026 |
| Skin Incision length (cm) | 10.08 (0.83) | 12.37 (2.24) | 15.03 (3.27) | <0.0001 | 0.005 | <0.0001 | 0.0008 |
| Pre-Hb (g/dL) | 12.62 (0.94) | 12.57 (0.90) | 12.60 (0.91) | 0.8913 | |||
| Post-Hb (g/dL) | 9.61 (1.08) | 9.19 (1.12) | 8.66 (1.11) | 0.0009 | 0.0377 | 0.0004 | 0.0033 |
| ΔHb (g/dL) | 3.01 (1.01) | 3.38 (0.80) | 3.93 (0.86) | ||||
| LoS (day) | 3.94 (1.00) | 4.26 (1.21) | 4.63 (1.34) | 0.0006 | 0.0441 | 0.0003 | 0.0021 |
| Outcome | Percentile | Group A | Group B | Group C |
|---|---|---|---|---|
| Duration of surgery (min) | 75th | 50 | 36 | 97 |
| 80th | 51 | 36 | 95 | |
| 85th | 52 | 35 | 87 | |
| 90th | 54 | 27 | 84 | |
| Skin incision length (cm) | 75th | 10.7 | 46 | 102 |
| 80th | 10.8 | 46 | 120 | |
| 85th | 11 | 45 | 100 | |
| 90th | 11.3 | 38 | 97 | |
| ΔHb (g/dL) | 75th | 3.5 | 13 | 75 |
| 80th | 3.8 | 9 | 47 | |
| 85th | 3.9 | 9 | 47 | |
| 90th | 4.2 | 3 | 5 | |
| LoS (day) | 75th | 5 | 12 | 16 |
| 80th | 5 | 12 | 16 | |
| 85th | 5 | 12 | 16 | |
| 90th | 5 | 12 | 16 |
| Complication | Group A | Group B | Group C |
|---|---|---|---|
| Hematoma | 5 (71.4%) | 28 (63.6%) | 61 (64.9%) |
| Fracture | 1 (14.3%) | 11 (25.0%) | 20 (21.3%) |
| Infection | 0 (0.0%) | 2 (4.5%) | 5 (5.3%) |
| Dislocation | 1 (14.3%) | 3 (6.8%) | 8 (8.5%) |
| HHS | Group A | Group B | Group C | Total | p-Value | A vs. B | A vs. C | B vs. C |
|---|---|---|---|---|---|---|---|---|
| HHS 6 mo | 80.36 (3.39) | 79.23 (2.80) | 77.84 (2.48) | 78.57 (2.83) | <0.001 | 0.00059 | <0.001 | <0.001 |
| HHS 1 y | 81.82 (2.54) | 80.94 (2.55) | 80.02 (2.74) | 80.54 (2.72) | <0.001 | 0.02078 | <0.001 | 0.00018 |
| HHS 2 y | 81.14 (2.29) | 80.97 (2.19) | 81.22 (2.25) | 81.14 (2.29) | 0.54 | 0.584 | 0.961 | 0.361 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maruccia, F.; Assaker, A.; Copetti, M.; Filoni, S.; Trivellin, G.; Perna, A.; Gorgoglione, F.; Elena, N. Surgeon Learning Curve for Minimally Invasive Hemiarthroplasty Using the Direct Anterior Approach for Treatment of Femoral Neck Fractures in Elderly Patients. Prosthesis 2025, 7, 102. https://doi.org/10.3390/prosthesis7040102
Maruccia F, Assaker A, Copetti M, Filoni S, Trivellin G, Perna A, Gorgoglione F, Elena N. Surgeon Learning Curve for Minimally Invasive Hemiarthroplasty Using the Direct Anterior Approach for Treatment of Femoral Neck Fractures in Elderly Patients. Prosthesis. 2025; 7(4):102. https://doi.org/10.3390/prosthesis7040102
Chicago/Turabian StyleMaruccia, Francesco, Assad Assaker, Massimiliano Copetti, Serena Filoni, Giacomo Trivellin, Andrea Perna, Franco Gorgoglione, and Nicholas Elena. 2025. "Surgeon Learning Curve for Minimally Invasive Hemiarthroplasty Using the Direct Anterior Approach for Treatment of Femoral Neck Fractures in Elderly Patients" Prosthesis 7, no. 4: 102. https://doi.org/10.3390/prosthesis7040102
APA StyleMaruccia, F., Assaker, A., Copetti, M., Filoni, S., Trivellin, G., Perna, A., Gorgoglione, F., & Elena, N. (2025). Surgeon Learning Curve for Minimally Invasive Hemiarthroplasty Using the Direct Anterior Approach for Treatment of Femoral Neck Fractures in Elderly Patients. Prosthesis, 7(4), 102. https://doi.org/10.3390/prosthesis7040102







