The Use of PEEK Barriers in Bone Regeneration Procedures: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Population–Context–Concept Framework
- Population: Human adults (aged ≥ 18) with vertical and/or horizontal bone resorption in the maxillofacial area or animals with bone defects, with the indication of bone augmentation techniques.
- Context: Laboratories, universities, dental clinics, and hospitals.
- Concept: Vertical and/or horizontal bone formation using PEEK scaffolds as a barrier in bone augmentation procedures.
2.2. Eligibility Criteria
- Human studies: (1) all primary studies in adult humans, including clinical (i.e., randomized clinical trials (RCTs)), prospective and retrospective cohort studies, case–control studies, case series and case reports reporting the use of PEEK scaffolds as a barrier in bone augmentation procedures in the maxillofacial area; (2) studies reporting the area of use and time of removal of the PEEK barriers; (3) studies reporting the surgical protocol of placement, including the use of grafts and their composition; and (4) studies reporting possible complications.
- Animal studies: (1) studies reporting the use of PEEK scaffolds as barriers in augmentation procedures or bone regeneration of defects and (2) studies reporting the number of specimens, defect area, characteristics of PEEK barriers, filler material, and postoperative endpoint.
2.3. Information Sources and Search Strategy
2.4. Study Selection/Screening
2.5. Data Extraction
2.6. Quality Assessment
3. Results
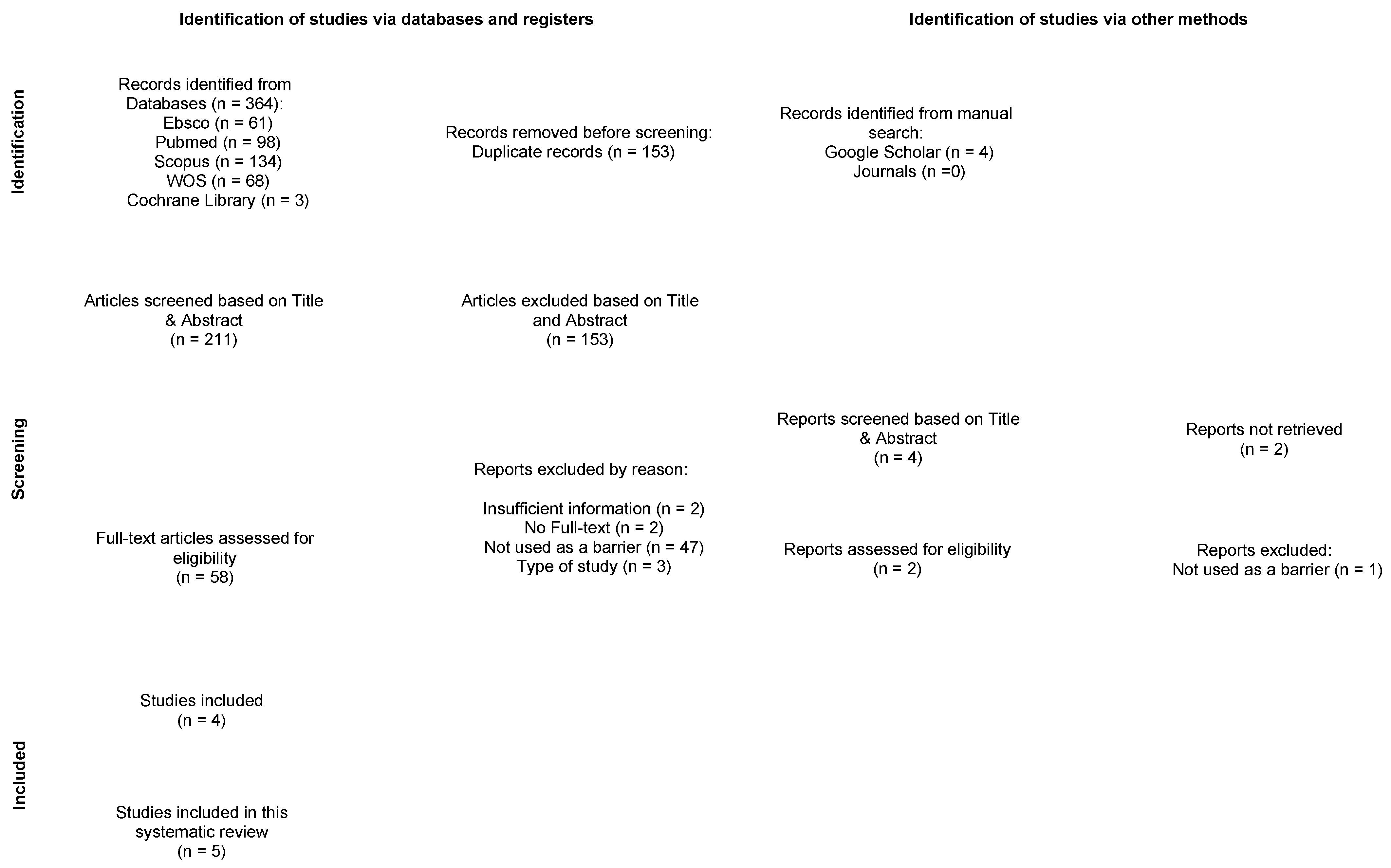
3.1. Study Selection
3.2. Characteristics of the Included Studies, Samples, and Bone Defects
3.3. Design, Manufacture, and Cleaning of PEEK Barriers
3.4. Characteristics of Bone Grafts, Fixation Methods, and Presence of Bone Perforations
3.5. Radiographic Analysis of Bone Formation
3.6. Histological Analysis
3.7. Complications
3.8. Biomechanical and Gene Expression Analysis
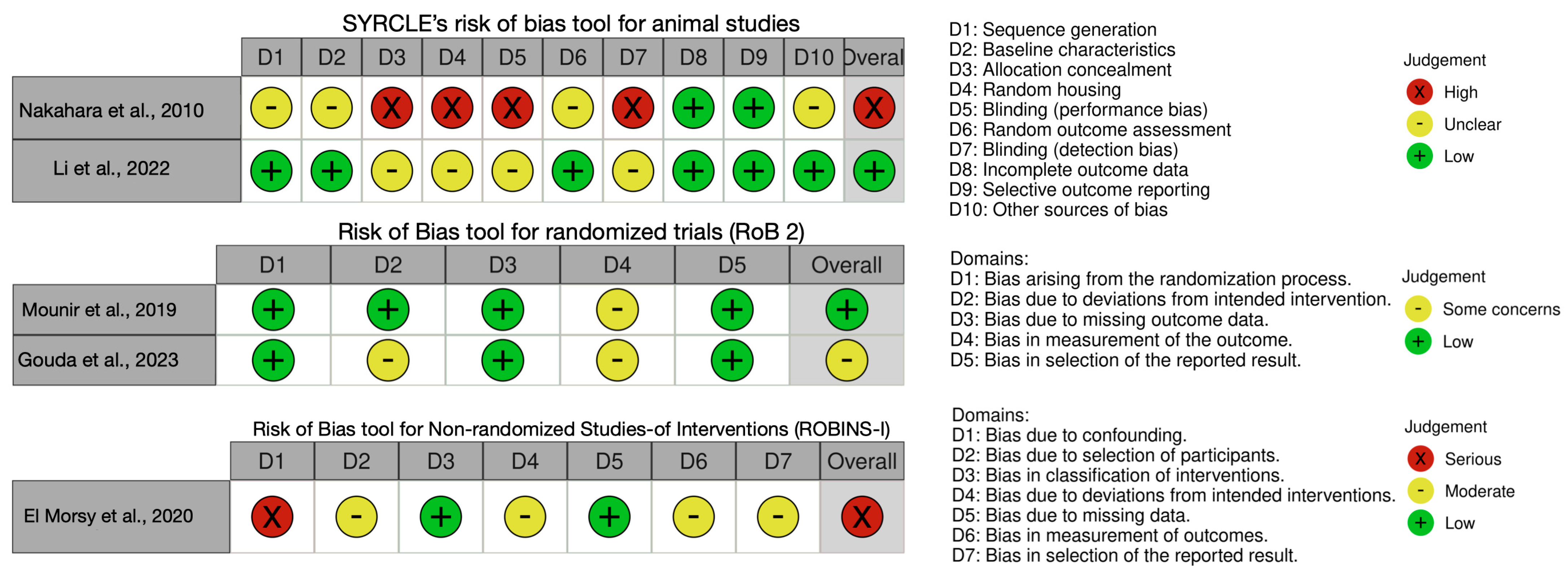
3.9. Risk of Bias Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological Principle and Therapeutic Applications. Clin. Oral Implant. Res. 2010, 21, 567–576. [Google Scholar] [CrossRef]
- Sanz, M.; Dahlin, C.; Apatzidou, D.; Artzi, Z.; Bozic, D.; Calciolari, E.; De Bruyn, H.; Dommisch, H.; Donos, N.; Eickholz, P.; et al. Biomaterials and Regenerative Technologies Used in Bone Regeneration in the Craniomaxillofacial Region: Consensus Report of Group 2 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46 (Suppl. S21), 82–91. [Google Scholar] [CrossRef]
- Uriarte, X.; Landázuri, A.; Marão, H.F.; Lucena, N.; Schiegnitz, E.; Díaz, L. Zirconia Barriers in Bone Regeneration Procedures: A Scoping Review. Clin. Oral Implants Res. 2025, 36, 411–422. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, J.; Du, M.; Zhang, S.; Liu, Y.; Yang, H.; Shi, R.; Guo, Y.; Song, F.; Zhao, Y.; et al. Customized Barrier Membrane (Titanium Alloy, Poly Ether-Ether Ketone and Unsintered Hydroxyapatite/Poly-l-Lactide) for Guided Bone Regeneration. Front. Bioeng. Biotechnol. 2022, 10, 916967. [Google Scholar] [CrossRef]
- Panayotov, I.V.; Orti, V.; Cuisinier, F.; Yachouh, J. Polyetheretherketone (PEEK) for Medical Applications. J. Mater. Sci. Mater. Med. 2016, 27, 118. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Devine, J.N. PEEK Biomaterials in Trauma, Orthopedic, and Spinal Implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [PubMed]
- Papia, E.; Brodde, S.A.C.; Becktor, J.P. Deformation of Polyetheretherketone, PEEK, with Different Thicknesses. J. Mech. Behav. Biomed. Mater. 2022, 125, 104928. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Khurshid, Z.; Matinlinna, J.P.; Siddiqui, F.; Nassani, M.Z.; Baroudi, K. Nanomodified Peek Dental Implants: Bioactive Composites and Surface Modification-A Review. Int. J. Dent. 2015, 2015, 381759. [Google Scholar] [CrossRef]
- Mounir, M.; Atef, M.; Abou-Elfetouh, A.; Hakam, M.M. Titanium and Polyether Ether Ketone (PEEK) Patient-Specific Sub-Periosteal Implants: Two Novel Approaches for Rehabilitation of the Severely Atrophic Anterior Maxillary Ridge. Int. J. Oral Maxillofac. Surg. 2018, 47, 658–664. [Google Scholar] [CrossRef]
- Uysal, I.; Tezcaner, A.; Evis, Z. Methods to Improve Antibacterial Properties of PEEK: A Review. Biomed. Mater. 2024, 19. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Liu, P.; Zhang, X.; Xin, J.; Wang, Y.; Zou, X.; Mei, X.; Zhang, S.; Zhang, S. Strategies to Improve Bioactive and Antibacterial Properties of Polyetheretherketone (PEEK) for Use as Orthopedic Implants. Mater. Today Bio 2022, 16, 100402. [Google Scholar] [CrossRef] [PubMed]
- Felice, P.; Pistilli, R.; Pellegrino, G.; Bonifazi, L.; Tayeb, S.; Simion, M.; Barausse, C. A Randomised Controlled Trial Comparing the Effectiveness of Guided Bone Regeneration with Polytetrafluoroethylene Titanium-Reinforced Membranes, CAD/CAM Semi-Occlusive Titanium Meshes and CAD/CAM Occlusive Titanium Foils in Partially Atrophic Arches. Int. J. Oral Implantol. 2024, 17, 285–296. [Google Scholar]
- Rocchietta, I.; Moreno, F.; Nisand, D. Management of Complications in Anterior Maxilla During Guided Bone Regeneration. In Bone Augmentation by Anatomical Region; Wiley: Hoboken, NJ, USA, 2020; pp. 235–254. [Google Scholar]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Nakahara, H.; Misawa, H.; Yoshida, A.; Hayashi, T.; Tanaka, M.; Furumatsu, T.; Tanaka, N.; Kobayashi, N.; Ozaki, T. Bone Repair Using a Hybrid Scaffold of Self-Assembling Peptide PuraMatrix and Polyetheretherketone Cage in Rats. Cell Transpl. 2010, 19, 791–797. [Google Scholar] [CrossRef]
- Li, L.; Gao, H.; Wang, C.; Ji, P.; Huang, Y.; Wang, C. Assessment of Customized Alveolar Bone Augmentation Using Titanium Scaffolds vs Polyetheretherketone (PEEK) Scaffolds: A Comparative Study Based on 3D Printing Technology. ACS Biomater. Sci. Eng. 2022, 8, 2028–2039. [Google Scholar] [CrossRef] [PubMed]
- Mounir, M.; Shalash, M.; Mounir, S.; Nassar, Y.; El Khatib, O. Assessment of Three Dimensional Bone Augmentation of Severely Atrophied Maxillary Alveolar Ridges Using Prebent Titanium Mesh vs Customized Poly-Ether-Ether-Ketone (PEEK) Mesh: A Randomized Clinical Trial. Clin. Implant Dent. Relat. Res. 2019, 21, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Gouda, A.; Mounir, M.; Noureldin, N.; El Khatib, O.; Mounir, S. Guided Bone Regeneration Using Patient Specific Titanium vs. Polyether-Ether Keton (PEEK) Meshes for Horizontal Maxillary Ridge Augmentation: A Histomorphometric Study. Egypt. Dent. J. 2023, 69, 75–84. [Google Scholar] [CrossRef]
- El Morsy, O.A.; Barakat, A.; Mekhemer, S.; Mounir, M. Assessment of 3-Dimensional Bone Augmentation of Severely Atrophied Maxillary Alveolar Ridges Using Patient-Specific Poly Ether-Ether Ketone (PEEK) Sheets. Clin. Implant Dent. Relat. Res. 2020, 22, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Nanba, K.; Murai, S. Effects of Bioabsorbable and Non-Resorbable Barrier Membranes on Bone Augmentation in Rabbit Calvaria. J. Periodontol. 1998, 69, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Naenni, N.; Schneider, D.; Jung, R.E.; Hüsler, J.; Hämmerle, C.H.F.; Thoma, D.S. Randomized Clinical Study Assessing Two Membranes for Guided Bone Regeneration of Peri-Implant Bone Defects: Clinical and Histological Outcomes at 6 Months. Clin. Oral Implant. Res. 2017, 28, 1309–1317. [Google Scholar] [CrossRef]
- Mandelli, F.; Traini, T.; Ghensi, P. Customized-3D Zirconia Barriers for Guided Bone Regeneration (GBR): Clinical and Histological Findings from a Proof-of-Concept Case Series. J. Dent. 2021, 114, 103780. [Google Scholar] [CrossRef] [PubMed]
- Schliephake, H.; Dard, M.; Planck, H.; Hierlemann, H.; Jakob, A. Guided Bone Regeneration around Endosseous Implants Using a Resorbable Membrane vs a PTFE Membrane. Clin. Oral Implants Res. 2000, 11, 230–241. [Google Scholar] [CrossRef]
- Buser, D.; Urban, I.; Monje, A.; Kunrath, M.F.; Dahlin, C. Guided Bone Regeneration in Implant Dentistry: Basic Principle, Progress over 35 Years, and Recent Research Activities. Periodontol. 2000 2023, 93, 9–25. [Google Scholar] [CrossRef]
- Jung, G.-U.; Jeon, J.-Y.; Hwang, K.-G.; Park, C.-J. Preliminary Evaluation of a Three-Dimensional, Customized, and Preformed Titanium Mesh in Peri-Implant Alveolar Bone Regeneration. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 181–187. [Google Scholar] [CrossRef]
- Sumida, T.; Otawa, N.; Kamata, Y.U.; Kamakura, S.; Mtsushita, T.; Kitagaki, H.; Mori, S.; Sasaki, K.; Fujibayashi, S.; Takemoto, M.; et al. Custom-Made Titanium Devices as Membranes for Bone Augmentation in Implant Treatment: Clinical Application and the Comparison with Conventional Titanium Mesh. J. Craniomaxillofac. Surg. 2015, 43, 2183–2188. [Google Scholar] [CrossRef]
- Oberoi, G.; Nitsch, S.; Edelmayer, M.; Janjić, K.; Müller, A.S.; Agis, H. 3D Printing-Encompassing the Facets of Dentistry. Front. Bioeng. Biotechnol. 2018, 6, 172. [Google Scholar] [CrossRef]
- Vaquette, C.; Mitchell, J.; Ivanovski, S. Recent Advances in Vertical Alveolar Bone Augmentation Using Additive Manufacturing Technologies. Front. Bioeng. Biotechnol. 2021, 9, 798393. [Google Scholar] [CrossRef]
- Ikawa, T.; Shigeta, Y.; Hirabayashi, R.; Hirai, S.; Hirai, K.; Harada, N.; Kawamura, N.; Ogawa, T. Computer Assisted Mandibular Reconstruction Using a Custom-Made Titan Mesh Tray and Removable Denture Based on the Top-down Treatment Technique. J. Prosthodont. Res. 2016, 60, 321–331. [Google Scholar] [CrossRef]
- Malmström, J.; Anderud, J.; Abrahamsson, P.; Wälivaara, D.-Å.; Isaksson, S.G.; Adolfsson, E. Guided Bone Regeneration Using Individualized Ceramic Sheets. Int. J. Oral Maxillofac. Surg. 2016, 45, 1246–1252. [Google Scholar] [CrossRef]
- Souidan, A.; Ayad, S.; Sweedan, A. Augmentation of the Anterior Maxillary Bony Defects Using Custom-Made Zirconia Membranes (A Case Series). Alex. Dent. J. 2022, 48, 35–43. [Google Scholar] [CrossRef]
- Schwitalla, A.; Müller, W.-D. PEEK Dental Implants: A Review of the Literature. J. Oral Implant. 2013, 39, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, X.; Zhang, W.; Wang, L.; Zhu, C.; Huang, Y.; Chen, R.; Chen, X.; Wang, M.; Pan, G.; et al. Bioclickable Mussel-Derived Peptides With Immunoregulation for Osseointegration of PEEK. Front. Bioeng. Biotechnol. 2021, 9, 780609. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Boyce, J.M.; Weber, D.J. Disinfection, Sterilization and Antisepsis: An Overview. Am. J. Infect. Control 2023, 51, A3–A12. [Google Scholar] [CrossRef]
- Savaris, M.; Carvalho, G.A.; Falavigna, A.; dos Santos, V.; Brandalise, R.N. Chemical and Thermal Evaluation of Commercial and Medical Grade PEEK Sterilization by Ethylene Oxide. Mater. Res. 2016, 19, 807–811. [Google Scholar] [CrossRef]
- Gutta, R.; Baker, R.A.; Bartolucci, A.A.; Louis, P.J. Barrier Membranes Used for Ridge Augmentation: Is There an Optimal Pore Size? J. Oral Maxillofac. Surg. 2009, 67, 1218–1225. [Google Scholar] [CrossRef]
- Zellin, G.; Linde, A. Effects of Different Osteopromotive Membrane Porosities on Experimental Bone Neogenesis in Rats. Biomaterials 1996, 17, 695–702. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Jiang, H.B.; Ryu, J.-H.; Kang, H.; Kim, K.-M.; Kwon, J.-S. Comparing Properties of Variable Pore-Sized 3D-Printed PLA Membrane with Conventional PLA Membrane for Guided Bone/Tissue Regeneration. Materials 2019, 12, 1718. [Google Scholar] [CrossRef] [PubMed]
| Article and Country | Study Design | Samples and Gender | Mean Age (Range) | Bone Defect Area | PEEK Barrier Characteristics | Bone Perforations | Filling Material | Cover Material | Postoperative Endpoint/ Removal Time | Bone Gained | Complications |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nakahara et al., 2010 [19] (Japan) | Animal model (in vivo study) | 30 female Wistar rats | 10 weeks | Left femur (n = 30) | PEEK scaffolds with a tubular structure Outer diameter = 5 mm Inner diameter = 3 mm Height = 5 mm Perforations = 4 (3 × 1.5 mm) Thickness = NR Fixation method = external fixator and four pins of 1.4 mm in diameter | No | G1: Peptide hydrogel (n = 10) G2: Autologous bone (n = 10) G3: Empty (n = 10) | No | 28 days | Average bridging ratios of bone defect: - G1: 78.9 ± 11.8% - G2: 96.5 ± 4.7% - G3: 29.5 ± 9.7% | NR |
| Li et al., 2022 [20] (China) | Animal model (in vivo study) | 3 Beagle dogs | 2 years | Mandible (bilateral premolars and first molars 7 × 7 mm defects) (n = 18) | PEEK barriers with a bone defect shape Perforations = 2.0 mm Thickness = 0.6 mm Fixation method = external fixator and 4 pins of 1.4 mm in diameter | No | G1: PEEK barrier with calcined bovine bone + autogenous bone (n = 6) G2: Titanium mesh with autologous bone (n = 6) G3: Collagen pericardium membrane and calcined bovine bone + autogenous bone (n = 6) | No | 3 months | Regenerated bone: - G1: 50.18 ± 7.26% - G2: 52.62 ± 3.61% - G3: 41.90 ± 5.20% Alveolar bone height: - G1: 0.29 ± 0.31 mm - G2: 0.28 ± 0.32 mm - G3: 0.99 ± 0.21 mm | NR |
| Mounir et al., 2019 [21] (Egypt) | Human study (RCT) | G1: 8 patients (6M/2F) G2: 8 patients (4M/4F) | G1: 38.0 years G2: 39.0 years | Severely atrophied anterior maxillary alveolar ridges <6 mm in height <2 mm in width | PEEK barriers with a bone defect shape Perforations = Yes (P/B) Thickness = 2 mm Fixation method = 3–4 fixation screws | Yes | G1: Titanium mesh with a 50:50 mixture of autogenous (IC) and xenogenic bone (n = 8). G2: PEEK barrier with 50:50 autogenous bone (IC) and xenogenic bone (n = 8). | Yes (collagen membrane) | 6 months | Three-dimensional bone gain: G1: 20.9 ± 13.3% G1: 31.8 ± 22.7% | G1: 1 case (mesh exposure) G2: 1 case (barrier exposure) |
| Gouda et al., 2023 [22] (Egypt) | Human study (RCT) | 8 patients (NR) | NR | Unilateral severe bone defect in the maxilla <6 mm in height. <2 mm in width | PEEK barriers with a bone defect shape Perforations: Yes (P/B) Thickness = 2 mm Fixation method: 3–4 micro titanium screws. | Yes | G1: Titanium mesh with 50:50 mixture of autogenous bone (IC) + xenogenic bone (n = 8). G2: PEEK barrier with 50:50 mixture of autogenous bone (IC) + xenogenic bone (n = 8). | Yes (collagen membrane) | 6 months | Percentage of newly formed bone: G1: 26.25 ± 4.35% G2: 19.5 ± 2.38% . | G1: 1 case (mesh exposure) G2: 1 case (barrier exposure) |
| El Morsy et al., 2020 [23] (Egypt) | Human study (Case series) | 14 patients (5M/9F) | 29.0 | Unilateral severe bone defect in the maxilla <6 mm in height <2 mm in width | PEEK barriers with a bone defect shape Perforations: No Thickness = NR Fixation method: micro titanium screws | Yes | PEEK barrier with 50:50 autogenous bone (chin or retromolar region) + xenogenic bone (n = 14). | No | 6 months | Average horizontal bone gain: 3.42 ± 1.10 mm. Average vertical bone gain: 3.47 ± 1.46 mm. | 1 case (barrier exposure). 1 case (very poor quality of bone and massive fibrointegration) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, L.; Uriarte, X.; Landázuri, A.; Marāo, H.F.; Urrutia, P.; Torres, A.; Fan, S. The Use of PEEK Barriers in Bone Regeneration Procedures: A Scoping Review. Prosthesis 2025, 7, 101. https://doi.org/10.3390/prosthesis7040101
Díaz L, Uriarte X, Landázuri A, Marāo HF, Urrutia P, Torres A, Fan S. The Use of PEEK Barriers in Bone Regeneration Procedures: A Scoping Review. Prosthesis. 2025; 7(4):101. https://doi.org/10.3390/prosthesis7040101
Chicago/Turabian StyleDíaz, Leonardo, Xavier Uriarte, Andrés Landázuri, Heloisa Fonseca Marāo, Pablo Urrutia, Alfredo Torres, and Shengchi Fan. 2025. "The Use of PEEK Barriers in Bone Regeneration Procedures: A Scoping Review" Prosthesis 7, no. 4: 101. https://doi.org/10.3390/prosthesis7040101
APA StyleDíaz, L., Uriarte, X., Landázuri, A., Marāo, H. F., Urrutia, P., Torres, A., & Fan, S. (2025). The Use of PEEK Barriers in Bone Regeneration Procedures: A Scoping Review. Prosthesis, 7(4), 101. https://doi.org/10.3390/prosthesis7040101